Development of a Novel Red Clay-Based Drug Delivery Carrier to Improve the Therapeutic Efficacy of Acyclovir in the Treatment of Skin Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Preparation of RC-SS Complex
2.2.2. Preparation of Nanocomplex F1 (ACV-Loaded RC-SS Complex)
2.2.3. Characterization of Nanocomplex F
2.2.4. In Vitro Permeation Study of ACV-RC-SS Complex
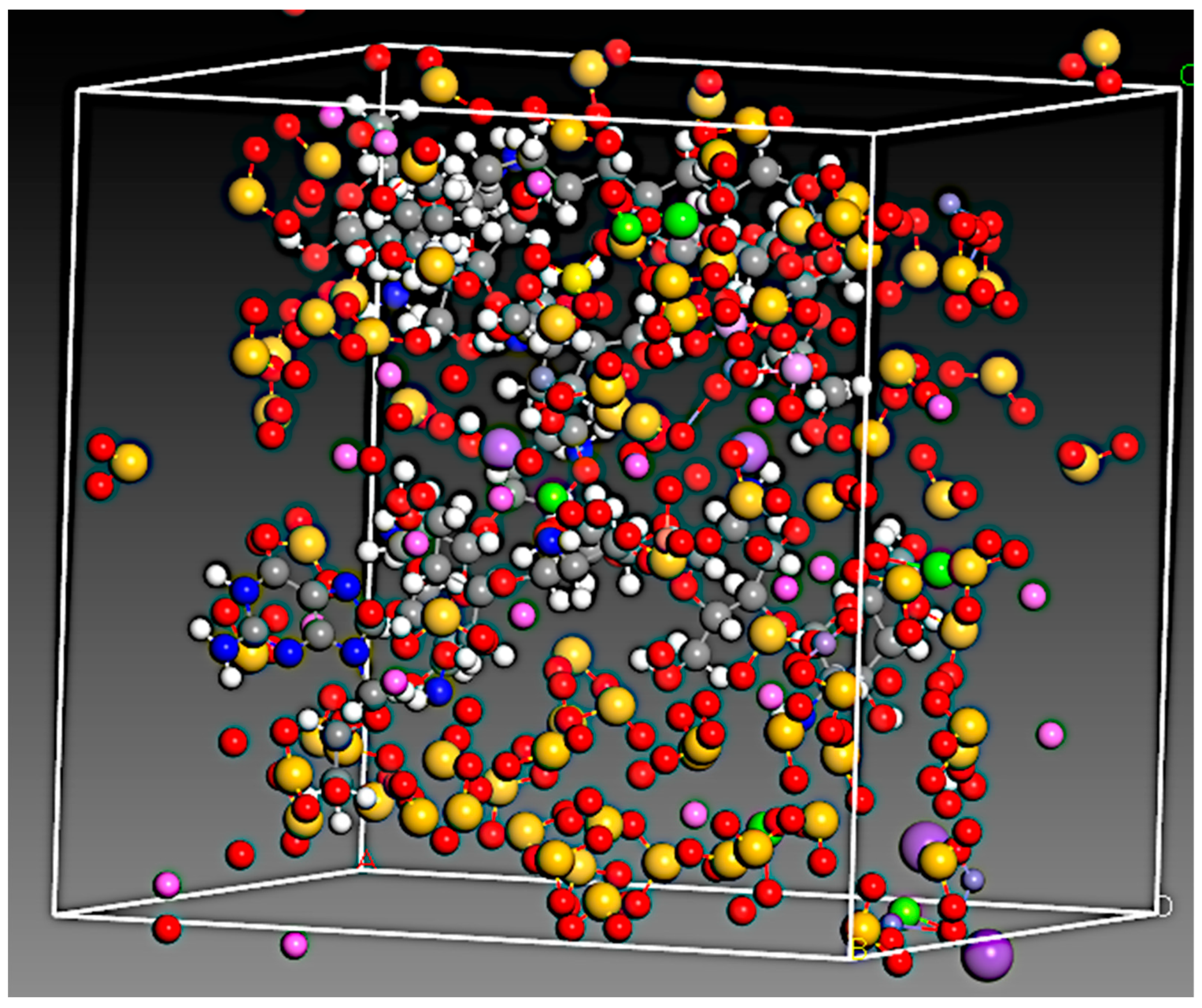
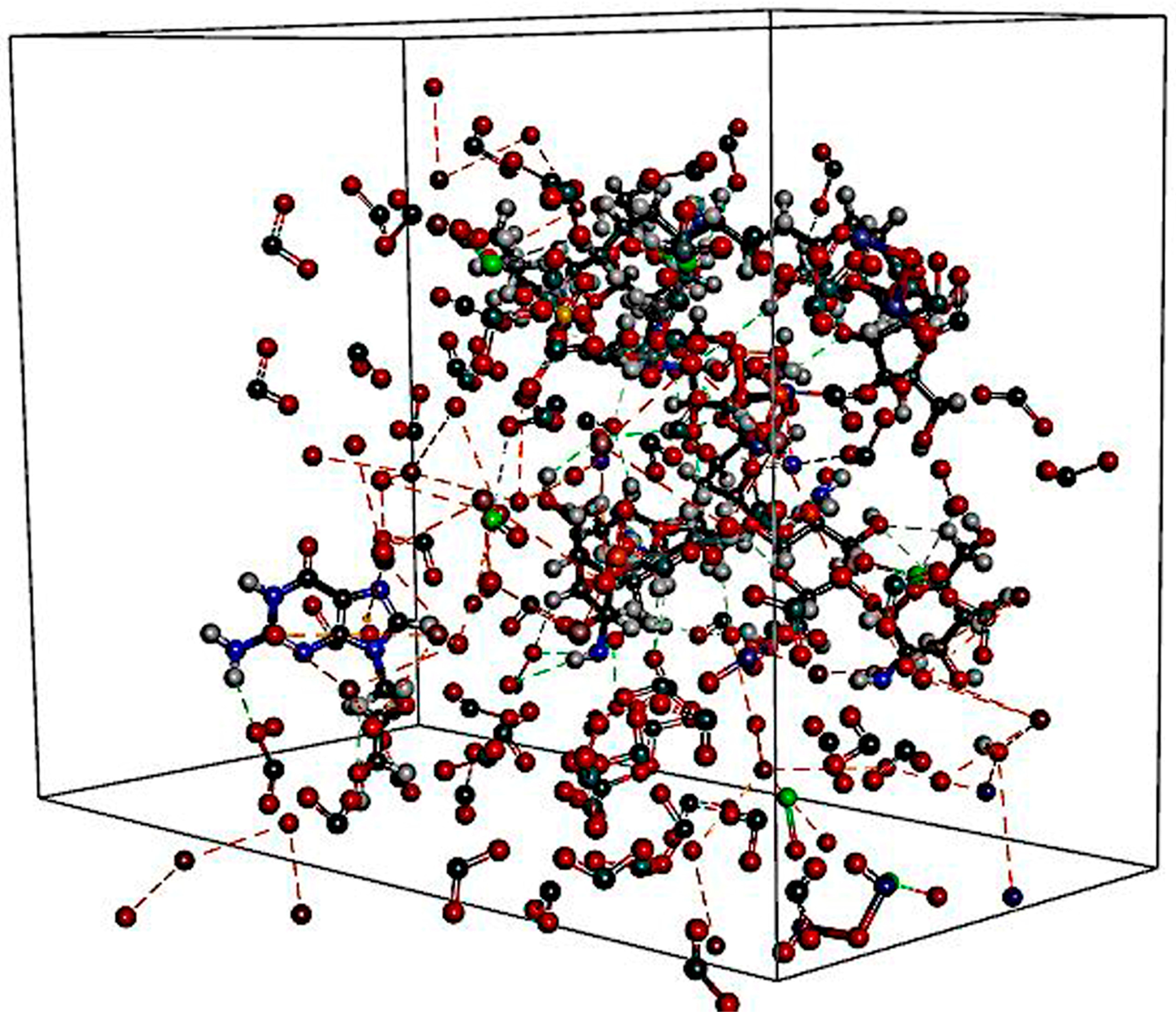
