Nanocellulose-Based Film-Forming Hydrogels for Improved Outcomes in Atopic Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SMEDDS
2.2.1. Solubility Study
2.2.2. HPLC Analysis
2.2.3. Construction of Phase Diagram
2.2.4. Loading of BMD in Microemulsion
2.3. Preparation of Hydrogel-Containing Microemulsion
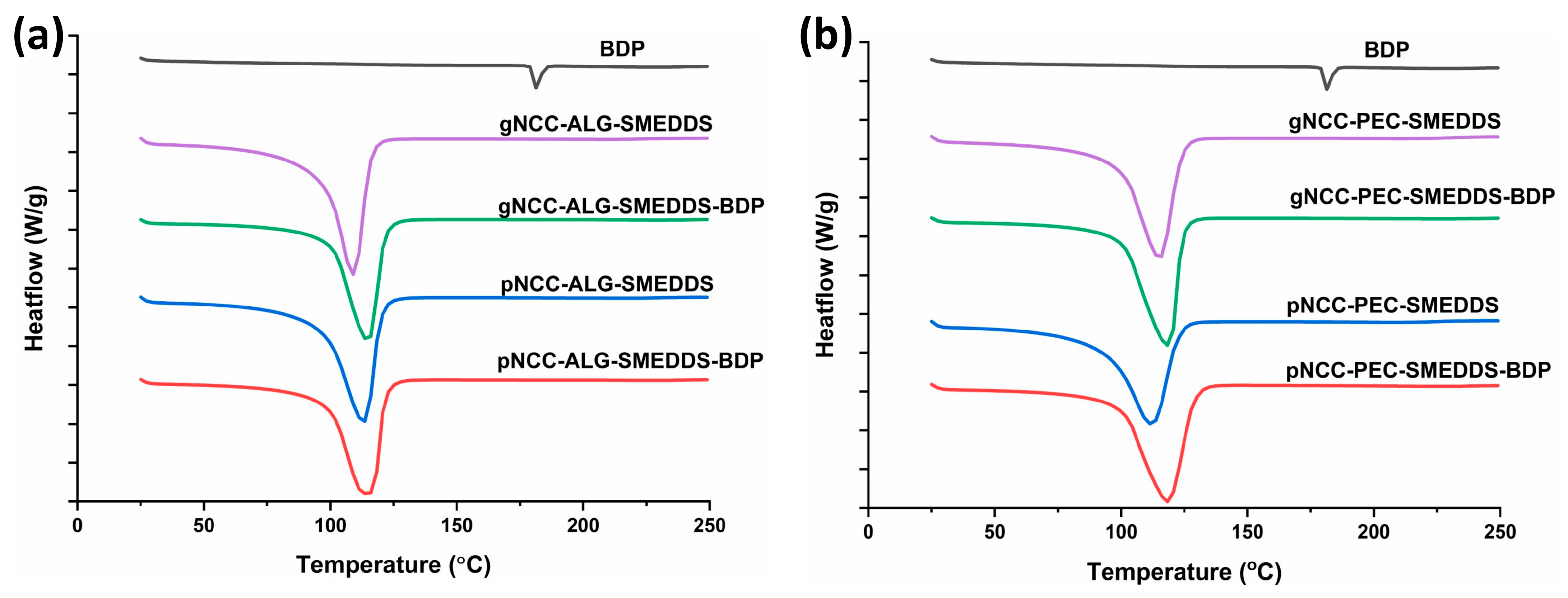
2.3.1. DSC (Differential Scanning Calorimetry) Analysis
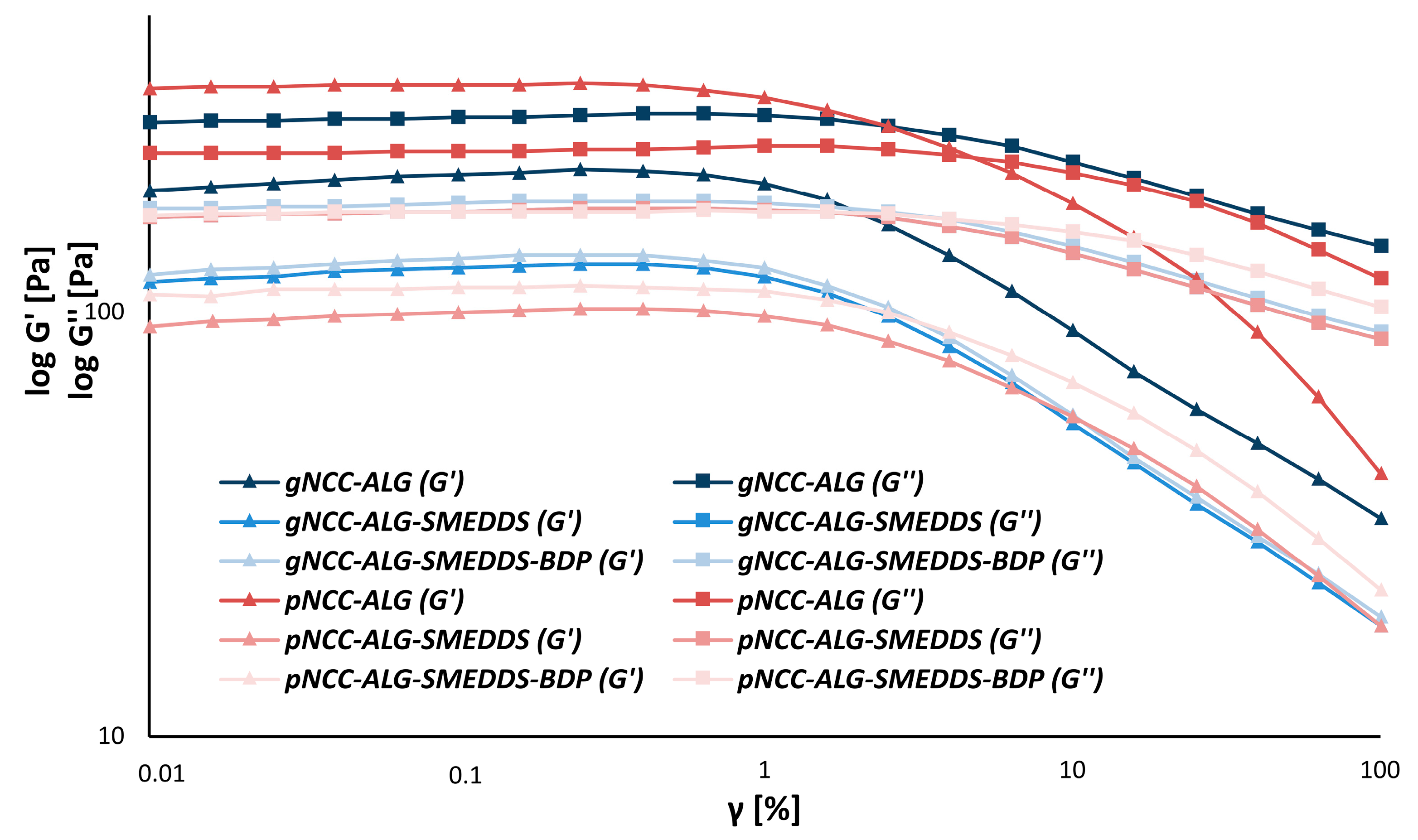
2.3.2. Rheological Study
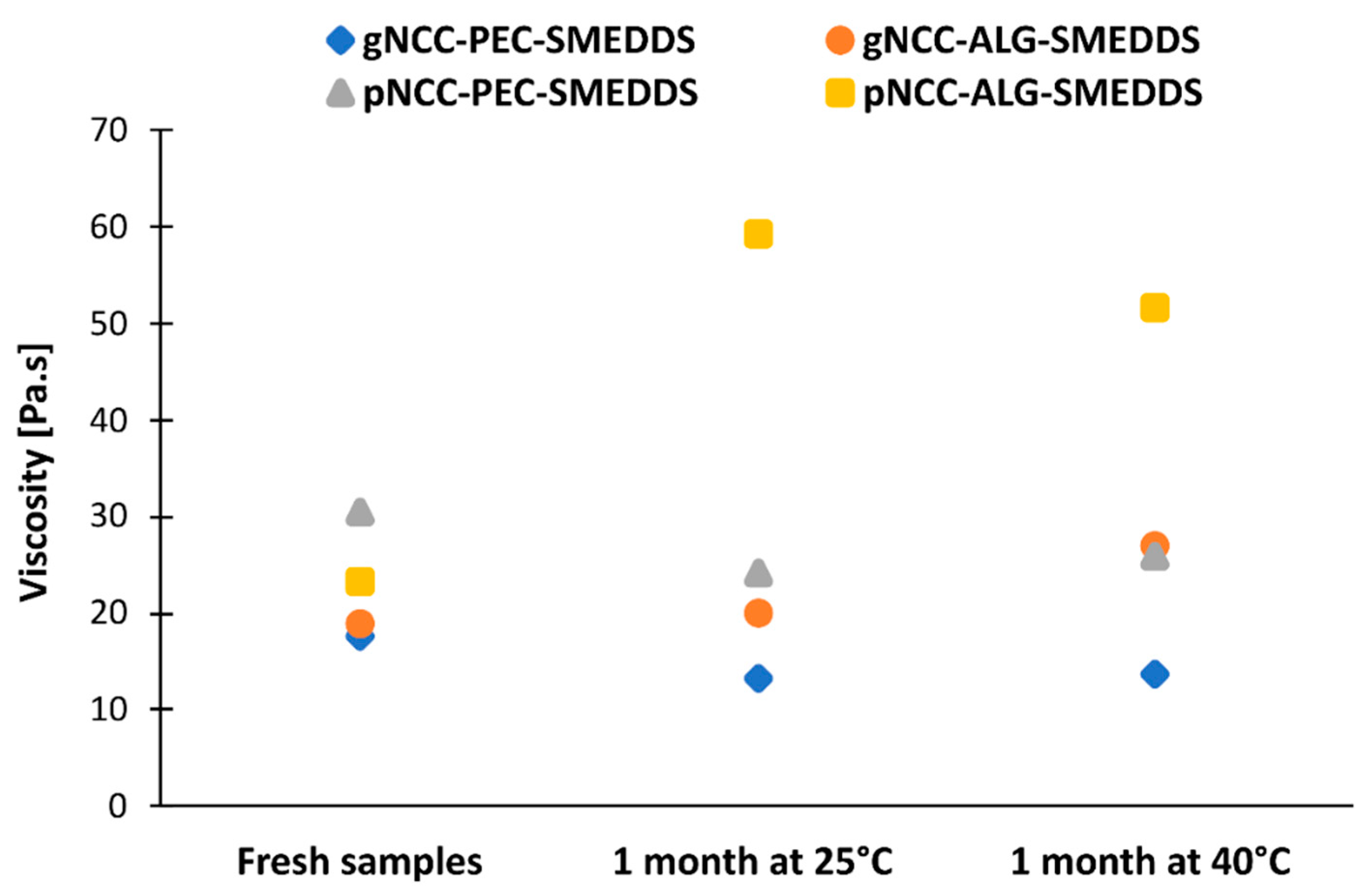
2.3.3. Stability Testing
2.4. Film Preparation
2.4.1. Film Residual Moisture Content Measurements
2.4.2. Film Thickness Measurements
2.4.3. Measurements of the Water Retention Capacity of the Films
2.4.4. Morphological Evaluation of Films by Scanning Electron Microscopy
2.5. Evaluation of Films and Hydrogels on Full-Thickness Pig Ear Skin
2.6. In Vitro Permeation Testing
2.7. Cell Culture and Cell Proliferation Assay
2.8. Statistical Analysis
3. Results and Discussion
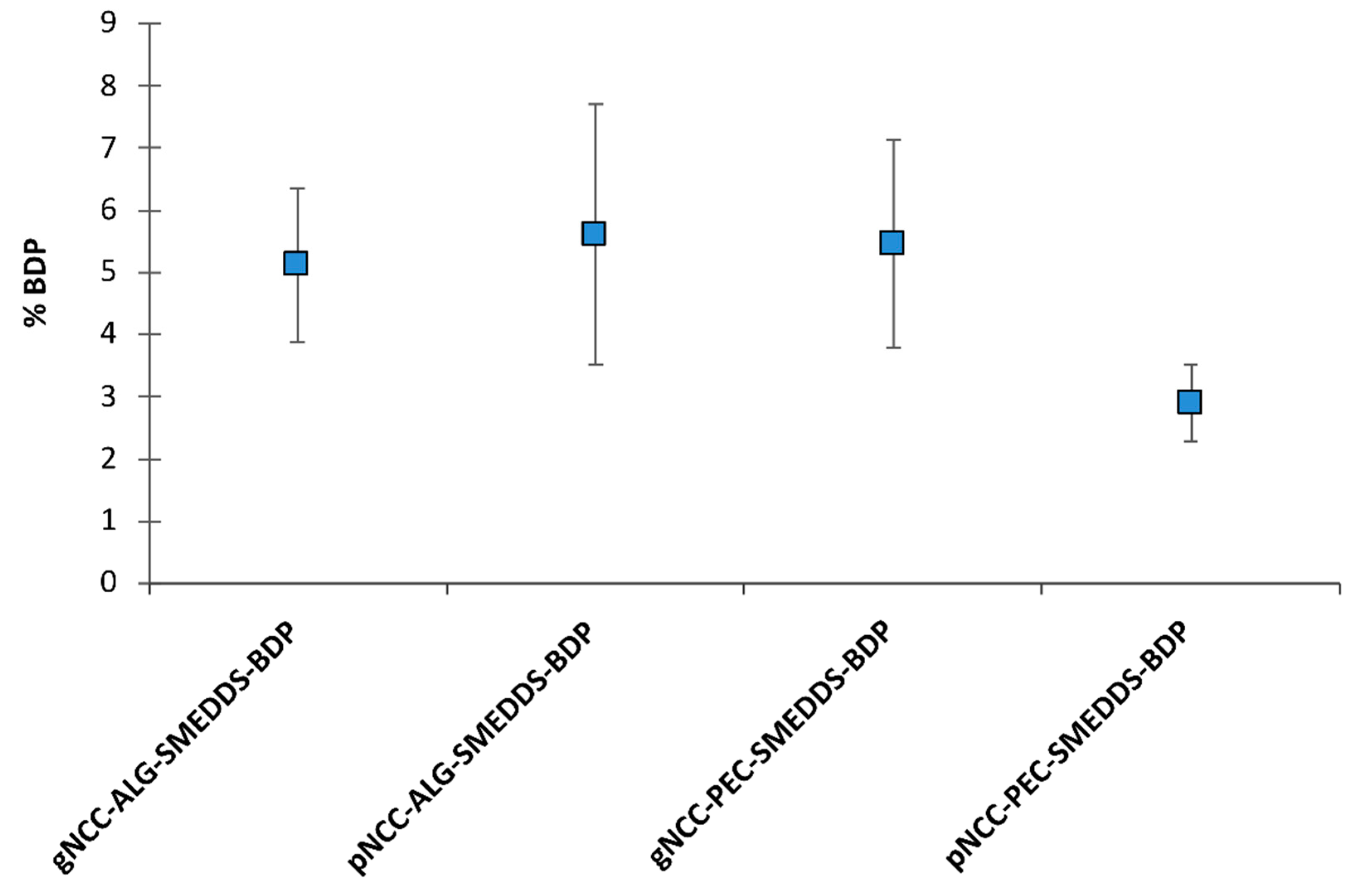
3.1. Hydrogel Preparation and Incorporation of BDP
3.2. DSC Analysis of Hydrogels
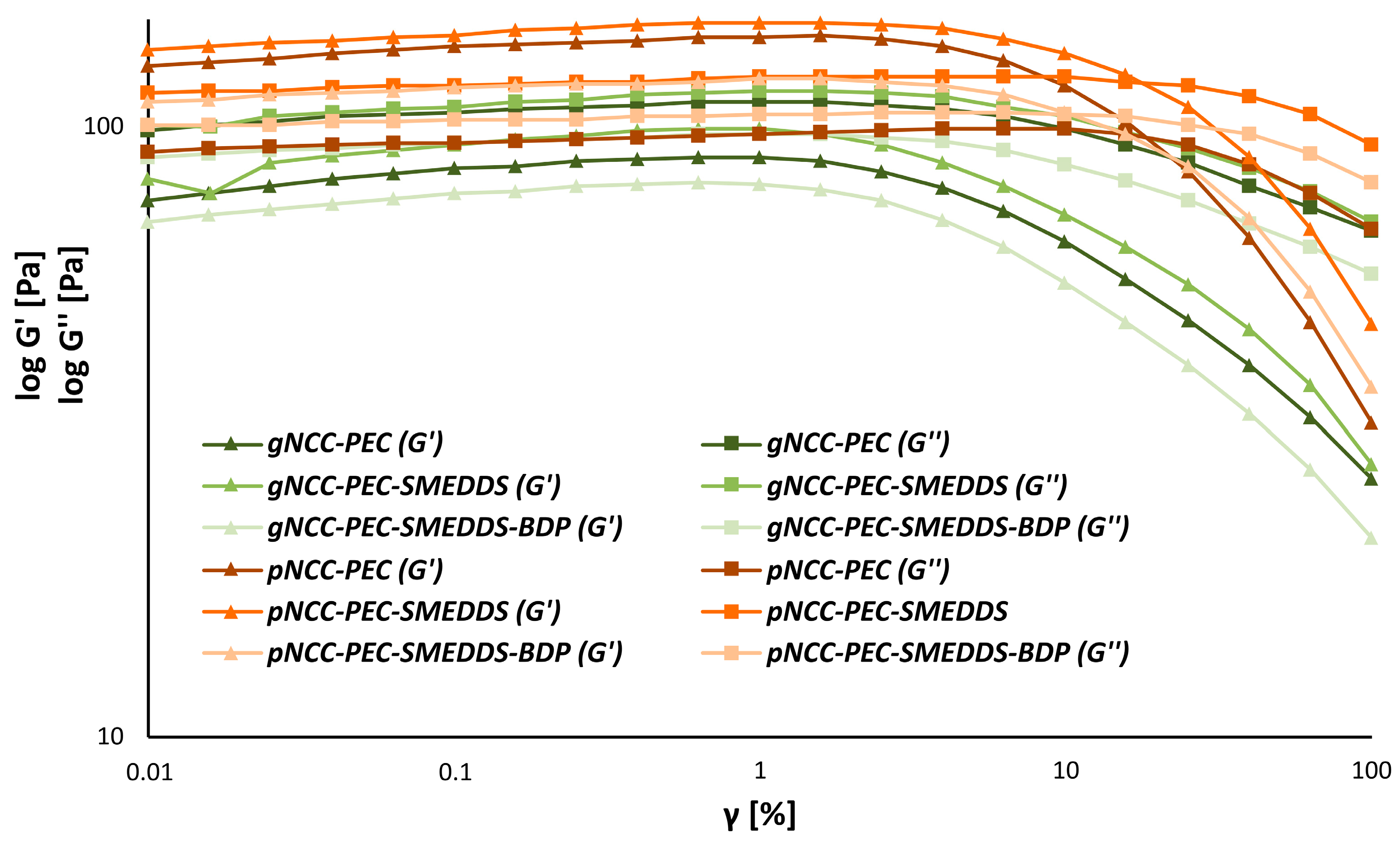
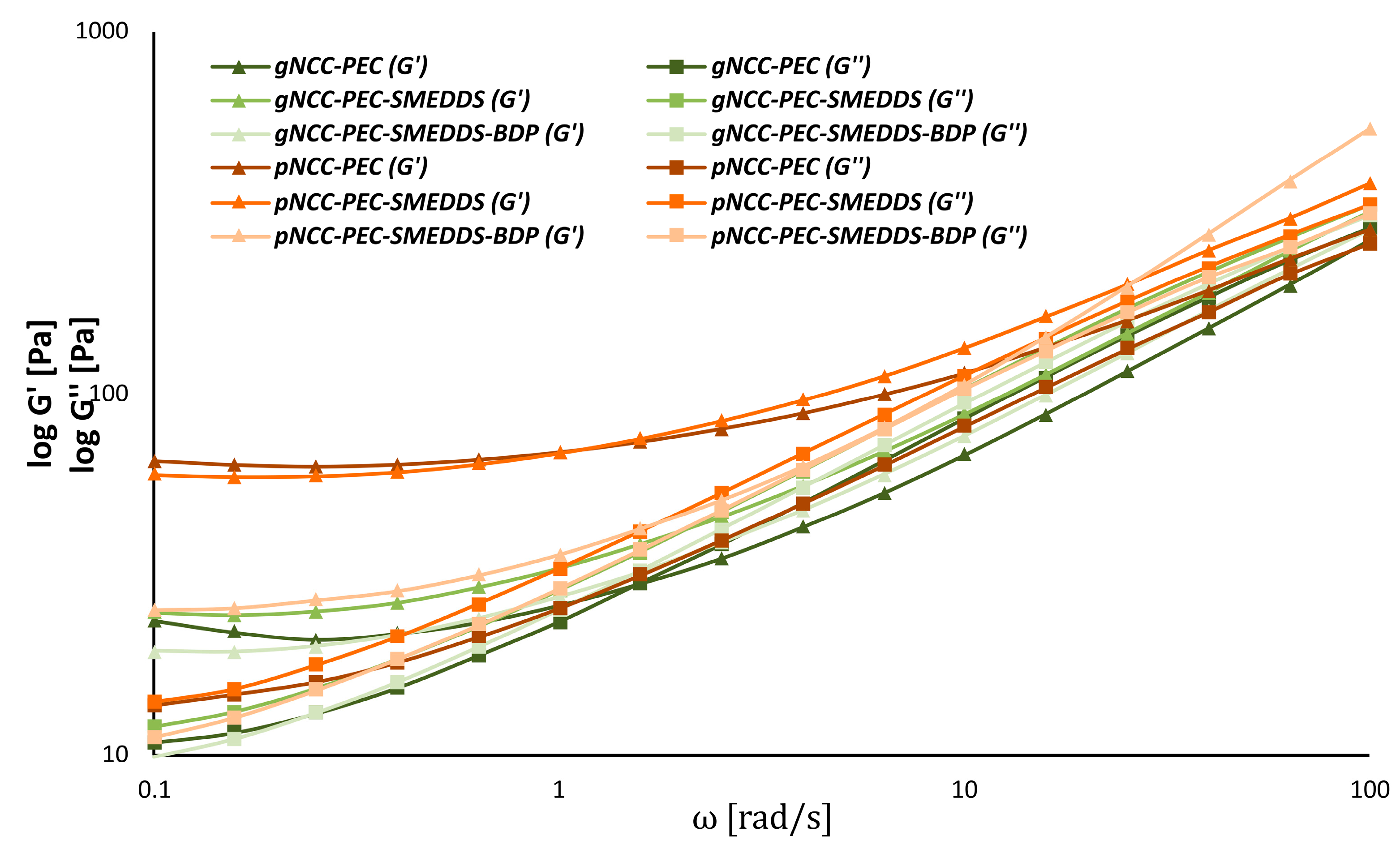
3.3. Rheology of the Hydrogel Formulations
3.4. Film Preparation and Evaluation
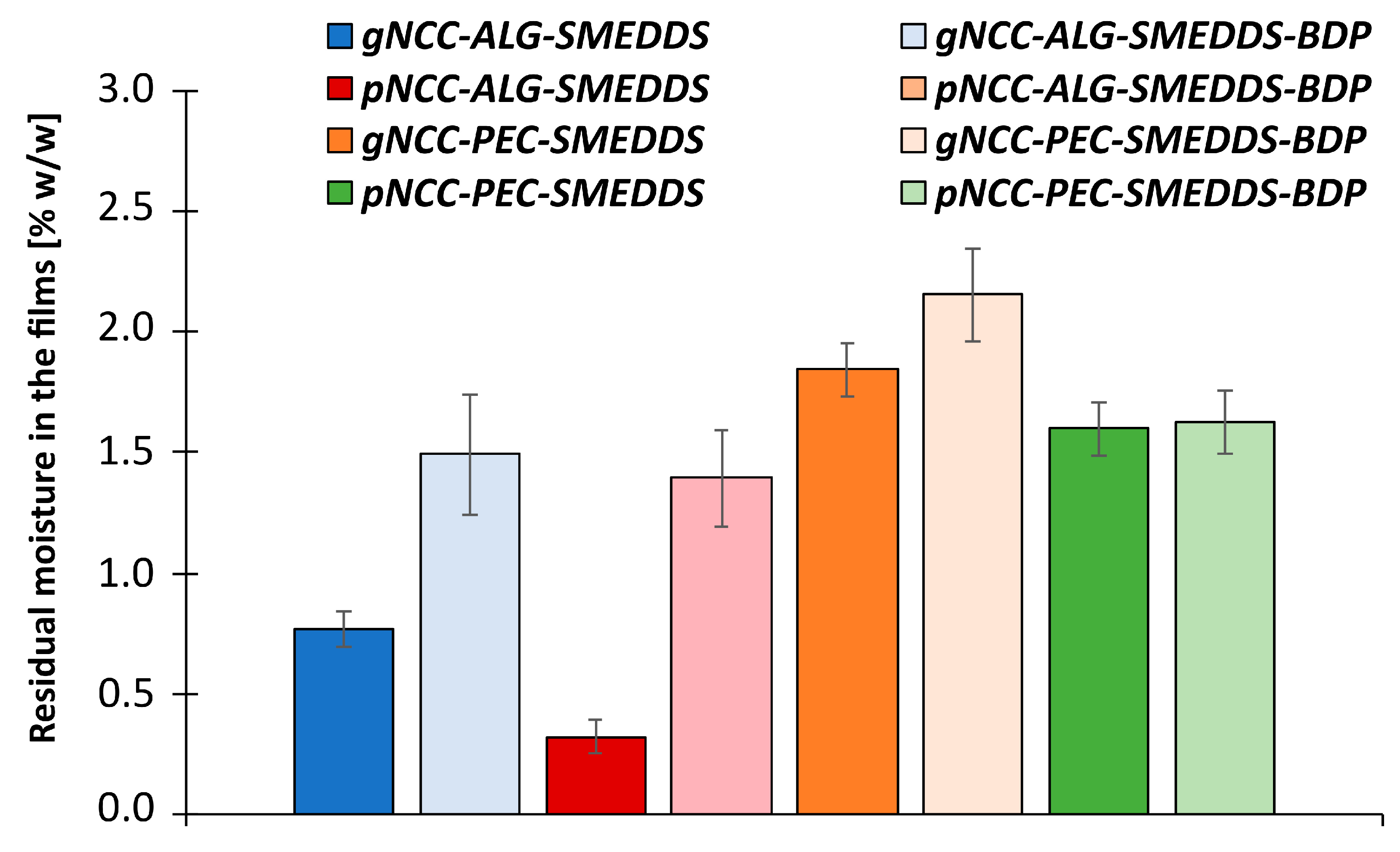
