New Highly Fluorescent Water Soluble Imidazolium-Perylenediimides: Synthesis and Cellular Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Singlet Oxygen Detection
2.3. Cell Culture
2.4. In Vitro Photocytotoxicity and Cell Viability Assays
2.5. Subcellular Localization and Fluorescence Microscopy
2.6. Starting Materials
2.7. Synthesis of PDI Derivatives
2.7.1. Synthesis of N,N′-di(ethylpropyl)-1-[2′,6′-(imidazol-1′′-ylmethyl)-4′-methylphenoxy]perylene-3,4:9,10-tetracarboxydiimide (PDI-3)
2.7.2. Synthesis of N,N′-di(ethylpropyl)-1,7(6)-di [2′,6′-(imidazol-1′′-ylmethyl)-4′-methylphenoxy]perylene-3,4:9,10-tetracarboxydiimide (PDI-4)
2.7.3. Synthesis of N,N′-di(ethylpropyl)-1-[2′,6′-(3′′-methylimidazolium-1′′-ylmethyl)-4′-methylphenoxy]perylene-3,4:9,10-tetracarboxydiimide diiodide (PDI-1)
2.7.4. Synthesis of N,N′-di(ethylpropyl)-1,7(6)-di [2′,6′-(3′′-methylimidazolium-1′′-ylmethyl)-4′-methylphenoxy]perylene-3,4:9,10-tetracarboxydiimide tetraiodide (PDI-2)
2.8. Statistical Analysis
3. Results
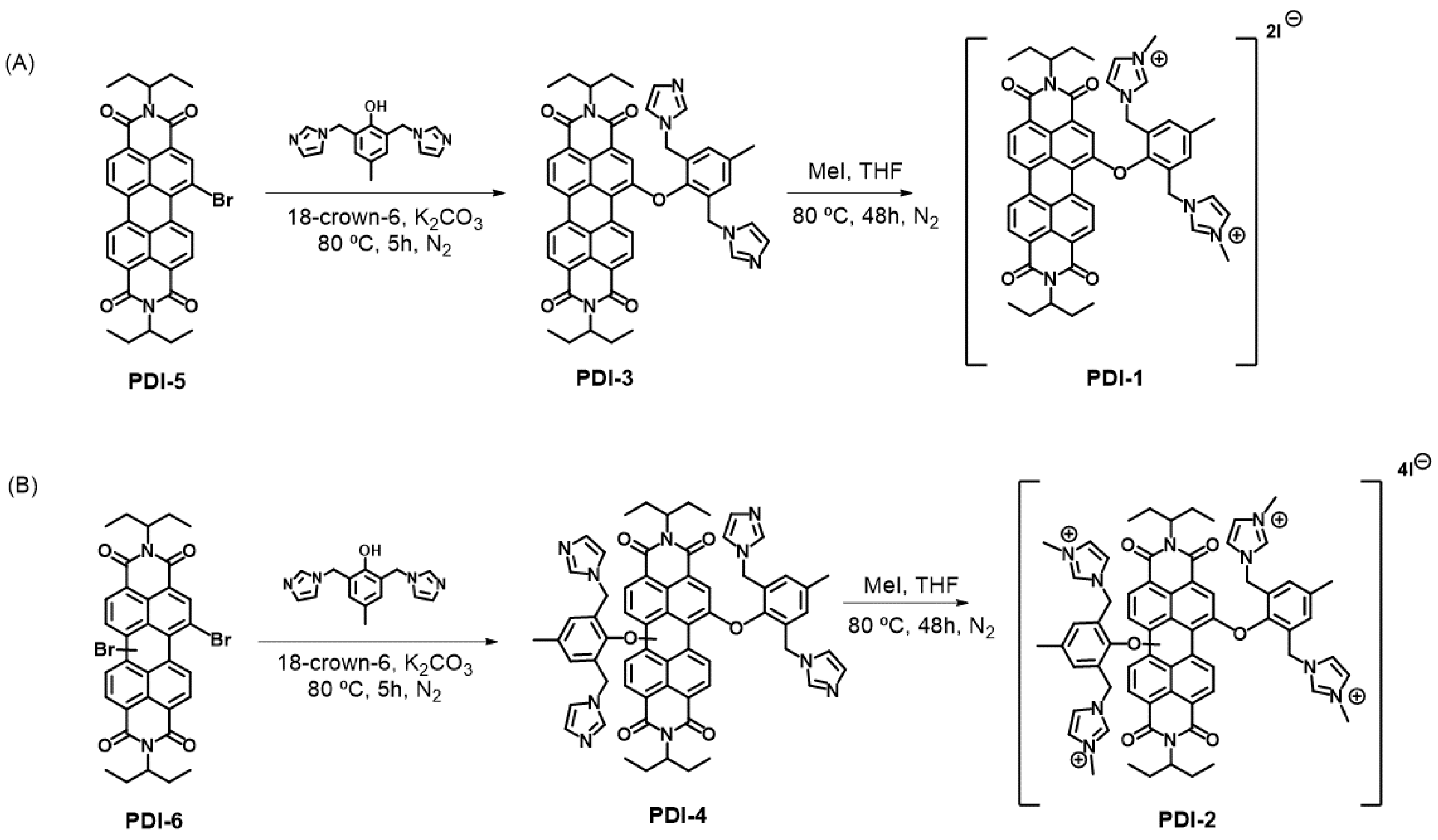
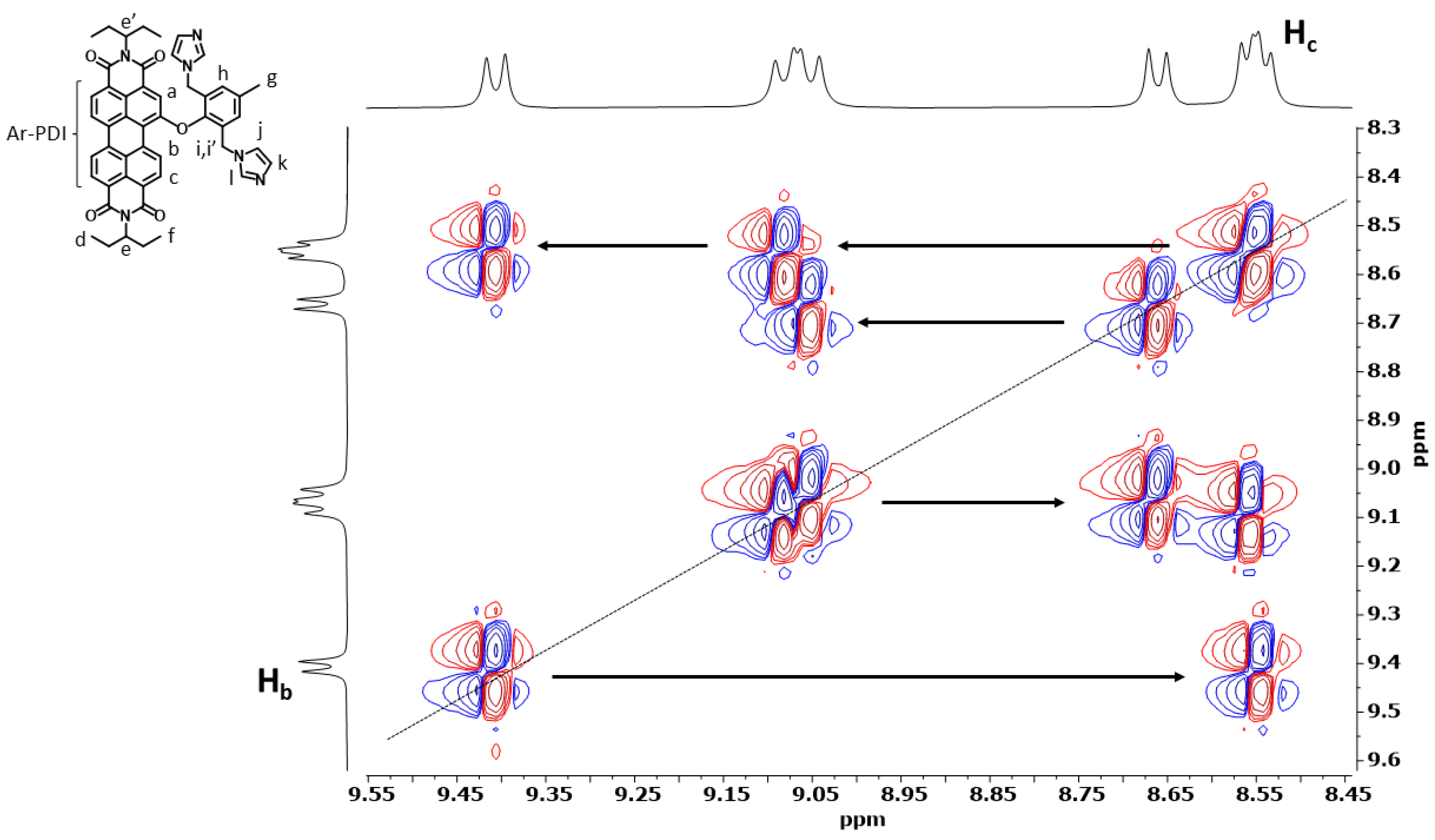
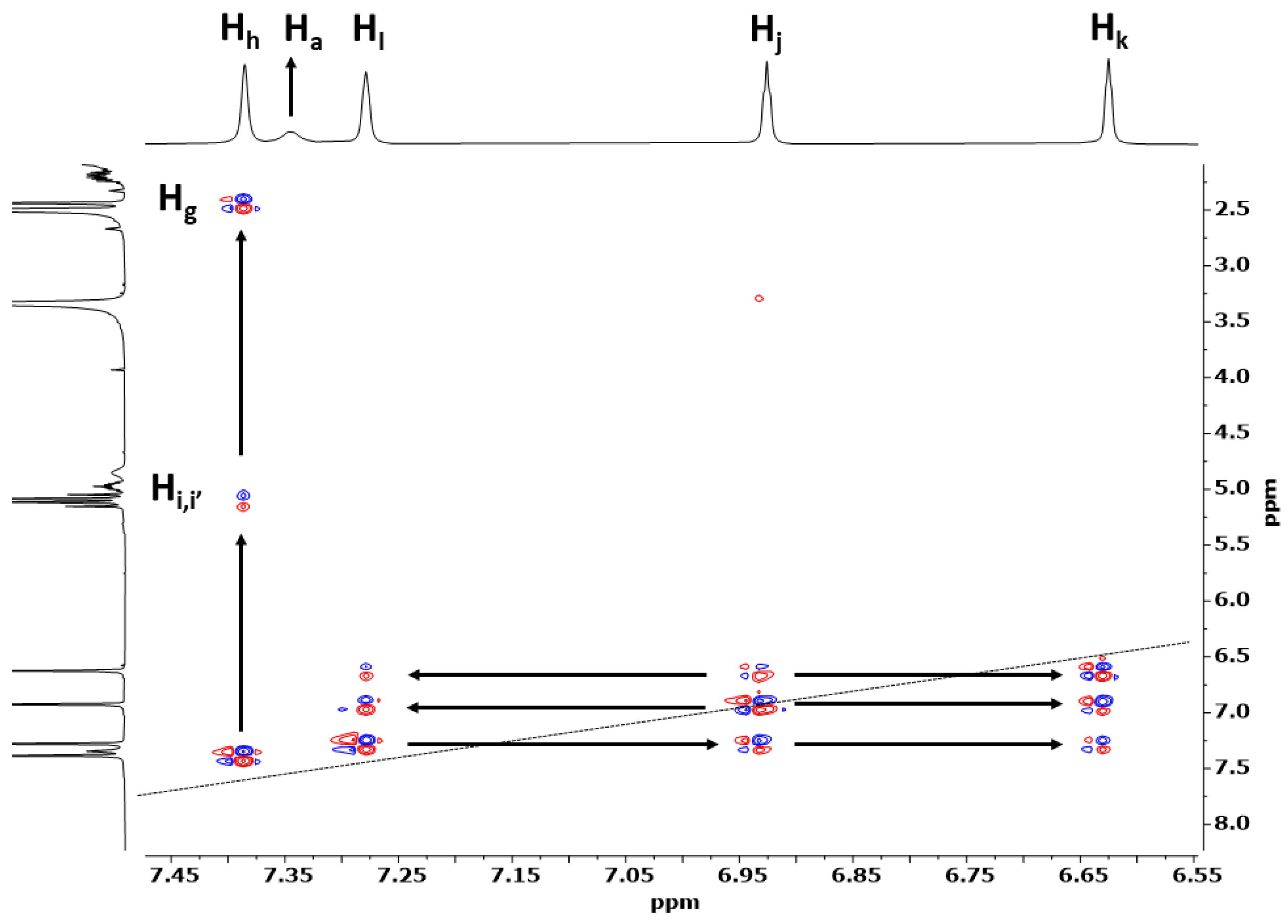
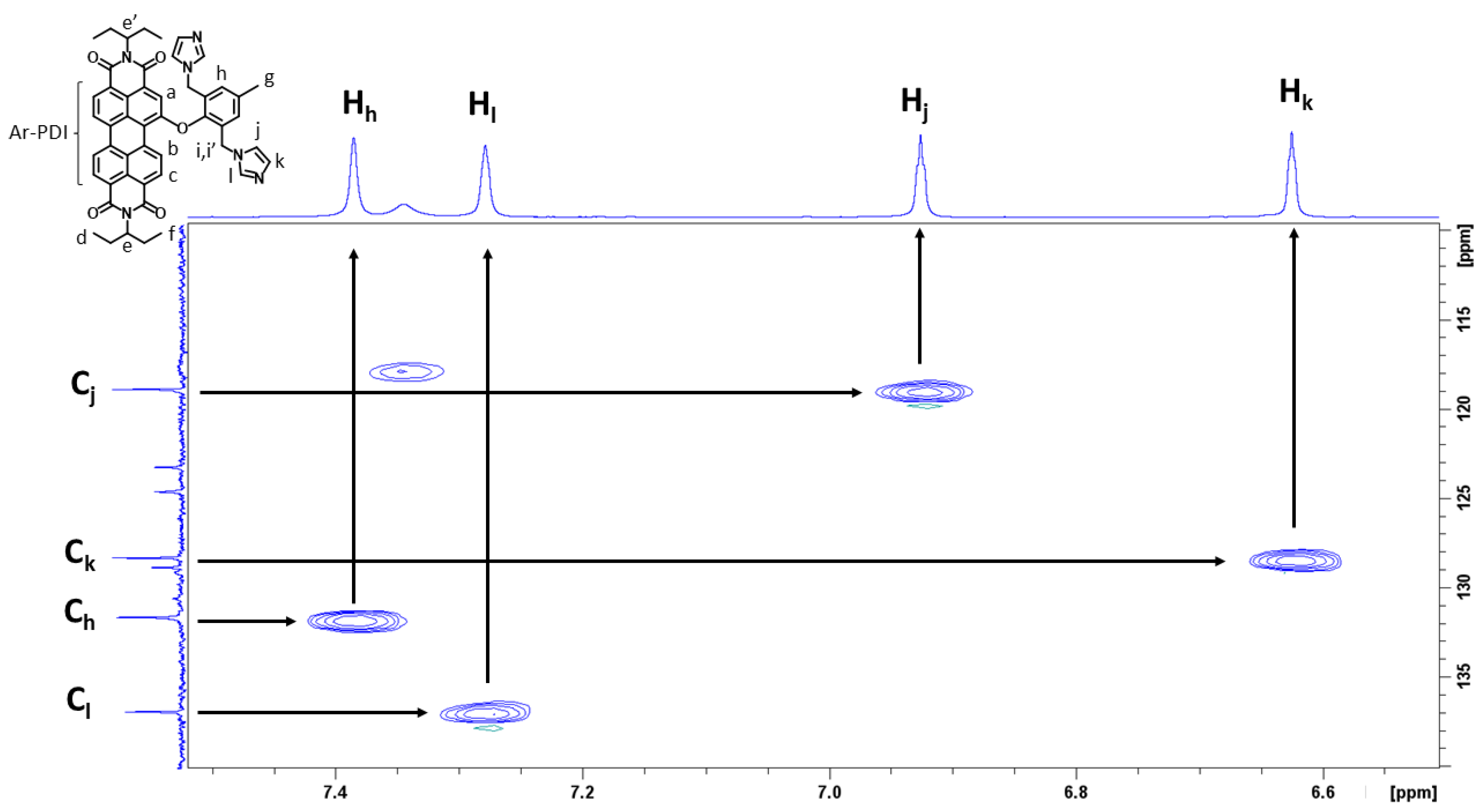
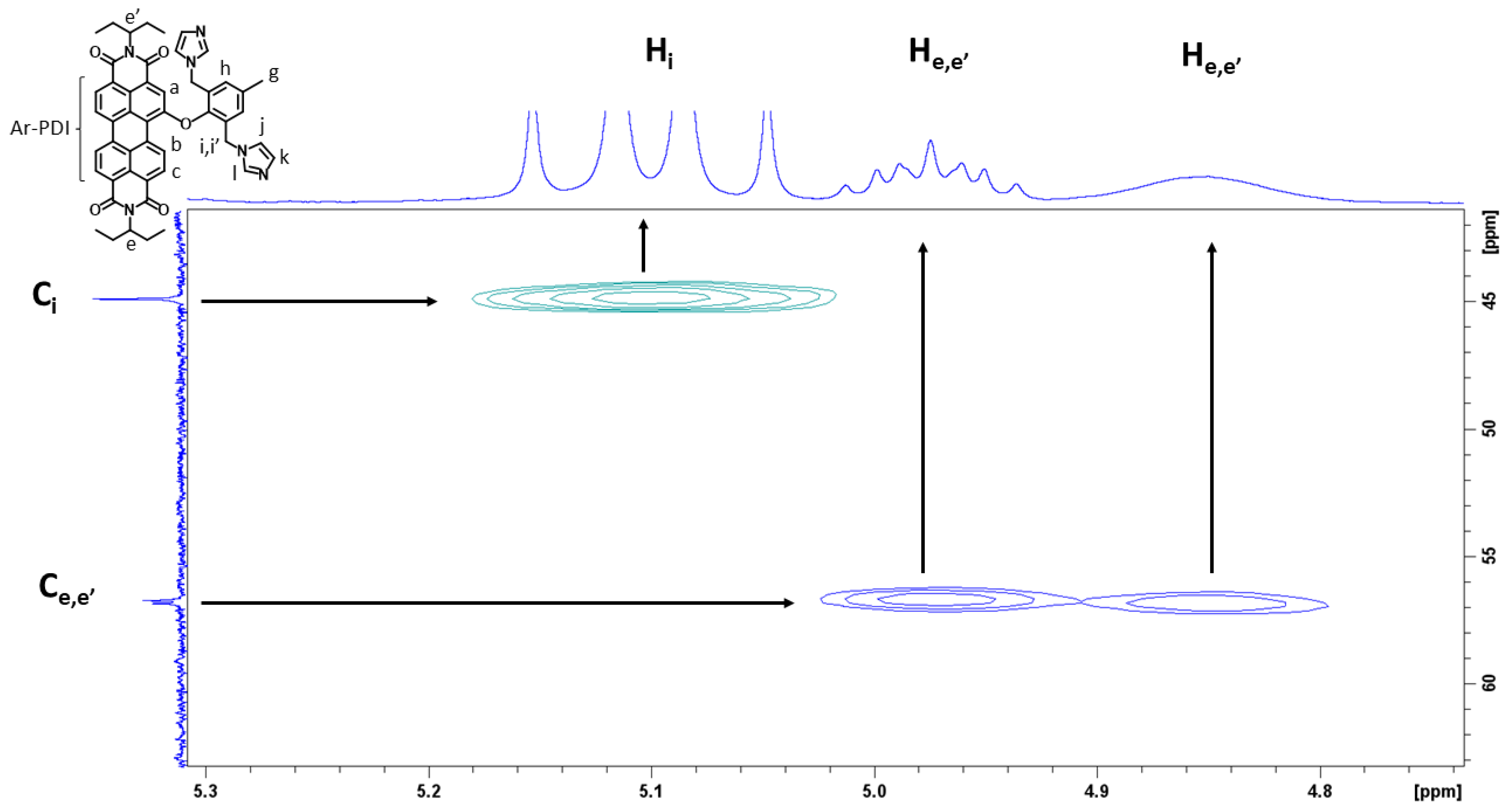
3.1. Preparation and Characterization of PDI-1–PDI-4
3.2. Optical Properties
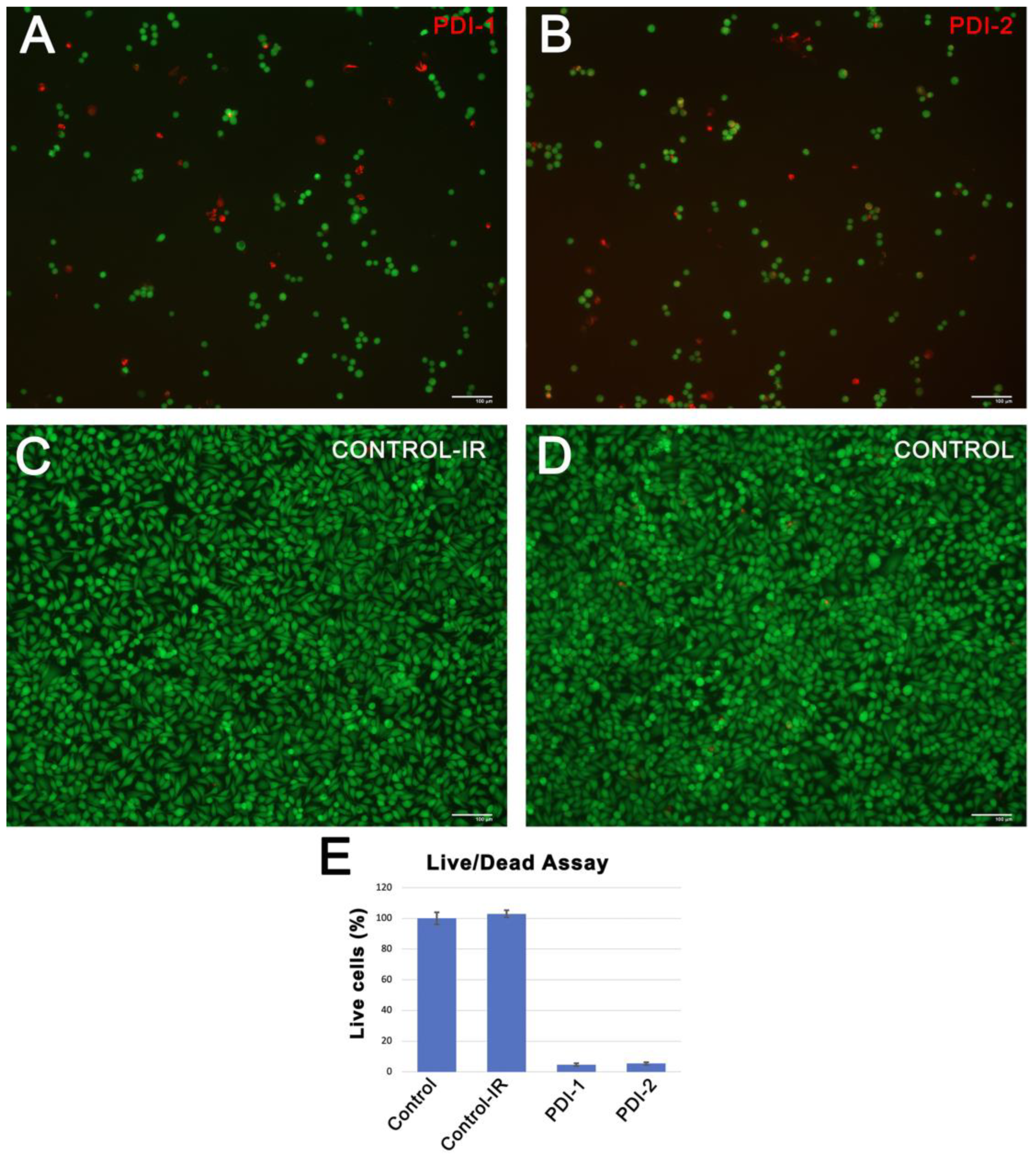
3.3. Cellular Uptake and Cytotoxicity of PDIs In Vitro
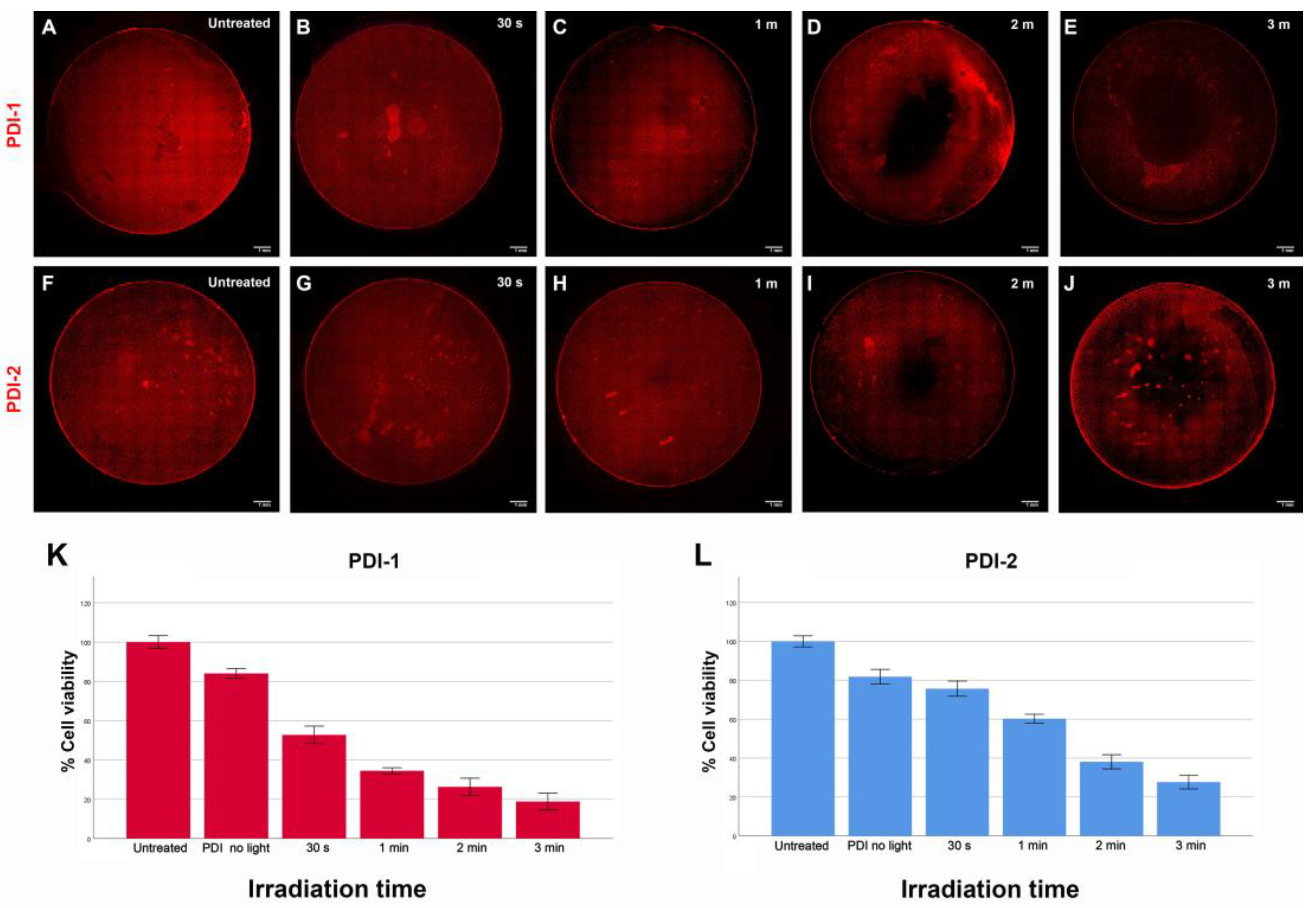
3.4. Phototoxicity of PDIs Induced by PDT
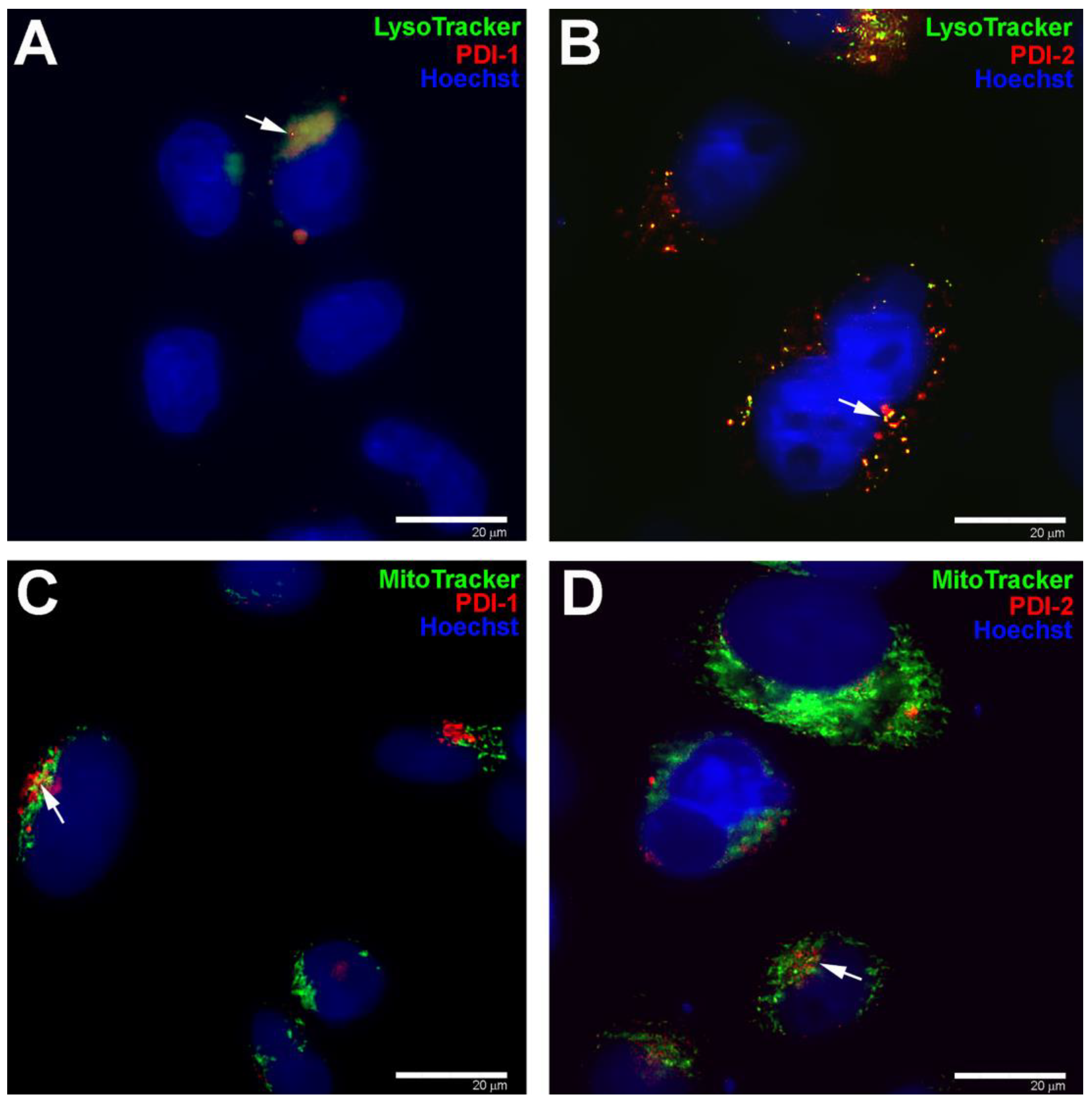
