Abstract
Amphibian secretions have been extensively investigated for the production of bioactive molecules. Salamandrin-I is an antioxidant peptide, isolated from the skin secretion of the fire salamander, that has induced no toxicity in microglia or erythrocytes. Importantly, the administration of antioxidants may constitute an adequate therapeutic approach to cancer treatment. Here, with the purpose of better characterizing the therapeutic potential of salamandrin-I, we investigated whether this antioxidant peptide also exerts anticancer activity, using the human leukemia cell line HL-60 as a cancer model. Salamandrin-I treatment induced a significant reduction in HL-60 proliferation, which was accompanied by cell cycle arrest. Furthermore, the peptide-induced cell death showed a significant increase in the LDH release in HL-60 cells. The cellular toxicity exerted by salamandrin-I is possibly related to pyroptosis, since the HL-60 cells showed loss of mitochondrial membrane potential and hyperexpression of inflammasome components following the peptide treatment. This is the first demonstration of the anticancer potential of the salamandrin-I peptide. Such results are important, as they offer relevant insights into the field of cancer therapy and allow the design of future bioactive molecules using salamandrin-I as a template.
1. Introduction
Salamanders are the second most diverse lineage of amphibians, with more than 760 living species. These amphibians have been used as models for scientific development in the most diverse fields of experimental biology. Importantly, they have served as classic models in the development of scientific research in the field of regenerative medicine due to their recognized ability to regenerate tissues, such as limbs, the heart, the spinal cord, lenses, and others. Furthermore, for hundreds of years, salamanders have served as a basis for the study of developmental biology because of the ease of access to their embryos. Additionally, salamanders have also been explored as important scientific models for studies of terrestrial locomotion, feeding, and toxicity [1,2].
As a defense mechanism against predators, salamanders are capable of producing a poisonous secretion through epidermal glands. Salamander venom has a diverse composition, but the presence of steroidal alkaloids of the salamandrine type stands out. Amphibian secretions have been extensively investigated for the production of other bioactive molecules that have important antimicrobial, anti-inflammatory, and antidiabetic properties [3,4,5]. In large part, the biological properties promoted by amphibian secretion are attributed to the presence of bioactive peptides, which include brevinins, bombesins, dermaseptins, esculentins, magainins, temporins, tigerinins, and salamandrins [6,7].
Recently, Plácido et al. isolated the salamandrin-I peptide from the skin secretion of the fire salamander (Salamandra salamandra). This peptide did not show antimicrobial activity against Gram-positive or -negative bacteria, did not promote in vitro cytotoxicity in microglia or erythrocytes, and did not show toxic effects in vivo when in contact with Galleria mellonella larvae. Interestingly, the authors of that study demonstrated that salamandrin-I showed antioxidant potential by scavenging DPPH and ABTS radicals [7]. Importantly, the excess of reactive oxygen species (ROSs) is associated with the pathogeneses of heart disease, neurodegenerative diseases, diabetes mellitus, and cancer [8,9,10,11]. Oxidative stress and excessive ROS production also seem to influence the pathogeneses of several hematological malignancies, such as myelodysplastic syndrome (MDS) [12], acute lymphoblastic leukemia (ALL) [13], and acute myeloid leukemia (AML) [14,15].
AML is the most common leukemia observed among the adult population, accounting for more than 80,000 deaths worldwide. It is characterized by the uncontrolled proliferation of myeloid blasts with impaired differentiation, resulting in ineffective hematopoiesis and life-threatening cytopenia. Cytogenetic abnormalities are also common in AML and have been used as criteria to define specific molecular subgroups [16,17]. The HL-60 cell line was derived from peripheral blood leucocytes obtained from a female adult patient who was initially diagnosed with acute promyelocytic leukemia (APL), a subtype of AML. APL is characterized by the presence of the PML/RARA gene, resulting from a reciprocal translocation, t(15;17), and leading to the fusion of the promyelocytic leukemia (PML) gene with the retinoic acid receptor alpha (RARA) gene. Additional karyotypic analysis of the HL-60 cell line revealed the absence of the t(15;17) translocation, leading to the classification of this cell line as AML M2. HL-60 is the most studied human acute myeloid leukemia cell line, representing a useful model for studying the processes of cell proliferation, death, and differentiation [18,19,20].
