In Situ Monitoring of Drug Precipitation from Digesting Lipid Formulations Using Low-Frequency Raman Scattering Spectroscopy
Abstract
1. Introduction
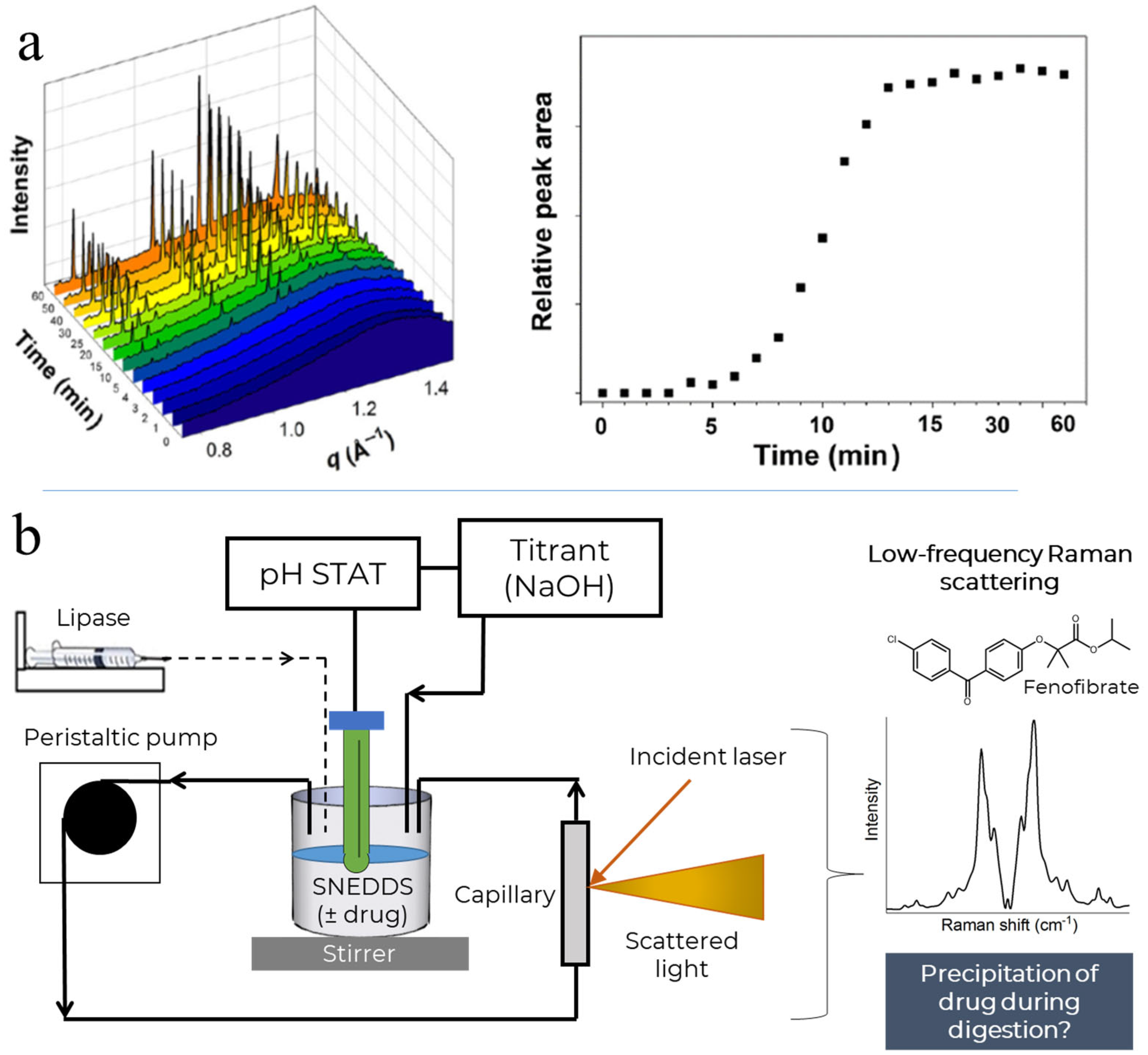
2. Materials and Methods
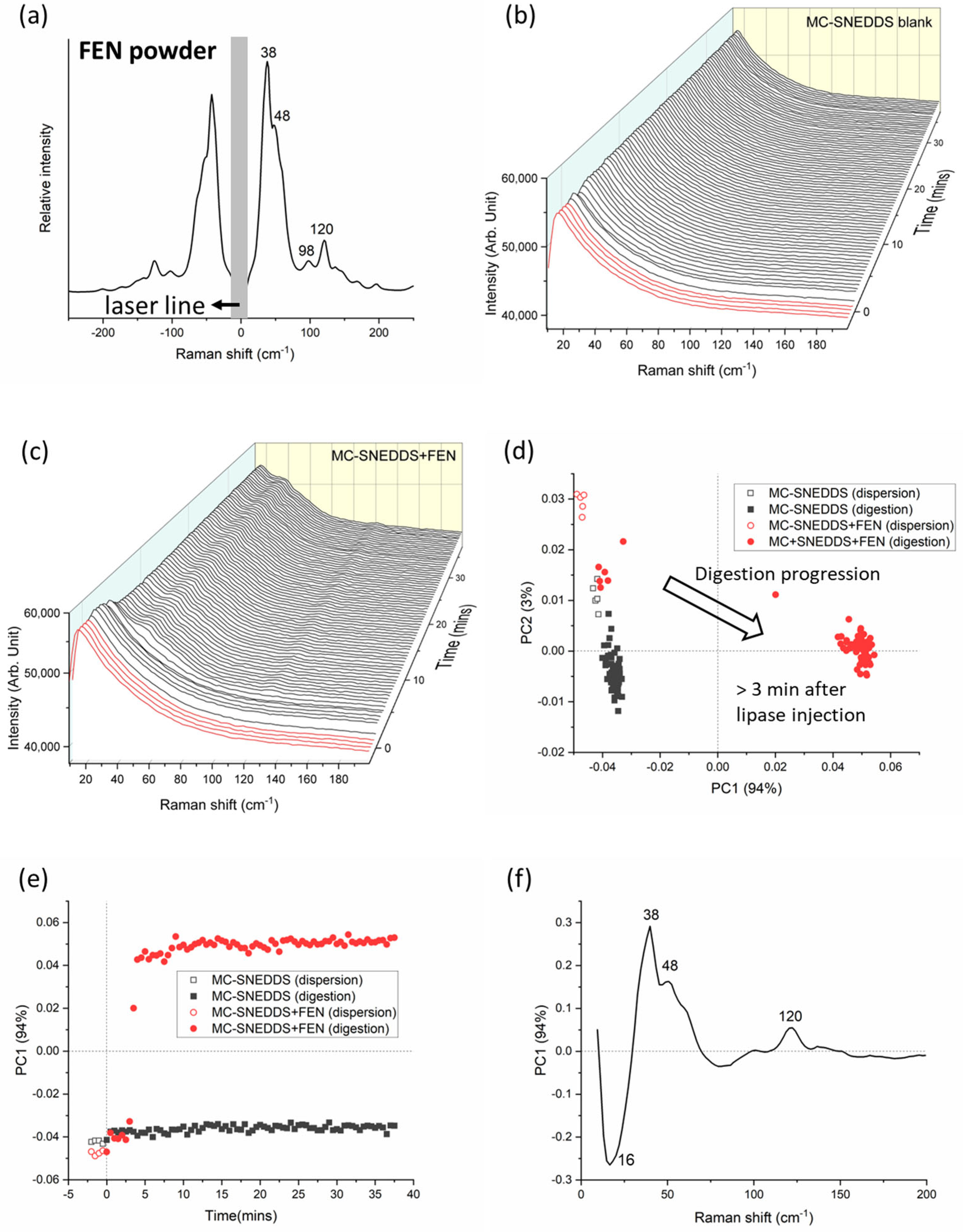
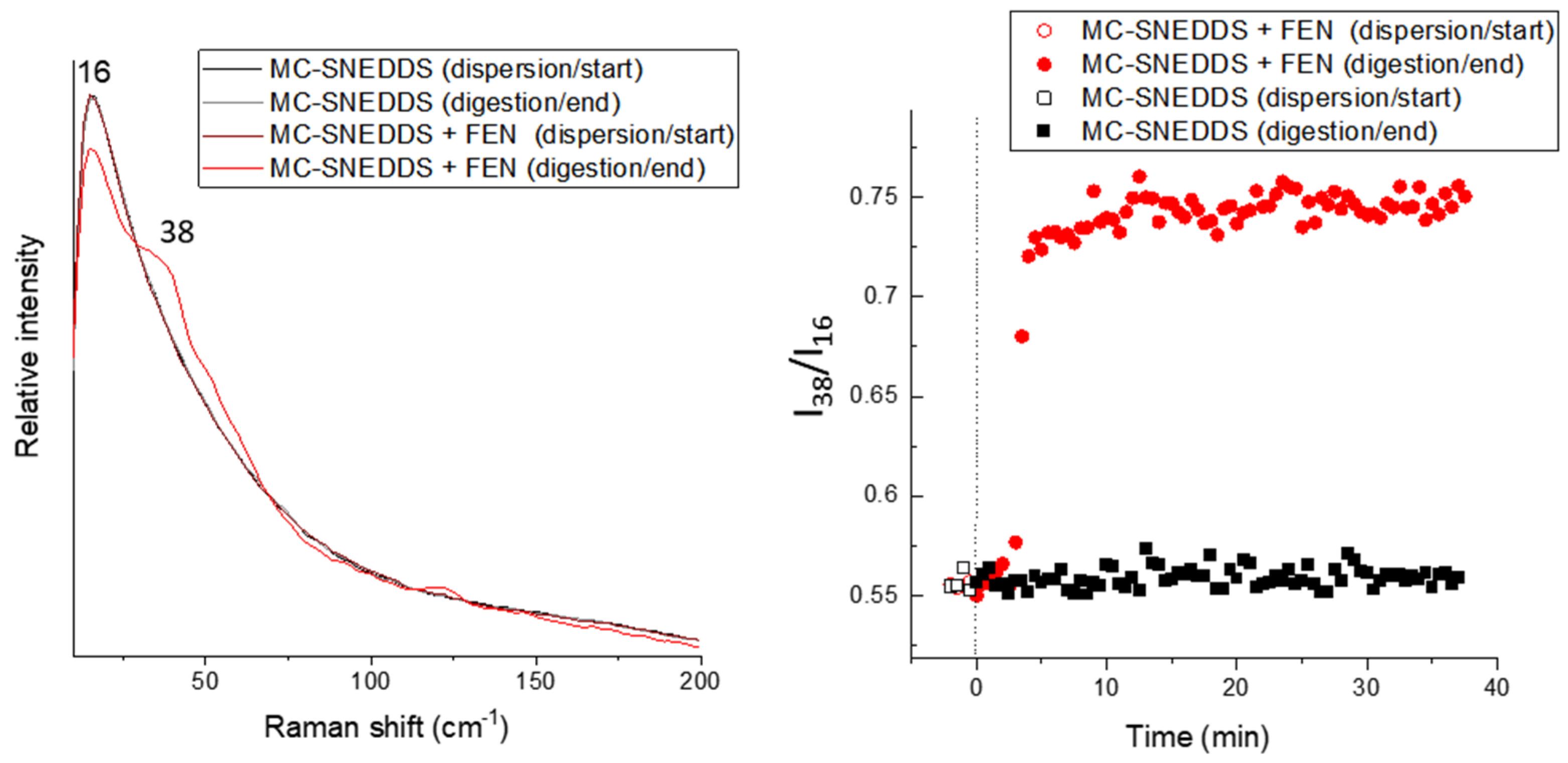
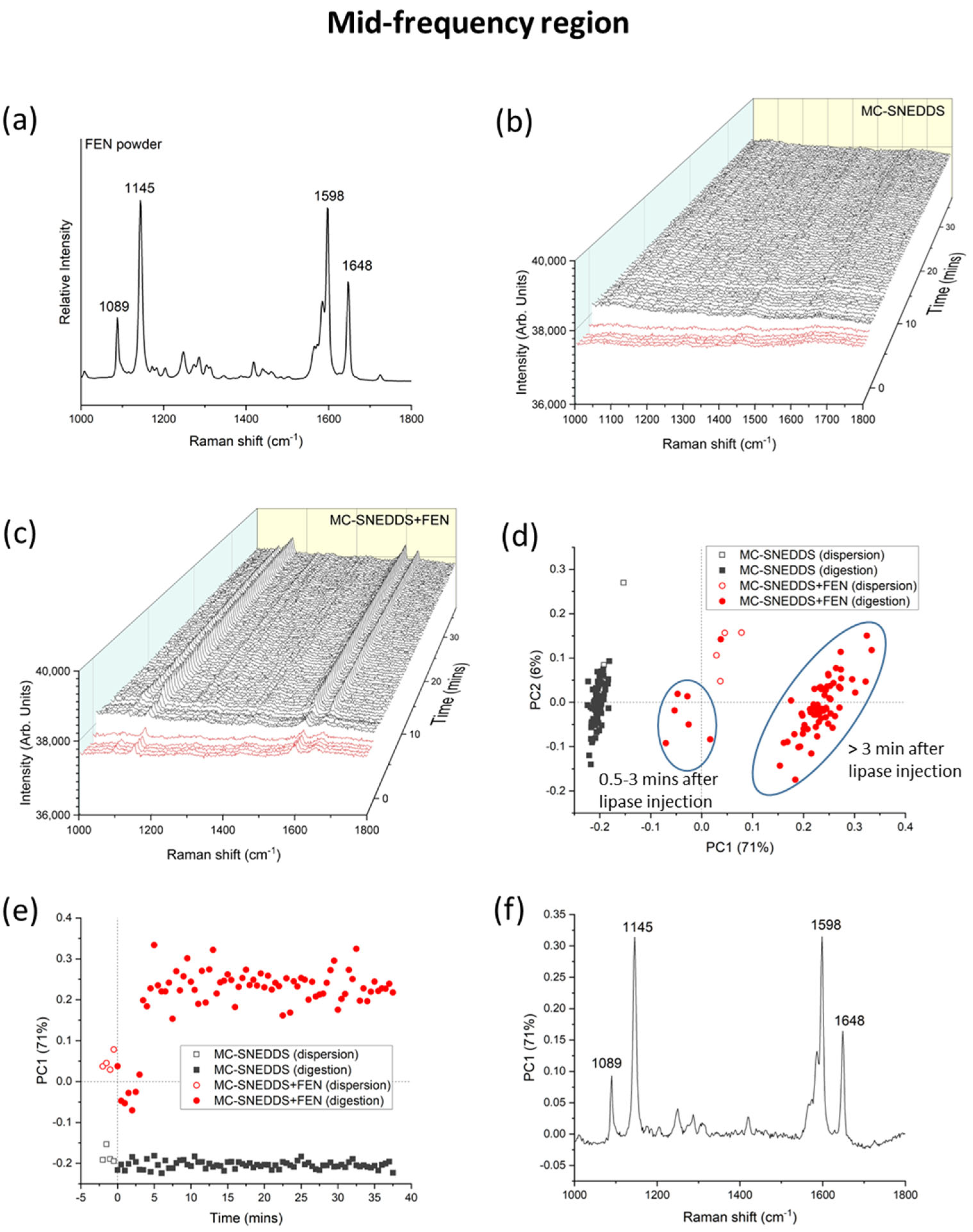
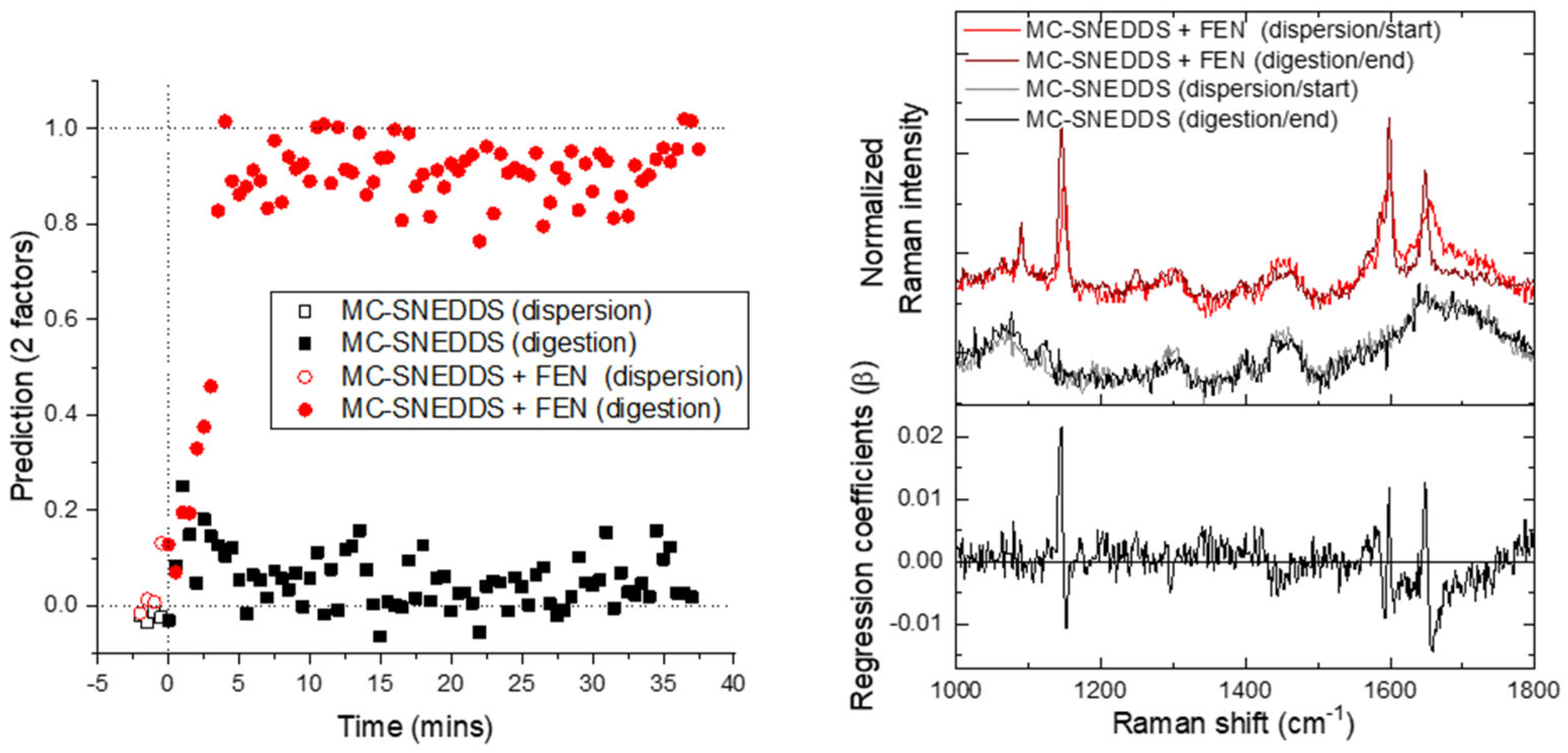
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauss, D.J. (Ed.) Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs. In Drugs and the Pharmaceutical Sciences; Informa Healthcare: Boca Raton, FL, USA, 2007; Volume 170. [Google Scholar]
- Siqueira, S.D.; Müllertz, A.; Gräeser, K.; Kasten, G.; Mu, H.; Rades, T. Influence of drug load and physical form of cinnarizine in new SNEDDS dosing regimens: In vivo and in vitro evaluations. AAPS J. 2017, 19, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Deen, G.R.; Arleth, L.; Bergenstahl, B.; Nielsen, F.S.; Pedersen, J.S.; Mullertz, A. Structural development of self nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small-angle X-ray scattering. Pharm. Res. 2007, 24, 1844–1853. [Google Scholar] [CrossRef]
- Warren, D.B.; Anby, M.U.; Hawley, A.; Boyd, B.J. Real Time Evolution of Liquid Crystalline Nanostructure during the Digestion of Formulation Lipids Using Synchrotron Small-Angle X-ray Scattering. Langmuir 2011, 27, 9528–9534. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Salim, M.; Clulow, A.J.; Ramirez, G.; Pham, A.C.; Hawley, A. The impact of digestion is essential to the understanding of milk as a drug delivery system for poorly water soluble drugs. J. Control. Release 2018, 292, 13–17. [Google Scholar] [CrossRef]