2.2.5. Interaction Study with In Silico Docking
2.2.6. Forcite Protocol
Amorphous Cell Protocol
Blend Protocol
2.2.7. Cell Culture Maintenance
2.2.8. Cytotoxicity Studies
2.2.9. Measurement of Apoptotic Induction Using Acridine Orange/Ethidium Bromide (AO/EB) Dual Staining Method
2.3. Statistical Analysis
3. Results
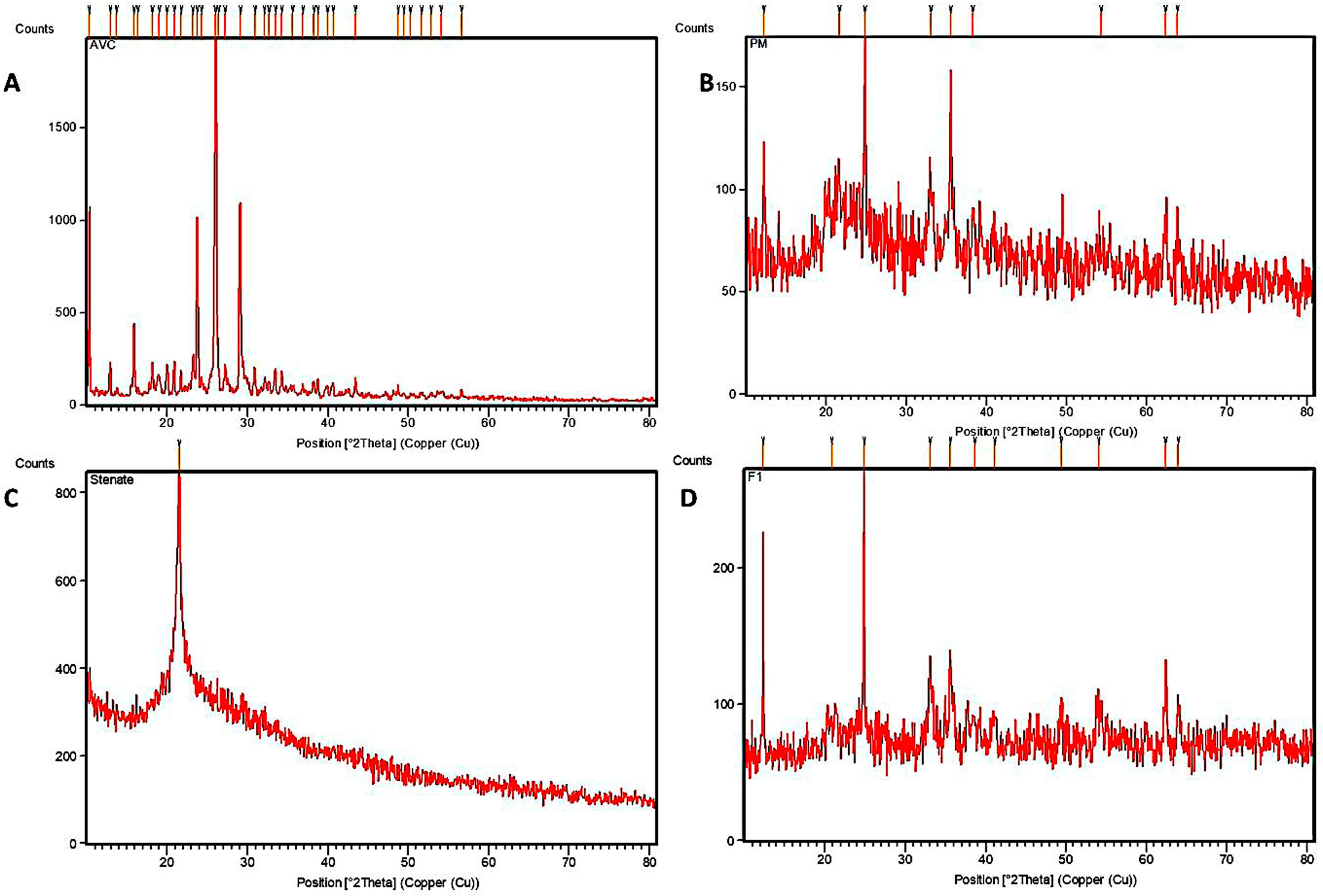
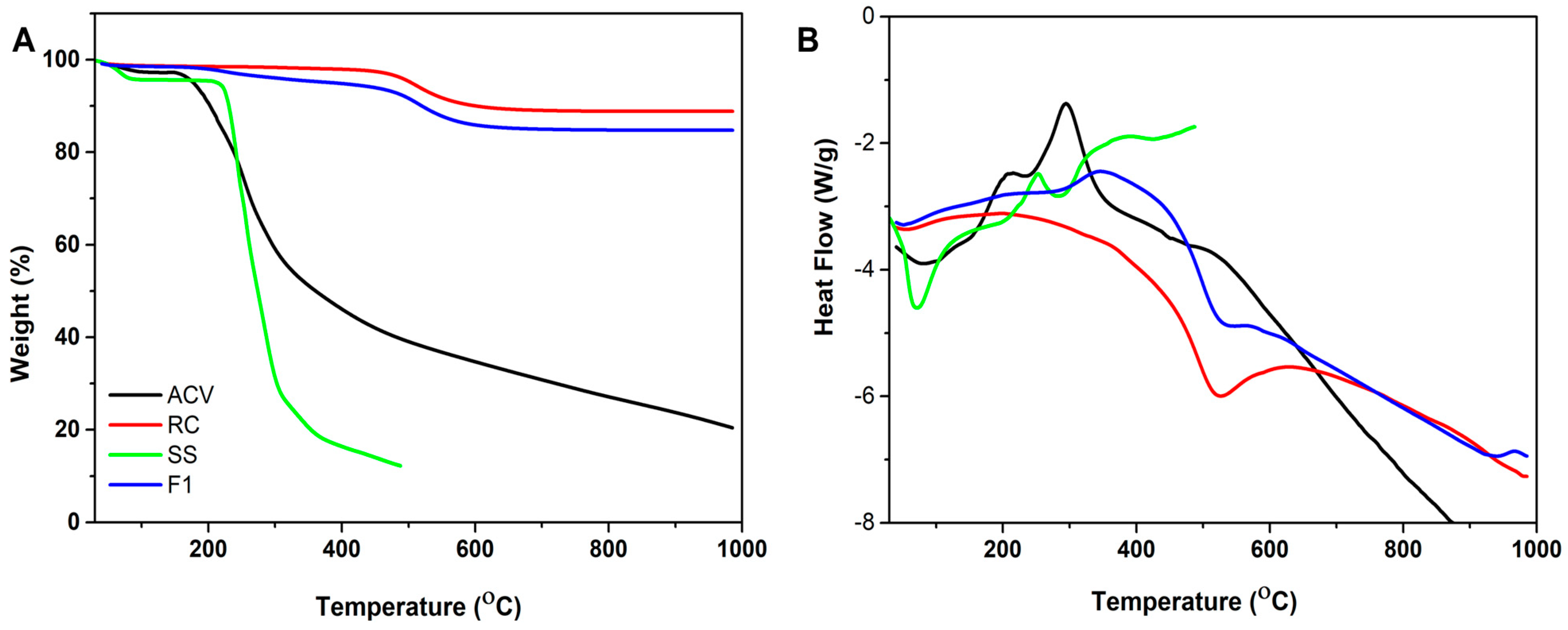
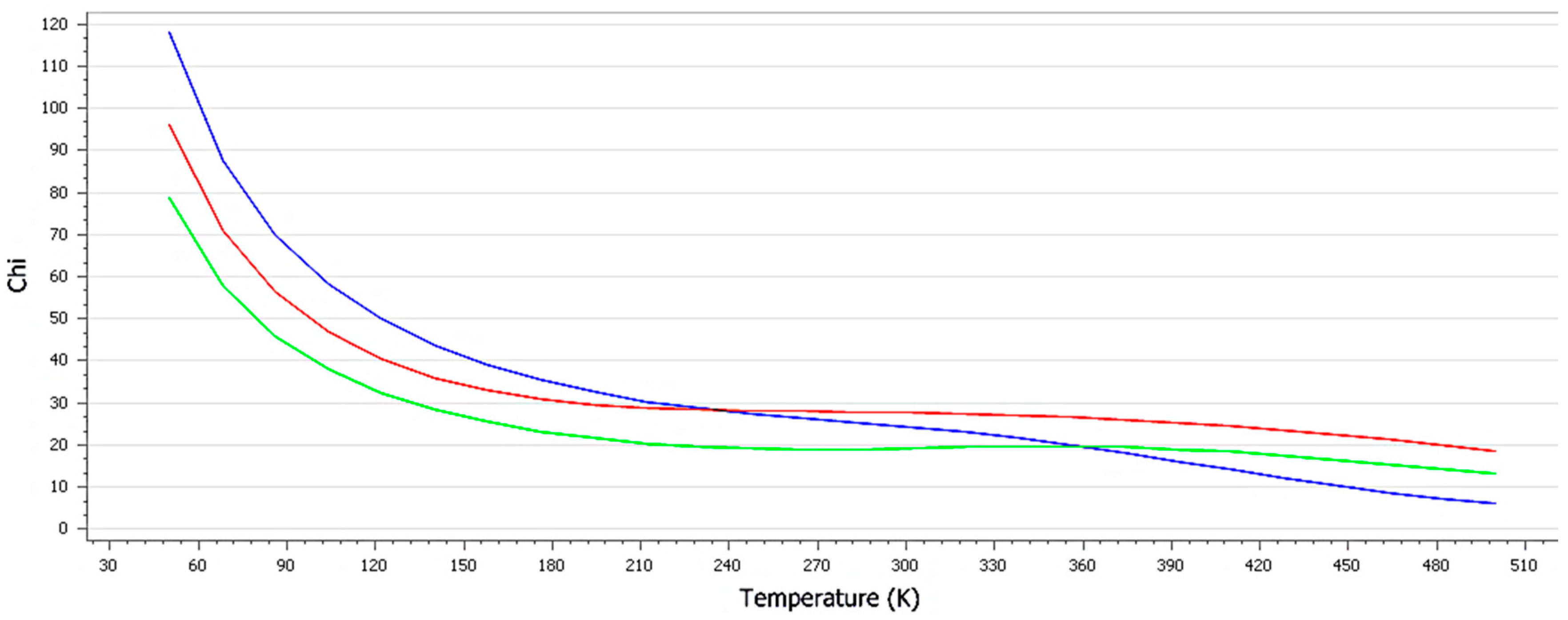
3.1. Characterization of the Formulation