Oxidative stress has been recognized as an intrinsic feature of AML relapse [14]. Primary blasts from AML patients seem to produce high ROS levels associated with impaired antioxidant defenses, contributing to blast proliferation [15]. Interestingly, azelaic acid treatment was able to suppress proliferation and induce apoptosis and cell cycle arrest in AML patient cells and AML cell lines by decreasing ROS levels and increasing GSH and SOD levels [21]. Such observations suggest that the administration of antioxidants may constitute an adequate therapeutic approach for the treatment of these pathologies [22]. Similar findings have been observed in nonhematological cancers. Antioxidant peptides isolated from some oysters species were able to induce apoptosis in prostate, colon, and oral cancer cell lines, demonstrating that—in addition to scavenging free radicals—these compounds may also bear anticancer potential [23,24,25].
Here, with the purpose of better characterizing the therapeutic potential of salamandrin-I, we have investigated whether this antioxidant peptide also exerts anticancer activity, using the HL-60 human leukemia cell line as a cancer model.
2. Materials and Methods
2.1. Peptide Isolation and Cell Culture
The salamandrin-I peptide was isolated as has been previously described [7], and its characterization is shown in Supplementary Figure S1. The peptide used in this study was 95% pure, and it was solubilized in DMSO (Sigma Aldrich, St. Louis, MO, USA) prior to dilution in a Roswell Park Memorial Institute medium (RPMI 1640, Gibco Waltham, MA, USA) for use in all experiments. The HL-60 cell line—a promyelocytic cell line derived from human leukemia—was kindly provided by Prof. João Agostinho Machado-Neto (University of São Paulo, São Paulo, Brazil). The cells were maintained in an RPMI 1640 medium supplemented with 10% FBS (Gibco, Waltham, MA, USA), plus 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cell cultures were maintained at 5% CO2 and 37 °C, and the culture medium was replaced every 48 h.
2.2. MTT Assay
The effect of salamandrin-I treatment on HL-60 growth (proliferation and/or viability) was assessed by measuring the 3-(4.5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma Aldrich, St. Louis, MO, USA) dye absorbance of the cells [26]. For this, a total of 5 × 104 cells were seeded into 96-well plates containing 100 µL of the culture medium. The cells were treated with different concentrations of salamandrin-I (0, 1, 5, 10, 25, 50, and 100 µM) for 48 h. After this period, the cells were incubated with 10 µL of MTT (5 mg/mL) for 4 h. The supernatant was removed after centrifuging of the plates at 400× g for 10 min. The reaction product was solubilized by the addition of DMSO (Sigma Aldrich, St. Louis, MO, USA). The optical density was read on a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA) at 570 nm. The IC25 and IC50 values were calculated using nonlinear regression analysis in GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA).
2.3. Proliferation Assay
In order to determine whether salamandrin-I controls HL-60 proliferation, cells were stained with 7.5 μM of carboxyfluorescein succinimidyl ester (CFSE; Sigma Aldrich, St. Louis, MO, USA) and treated with IC25 and IC50 peptide doses for 48 h. Then, the cells were recovered and used to determine the percentage of CFSE+ cells with flow cytometry (FACSCalibur; BD Biosciences, East Rutherford, NJ, USA). Ten thousand events were recorded for each sample, and the data were analyzed using FlowJo software 10.0.7 (Treestar, Inc., Ashland, OR, USA).
2.4. Cell Cycle Analysis
A total of 1 × 106 cells per well were seeded in 24-well plates and treated with IC25 and IC50 doses of salamandrin-I for 48 h. Then, the cells were fixed with 70% ethanol and stored at 4 °C for at least 2 h before analysis. For the flow cytometric analysis, the fixed cells were treated with 100 µg/mL of RNAse A for 10 min and then stained with 50 μg/mL of Propidium Iodide (PI) for 20 min. One hundred thousand events were collected using a FACSCalibur flow cytometer (BD Biosciences, East Rutherford, NJ, USA), and the data were analyzed using FlowJo software 10.0.7 (Treestar, Inc., Ashland, OR, USA).
2.5. Apoptosis Detection
The percentage of apoptotic cells was determined using the Annexin V/PI Apoptosis Detection Kit (BD Biosciences, East Rutherford, NJ, USA). For this, the HL-60 cell line was treated with IC25 and IC50 doses of salamandrin-I for 48 h. Then, the cells were recovered and stained with Annexin V and PI, following the manufacturer’s instructions. Ten thousand events were recorded from each sample with a FACSCalibur flow cytometer (BD Biosciences, East Rutherford, NJ, USA). The annexin V−/PI− cell population was considered viable, while the annexin V+/PI− and annexin V+/PI+ cell populations were considered apoptotic. The data were analyzed using FlowJo software 10.0.7 (Treestar, Inc., Ashland, OR, USA).