3.4.1. Residual Moisture Content in the Films
3.4.2. The Thickness of the Films
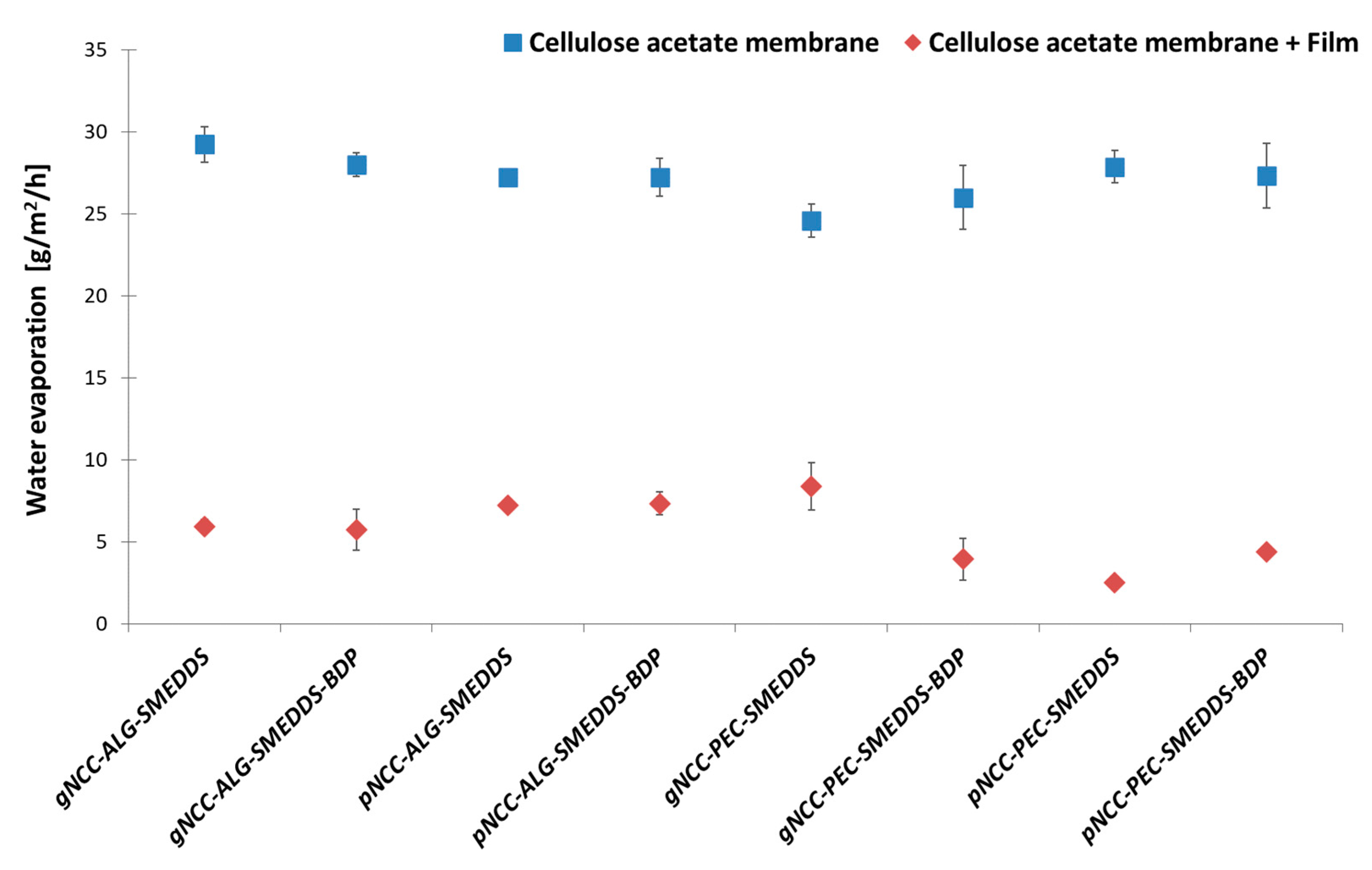
3.4.3. Water Retention Capacity of the Films
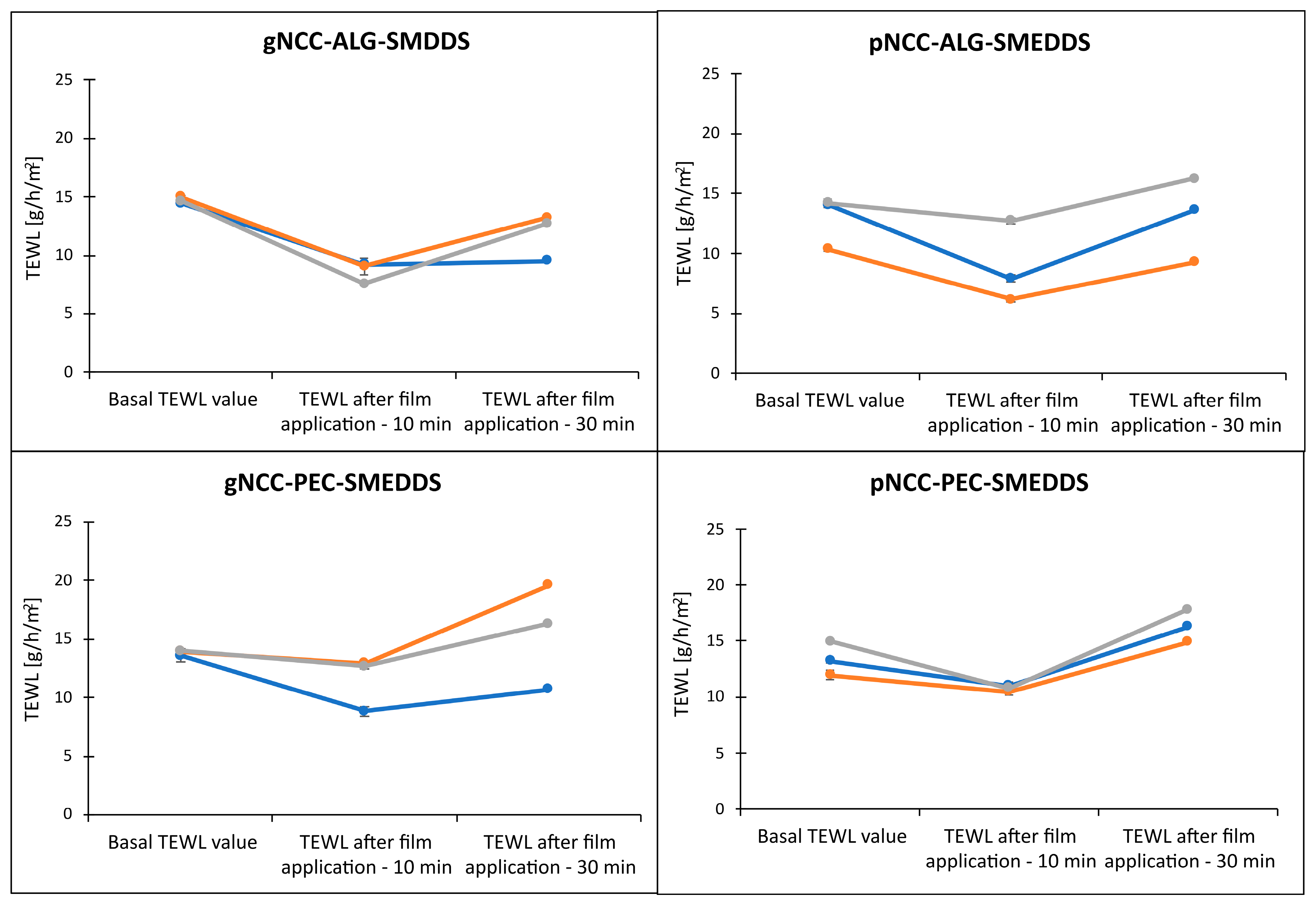
3.5. Evaluation of Transepidermal Water Loss after Film Application and Hydrogel Irritation Potential on Pig Ear Skin
3.6. In Vitro Permeation Test
3.7. Cell Proliferation Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Exploring Preclinical and Clinical Effectiveness of Nanoformulations in the Treatment of Atopic Dermatitis: Safety Aspects and Patent Reviews. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Silverberg, J.I. Atopic Dermatitis in Adults. Med. Clin. 2020, 104, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Dias-Ferreira, J.; Oliveira, J.; Sanchez-Lopez, E.; Lopez-Machado, A.; Espina, M.; Garcia, M.L.; Souto, S.B.; Martins-Gomes, C.; Silva, A.M. Trends in Atopic Dermatitis—From Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659. [Google Scholar] [CrossRef] [PubMed]
- Parekh, K.; Hariharan, K.; Qu, Z.; Rewatkar, P.; Cao, Y.; Moniruzzaman, M.; Pandey, P.; Popat, A.; Mehta, T. Tacrolimus Encapsulated Mesoporous Silica Nanoparticles Embedded Hydrogel for the Treatment of Atopic Dermatitis. Int. J. Pharm. 2021, 608, 121079. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.; Del Rosso, J. A Novel Hydrogel Vehicle Formulated for the Treatment of Atopic Dermatitis. J. Drugs Derm. 2007, 6, 718–722. [Google Scholar]
