The Peptide Salamandrin-I Modulates Components Involved in Pyroptosis and Induces Cell Death in Human Leukemia Cell Line HL-60
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Isolation and Cell Culture
2.2. MTT Assay
2.3. Proliferation Assay
2.4. Cell Cycle Analysis
2.5. Apoptosis Detection
2.6. Determination of Lactate Dehydrogenase (LDH) Release
2.7. Measurement of Mitochondrial Membrane Potential (ΔΨm)
2.8. Caspase-1 Inflammasome Assay
2.9. RNA Extraction, cDNA Synthesis, and Real-Time PCR
2.10. Statistical Analysis
3. Results
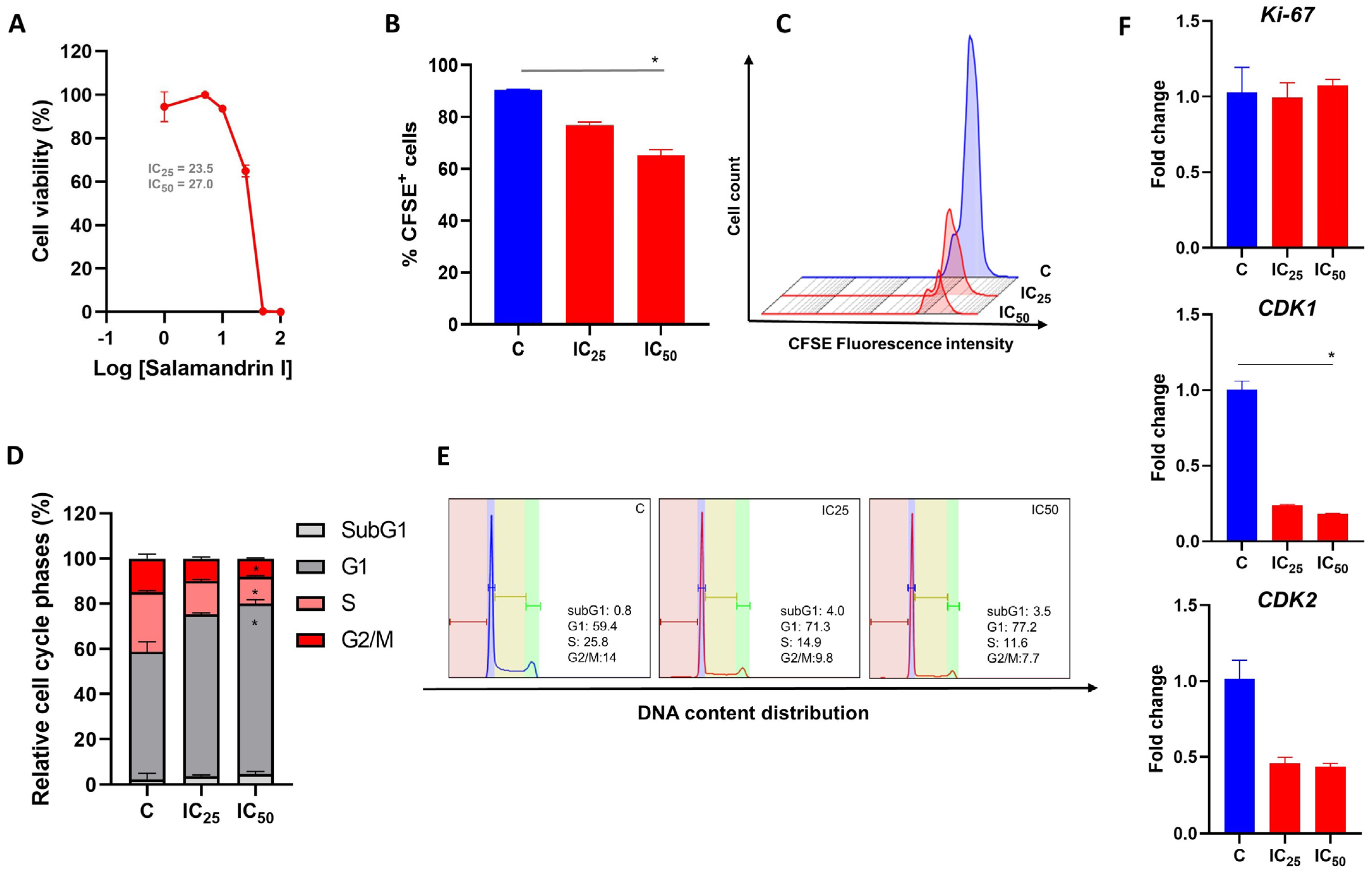
3.1. Salamandrin-I Treatment Decreases Human Leukemia Cell Viability
3.2. Salamandrin-I Controls Cell Proliferation and Promotes Cell Cycle Arrest
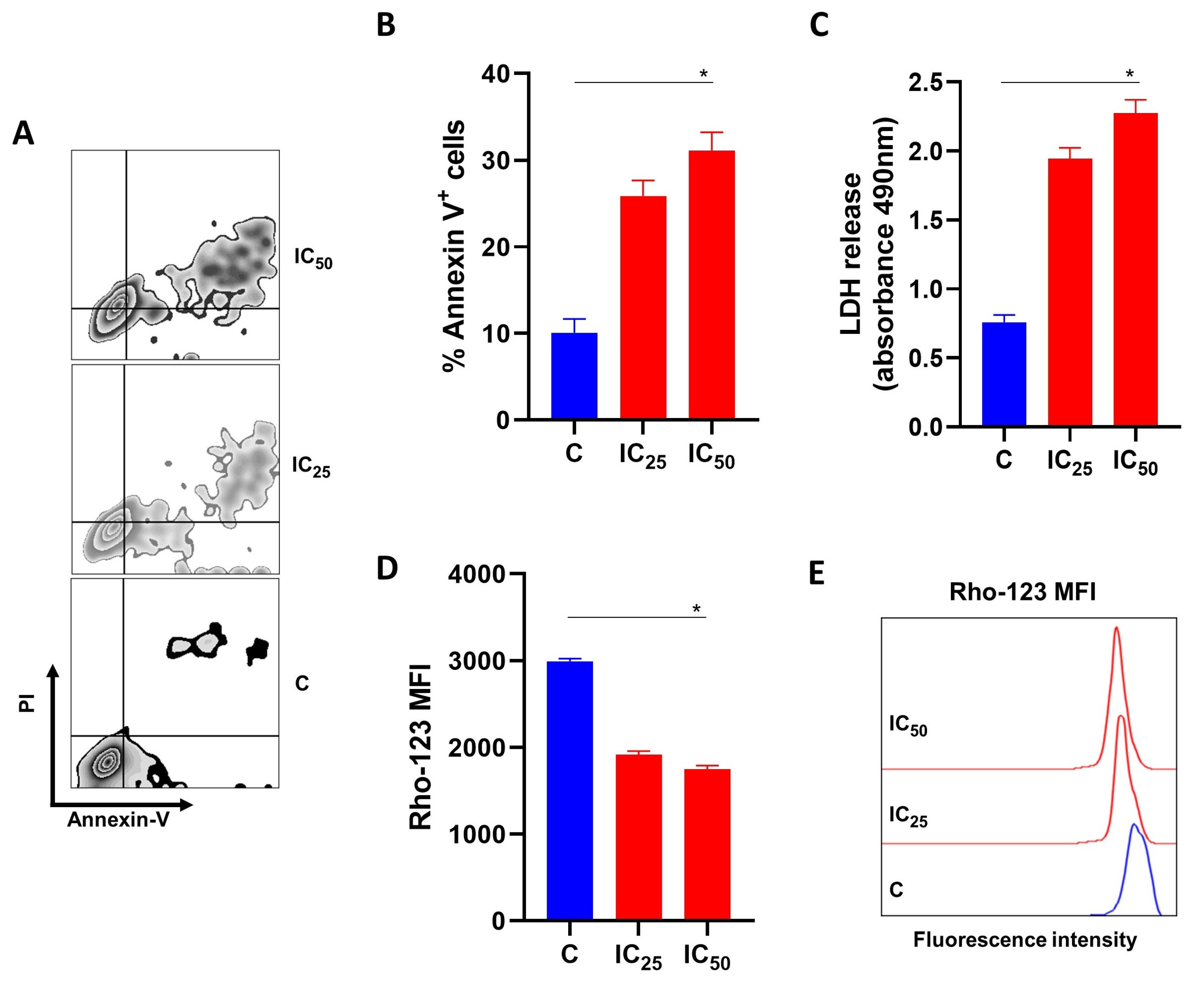
3.3. Salamandrin-I Induces Cell Death in Human Leukemia Cell Line
3.4. Salamandrin-I Induces LDH Releases and Compromises the Mitochondrial Membrane Potential of Human Leukemia Cell Lines
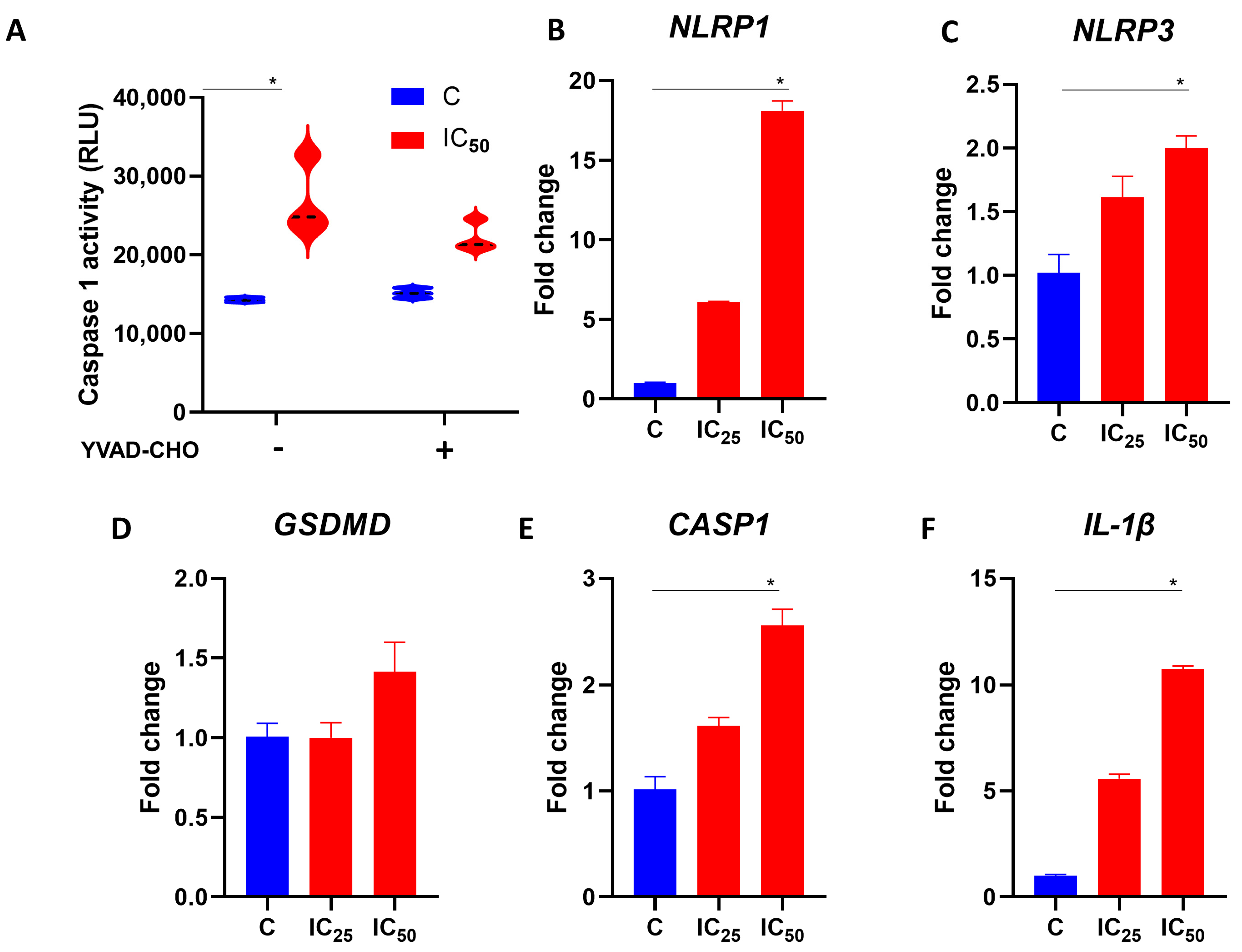
3.5. Salamandrin-I Activates Caspase-1 and Modulates Inflammasome Components in Human Leukemia Cell Line
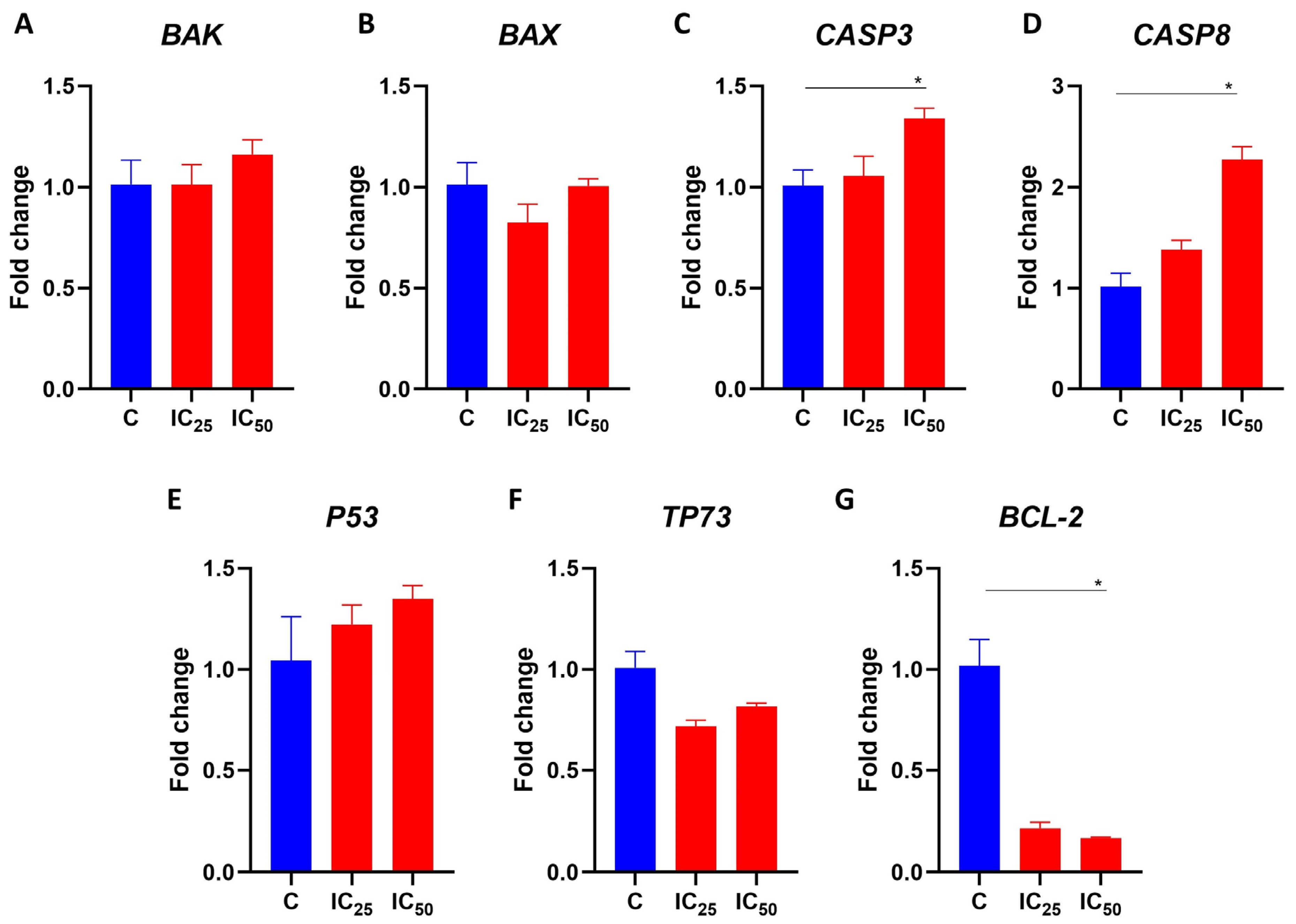
3.6. Salamandrin-I Increases Transcriptional Levels of CASP8 and Inhibits BCL-2 in HL-60 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, M.E.H.; Benson, R.B.J.; Skutschas, P.; Hill, L.; Panciroli, E.; Schmitt, A.D.; Walsh, S.A.; Evans, S.E. Middle Jurassic Fossils Document an Early Stage in Salamander Evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2114100119. [Google Scholar] [CrossRef]