2.6. Determination of Lactate Dehydrogenase (LDH) Release
A total of 2 × 105 cells were treated with IC25 and IC50 doses of salamandrin-I for 48 h. Then, supernatants from the cell cultures were collected and the LDH release measurement was performed using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit according to the manufacturer’s instructions (Promega Corp., Madison, WI, USA). Absorbance was determined using a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA) at 490 nm.
2.7. Measurement of Mitochondrial Membrane Potential (ΔΨm)
To evaluate the mitochondrial membrane potential, 2 × 105 cells were treated with IC25 and IC50 doses of salamandrin-I for 48 h. After this period, the cells were incubated with 5 µg/mL of rhodamine 123 for 20 min at room temperature. Then, the cells were washed with PBS and immediately analyzed with the FACSCalibur flow cytometer (BD Biosciences, East Rutherford, NJ, USA). Ten thousand events were acquired from each sample, and data analysis was performed using FlowJo software 10.0.7 (Treestar, Inc., Ashland, OR, USA).
2.8. Caspase-1 Inflammasome Assay
Caspase-1 activity was determined using a Caspase-Glo®1 inflammasome assay kit (Promega Corp., Madison, WI, USA). Briefly, 5 × 104 cells were treated with an IC50 dose of salamandrin-I for 48 h. After incubation, 100 μL of the Caspase-Glo 1 reagent, with and without the caspase-1 inhibitor YVAD-CHO, was added to the plate and mixed using a plate shaker at 300 rpm for 30 s. The samples were incubated at room temperature, and luminescence was measured after 60 min using a Multimode Plate Reader (PerkinElmer, Waltham, MA, USA).
2.9. RNA Extraction, cDNA Synthesis, and Real-Time PCR
RNA extraction was performed using the TRI reagent (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The amount and quality of the RNA samples were determined using Nanodrop One (Thermo Scientific, Waltham, MA, USA). One microgram of total RNA was converted to single-stranded cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied BioSystems, Waltham, MA, USA).
The PCR primers used to amplify the CASP1, CASP3, CASP8, P53, TP73, BAX, BAK, NLRP1, NLRP3, GSDMD, IL-1β, CDK1, CDK2, KI-67, and GAPDH genes are listed in Table 1. Real-time PCRs were performed using SYBR Green MasterMix (Thermo Scientific, Waltham, MA, USA) and a QuantStudio 1 Real-Time PCR System (Thermo Scientific, Waltham, MA, USA). All reactions were performed in technical duplicate, and the relative fold changes were obtained with the 2−ΔΔCt method [27]. Median Ct values obtained from untreated cell lines were used as a reference.

Table 1.
qPCR primer sequences.
2.10. Statistical Analysis
Data were tested for normal distribution with the Shapiro–Wilk normality test and analyses of skewness and kurtosis when applicable. All experiments were performed in triplicate, and values are expressed as means ± SEMs. All analyses were performed using Prism 9 software (GraphPad Software Inc., San Diego, CA, USA). The nonparametric Kruskal–Wallis test, followed by Dunn’s multiple comparison test, was used for numerical comparisons between groups. Probability values of p < 0.05 were accepted as indications of statistically significant differences.
3. Results
3.1. Salamandrin-I Treatment Decreases Human Leukemia Cell Viability
To determine whether salamandrin-I could compromise the viability of HL-60 cells, an MTT assay was performed. After 48 h of peptide treatment, the HL-60 cells showed a dose-dependent reduction in viability (Figure 1A). Nonlinear regression revealed that the IC25 value of the salamandrin-treated HL-60 cells was 23.5 µM and the corresponding IC50 value was 27 µM. Other myeloid leukemia cell lines were also evaluated and showed reduced cell viability after treatment with salamandrin-I (Supplementary Table S1).
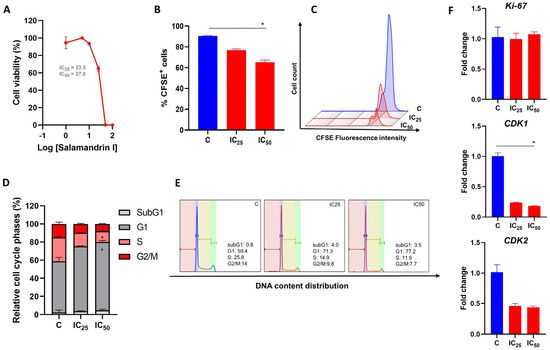
Figure 1.