3.5. Subcellular Localization of PDIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy An Up-to-Date Review. Appl. Sci. 2021, 11, 3636. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L.J. Photodynamic therapy in cancer treatment-an update review. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 10291. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Sato, K.; Higio, S.; Tani, Y.; Mori, T.; Ohkubo, K.; Osakada, Y.; Fujitsuka, M.; Kida, T. Amphiphilic Rhodamine Nano-assembly as Type I Supramolecular Photosensitizer for Photodynamic Therapy. ACS Appl. Nano Mater. 2022, 5, 14954–14960. [Google Scholar]
- Linares, I.A.P.; Martinelli, L.P.; Moritz, M.N.O.; Selistre-de-Arujo, H.S.; de Oliveira, K.T.; Perussi, J.R.J. Cytotoxicity of structurally-modified chlorins aimed for photodynamic therapy applications. Photochem. Photobiol. 2022, 425, 113647. [Google Scholar] [CrossRef]
- Huang, L.; Asghar, S.; Zhu, T.; Ye, P.; Hu, Z.; Chen, Z.; Xiao, Y. Advances in chlorin-based photodynamic therapy with nanoparticle delivery system for cancer treatment. Expert. Opin. Drug. Deliv. 2021, 18, 1473–1500. [Google Scholar] [CrossRef] [PubMed]
- Pratavieira, S.; Uliana, M.C.; dos Santos Lopes, N.S.; Donatoni, C.; Rodriguez-Linares, D.; da Freitas Anibal, F.; Thiago de Oliveira, K.; Kurachi, C.; Oliveira da Souza, C.W. Photodynamic therapy with a new bacteriochlorin derivative: Characterization and in vitro studies. Photodiagnosis. Photodyn. Ther. 2021, 34, 102251. [Google Scholar] [CrossRef]
- Luo, T.; Ni, K.; Culbert, A.; Lan, G.; Li, Z.; Jiang, X.; Kaufmann, M.; Lin, W. Nanoscale Metal-Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 7334–7339. [Google Scholar] [CrossRef]
- Wolfsen, H.C. Porfimer sodium photodynamic therapy: The long road to acceptance in America. Photodiagnosis Phtodyn. Ther. 2007, 4, 242–243. [Google Scholar] [CrossRef]
- Roguin, L.P.; Chiarante, N.; García-Vior, M.C.; Marino, J. Zinc (II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef]