- Thomas, N.; Holm, R.; Garmer, M.; Karlsson, J.J.; Müllertz, A.; Rades, T. Supersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013, 15, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sassene, P.J.; Knopp, M.M.; Hesselkilde, J.Z.; Koradia, V.; Larsen, A.; Rades, T.; Müllertz, A. Precipitation of a Poorly Soluble Model Drug during In Vitro Lipolysis: Characterization and Dissolution of the Precipitate. J. Pharm. Sci. 2010, 99, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Richter, K.; Pedersen, T.B.; Holm, R.; Müllertz, A.; Rades, T. In vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014, 16, 539–549. [Google Scholar] [CrossRef]
- Khan, J.; Hawley, A.; Rades, T.; Boyd, B.J. In Situ Lipolysis and Synchrotron Small-Angle X-ray Scattering for the Direct Determination of the Precipitation and Solid-State Form of a Poorly Water-Soluble Drug During Digestion of a Lipid-Based Formulation. J. Pharm. Sci. 2016, 105, 2631–2639. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Gao, Y.; Zhou, D.; Mo, H.; Zhang, G.G.; Taylor, L.S. Dissolution and precipitation behavior of amorphous solid dispersions. J. Pharm. Sci. 2011, 100, 3316–3331. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Improved Oral Bioavailability of Chlorpromazine: In Vitro and In Vivo Evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Brittain, H.G.; Suryanarayanan, R. Thermoanalytical and crystallographic methods. In Polymorphism in Pharmaceutical Solids; CRC Press: Boca Raton, FL, USA, 2018; pp. 330–358. [Google Scholar]
- Tishmack, P.A. Solid-State Nuclear Magnetic Resonance Spectroscopy. In Polymorphism in Pharmaceutical Solids; CRC Press: Boca Raton, FL, USA, 2018; pp. 393–447. [Google Scholar]