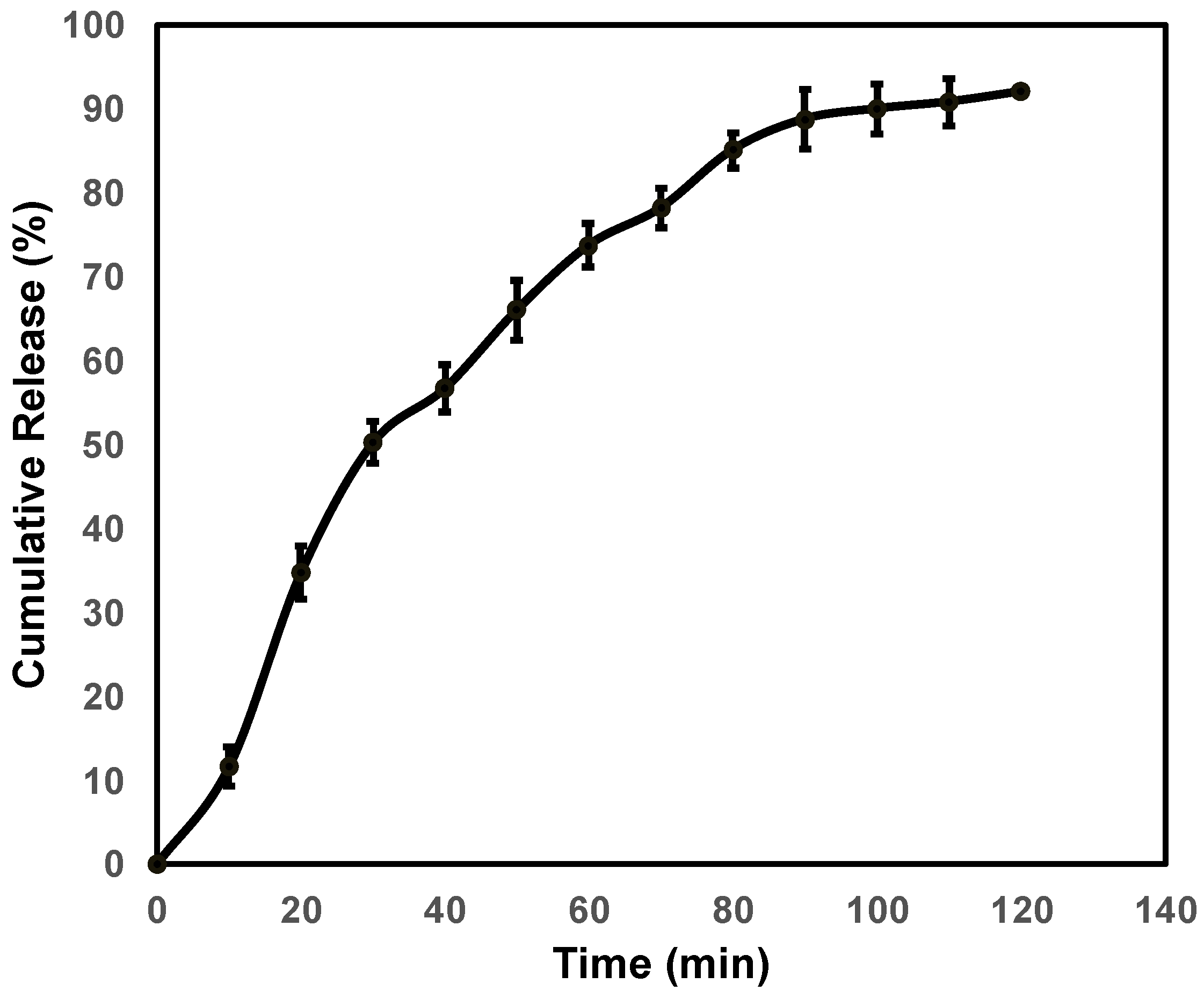
3.2. In Vitro Permeation Study of ACV-RC-SS Complex
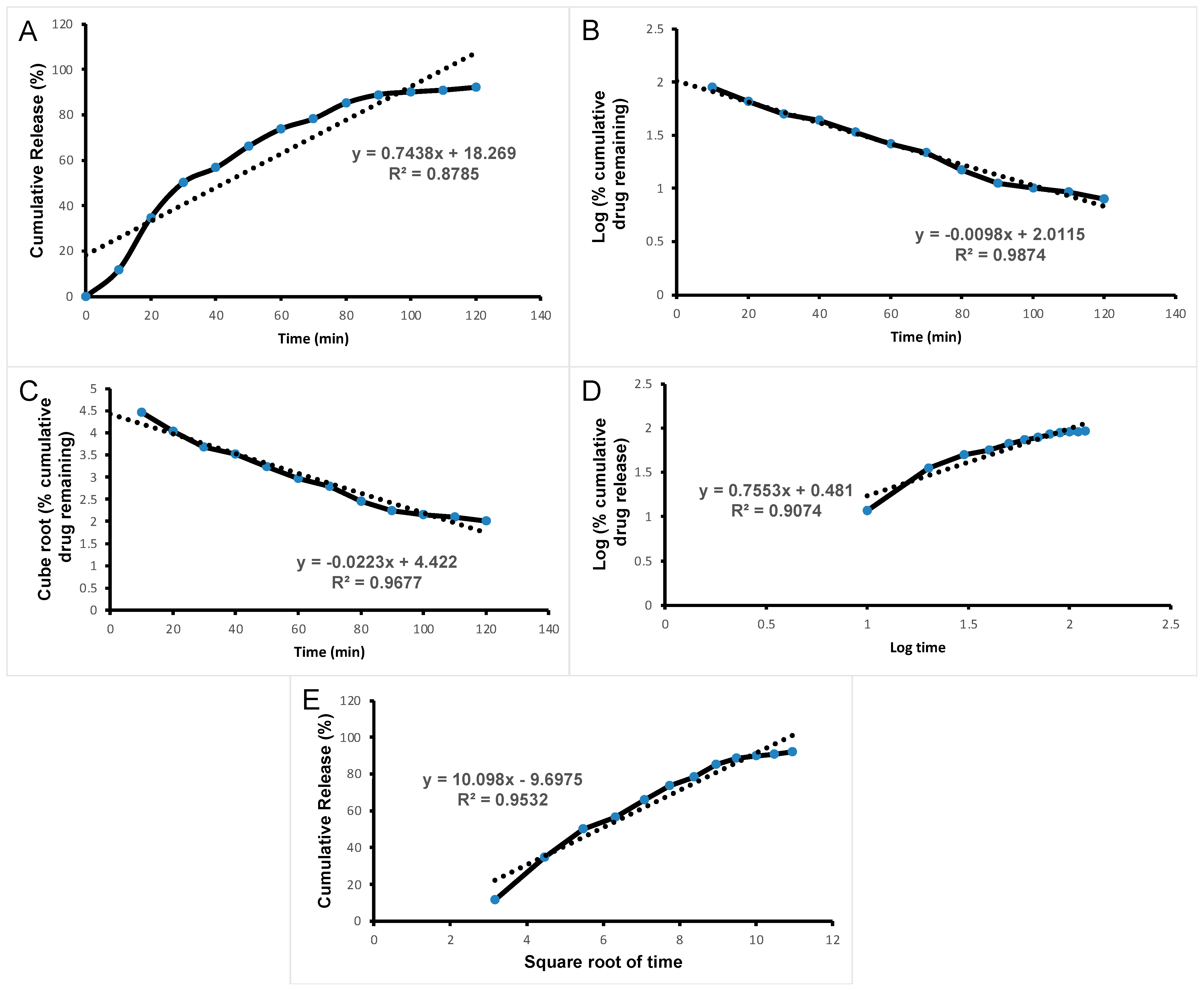
3.3. Drug Release Kinetics
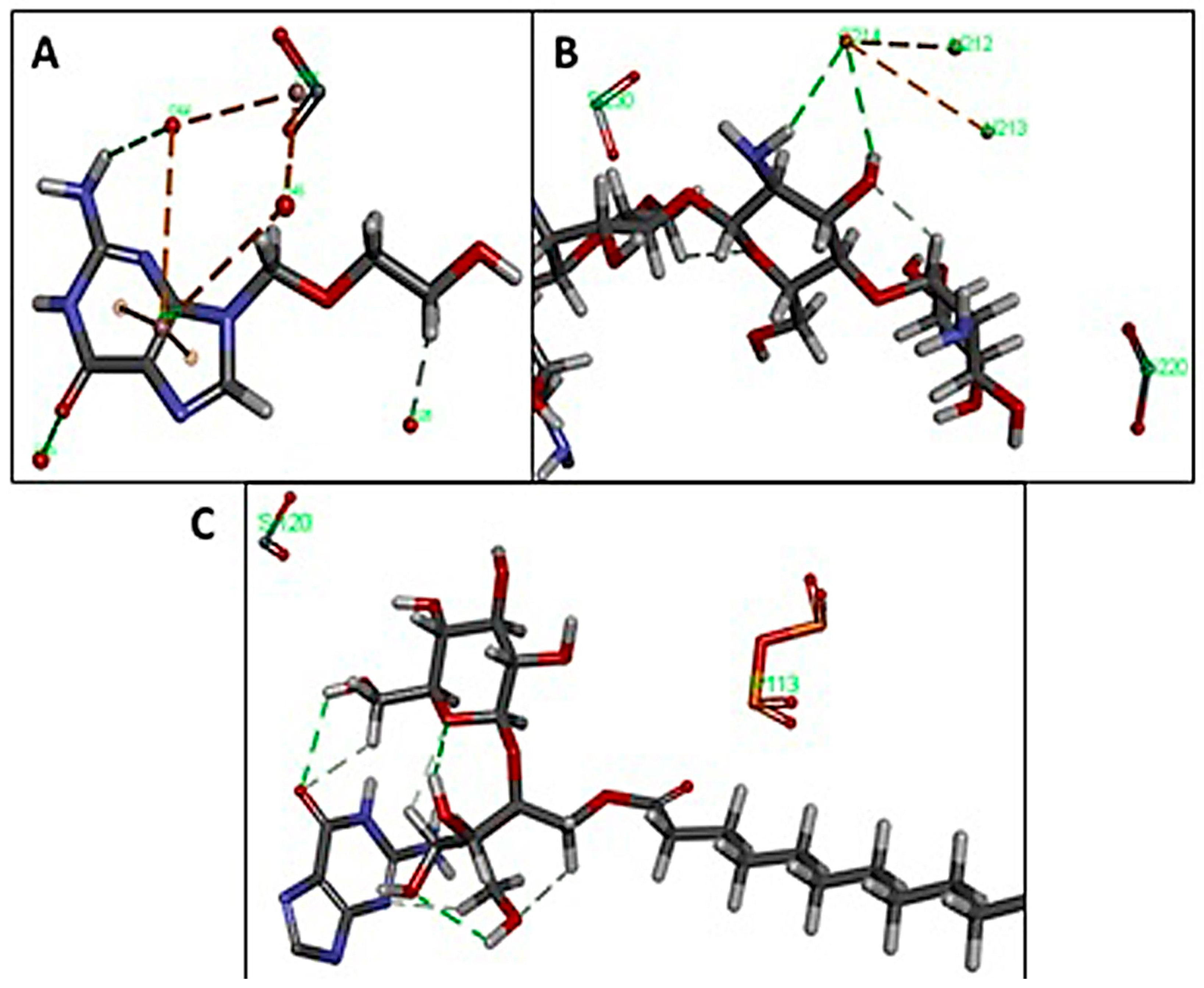
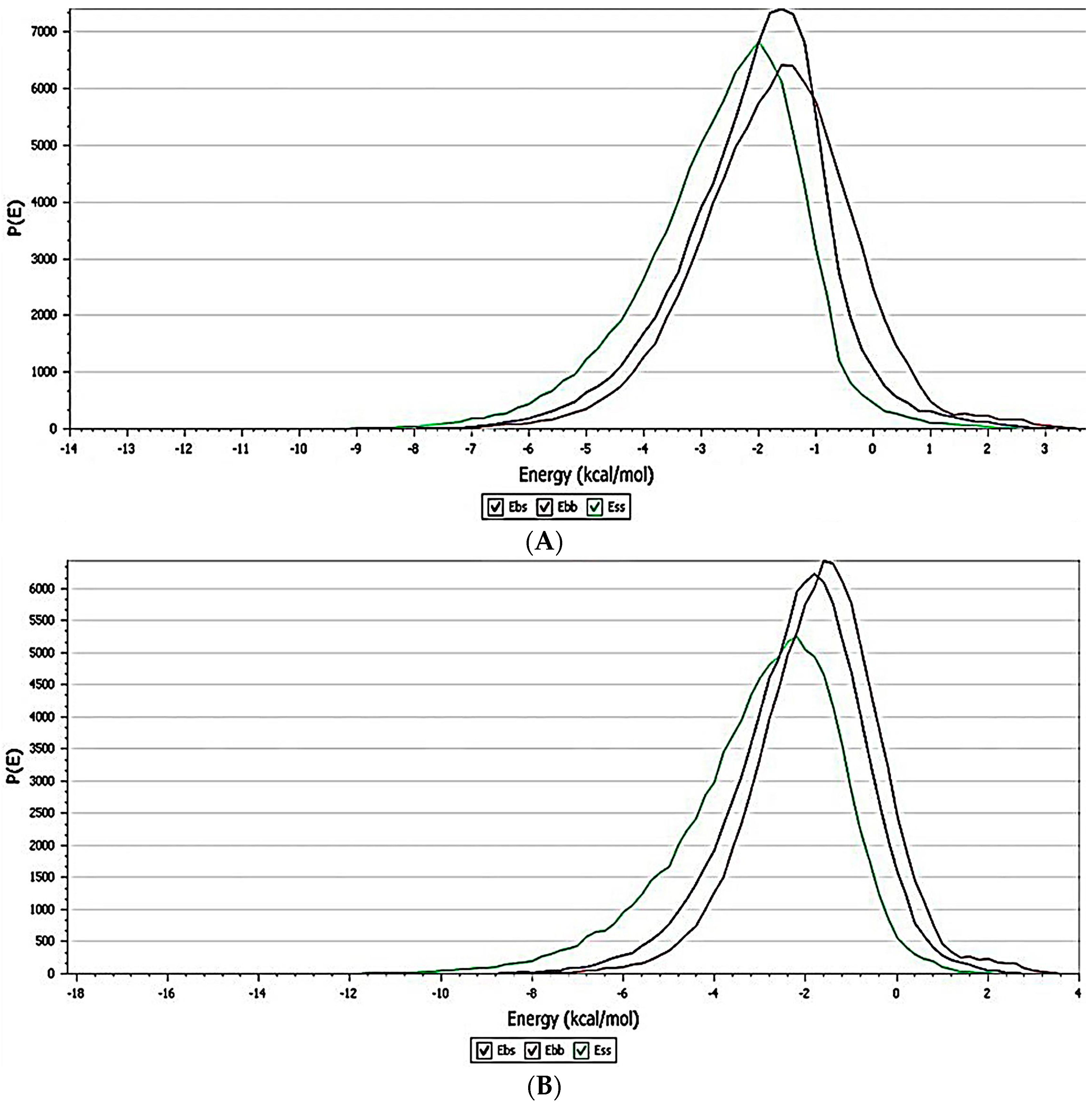
3.4. Interaction Study with In Silico Docking
3.4.1. Amorphous Cell
3.4.2. Blend Mixing
3.5. Cytotoxicity Study Using MTT
3.6. Detection of Apoptosis with AO/EB Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skin