- Ezquerra-Riega, S.D.; Valli, F.; Rodriguez, H.B.; Marino, J.; Roguin, L.P.; Lantaño, B.; García-Vior, M.C. Chalcogen bearning tetrasubstituted zinc (II) phthalocyanines for CT26 colon carcinoma cells photodynamic therapy. Dyes Pigm. 2022, 201, 110110. [Google Scholar] [CrossRef]
- Yurttaş, A.G.; Sevim, A.M.; Çinar, K.; Atmaca, G.Y.; Erdoğmuş, A.; Gül, A. The effects of zinc (II) phthalocyanine photosensitizers on biological activities of epitheloid cérvix carcinoma cells and precise determination of absorbed fluence at a specific wavelenght. Dyes Pigm. 2022, 198, 110012. [Google Scholar] [CrossRef]
- Zhang, W.; Ahmed, A.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Application of multifunctional BODIPY in photodynamic therapy. Dyes Pigm. 2021, 185, 108937. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Ballatore, M.B.; Durantini, A.M.; Durantini, E.N.; Tomé, A.C.J. BODIPYs in antitumoral and antimicrobial photodynamic therapy: An integrating review. Photochem. Photobiol. 2019, 40, 21–48. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwilkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Atchison, J.; Kamila, S.; Nesbitt, H.; Logan, K.A.; Nicholas, D.M.; Fowley, C.; Davis, J.; Callan, B.; McHale, A.; Callan, J.F. Iodinated cyanine dyes: A new class of sensitisers for use in NIR activated photodynamic therapy (PDT). Chem. Commun. 2017, 53, 2009–2012. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y.J. Regiosiomer-manipulating thio-perylenediimide nanoagents for phtothermal/photodynamic theranostics. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-C.; Wang, C.-H.; Chang, K.-H.; Ho, J.