- Salim, M.; Fraser-Miller, S.J.; Bērziņš, K.; Sutton, J.J.; Ramirez, G.; Clulow, A.J.; Hawley, A.; Beilles, S.; Gordon, K.C.; Boyd, B.J. Low-Frequency Raman Scattering Spectroscopy as an Accessible Approach to Understand Drug Solubilization in Milk-Based Formulations during Digestion. Mol. Pharm. 2020, 17, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Ramirez, G.; Clulow, A.J.; Hawley, A.; Boyd, B.J. Implications of the Digestion of Milk-Based Formulations for the Solubilization of Lopinavir/Ritonavir in a Combination Therapy. Mol. Pharm. 2023, 20, 2256–2265. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Rades, T.; Gordon, K.C. Low-Frequency Raman Spectroscopy as an Avenue to Determine the Transition Temperature of β- and γ-Relaxation in Pharmaceutical Glasses. Anal. Chem. 2022, 94, 8241–8248. [Google Scholar] [CrossRef]
- Bērziņš, K.; Sales, R.E.; Barnsley, J.E.; Walker, G.; Fraser-Miller, S.J.; Gordon, K.C. Low-wavenumber Raman spectral database of pharmaceutical excipients. Vib. Spectrosc. 2020, 107, 103021. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Rades, T.; Gordon, K.C. Low-Frequency Raman Spectroscopic Study on Compression-Induced Destabilization in Melt-Quenched Amorphous Celecoxib. Mol. Pharm. 2019, 16, 3678–3686. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Fraser-Miller, S.J.; Bērziņš, K.; Okeyo, P.O.; Rantanen, J.; Rades, T.; Gordon, K.C. Monitoring the Isothermal Dehydration of Crystalline Hydrates Using Low-Frequency Raman Spectroscopy. Mol. Pharm. 2021, 18, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C. Recent advances in low-frequency Raman spectroscopy for pharmaceutical applications. Int. J. Pharm. 2021, 592, 120034. [Google Scholar] [CrossRef]
