Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation
Abstract
1. Introduction
2. Methods
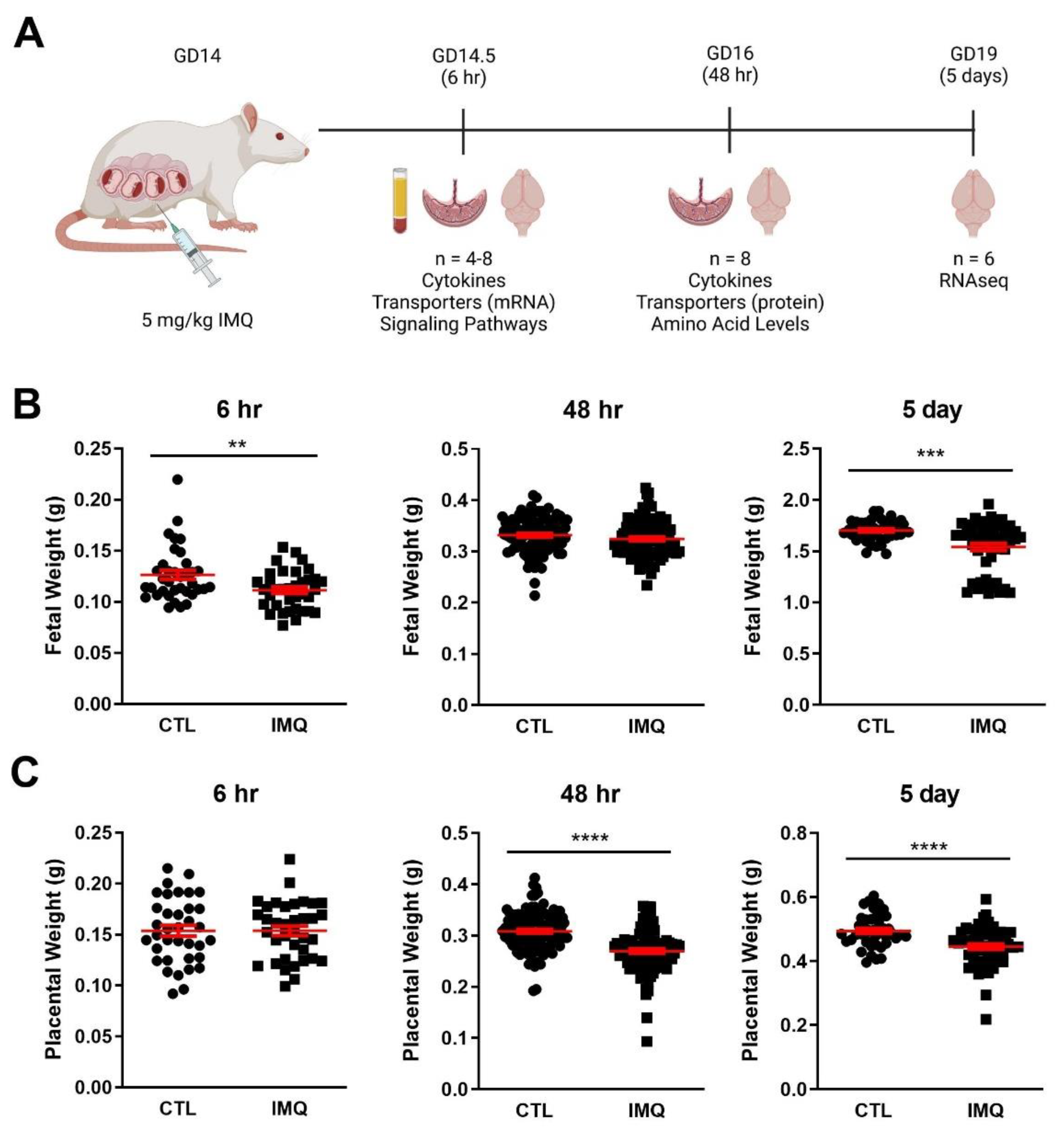
2.1. Animal Experiments
2.2. Sexing Rat Embryos
2.3. Human Placental Sample Acquisition
2.4. Quantification of Cytokines in Rat Serum