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- Semeraro, P.; Syrgiannis, Z.; Bettini, S.; Giancane, G.; Guerra, F.; Fraix, A.; Bucci, C.; Sortino, S.; Prato, M.; Valli, L.J. Singlet oxygen photo-production by perylene bisimide derivative Langmuir-Schaefer films for photodynamic therapy applications. Colloid Interface Sci. 2019, 553, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, X.; Wang, G.; Deng, W.; Shen, Q.; Jiang, R.; Wang, W.; Fan, Q.; Huang, W.J. A perylene diimide zwitterionic polymer for photoacoustic imaging guieded photothermal/photodynamic synergistic therapy with single near-infrared irradiation. Mater. Chem. B 2018, 6, 3395–3403. [Google Scholar] [CrossRef]
- Aksakal, N.E.; Kazan, H.H.; Eçik, E.T.; Yuksel, F. A novel photosensitizer based on a ruthenium (II) phenanthroline bis(perylenediimide) dyad: Synthesis, generation of singlet oxygen and in vitro photodynamic therapy. New J. Chem. 2018, 42, 17538–17545. [Google Scholar] [CrossRef]
- Tiburcio, M.A.; Rocha, A.R.; Romano, R.A.; Inada, N.M.; Bagnato, V.S.; Carlos, R.M.; Buzzá, H.H.J. In vitro evaluation of the cis-[Ry(phen)2(pPDIp)]2+ complex for antimicrobial photodynamic therapy against Sporothrix brasiliensis and Candida albicans. Photochem. Photobiol. B 2022, 229, 112414. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Myong, M.S.; Qi, Y.; Stern, C.; Wasielewski, M.R.J. Ultrafast photo-driven charge transfer exciton dynamics in mixed-stack pyrene-perylenediimide single co-crystals. Mater. Chem. C 2021, 9, 16911–16917. [Google Scholar] [CrossRef]
- Kaufmann, C.; Kim, W.; Nowak-Król, A.; Hong, Y.; Kim, D.; Würthner, F. Ultrafast Exciton Delocalization. Localization. And Excimer Formation Dynamics in a Highly Defined Perylene Bisimide Quadruple π-Stack. J. Am. Chem. Soc. 2018, 140, 4253–4258. [Google Scholar] [CrossRef]
- Smith, A.G.; Shuford, L.J. Photovoltaic and charge transfer analysis of perylene diimide dimer systems. Photochem. Photobiol. 2018, 367, 115–123. [Google Scholar] [CrossRef]