- Arenas Gómez, C.M.; Echeverri, K. Salamanders: The Molecular Basis of Tissue Regeneration and Its Relevance to Human Disease. Curr. Top. Dev. Biol. 2021, 145, 235–275. [Google Scholar]
- Lüddecke, T.; Schulz, S.; Steinfartz, S.; Vences, M. A Salamander’s Toxic Arsenal: Review of Skin Poison Diversity and Function in True Salamanders, Genus Salamandra. Naturwissenschaften 2018, 105, 56. [Google Scholar] [CrossRef]
- Mechkarska, M.; Attoub, S.; Sulaiman, S.; Pantic, J.; Lukic, M.L.; Conlon, J.M. Anti-Cancer, Immunoregulatory, and Antimicrobial Activities of the Frog Skin Host-Defense Peptides Pseudhymenochirin-1Pb and Pseudhymenochirin-2Pa. Regul. Pept. 2014, 194–195, 69–76. [Google Scholar] [CrossRef]
- Ojo, O.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Frog Skin Peptides (tigerinin-1R, Magainin-AM1, -AM2, CPF-AM1, and PGla-AM1) Stimulate Secretion of Glucagon-like Peptide 1 (GLP-1) by GLUTag Cells. Biochem. Biophys. Res. Commun. 2013, 431, 14–18. [Google Scholar] [CrossRef]
- Indriani, S.; Karnjanapratum, S.; Nirmal, N.P.; Nalinanon, S. Amphibian Skin and Skin Secretion: An Exotic Source of Bioactive Peptides and Its Application. Foods 2023, 12, 1282. [Google Scholar] [CrossRef]