- Arkwright, P.D.; Motala, C.; Subramanian, H.; Spergel, J.; Schneider, L.C.; Wollenberg, A.; Atopic Dermatitis Working Group of the Allergic Skin Diseases Committee of the AAAAI. Management of Difficult-to-Treat Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 142–151. [Google Scholar] [CrossRef]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.; et al. Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) Part I. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1045–1060. [Google Scholar] [CrossRef]
- Vitek, M.; Pobirk, A.Z.; Gašperlin, M.; Matjaž, M.G. Izzivi in sodobni pristopi k zdravljenju atopijskega dermatitisa. Slov. Med. J. 2022, 92, 1–14. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film Forming Systems for Topical and Transdermal Drug Delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering Nanocellulose Hydrogels for Biomedical Applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-Based Films and Their Emerging Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- González-Paredes, A.; Manconi, M.; Caddeo, C.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M.; Fadda, A.M. Archaeosomes as Carriers for Topical Delivery of Betamethasone Dipropionate: In Vitro Skin Permeation Study. J. Liposome Res. 2010, 20, 269–276. [Google Scholar] [CrossRef]
- Kizu, J.; Ichihara, W.; Tomonaga, E.; Abe, J.; Watanabe, T.; Inoue, T.; Hori, S. Survey of Mixing Commercially Available Corticosteroid Ointments with Other Ointments and the Anti-Inflammatory Activity of the Admixtures. Yakugaku Zasshi 2004, 124, 93–97. [Google Scholar] [CrossRef]
- Fahr, A.; Liu, X. Drug Delivery Strategies for Poorly Water-Soluble Drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Gu, D.; O’Connor, A.J.; Qiao, G.H.G.; Ladewig, K. Hydrogels with Smart Systems for Delivery of Hydrophobic Drugs. Expert Opin. Drug Deliv. 2017, 14, 879–895. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.W.; Pan, W.; Yang, Z. Thermoreversible Pluronic® F127-Based Hydrogel Containing Liposomes for the Controlled Delivery of Paclitaxel: In Vitro Drug Release, Cell Cytotoxicity, and Uptake Studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, X.; Lu, W.; Zhang, Q. A Novel Mixed Micelle Gel with Thermo-Sensitive Property for the Local Delivery of Docetaxel. J. Control. Release 2009, 135, 175–182. [Google Scholar] [CrossRef]
- Sabale, V.; Vora, S. Formulation and Evaluation of Microemulsion-Based Hydrogel for Topical Delivery. Int. J. Pharm. Investig. 2012, 2, 140. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.-T.; Vu, V.