- Papadopoulos, I.; Gutierrez-Moreno, D.; Bo, Y.; Casillas, R.; Greibel, P.M.; Clark, T.; Fernández-Lázaro, F.; Guldi, D.M. Altering singlet fission pathways in perylene-dimers; perylene-diimide versus perylene-monoimide. Nanoscale 2022, 14, 5194–5203. [Google Scholar] [CrossRef]
- Carlotti, B.; Madu, I.K.; Kim, H.; Cai, Z.; Jiang, H.; Muthike, A.; Yu, L.; Zimmerman, P.M.; Goodsin, T. Activating intramolecular singlet exciton fission by altering π-bridge flexibility in perylene diimide trimers for organic solar cells. Chem. Sci. 2020, 11, 8757–8770. [Google Scholar] [CrossRef]
- Muñoz-Mármol, R.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Zink-Lorre, N.; Sastre-Santos, Á.; Aragó, J.; Ortí, E.; Baronas, P.; Litvinas, D.; et al. Effect of Substituents at Imide Position on the Laser Performance of 1,7-Bay –Substituted Perylenediimide Dyes. J. Phys. Chem. C 2021, 125, 12277–12288. [Google Scholar] [CrossRef]
- Li, H.; Wenger, O. Photophysics of Perylene Diimide Dianions and Therir Application in Photoredox Catalysis. Angew. Chem. 2022, 134, e202110491. [Google Scholar]
- Koenig, J.D.B.; Piers, W.E.; Welch, G.C. Promoting photocatalytic CO2 reduction through facile electronic modification of N-annulated perylene diimide rhenium bipyridine dyads. Chem. Sci. 2022, 13, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Gredilla, P.; García-Calvo, J.; Cuevas, J.V.; Torroba, T.; Pablos, J.L.; García, F.C.; García, J.M.; Zink-Lorre, N.; Font-Sanchis, E.; Sastre-Santos, Á.; et al. Solvent-Free Off-On Detection of the Improvised Explosive Triacetone (TATP) with Fluorogenic Materials. Chem. Eur. J. 2017, 23, 13973–13979. [Google Scholar] [CrossRef]