Antiproliferative effect of salamandrin-I. (A) HL-60 cells treated with increasing concentrations of salamandrin-I presented decreased viability, detected with an MTT assay. (B) Cell proliferation of HL-60 cells untreated (control, C) and treated with salamandrin-I at IC25 and IC50 doses. (C) Representative flow cytometry histogram showing CFSE+ in HL-60 cells treated with salamandrin-I at IC25 and IC50 doses. (D,E) Phases of the cell cycle, determined with flow cytometry, in C and HL-60 cells treated with salamandrin-I and exposed to IC25 and IC50 doses. (F) Expression of the genes Ki-67, CDK1, and CDK2 in C and HL-60 cells treated with salamandrin-I at IC25 and IC50 doses. Real-time PCRs were performed in technical duplicate, and the relative fold change was obtained with the 2−ΔΔCt method. Median Ct values obtained from untreated cell lines were used as a reference. Results are presented as means ± SEMs. * Asterisks indicate results that are statistically significant. * p < 0.05.
3.2. Salamandrin-I Controls Cell Proliferation and Promotes Cell Cycle Arrest
The effect of salamandrin-I on HL-60 cell proliferation was determined after cell treatment with IC25 and IC50 doses for 48 h. Importantly, a reduction in the proliferation of the HL-60 lineage was observed with the IC50 dose (p = 0.02) and was accompanied by cell cycle arrest in the G1 phase (p = 0.02) as well as decreases in the percentages of cells in the S and G2/M phases (p = 0.02 and p = 0.02, respectively) (Figure 1B–E). In line, after the salamandrin-I treatment, the HL-60 cells showed significant reductions in their transcriptional levels of CDK1 (p = 0.02) (Figure 1F).
3.3. Salamandrin-I Induces Cell Death in Human Leukemia Cell Line
To evaluate the mechanisms involved in the reduction in cell viability promoted by salamandrin-I, the HL-60 cells were treated with IC25 and IC50 doses of the peptide for 48 h and the percentage of cells that were positive for Annexin-V was determined with flow cytometry. Importantly, the treatment with salamandrin-I at the IC50 dose significantly promoted HL-60 cell death (p = 0.05) (Figure 2A,B).
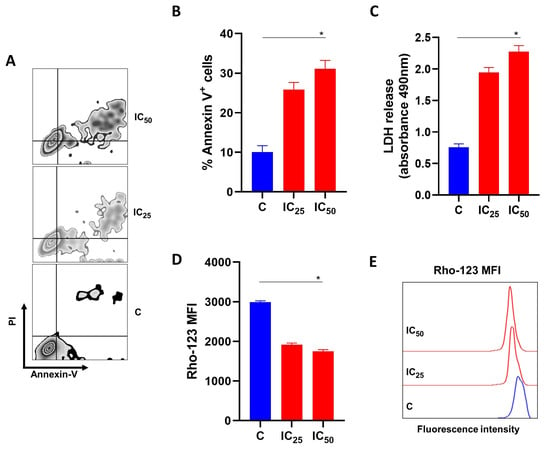
Figure 2.
Effects of salamandrin-I on the viability and mitochondrial health of HL-60 cells. (A) Representative zebra plot showing the expressions of annexin-V and PI in C and HL-60 cells treated with salamandrin-I at IC25 and IC50 doses. (B) Annexin V+ HL-60 cells after treatment with salamandrin-I at IC25 and IC50 doses. (C) LDH releases in culture media for C and HL-60 cells treated with salamandrin-I at IC25 and IC50 doses. (D,E) Rhodamine 123 staining in C and HL-60 cells treated with salamandrin-I at IC25 and IC50 doses. Results are presented as means ± SEMs. Asterisks indicate results that are statistically significant. * p < 0.05.
3.4. Salamandrin-I Induces LDH Releases and Compromises the Mitochondrial Membrane Potential of Human Leukemia Cell Lines
After determining of the percentage of annexin-V-positive cells, the culture supernatant was collected to measure the LDH release. A significant increase in the LDH release was observed after the treatment of the HL-60 cell line with the salamandrin-I at the IC50 dose (p = 0.02) (Figure 2C). Importantly, the treatment with the salamandrin-I significantly impaired the mitochondrial membrane potential of the HL-60 cells (p = 0.02) (Figure 2D,E).