-D.; Nguyen, P.-L. DoE-Based Development, Physicochemical Characterization, and Pharmacological Evaluation of a Topical Hydrogel Containing Betamethasone Dipropionate Microemulsion. Colloids Surf. B Biointerfaces 2019, 181, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Rozman, B.; Gosenca, M.; Gasperlin, M.; Padois, K.; Falson, F. Dual Influence of Colloidal Silica on Skin Deposition of Vitamins C and E Simultaneously Incorporated in Topical Microemulsions. Drug Dev. Ind. Pharm. 2010, 36, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Rozman, B.; Gosenca, M.; Falson, F.; Gašperlin, M. The Influence of Microemulsion Structure on Their Skin Irritation and Phototoxicity Potential. Int. J. Pharm. 2016, 499, 228–235. [Google Scholar] [CrossRef]
- Fentem, J.H.; Briggs, D.; Chesné, C.; Elliott, G.R.; Harbell, J.W.; Heylings, J.R.; Portes, P.; Roguet, R.; van de Sandt, J.J.; Botham, P.A. A Prevalidation Study on in Vitro Tests for Acute Skin Irritation. Results and Evaluation by the Management Team. Toxicol. Vitr. 2001, 15, 57–93. [Google Scholar] [CrossRef]
- Baboota, S.; Alam, M.S.; Sharma, S.; Sahni, J.K.; Kumar, A.; Ali, J. Nanocarrier-Based Hydrogel of Betamethasone Dipropionate and Salicylic Acid for Treatment of Psoriasis. Int. J. Pharm. Investig. 