- Céspedes-Guirao, F.J.; Ropero, A.B.; Font-Sanchis, E.; Nadal, Á.; Fernández-Lázaro, F.; Sastre-Santos, Á. A water-soluble perylenedye functionalised with a 17β-estradiol: A new fluorescent tool for steroid hormones. Chem. Commun. 2011, 47, 8307–8309. [Google Scholar] [CrossRef]
- Gálvez, N.; Kedracka, E.J.; Carmona, F.; Céspedes-Guirao, F.J.; Font-Sanchis, E.; Fernández-Lázaro, F.; Sastre-Santos, F.; Domínguez-Vera, J.M.J. Water soluble fluorescent-magnetic perylenediimide-containing maghemite-nanoparticles for bimodal MRI/OI imaging. Inorg. Biochem. 2012, 117, 205–211. [Google Scholar] [CrossRef]
- Matussek, M.; Filapek, M.; Gancarz, P.; Krompiec, S.; Malecki, J.G.; Kotowicz, S.; Siwy, M.; Mackoski, S.; Chrobok, A.; Schab-Balcezark, E.; et al. Synthesis and photophysical properties of new perylene bisimide derivatives for application as emitting materials in OLEDs. Dyes Pigm. 2018, 159, 590–599. [Google Scholar] [CrossRef]
- Giri, D.; Raut, S.K.; Patra, S.K. Diketopyrrolopyrrole/perylene-diimide and thiophene based D-π-A low bandgap polymer sensitizers for application in dye sensitized solar cells. Dyes Pigm. 2020, 174, 10832. [Google Scholar] [CrossRef]
- Tilley, A.J.; Guo, C.; Miltenburg, M.B.; Schon, T.B.; Yan, H.; Li, Y.; Seferos, D.S. Thionation Enhances the Electron Mobility of Perylene Diimide for High Performance n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2015, 25, 3321–3329. [Google Scholar] [CrossRef]
- Deger, S.N.; Weishäupl, S.J.; Pöthig, A.; Fischer, R.A. A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation. Energies 2022, 15, 2437. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wei, J.; Yin, M. Perylenediimide chromophore as an efficient photothermal agent for cancer therapy. Sci. Bull. 2018, 63, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Zimmerman, S.C. Polyglycerol-Dendronized Perylenediimides as Stable, Water-Soluble Fluorophores. Adv. Funct. Mater. 