3.5. Salamandrin-I Activates Caspase-1 and Modulates Inflammasome Components in Human Leukemia Cell Line
Using the Caspase-Glo 1 inflammasome assay kit, we evaluated whether salamandrin-I could modulate inflammasome components in HL-60 cells. Importantly, at the IC50 dose, there was an increase in caspase activity in the HL-60 cells (p = 0.02). Interestingly, in treatment with the caspase 1 inhibitor YVAD-CHO, no statistically significant changes were observed regarding caspase activity in the HL-60 cell line (Figure 3A). In line with the possible involvement of the inflammasome in the observed cell death process, the HL-60 cells showed elevated expressions of NLRP1 (p = 0.02), NLRP3 (p = 0.02), CASP-1 (p = 0.02), and IL-1β (p = 0.02) after treatment with salamandrin-I (Figure 3B–F).
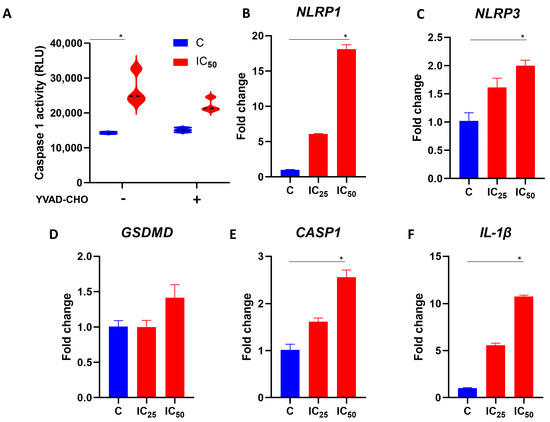
Figure 3.
Modulation of inflammasome components with salamandrin-I. (A) Caspase 1 activity of C and HL-60 cells treated with salamandrin-I at IC50 dose. These experiments were conducted in the absence and presence of caspase 1 inhibitor YVAD-CHO. (B–F) Expression of the inflammasome components NLRP-1, NLRP-3, GSDMD, CASP-1, and IL-1β. Real-time PCRs were performed in technical duplicate, and the relative fold change was obtained with the 2−ΔΔCt method. Median Ct values obtained from untreated cell lines (C, control) were used as a reference. Results are presented as means ± SEMs. * Asterisks indicate results that are statistically significant. * p < 0.05.
3.6. Salamandrin-I Increases Transcriptional Levels of CASP8 and Inhibits BCL-2 in HL-60 Cells
To better investigate the molecular effects that the salamandrin-I promoted in the HL-60 cells, these cells were cultured for 48 h with the peptide at the IC25 and IC50 doses, and their RNA was obtained for gene expression analysis. Real-time PCRs revealed that the transcript levels of CASP8 (p = 0.03) and CASP3 (p = 0.03) were significantly increased after the treatment of the HL-60 cells with the salamandrin-I at the IC50 dose. In addition, the HL-60 cells showed a lower expression of BCL2 when treated with the salamandrin-I at the IC50 (p = 0.05) dose. No statistically significant changes were observed in the transcriptional levels of BAK, BAX, P53, or TP73 (Figure 4).
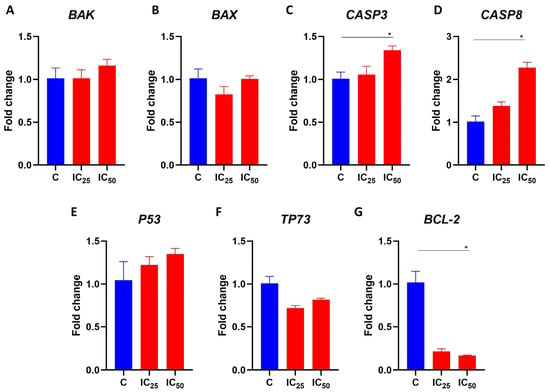
Figure 4.
Gene expression analysis of selected transcripts. (A–G) Transcriptional levels of BAK, BAX, CASP3, CASP8, P53, TP73, and BCL-2. Real-time PCRs were performed in technical duplicate, and the relative fold change was obtained with the 2−ΔΔCt method. Median Ct values obtained from untreated cell lines (C) were used as a reference. Results are presented as means ± SEMs. Asterisks indicate results that are statistically significant. * p < 0.05.
4. Discussion
Along with cardiovascular disease, cancer is emerging as one of the leading causes of death worldwide. According to recent GLOBOCAN estimates produced by the International Agency for Research on Cancer, around 19 million new cases of cancer and approximately 10 million deaths have occurred annually in the world. Of this total, almost 475,000 new cases are leukemia, with approximately 300,000 deaths from this pathology being recorded [28]. The use of bioactive peptides has been explored as a promising approach for cancer therapy, especially for their selectivity, their high potency, and the possibility of reducing the toxic effects promoted by chemotherapy [29]. Here, we have evaluated for the first time the anticancer effect of the salamandrin-I peptide, using it in a cellular model of human myeloid leukemia, and we have demonstrated that in addition to controlling the growth of the HL-60 line, this peptide can efficiently promote its cell death.