2011, 1, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.A.; Ghorab, M.M.; Gad, S. Development of Betamethasone Dipropionate-Loaded Nanostructured Lipid Carriers for Topical and Transdermal Delivery. Antiinflamm. Antiallergy Agents. Med. Chem. 2019, 18, 26–44. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and Diffusion Studies of Calcium Polysaccharide Gels Intended for Film Coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Suchenek, A.; Wilkowski, D. Optimization and Evaluation of the In Vitro Permeation Parameters of Topical Products with Non-Steroidal Anti-Inflammatory Drugs through Strat-M® Membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of Skin Permeation by Chemical Compounds Using the Artificial Membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Tuerxun, D.; Pulingam, T.; Nordin, N.I.; Chen, Y.W.; Kamaldin, J.B.; Julkapli, N.B.M.; Lee, H.V.; Leo, B.F.; Johan, M.R.B. Synthesis, Characterization and Cytotoxicity Studies of Nanocrystalline Cellulose from the Production Waste of Rubber-Wood and Kenaf-Bast Fibers. Eur. Polym. J. 2019, 116, 352–360. [Google Scholar] [CrossRef]
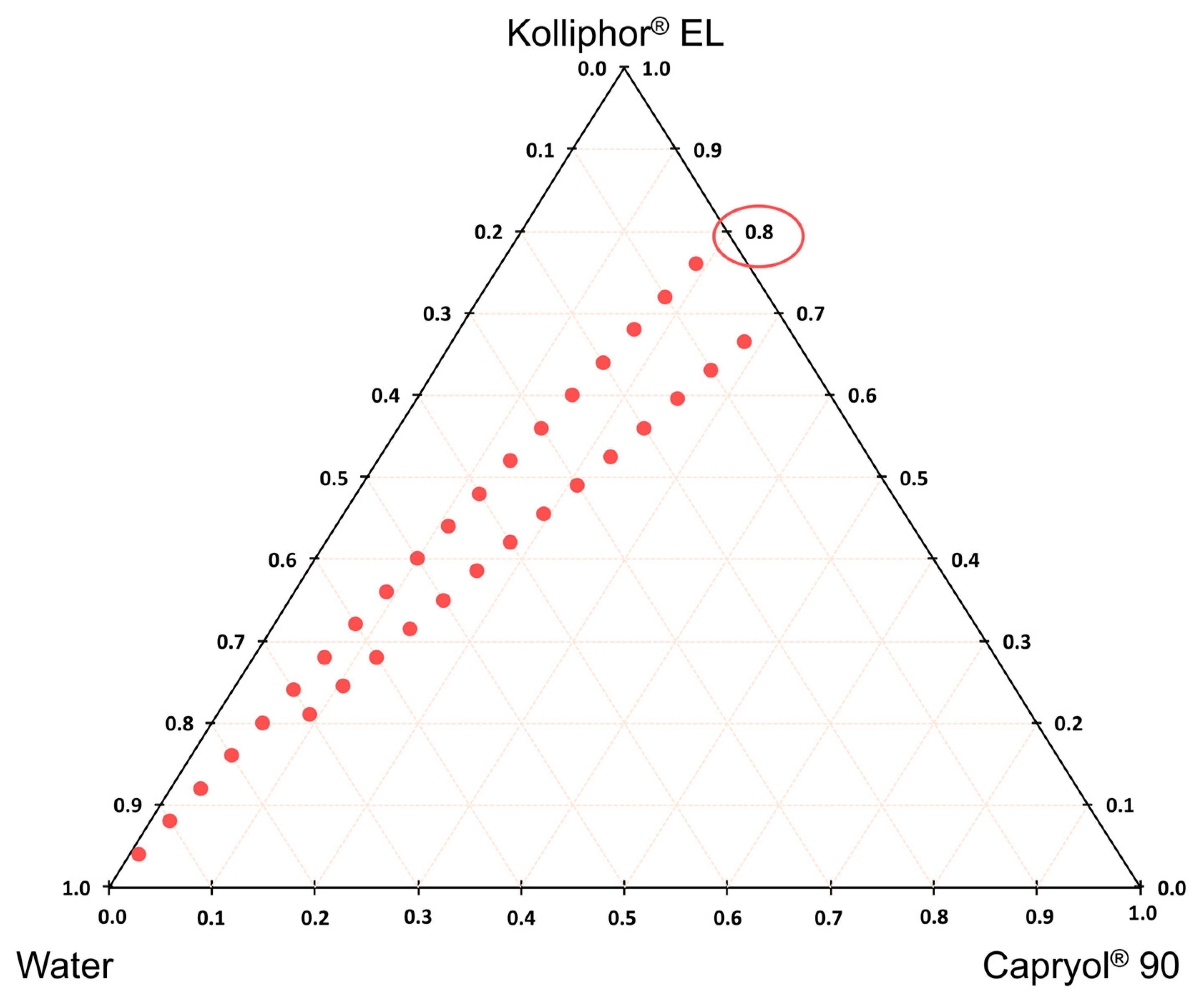






| Oil Phase | Surfactant Phase | Surfactant/Oil Phase Ratio |
|---|---|---|
| Capryol®90 | Tween® 20 | 7/3 and 8/2 |
| Tween® 80 | ||
| Labrasol® | ||
| Polyethylene glycol 400 | ||
| Kolliphor® EL |