2012, 22, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Donnier-Maréchal, M.; Galanos, N.; Grandjean, T.; Pascla, Y.; Ji, D.K.; Dong, L.; Gillon, E.; He, X.P.; Imberty, A.; Kipins, E.; et al. Perylenediimide-based glycoclusters as high affinity ligands of bacterial lectins: Synthesis, binding studied and anti-adhesive properties. Org. Biomol. Chem. 2017, 15, 10037–10043. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, K.; Yu, J.; Sun, H.; Tang, J.; Shen, J.; Müllen, K.; Yang, W.; Yin, M. A Unique Perylene-Based DNA intercalator: Localization in Cell Nuclei and Inhibition of Cancer Cells and Tumors. Small 2014, 10, 4087–4092. [Google Scholar] [CrossRef]
- Battagliarin, G.; Davies, M.; Mackowiak, S.; Li, C.; Müllen, K. Ortho-Functionalized Perylenediimides for Highly Fluorescent Water-Soluble Dyes. ChemPhysChem 2012, 13, 923–926. [Google Scholar] [CrossRef]
- Zhong, L.; Xing, F.; Shi, W.; Yan, L.; Xie, L.; Zhu, S. Synthesis, Spectra, and Electron-Transfer Reaction of Aspartic Acid-Functionalized Water-Soluble Perylene Bisimide in Aqueous Solution. ACS Appl. Mater. Interfaces 2013, 5, 3401–3407. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchiski, B.J. Selective Bromination of Perylene Diimides under Mild Conditions. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, L.; Zhang, S.; Su, X.; Lan, J.; Chen, C.T.; You, J. Self-assembly from metal-organic vesicles to globular networks: Metallogel-mediated phenylation of indole with phenyl boronic acid. Chem. Commun. 2010, 46, 3938–3940. [Google Scholar] [CrossRef]
- Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 17 November 2022).