Cancer Statistics. In WCRF International. Available online: https://www.wcrf.org/cancer-trends/skin-cancer-statistics/ (accessed on 4 May 2023).
- Labani, S.; Asthana, S.; Rathore, K.; Sardana, K. Incidence of melanoma and nonmelanoma skin cancers in Indian and the global regions. J. Cancer Res. Ther. 2021, 17, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Yu, B.; Ouyang, L. Drug repurposing: An effective strategy to accelerate contemporary drug discovery. Drug Discov. Today 2022, 27, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Pfab, C.; Schnobrich, L.; Eldnasoury, S.; Gessner, A.; El-Najjar, N. Repurposing of Antimicrobial Agents for Cancer Therapy: What Do We Know? Cancers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Taylor, M.; Gerriets, V. Acyclovir; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shaimerdenova, M.; Karapina, O.; Mektepbayeva, D.; Alibek, K.; Akilbekova, D. The effects of antiviral treatment on breast cancer cell line. Infect. Agent Cancer 2017, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G.; Martinez, M.N. Applying Biopharmaceutical Classification System (BCS) Criteria to Predict Oral Absorption of Drugs in Dogs: Challenges and Pitfalls. AAPS J. 2015, 17, 948–964. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Z.; Tan, S.; Zhu, Q. Clay nanoparticles as pharmaceutical carriers in drug delivery systems. Expert Opin. Drug Deliv. 2021, 18, 695–714. [Google Scholar] [CrossRef]
- Rakhila, Y.; Ezzahi, A.; Elmchaouri, A.; Mestari, A. Synthesis and Characterization of a Red Clay Based New Composite Ceramic Material. Adv. Mater. Phys. Chem. 2018, 8, 295–310. [Google Scholar] [CrossRef]
- Roy A French Red Clay Benefits—Skin Care Ingredients—Mirah Belle. In Magento2 Store. 2020. Available online: https://mirahbelle.com/blog/post/french-red-clay/# (accessed on 4 May 2023).