2.5. RNA Extraction and qRT-PCR
2.6. Protein Extraction
2.7. Western Blotting and Simple Western
2.8. Quantification of Amino Acids by HPLC
2.9. RNAseq
2.10. Statistical Analysis
3. Results
3.1. Animal Studies: IMQ Reduces Fetal and Placental Weight
3.2. IMQ Induces IL-6 in Maternal Serum and Placenta
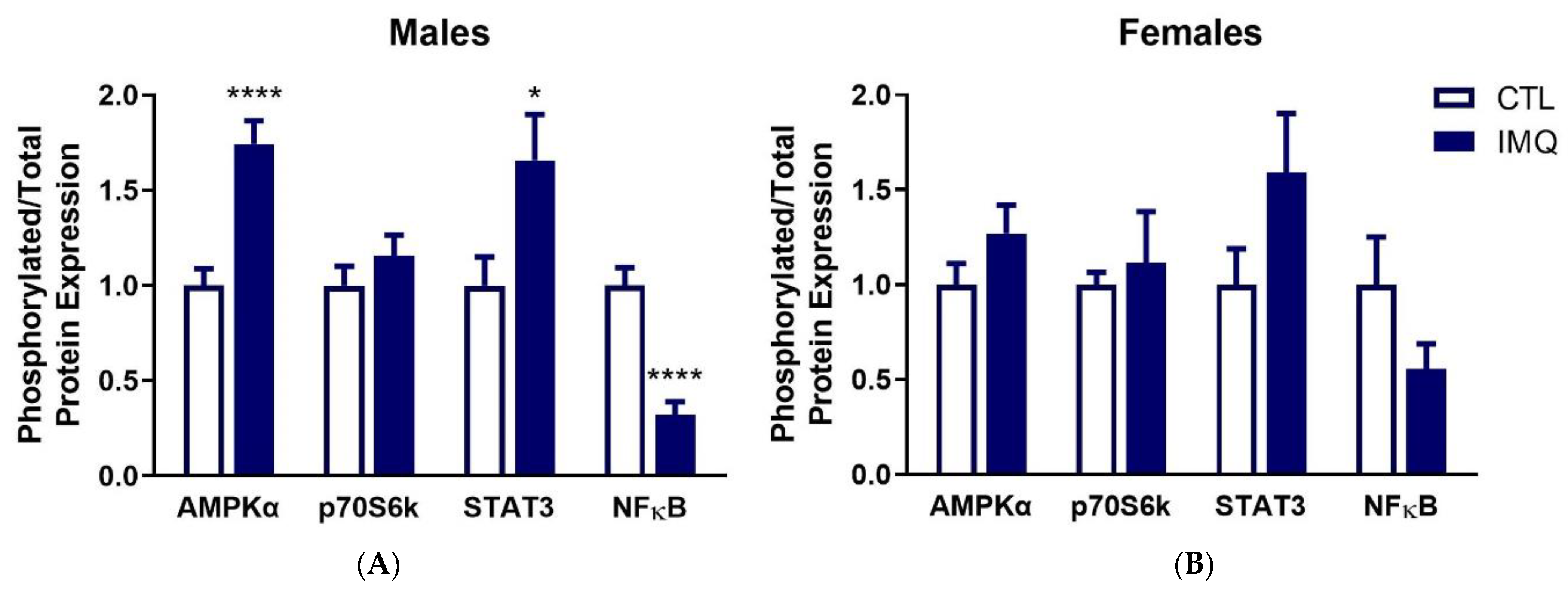
3.3. IMQ Alters Signaling Pathways in Placenta
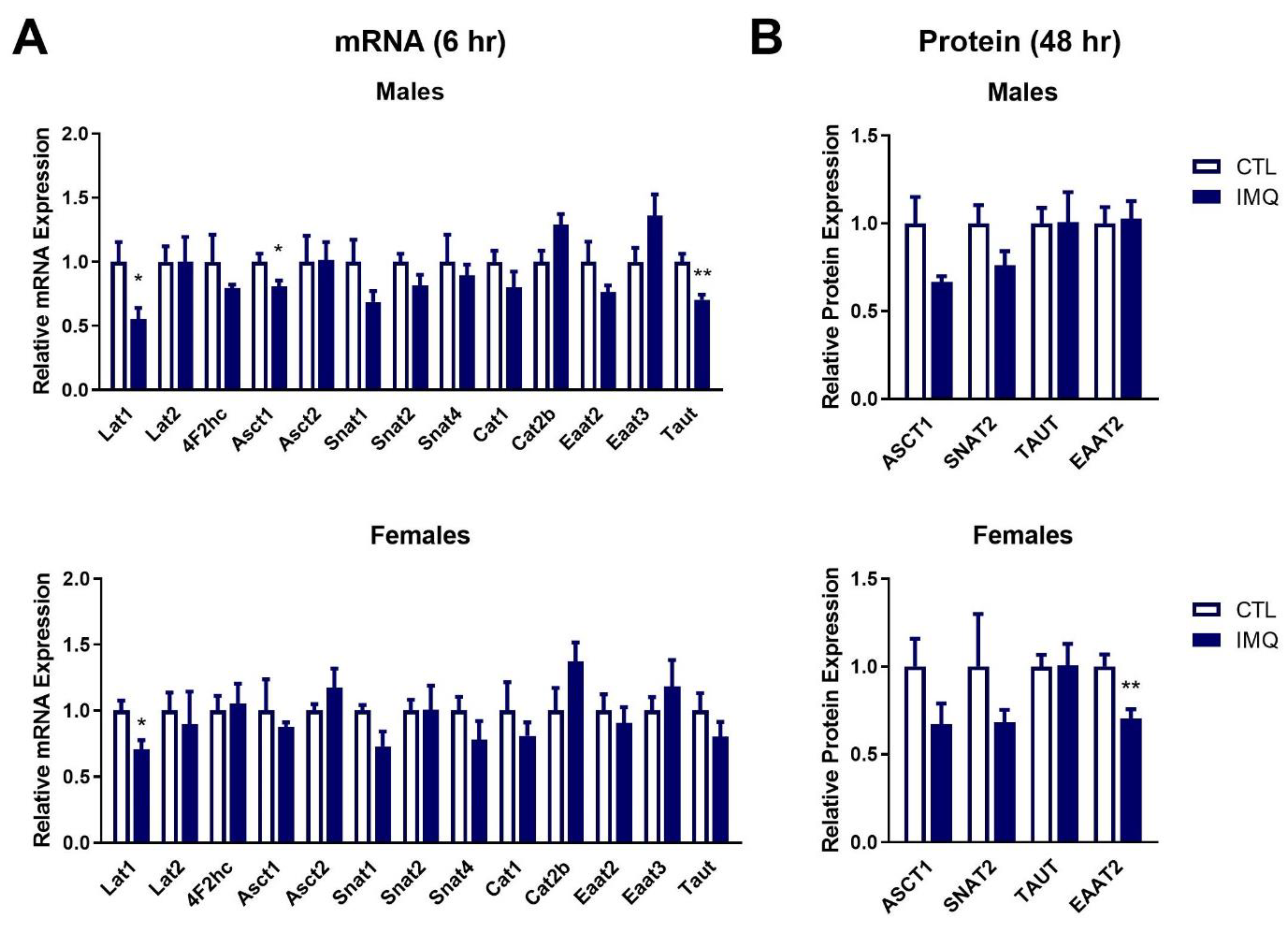
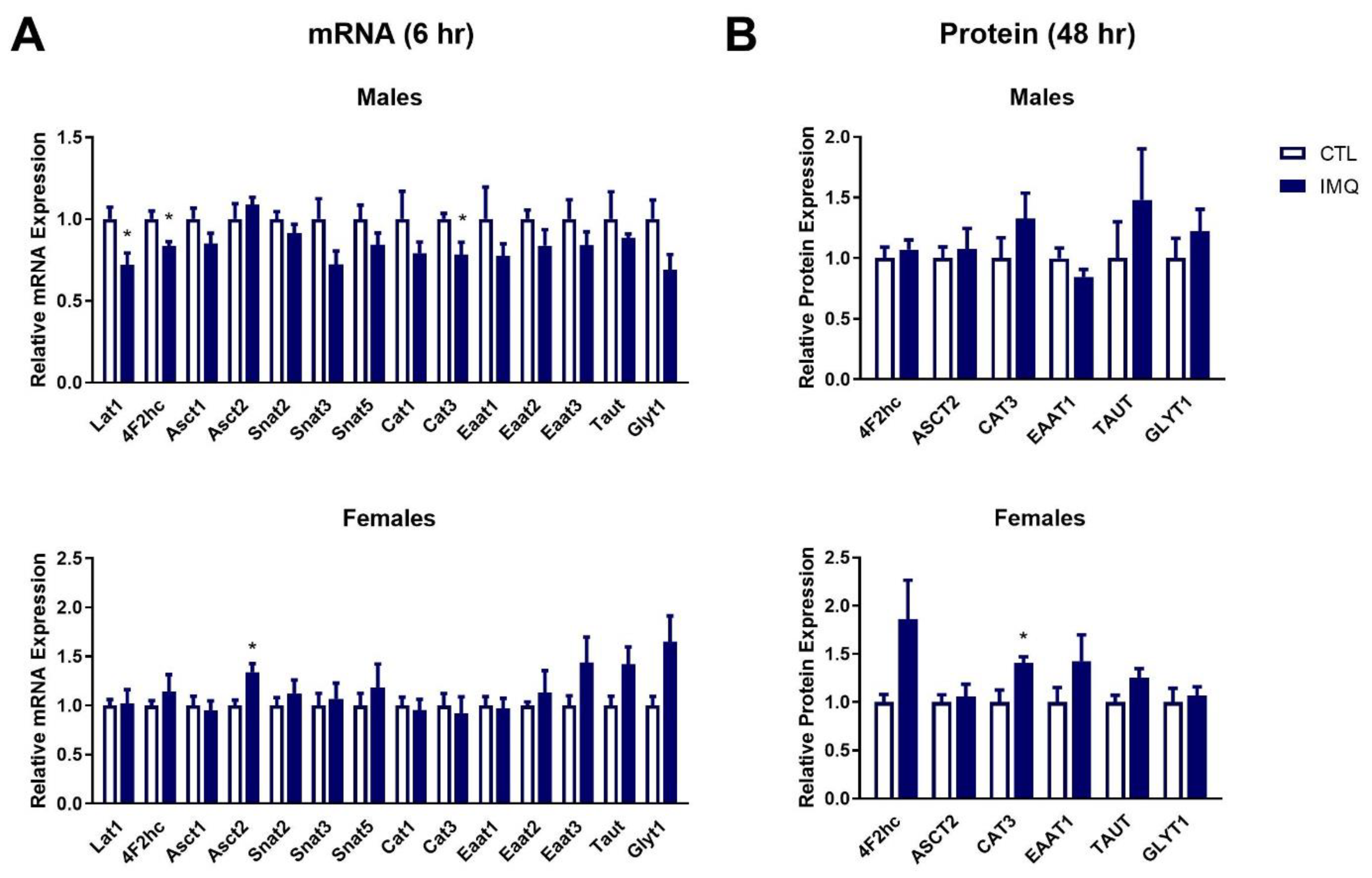