Uncontrolled and independent proliferation of cancer cells is one of the main characteristics of tumors. Therefore, the development of new therapeutic approaches that can control tumor growth is of fundamental importance for cancer treatment [30]. The cell division process is controlled by several regulatory mechanisms to ensure the correct segregation of genetic material into new daughter cells. In eukaryotes, the cell cycle is composed of four phases, G1, S, G2, and M, each characterized by specific cellular activities and checkpoints. These checkpoints are crucial to verifying DNA integrity and activating mechanisms to either delay cell cycle progression or initiate programmed cell death. DNA replication happens during the S phase, while genetic material segregation is divided into two new daughter cells during the M phase. The G1 and G2 phases act as decision points for the cell’s commitment to DNA replication and cell division. In cancer cells, some of these aspects are disrupted, enabling the cells to escape repair mechanisms and undergo uncontrolled proliferation [31,32]. Interestingly, salamandrin-I was able to significantly control HL-60 proliferation in a dose-dependent manner. After salamandrin-I treatment, a high percentage of HL-60 cells remained in the G1 phase of the cell cycle, and there were reductions in the numbers of cells in the S and G2/M phases. Cell cycle progression is driven by cyclin-dependent kinases (CDKs), which bind to different cyclins and allow coordinated cell cycle progression. However, it seems that only some complexes can regulate the cell cycle [33]. CDK1 and CDK2 are essential for controlling the cell cycle, acting in the G1-S and G2-M phase transitions of eukaryotic cells [34,35]. The complex formed of cyclin E and CDK2 mediates the initiation of the S phase and DNA synthesis. At the end of the S phase, cyclin A forms a complex with CDK2, enabling the transition to the G2 phase. A subsequent complex formed of cyclin A and CDK1 leads cells to the M phase [36,37]. Corroborating the functional tests carried out, the salamandrin-I drastically inhibited the CDK1 and CDK2 transcriptional levels in the HL-60 cells.
The identification of antioxidant peptides with important anticancer properties has been reported in the literature [38,39]. Interestingly, it was previously demonstrated that at up to a 100 µM concentration, salamandrin-I was not toxic when cultured with human microglia and red blood cells [7]. However, using an in vitro model of human leukemia, we identified that in addition to controlling cell division, salamandrin-I can induce apoptosis of the HL-60 cell line, which is accompanied by greater LDH release and loss of mitochondrial transmembrane potential. In addition, the treated cells also showed increased transcriptional levels of CASP-3 and CASP-8 and decreased levels of antiapoptotic factor BCL-2. The BCL-2 protein family includes both proapoptotic and antiapoptotic members, which play an important role in cell survival by modulating the mitochondrial pathway of apoptosis [40,41]. This intrinsic pathway of apoptosis is triggered by a variety of stress signals, leading to BAX and BAK activation and subsequent damage of the mitochondrial outer membrane (MOMP) [42]. This disrupted membrane allows the release of several apoptotic factors, including cytochrome c, that are able to activate members of the caspase family [43,44]. Initiator caspase-9 is activated by conformational changes induced by apoptosomes and further processes the downstreams of some effector caspases, such as CASP-3 and CASP-7, leading to apoptosis [45,46]. BCL-2 and BCL-xL are able to counter these proapoptotic events by binding and sequestering activated BAX and BAK proteins [47]. Despite the observation of a decrease in the mitochondrial membrane potential in HL-60 cells after treatment with this peptide, we did not observe changes in the BAX or BAK expressions. At the molecular level, we noticed that salamandrin-I treatment provoked a trend of P53 increase in HL-60 cells, although this difference was not statistically significant.
With the purpose of advancing the identification of mechanisms associated with cell death promoted by salamandrin-I, we evaluated whether this peptide could modulate components involved in pyroptosis, an inflammatory programmed cell death pathway. Pyroptosis is a caspase-1-dependent mechanism that leads to cell swelling, membrane pore formation, and plasma membrane rupture [48]. Caspase-1 activation induces gasdermin D (GSDMD) cleavage and activation, leading to the oligomerization of the fragments into the plasma membrane, forming pores. Additionally, caspase-1 is involved in the subsequent proteolytic cleavage of the IL-1β and IL-18 precursor forms. After membrane rupture, these inflammatory cytokines and several cytosolic contents are released into extracellular space, characterizing the inflammatory potential of pyroptosis [48,49,50,51].