- Plácido, A.; Bueno, J.; Barbosa, E.A.; Moreira, D.C.; do Dias, J.N.; Cabral, W.F.; Albuquerque, P.; Bessa, L.J.; Freitas, J.; Kuckelhaus, S.A.S.; et al. The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra). Biomolecules 2020, 10, 512. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Plácido, A.; do Pais do Amaral, C.; Teixeira, C.; Nogueira, A.; Brango-Vanegas, J.; Alves Barbosa, E.; Moreira, D.C.; Silva-Carvalho, A.É.; da Gloria da Silva, M..; do Nascimento Dias, J.; et al. Neuroprotective Effects on Microglia and Insights into the Structure-Activity Relationship of an Antioxidant Peptide Isolated from Pelophylax Perezi. J. Cell. Mol. Med. 2022, 26, 2793–2807. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Nascimento Costa, J.M.; et al. Oxidative Stress and Mitochondrial Dysfunction Play a Role in Myelodysplastic Syndrome Development, Diagnosis, and Prognosis: A Pilot Study. Free Radic. Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; do Araújo, M.C.; Schetinger, M.R.C.; Morsch, V.M. Measurement of Oxidative Stress and Antioxidant Status in Acute Lymphoblastic Leukemia Patients. Clin. Biochem. 2008, 41, 511–518. [Google Scholar] [CrossRef]
- Zhou, F.-L.; Zhang, W.-G.; Wei, Y.-C.; Meng, S.; Bai, G.-G.; Wang, B.-Y.; Yang, H.-Y.; Tian, W.; Meng, X.; Zhang, H.; et al. Involvement of Oxidative Stress in the Relapse of Acute Myeloid Leukemia. J. Biol. Chem. 2010, 285, 15010–15015. [Google Scholar] [CrossRef]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.K.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-Derived ROS in AML Promotes Proliferation and Is Associated with Defective Oxidative Stress Signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute Myeloid Leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef]