- Makhseed, S.; Macháček, M.; Alfadly, W.; Tuhl, A.; Vinodh, M.; Šimunek, T.; Novakova, V.; Kubat, P.; Rudolf, E.; Zimčík, P. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem. Commun. 2013, 49, 11149–11151. [Google Scholar] [CrossRef]
- Macháček, M.; Demuth, J.; Cermak, P.; Vavreckova, M.; Hruba, L.; Jedlickova, A.; Kubat, P.; Šimunek, T.; Novakova, V.; Zimčík, P.J. Tetra(3,4-pyrido)porphyrazines Caught in the Cationinc Cage: Toward Nanomolar Active Photosensitizers. Med. Chem. 2016, 59, 9443–9456. [Google Scholar] [CrossRef]
- Macháček, M.; Carter, K.A.; Kostelanský, F.; Miranda, D.; Seffouh, A.; Ortega, J.; Šimunek, T.; Zimčík, P.; Lovell, J.F.J. Binding of an amphilic phthalocyanine to pre-formed liposomes confers light-triggered cargo release. Mater. Chem. B 2018, 6, 7298–7305. [Google Scholar] [CrossRef]
- Gorman, A.; Rodgers, M.A. Current perspectives of singlet oxygen detection in biological environments. J. Photochem. Photobiol. B 1992, 14, 159–176. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Improving the Phototherapeutic Efficiencies of Molecular and Nanoscale Materials by Targeting Mitochondria. Molecules 2018, 23, 3016. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Huang, R.; Fang, L.; Sun, Y.; Yang, Y.; Gou, S.; Zhao, J. An Effective Supramolecular Approach to Boost the Photodynamic Therapy Efficacy of a Near-Infrared Activating Perylene Diimide-Based Photosensitizer. ACS Materials Lett. 2022, 4, 657–664. [Google Scholar] [CrossRef]
- Nascimento, B.F.O.; Laranjo, M.; Pereira, N.A.M.; Dias-Ferreira, J.; Piñeiro, M.; Botelho, M.F.; Pinho e Melo, T.M. Ring-Fused Diphenylchlorins as Potent Photosensitizers for Photodynamic Therapy Applications: In Vitro Tumor Cell Biology and in Vivo Chick Embryo Chorioallantoic Membrane Studies. ACS Omega 2019, 4, 17244–17250. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Turchiello, R.; Kowaltowski, A.J.; Indig, G.L.; Baptista, M.S. Major determinants of photoinduced cell death: Subcellular localization versus photosensitization efficiency. Free Radic. Biol. Med. 2011, 51, 824–833. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C.J. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Röder, B. Singlet oxyen mediated photodynamic effects. Photonics Lasers Med. 2015, 4, 271–272. [Google Scholar] [CrossRef]
- Bagnyukova, T.V.; Serebriiskii, I.G.; Zhou, Y.; Hopper-Borge, E.A.; Golemis, E.A.; Astsaturov, I. How can targeted therapies supercharge cytotoxic agents? Cancer Biol. Ther. 2010, 10, 839–853. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Vasir, J.K.; Labhasetwar, V. Targeted drug delivery in cancer therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef] [PubMed]



| Absorption Maxima (λmax, nm) | Emission (λmax, nm) | Fluorescence Quantum Yield (%) | |
|---|---|---|---|
| PDI-1 | 506, 544 | 561, 604 | 31 |
| PDI-2 | 512, 551 | 571, 603 | 53 |
| PDI-3 | 470, 501, 538 | 550, 594 | 75 |
| PDI-4 | 477, 508, 547 | 560, 602 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés-Garcés, J.; Sánchez-Martos, M.; Martinez-Navarrete, G.; Fernández-Jover, E.; Encheva, M.; León, M.; Ortiz, J.; Sastre-Santos, Á.; Fernández-Lázaro, F. New Highly Fluorescent Water Soluble Imidazolium-Perylenediimides: Synthesis and Cellular Response. Pharmaceutics 2023, 15, 1892. https://doi.org/10.3390/pharmaceutics15071892
Garcés-Garcés J, Sánchez-Martos M, Martinez-Navarrete G, Fernández-Jover E, Encheva M, León M, Ortiz J, Sastre-Santos Á, Fernández-Lázaro F. New Highly Fluorescent Water Soluble Imidazolium-Perylenediimides: Synthesis and Cellular Response. Pharmaceutics. 2023; 15(7):1892. https://doi.org/10.3390/pharmaceutics15071892
Chicago/Turabian StyleGarcés-Garcés, José, Miguel Sánchez-Martos, Gema Martinez-Navarrete, Eduardo Fernández-Jover, Mirela Encheva, Martín León, Javier Ortiz, Ángela Sastre-Santos, and Fernando Fernández-Lázaro. 2023. "New Highly Fluorescent Water Soluble Imidazolium-Perylenediimides: Synthesis and Cellular Response" Pharmaceutics 15, no. 7: 1892. https://doi.org/10.3390/pharmaceutics15071892
APA StyleGarcés-Garcés, J., Sánchez-Martos, M., Martinez-Navarrete, G., Fernández-Jover, E., Encheva, M., León, M., Ortiz, J., Sastre-Santos, Á., & Fernández-Lázaro, F. (2023). New Highly Fluorescent Water Soluble Imidazolium-Perylenediimides: Synthesis and Cellular Response. Pharmaceutics, 15(7), 1892. https://doi.org/10.3390/pharmaceutics15071892