The canonical pyroptosis pathway is mediated by inflammasome assembly. An inflammasome is a multiprotein signaling complex responsible for controlling and coordinating inflammatory responses in the presence of pathogens and signs of damage to the cell or cellular structures such as the mitochondria [52,53]. NLRP3, a member of the nucleotide-binding domain (NOD) receptor family, is one of the receptor proteins responsible for inflammasome formation, which responds to several types of stimuli and signals that are related to PAMP and DAMP as well as being related to the mechanisms of pyroptosis and mitochondrial damage [53,54,55]. Interestingly, salamandrin-I-treated HL-60 cells presented significantly increased levels of NLRP-1, NLRP-3, CASP-1, and IL-1β transcripts, as well as caspase 1 activity, suggesting that the cell death observed was influenced by inflammasome assembly [56]. Importantly, it has been reported that inflammasome activation can be triggered by CASP-8 and mitochondrial injury [57,58,59], which is in line with our results.
This is the first demonstration of the anticancer role of salamandrin-I. These results are important, as they offer important insights into the field of anticancer therapy and allow the design of future bioactive molecules using salamandrin-I as a template.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15071864/s1, Figure S1: MS/MS spectrum of synthetic salamandrin-I; Table S1: IC25 and IC50 values for salamandrin-I in human myeloid cell lines.
Author Contributions
A.É.S.-C., N.N.d.O., J.V.L.M. and D.C.M.: conceptualization; methodology; investigation; analysis; and writing—original draft. G.D.B. and A.P.: methodology; investigation; and analysis. J.R.S.A.L., P.E. and F.S.-A.: conceptualization; resources; writing—review and editing; and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was Funded by Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF), Conselho Nacional de Desenvolvimento Cientifico e Tecnoloógico (CNPq), and by national funds through the FCT—Fundação para a Ciência e a Tecnologia, I.P., within the project No. PTDC/BII_BIO/31158/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that all data underlying these findings are fully available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, M.E.H.; Benson, R.B.J.; Skutschas, P.; Hill, L.; Panciroli, E.; Schmitt, A.D.; Walsh, S.A.; Evans, S.E. Middle Jurassic Fossils Document an Early Stage in Salamander Evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2114100119. [Google Scholar] [CrossRef]
- Arenas Gómez, C.M.; Echeverri, K. Salamanders: The Molecular Basis of Tissue Regeneration and Its Relevance to Human Disease. Curr. Top. Dev. Biol. 2021, 145, 235–275. [Google Scholar]
- Lüddecke, T.; Schulz, S.; Steinfartz, S.; Vences, M. A Salamander’s Toxic Arsenal: Review of Skin Poison Diversity and Function in True Salamanders, Genus Salamandra. Naturwissenschaften 2018, 105, 56. [Google Scholar] [CrossRef]
- Mechkarska, M.; Attoub, S.; Sulaiman, S.; Pantic, J.; Lukic, M.L.; Conlon, J.M. Anti-Cancer, Immunoregulatory, and Antimicrobial Activities of the Frog Skin Host-Defense Peptides Pseudhymenochirin-1Pb and Pseudhymenochirin-2Pa. Regul. Pept. 2014, 194–195, 69–76. [Google Scholar] [CrossRef]
- Ojo, O.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Frog Skin Peptides (tigerinin-1R, Magainin-AM1, -AM2, CPF-AM1, and PGla-AM1) Stimulate Secretion of Glucagon-like Peptide 1 (GLP-1) by GLUTag Cells. Biochem. Biophys. Res. Commun. 2013, 431, 14–18. [Google Scholar] [CrossRef]
- Indriani, S.; Karnjanapratum, S.; Nirmal, N.P.; Nalinanon, S. Amphibian Skin and Skin Secretion: An Exotic Source of Bioactive Peptides and Its Application. Foods 2023, 12, 1282. [Google Scholar] [CrossRef]