| gNCC | ALG | PEC | SMEDDS | SMEDDS-BDP | Glyc. | ||
|---|---|---|---|---|---|---|---|
| ALG or PEC | |||||||
| gNCC-ALG/PEC | 2.5% | 1.75% | 5% | 3.5% | / | / | 5% |
| gNCC-ALG/PEC-SMEDDS | 2.5% | 1.75% | 5% | 3.5% | 3% | / | 5% |
| gNCC-ALG/PEC-SMEDDS-BDP | 2.5% | 1.75% | 5% | 3.5% | / | 3% | 5% |
| pNCC | |||||||
| pNCC-ALG/PEC | 2.5% | 1.75% | 5% | 3.5% | / | / | 5% |
| pNCC-ALG/PEC-SMEDDS | 2.5% | 1.75% | 5% | 3.5% | 3% | / | 5% |
| gNCC-ALG/PEC-SMEDDS-BDP | 2.5% | 1.75% | 5% | 3.5% | / | 3% | 5% |
| Scheme | Saturated Solubility of BDP (mg/g) | |
|---|---|---|
| Oil phase | Capmul MCM C8 | 24.8 |
| Capryol 90 | 55.8 | |
| Isopropyl myristate | * | |
| Oleic acid | * | |
| Plurol oleique | 4.3 | |
| Surfactant phase | Tween 20 | 24.9 |
| Tween 80 | 22.9 | |
| Labrasol | 33.5 | |
| Kolliphor EL | 20.7 | |
| Hydrophilic cosolvent | Polyethylene glycol 400 | 23.4 |
| Sample Name | Film Thickness (mm) |
|---|---|
| gNCC-ALG-SMEDDS | 0.058 ± 0.004 |
| gNCC-ALG-SMEDDS-BDP | 0.064 ± 0.015 |
| pNCC-ALG-SMEDDS | 0.062 ± 0.008 |
| pNCC-ALG-SMEDDS-BDP | 0.078 ± 0.008 |
| gNCC-PEC-SMEDDS | 0.052 ± 0.004 |
| gNCC-PEC-SMEDDS-BDP | 0.068 ± 0.008 |
| pNCC-PEC-SMEDDS | 0.048 ± 0.005 |
| pNCC-PEC-SMEDDS-BDP | 0.060 ± 0.007 |
| Ingredient | Toxicity | Irritancy | |
|---|---|---|---|
| LD50 Oral | LD50 Dermal | ||