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Arjunan, A.; Pajaniradje, S.; Francis, A.P.; Subramanian, S.; Chandramohan, S.; Parthasarathi, D.; Sajith, A.M.; Padusha, M.S.A.; Mathur, P.P.; Rajagopalan, R. Epigenetic modulation and apoptotic induction by a novel imidazo-benzamide derivative in human lung adenocarcinoma cells. Daru 2021, 29, 377–387. [Google Scholar] [CrossRef]
- Farshi Azhar, F.; Olad, A. A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate-chitosan/montmorillonite nanocomposite systems. Appl. Clay Sci. 2014, 101, 288–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, M.; Huang, P.; Yang, H.; Chang, S.; Hu, Y.; Tang, A.; Mao, L. Intercalated 2D nanoclay for emerging drug delivery in cancer therapy. Nano Res. 2017, 10, 2633–2643. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Zhang, Y.; He, H. Thermal effect on fluorine emission in coal and clay minerals. Environ. Earth Sci. 2017, 76, 579. [Google Scholar] [CrossRef]
- Ng, S.P.; Khor, Y.P.; Lim, H.K.; Lai, O.M.; Wang, Y.; Wang, Y.; Tan, C.P. Improved thermal properties and flow behavior of palm olein-based diacylglycerol: Impact of sucrose stearate incorporation. Processes 2021, 9, 604. [Google Scholar] [CrossRef]
- Massaro, M.; Poma, P.; Cavallaro, G.; García-Villén, F.; Lazzara, G.; Notarbartolo, M.; Muratore, N.; Sánchez-Espejo, R.; Iborra, C.V.; Riela, S. Prodrug based on halloysite delivery systems to improve the antitumor ability of methotrexate in leukemia cell lines. Colloids Surf. B Biointerfaces 2022, 213, 112385. [Google Scholar] [CrossRef]
- Leal, D.A.; Kuznetsova, A.; Silva, G.M.; Tedim, J.; Wypych, F.; Marino, C.E.B. Layered materials as nanocontainers for active corrosion protection: A brief review. Appl. Clay Sci. 2022, 225, 106537. [Google Scholar] [CrossRef]
- Huang, H.J.; Huang, S.Y.; Wang, T.H.; Lin, T.-Y.; Huang, N.-C.; Shih, O.; Jeng, U.-S.; Chu, C.-Y.; Chiang, W.-H. Clay nanosheets simultaneously intercalated and stabilized by PEGylated chitosan as drug delivery vehicles for cancer chemotherapy. Carbohydr. Polym. 2023, 302, 120390. [Google Scholar] [CrossRef] [PubMed]
- Abduljauwad, S.N.; Ahmed, H.U.R.; Moy, V.T. Melanoma treatment via non-specific adhesion of cancer cells using charged nano-clays in pre-clinical studies. Sci. Rep. 2021, 11, 2737. [Google Scholar] [CrossRef] [PubMed]
- Senthilvel, C.K.; Karuppaiyan, K.; Pothumani, A.; Vedharethinam, A.; Jose, A.W.; Mohamed, J.M.M.; El Sherbiny, M.; Ebrahim, H.A.; El Shafey, M.; Dejene, M. Development of Atorvastatin Calcium Biloaded Capsules for Oral Administration of Hypercholesterolemia. Evid.-Based Complement. Alternat. Med. 2022, 2022, 4995508. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Handbook of Clay Science Part A. Fundamentals, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar]
- Pajaniradje, S.; Mohankumar, K.; Pamidimukkala, R.; Subramanian, S.; Rajagopalan, R. Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. BioMed Res. Int. 2014, 2014, 474953. [Google Scholar] [CrossRef] [PubMed]




| Component | Percentage | Component | Percentage |
|---|---|---|---|
| CuO | 0.01% | K2O | 0.18% |
| ZnO | 0.02% | MgO | 1.57% |
| CaO | 2.10% | Na2O | 0.80% |
| P2O5 | 0.12% | Fe2O3 | 4.29% |
| SO3 | 0.75% | MnO2 | 0.09% |
| Al2O3 | 11.44% | SiO2 | 59.32% |
| Sample | Zeta Potential (mV) | Particle Size (nm) |
|---|---|---|
| RC | −19.0 ± 7.6 | 938.0 ± 97.5 |
| SS | −39.3 ± 7.9 | 851.0 ± 87.3 |
| ACV | −16.9 ± 7.3 | 193.4 ± 20.0 |
| Nanocomplex F1 | −22.2 ± 5.1 | 426.0 ± 31.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, A.P.; Ahmad, A.; Nagarajan, S.D.D.; Yogeeswarakannan, H.S.; Sekar, K.; Khan, S.A.; Meenakshi, D.U.; Husain, A.; Bazuhair, M.A.; Selvasudha, N. Development of a Novel Red Clay-Based Drug Delivery Carrier to Improve the Therapeutic Efficacy of Acyclovir in the Treatment of Skin Cancer. Pharmaceutics 2023, 15, 1919. https://doi.org/10.3390/pharmaceutics15071919
Francis AP, Ahmad A, Nagarajan SDD, Yogeeswarakannan HS, Sekar K, Khan SA, Meenakshi DU, Husain A, Bazuhair MA, Selvasudha N. Development of a Novel Red Clay-Based Drug Delivery Carrier to Improve the Therapeutic Efficacy of Acyclovir in the Treatment of Skin Cancer. Pharmaceutics. 2023; 15(7):1919. https://doi.org/10.3390/pharmaceutics15071919
Chicago/Turabian StyleFrancis, Arul Prakash, Aftab Ahmad, Sri Durga Devi Nagarajan, Harish Sundar Yogeeswarakannan, Krishnaraj Sekar, Shah Alam Khan, Dhanalekshmi Unnikrishnan Meenakshi, Asif Husain, Mohammed A. Bazuhair, and Nandakumar Selvasudha. 2023. "Development of a Novel Red Clay-Based Drug Delivery Carrier to Improve the Therapeutic Efficacy of Acyclovir in the Treatment of Skin Cancer" Pharmaceutics 15, no. 7: 1919. https://doi.org/10.3390/pharmaceutics15071919
APA StyleFrancis, A. P., Ahmad, A., Nagarajan, S. D. D., Yogeeswarakannan, H. S., Sekar, K., Khan, S. A., Meenakshi, D. U., Husain, A., Bazuhair, M. A., & Selvasudha, N. (2023). Development of a Novel Red Clay-Based Drug Delivery Carrier to Improve the Therapeutic Efficacy of Acyclovir in the Treatment of Skin Cancer. Pharmaceutics, 15(7), 1919. https://doi.org/10.3390/pharmaceutics15071919