- Plácido, A.; Bueno, J.; Barbosa, E.A.; Moreira, D.C.; do Dias, J.N.; Cabral, W.F.; Albuquerque, P.; Bessa, L.J.; Freitas, J.; Kuckelhaus, S.A.S.; et al. The Antioxidant Peptide Salamandrin-I: First Bioactive Peptide Identified from Skin Secretion of Salamandra Genus (Salamandra salamandra). Biomolecules 2020, 10, 512. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Plácido, A.; do Pais do Amaral, C.; Teixeira, C.; Nogueira, A.; Brango-Vanegas, J.; Alves Barbosa, E.; Moreira, D.C.; Silva-Carvalho, A.É.; da Gloria da Silva, M..; do Nascimento Dias, J.; et al. Neuroprotective Effects on Microglia and Insights into the Structure-Activity Relationship of an Antioxidant Peptide Isolated from Pelophylax Perezi. J. Cell. Mol. Med. 2022, 26, 2793–2807. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Nascimento Costa, J.M.; et al. Oxidative Stress and Mitochondrial Dysfunction Play a Role in Myelodysplastic Syndrome Development, Diagnosis, and Prognosis: A Pilot Study. Free Radic. Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; do Araújo, M.C.; Schetinger, M.R.C.; Morsch, V.M. Measurement of Oxidative Stress and Antioxidant Status in Acute Lymphoblastic Leukemia Patients. Clin. Biochem. 2008, 41, 511–518. [Google Scholar] [CrossRef]
- Zhou, F.-L.; Zhang, W.-G.; Wei, Y.-C.; Meng, S.; Bai, G.-G.; Wang, B.-Y.; Yang, H.-Y.; Tian, W.; Meng, X.; Zhang, H.; et al. Involvement of Oxidative Stress in the Relapse of Acute Myeloid Leukemia. J. Biol. Chem. 2010, 285, 15010–15015. [Google Scholar] [CrossRef]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.K.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-Derived ROS in AML Promotes Proliferation and Is Associated with Defective Oxidative Stress Signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute Myeloid Leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef]
- de Thé, H.; Chomienne, C.; Lanotte, M.; Degos, L.; Dejean, A. The t(15;17) Translocation of Acute Promyelocytic Leukaemia Fuses the Retinoic Acid Receptor Alpha Gene to a Novel Transcribed Locus. Nature 1990, 347, 558–561. [Google Scholar] [CrossRef]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Zhou, G.-B.; Zhang, J.; Wang, Z.-Y.; Chen, S.-J.; Chen, Z. Treatment of Acute Promyelocytic Leukaemia with All-Trans Retinoic Acid and Arsenic Trioxide: A Paradigm of Synergistic Molecular Targeting Therapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 959–971. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Z.; Jin, Y.; Chen, Y.; Yang, T.; Yang, Q.; Wu, B.; Shang, Y.; Liu, X.; Wei, Y.; et al. Azelaic Acid Exerts Antileukemia Effects against Acute Myeloid Leukemia by Regulating the Prdxs/ROS Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1295984. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenserbaerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer Peptide: Physicochemical Property, Functional Aspect and Trend in Clinical Application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-Cycle Checkpoints and Cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Mammalian Cyclin-Dependent Kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To Cycle or Not to Cycle: A Critical Decision in Cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef]
- Kalous, J.; Jansová, D.; Šušor, A. Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase. Cells 2020, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-Dependent Kinases and Cell-Cycle Transitions: Does One Fit All? Nat. Rev. Mol. Cell Biol. 2008, 9, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-Cancer Activity of Maize Bioactive Peptides. Front Chem 2017, 5, 44. [Google Scholar] [CrossRef]
- Zhan, W.; Liao, X.; Li, L.; Chen, Z.; Tian, T.; Yu, L.; Chen, Z. In Vitro Mitochondrial-Targeted Antioxidant Peptide Induces Apoptosis in Cancer Cells. Onco. Targets. Ther. 2019, 12, 7297–7306. [Google Scholar] [CrossRef]
- Luna-Vargas, M.P.A.; Chipuk, J.E. The Deadly Landscape of pro-Apoptotic BCL-2 Proteins in the Outer Mitochondrial Membrane. FEBS J. 2016, 283, 2676–2689. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond - Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Dewson, G.; Kluck, R.M. Mechanisms by Which Bak and Bax Permeabilise Mitochondria during Apoptosis. J. Cell Sci. 2009, 122, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Peña-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The Interplay between BAX and BAK Tunes Apoptotic Pore Growth to Control Mitochondrial-DNA-Mediated Inflammation. Mol. Cell 2022, 82, 933–949.e9. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kang, T.-B. The Molecular Links between Cell Death and Inflammasome. Cells 2019, 8, 1057. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and Diseases. Signal Transduct Target Ther 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 Promotes NLRP1/NLRP3 Inflammasome Activation and IL-1β Production in Acute Glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).