- de Thé, H.; Chomienne, C.; Lanotte, M.; Degos, L.; Dejean, A. The t(15;17) Translocation of Acute Promyelocytic Leukaemia Fuses the Retinoic Acid Receptor Alpha Gene to a Novel Transcribed Locus. Nature 1990, 347, 558–561. [Google Scholar] [CrossRef]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Zhou, G.-B.; Zhang, J.; Wang, Z.-Y.; Chen, S.-J.; Chen, Z. Treatment of Acute Promyelocytic Leukaemia with All-Trans Retinoic Acid and Arsenic Trioxide: A Paradigm of Synergistic Molecular Targeting Therapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 959–971. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Z.; Jin, Y.; Chen, Y.; Yang, T.; Yang, Q.; Wu, B.; Shang, Y.; Liu, X.; Wei, Y.; et al. Azelaic Acid Exerts Antileukemia Effects against Acute Myeloid Leukemia by Regulating the Prdxs/ROS Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1295984. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenserbaerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer Peptide: Physicochemical Property, Functional Aspect and Trend in Clinical Application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-Cycle Checkpoints and Cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Mammalian Cyclin-Dependent Kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To Cycle or Not to Cycle: A Critical Decision in Cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef]
- Kalous, J.; Jansová, D.; Šušor, A. Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase. Cells 2020, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-Dependent Kinases and Cell-Cycle Transitions: Does One Fit All? Nat. Rev. Mol. Cell Biol. 2008, 9, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-Cancer Activity of Maize Bioactive Peptides. Front Chem 2017, 5, 44. [Google Scholar] [CrossRef]
- Zhan, W.; Liao, X.; Li, L.; Chen, Z.; Tian, T.; Yu, L.; Chen, Z. In Vitro Mitochondrial-Targeted Antioxidant Peptide Induces Apoptosis in Cancer Cells. Onco. Targets. Ther. 2019, 12, 7297–7306. [Google Scholar] [CrossRef]
- Luna-Vargas, M.P.A.; Chipuk, J.E. The Deadly Landscape of pro-Apoptotic BCL-2 Proteins in the Outer Mitochondrial Membrane. FEBS J. 2016, 283, 2676–2689. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond - Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Dewson, G.; Kluck, R.M. Mechanisms by Which Bak and Bax Permeabilise Mitochondria during Apoptosis. J. Cell Sci. 2009, 122, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Peña-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The Interplay between BAX and BAK Tunes Apoptotic Pore Growth to Control Mitochondrial-DNA-Mediated Inflammation. Mol. Cell 2022, 82, 933–949.e9. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kang, T.-B. The Molecular Links between Cell Death and Inflammasome. Cells 2019, 8, 1057. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and Diseases. Signal Transduct Target Ther 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 Promotes NLRP1/NLRP3 Inflammasome Activation and IL-1β Production in Acute Glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | TCAACGACCACTTTGTCAAGCTCAGCT |
| Reverse | GGTGGTCCAGGGGTCTTAC | |
| CASP1 | Forward | AAGACCCGAGCTTTGATTGACTC |
| Reverse | AAATCTCTGCCGACTTTTGTTTCC | |
| CASP3 | Forward | CTAGCGGATGGGTGCTATTG |
| Reverse | GATACACAGCCACAGGTATGAG | |
| CASP8 | Forward | GGATGGCCACTGTGAATAACTG |
| Reverse | TCGAGGACATCGCTCTCTCA | |
| P53 | Forward | AGAAAACCTACCAGGGCAGC |
| Reverse | ACATCTTGTTGAGGGCAGGG | |
| TP73 | Forward | GGAAGATGGCCCAGTCCAC |
| Reverse | GGGAAGGTCGAAGTAGGTGC | |
| BAX | Forward | CAGACCGTGACCATCTTTGT |
| Reverse | GCCTCAGCCCATCTTCTTC | |
| BAK | Forward | GTTTTCCGCAGCTACGTTTTT |
| Reverse | GCAGAGGTAAGGTGACCATCTC | |
| NLRP1 | Forward | AAGGGGCAGGCCACTCTCCCTC |
| Reverse | TGAGGCAGAGATTTCTCTCCAG | |
| NLRP3 | Forward | TCCTCGGTACTCAGCACTAATCAG |
| Reverse | GGTCGCCCAGGTCATTGTTG | |
| GSDMD | Forward | ATGAGGTGCCTCCACAACTTCC |
| Reverse | CCAGTTCCTTGGAGATGGTCTC | |
| IL-1β | Forward | AGAAGTACCTGAGCTCGCCA |
| Reverse | TGTTTAGGGCCATCAGCTTCA | |
| CDK1 | Forward | CTTGGCTTCAAAGCTGGCTC |
| Reverse | GGGTATGGTAGATCCCGGCT | |
| CDK2 | Forward | CCAGGAGTTACTTCTATGCCTGA |
| Reverse | TTCATCCAGGGGAGGTACAAC | |
| KI-67 | Forward | TAACACCATCAGCAGGGAAAG |