| Alginate | >5 g/kg (rat) | / | No skin irritation |
| Pectin | >5 g/kg (rat) | / | Prolonged contact with dry powder may cause mild skin irritation. |
| Glycerol | 12.6 g/kg (rat) | >10 g/kg (rabbit) | Mild skin irritation (rabbit) |
| Capryol 90 | >5 g/kg (rat) | >5 g/kg (rat) | Mild skin irritation (rabbit) |
| Kolliphor EL | >6.4 g/kg (rat) | >5 g/kg (rat) | No skin irritation (rabbit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolko Seljak, K.; Sterle Zorec, B.; Gosenca Matjaž, M. Nanocellulose-Based Film-Forming Hydrogels for Improved Outcomes in Atopic Skin. Pharmaceutics 2023, 15, 1918. https://doi.org/10.3390/pharmaceutics15071918
Bolko Seljak K, Sterle Zorec B, Gosenca Matjaž M. Nanocellulose-Based Film-Forming Hydrogels for Improved Outcomes in Atopic Skin. Pharmaceutics. 2023; 15(7):1918. https://doi.org/10.3390/pharmaceutics15071918
Chicago/Turabian StyleBolko Seljak, Katarina, Barbara Sterle Zorec, and Mirjam Gosenca Matjaž. 2023. "Nanocellulose-Based Film-Forming Hydrogels for Improved Outcomes in Atopic Skin" Pharmaceutics 15, no. 7: 1918. https://doi.org/10.3390/pharmaceutics15071918
APA StyleBolko Seljak, K., Sterle Zorec, B., & Gosenca Matjaž, M. (2023). Nanocellulose-Based Film-Forming Hydrogels for Improved Outcomes in Atopic Skin. Pharmaceutics, 15(7), 1918. https://doi.org/10.3390/pharmaceutics15071918