- Mah, P.T.; Fraser, S.J.; Reish, M.E.; Rades, T.; Gordon, K.C.; Strachan, C.J. Use of low-frequency Raman spectroscopy and chemometrics for the quantification of crystallinity in amorphous griseofulvin tablets. Vib. Spectrosc. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Larkin, P.J.; Dabros, M.; Sarsfield, B.; Chan, E.; Carriere, J.T.; Smith, B.C. Polymorph Characterization of Active Pharmaceutical Ingredients (APIs) Using Low-Frequency Raman Spectroscopy. Appl. Spectrosc. 2014, 68, 758–776. [Google Scholar] [CrossRef]
- Larkin, P.J.; Wasylyk, J.; Raglione, M. Application of Low- and Mid-Frequency Raman Spectroscopy to Characterize the Amorphous-Crystalline Transformation of Indomethacin. Appl. Spectrosc. 2015, 69, 1217–1228. [Google Scholar] [CrossRef]
- Walker, G.; Römann, P.; Poller, B.; Löbmann, K.; Grohganz, H.; Rooney, J.S.; Huff, G.S.; Smith, G.P.S.; Rades, T.; Gordon, K.C.; et al. Probing Pharmaceutical Mixtures during Milling: The Potency of Low-Frequency Raman Spectroscopy in Identifying Disorder. Mol. Pharm. 2017, 14, 4675–4684. [Google Scholar] [CrossRef] [PubMed]
- Hédoux, A.; Guinet, Y.; Derollez, P.; Dudognon, E.; Correia, N.T. Raman spectroscopy of racemic ibuprofen: Evidence of molecular disorder in phase II. Int. J. Pharm. 2011, 421, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hisada, H.; Koide, T.; Fukami, T.; Roy, A.; Carriere, J.; Heyler, R. Transmission low-frequency Raman spectroscopy for quantification of crystalline polymorphs in pharmaceutical tablets. Anal. Chem. 2019, 91, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Lipiäinen, T.; Fraser-Miller, S.J.; Gordon, K.C.; Strachan, C.J. Direct comparison of low- and mid-frequency Raman spectroscopy for quantitative solid-state pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 149, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hisada, H.; Inoue, M.; Koide, T.; Carriere, J.; Heyler, R.; Fukami, T. Direct high-resolution imaging of crystalline components in pharmaceutical dosage forms using low-frequency Raman spectroscopy. Org. Process Res. Dev. 2015, 19, 1796–1798. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C. Pseudo-3D Subsurface Imaging of Pharmaceutical Solid Dosage Forms Using Micro-spatially Offset Low-Frequency Raman Spectroscopy. Anal. Chem. 2021, 93, 8986–8993. [Google Scholar] [CrossRef]
- Hubert, S.; Briancon, S.; Hedoux, A.; Guinet, Y.; Paccou, L.; Fessi, H.; Puel, F. Process induced transformations during tablet manufacturing: Phase transition analysis of caffeine using DSC and low frequency micro-Raman spectroscopy. Int. J. Pharm. 2011, 420, 76–83. [Google Scholar] [CrossRef]
- Hédoux, A.; Paccou, L.; Guinet, Y.; Willart, J.-F.; Descamps, M. Using the low-frequency Raman spectroscopy to analyze the crystallization of amorphous indomethacin. Eur. J. Pharm. Sci. 2009, 38, 156–164. [Google Scholar] [CrossRef]
- Hédoux, A.; Guinet, Y.; Paccou, L.; Danède, F.; Derollez, P. Polymorphic transformation of anhydrous caffeine upon grinding and hydrostatic pressurizing analyzed by low-frequency raman spectroscopy. J. Pharm. Sci. 2013, 102, 162–170. [Google Scholar] [CrossRef]