| Reverse | CTGCACTGGAGTTCCCATAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Carvalho, A.É.; Oliveira, N.N.d.; Machado, J.V.L.; Moreira, D.C.; Brand, G.D.; Leite, J.R.S.A.; Plácido, A.; Eaton, P.; Saldanha-Araujo, F. The Peptide Salamandrin-I Modulates Components Involved in Pyroptosis and Induces Cell Death in Human Leukemia Cell Line HL-60. Pharmaceutics 2023, 15, 1864. https://doi.org/10.3390/pharmaceutics15071864
Silva-Carvalho AÉ, Oliveira NNd, Machado JVL, Moreira DC, Brand GD, Leite JRSA, Plácido A, Eaton P, Saldanha-Araujo F. The Peptide Salamandrin-I Modulates Components Involved in Pyroptosis and Induces Cell Death in Human Leukemia Cell Line HL-60. Pharmaceutics. 2023; 15(7):1864. https://doi.org/10.3390/pharmaceutics15071864
Chicago/Turabian StyleSilva-Carvalho, Amandda Évelin, Nakaly Natiely de Oliveira, Julia Viana Lafetá Machado, Daniel Carneiro Moreira, Guilherme Dotto Brand, José Roberto S. A. Leite, Alexandra Plácido, Peter Eaton, and Felipe Saldanha-Araujo. 2023. "The Peptide Salamandrin-I Modulates Components Involved in Pyroptosis and Induces Cell Death in Human Leukemia Cell Line HL-60" Pharmaceutics 15, no. 7: 1864. https://doi.org/10.3390/pharmaceutics15071864
APA StyleSilva-Carvalho, A. É., Oliveira, N. N. d., Machado, J. V. L., Moreira, D. C., Brand, G. D., Leite, J. R. S. A., Plácido, A., Eaton, P., & Saldanha-Araujo, F. (2023). The Peptide Salamandrin-I Modulates Components Involved in Pyroptosis and Induces Cell Death in Human Leukemia Cell Line HL-60. Pharmaceutics, 15(7), 1864. https://doi.org/10.3390/pharmaceutics15071864