- Hédoux, A.; Decroix, A.-A.; Guinet, Y.; Paccou, L.; Derollez, P.; Descamps, M. Low-and high-frequency Raman investigations on caffeine: Polymorphism, disorder and phase transformation. J. Phys. Chem. B 2011, 115, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Remoto, P.I.J.G.; Bērziņš, K.; Fraser-Miller, S.J.; Korter, T.M.; Rades, T.; Rantanen, J.; Gordon, K.C. Exploring the Solid-State Landscape of Carbamazepine during Dehydration: A Low Frequency Raman Spectroscopy Perspective. Pharmaceutics 2023, 15, 1526. [Google Scholar] [CrossRef] [PubMed]
- Remoto, P.J.G.; Bērziņš, K.; Fraser-Miller, S.J.; Korter, T.M.; Rades, T.; Rantanen, J.; Gordon, K.C. Elucidating the Dehydration Mechanism of Nitrofurantoin Monohydrate II Using Low-Frequency Raman Spectroscopy. Cryst. Growth Des. 2022, 22, 2733–2741. [Google Scholar] [CrossRef]
- Latreche, M.; Willart, J.-F.; Paccou, L.; Guinet, Y.; Danède, F.; Hédoux, A. Contribution of low-frequency Raman spectroscopy to determine the solubility curves of drugs in polymers: The case of paracetamol/PVP. Eur. J. Pharm. Biopharm. 2020, 154, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bērziņš, K.; Fraser-Miller, S.J.; Walker, G.F.; Rades, T.; Gordon, K.C. Investigation on formulation strategies to mitigate compression-induced destabilization in supersaturated celecoxib amorphous solid dispersions. Mol. Pharm. 2021, 18, 3882–3893. [Google Scholar] [CrossRef]
- Iwata, K.; Karashima, M.; Ikeda, Y.; Inoue, M.; Fukami, T. Discrimination and quantification of sulfathiazole polytypes using low-frequency Raman spectroscopy. CrystEngComm 2018, 20, 1928–1934. [Google Scholar] [CrossRef]
- Inoue, M.; Osada, T.; Hisada, H.; Koide, T.; Fukami, T.; Roy, A.; Carriere, J.; Heyler, R. Solid-state quantification of cocrystals in pharmaceutical tablets using transmission low-frequency Raman spectroscopy. Anal. Chem. 2019, 91, 13427–13432. [Google Scholar] [CrossRef]
- Salim, M.; Fraser-Miller, S.J.; Sutton, J.J.; Bērziņš, K.; Hawley, A.; Clulow, A.J.; Beilles, S.; Gordon, K.C.; Boyd, B.J. Application of Low-Frequency Raman Scattering Spectroscopy to Probe in Situ Drug Solubilization in Milk during Digestion. J. Phys. Chem. Lett. 2019, 10, 2258–2263. [Google Scholar] [CrossRef]
- Stillhart, C.; Imanidis, G.; Kuentz, M. Insights into Drug Precipitation Kinetics during In Vitro Digestion of a Lipid-Based Drug Delivery System Using In-Line Raman Spectroscopy and Mathematical Modeling. Pharm. Res. 2013, 30, 3114–3130. [Google Scholar] [CrossRef]
- Alskär, L.C.; Keemink, J.; Johannesson, J.; Porter, C.J.H.; Bergström, C.A.S. Impact of Drug Physicochemical Properties on Lipolysis-Triggered Drug Supersaturation and Precipitation from Lipid-Based Formulations. Mol. Pharm. 2018, 15, 4733–4744. [Google Scholar] [CrossRef]
- Koskela, J.; Sutton, J.J.; Lipiäinen, T.; Gordon, K.C.; Strachan, C.J.; Fraser-Miller, S.J. Low- versus Mid-frequency Raman Spectroscopy for in Situ Analysis of Crystallization in Slurries. Mol. Pharm. 2022, 19, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.S.; McGoverin, C.M.; Fraser, S.J.; Gordon, K.C. Raman imaging of drug delivery systems. Adv. Drug Deliv. Rev. 2015, 89, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Eason, T.; Boyd, B.J. Opportunities for milk and milk-related systems as ‘new’ low-cost excipient drug delivery materials. Adv. Drug Deliv. Rev. 2022, 183, 114139. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A. Orange: Data mining toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Porter, C.J.H.; Kaukonen, A.M.; Boyd, B.J.; Edwards, G.A.; Charman, W.N. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm. Res. 2004, 21, 1405–1412. [Google Scholar] [CrossRef]
- Heinz, A.; Gordon, K.C.; McGoverin, C.M.; Rades, T.; Strachan, C.J. Understanding the solid-state forms of fenofibrate—A spectroscopic and computational study. Eur. J. Pharm. Biopharm. 2009, 71, 100–108. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim, M.; Fraser-Miller, S.J.; Bērziņš, K.; Sutton, J.J.; Gordon, K.C.; Boyd, B.J. In Situ Monitoring of Drug Precipitation from Digesting Lipid Formulations Using Low-Frequency Raman Scattering Spectroscopy. Pharmaceutics 2023, 15, 1968. https://doi.org/10.3390/pharmaceutics15071968
Salim M, Fraser-Miller SJ, Bērziņš K, Sutton JJ, Gordon KC, Boyd BJ. In Situ Monitoring of Drug Precipitation from Digesting Lipid Formulations Using Low-Frequency Raman Scattering Spectroscopy. Pharmaceutics. 2023; 15(7):1968. https://doi.org/10.3390/pharmaceutics15071968
Chicago/Turabian StyleSalim, Malinda, Sara J. Fraser-Miller, Kārlis Bērziņš, Joshua J. Sutton, Keith C. Gordon, and Ben J. Boyd. 2023. "In Situ Monitoring of Drug Precipitation from Digesting Lipid Formulations Using Low-Frequency Raman Scattering Spectroscopy" Pharmaceutics 15, no. 7: 1968. https://doi.org/10.3390/pharmaceutics15071968
APA StyleSalim, M., Fraser-Miller, S. J., Bērziņš, K., Sutton, J. J., Gordon, K. C., & Boyd, B. J. (2023). In Situ Monitoring of Drug Precipitation from Digesting Lipid Formulations Using Low-Frequency Raman Scattering Spectroscopy. Pharmaceutics, 15(7), 1968. https://doi.org/10.3390/pharmaceutics15071968