3.4. IMQ Alters Amino Acid Transporter Expression
3.5. IMQ Alters Amino Acid Levels in Fetal Brains
3.6. IMQ Alters Fetal Cerebral Cortex Expression of Genes Associated with ASD
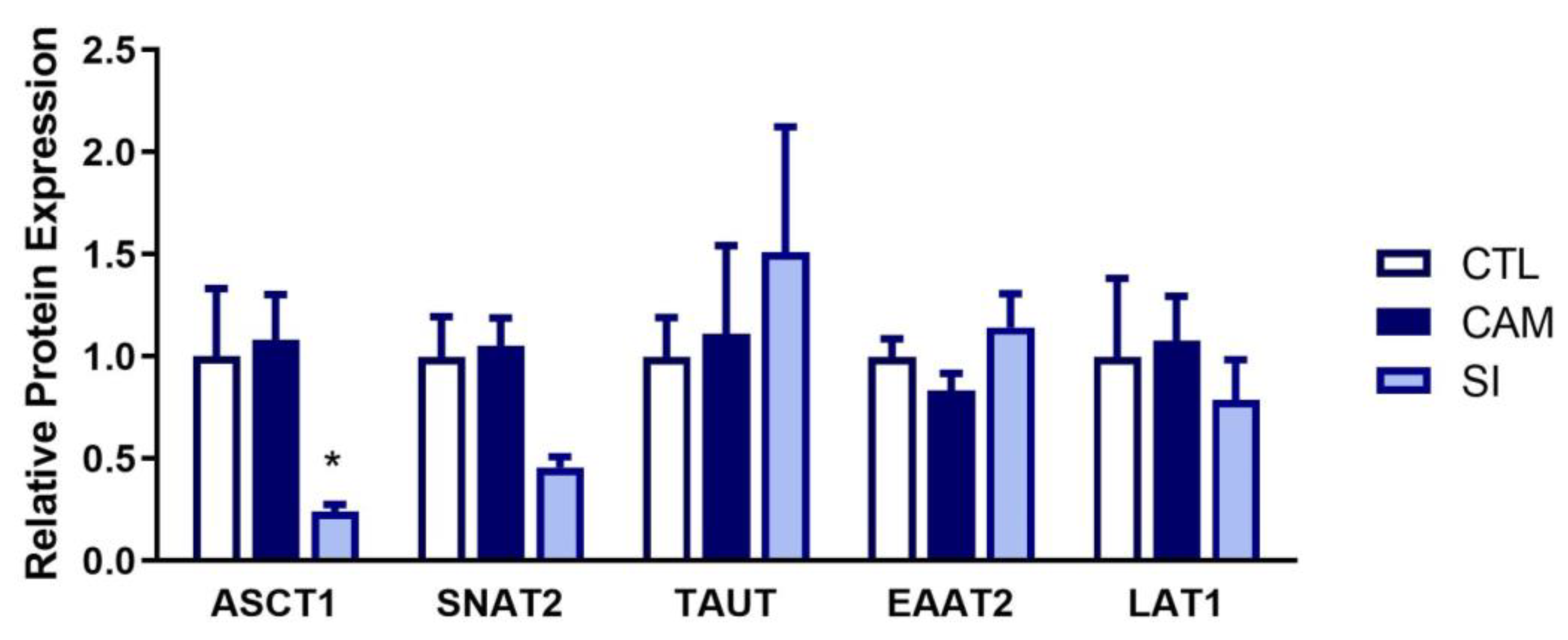
3.7. Human Placenta: Suspected Infection Decreases ASCT1 and SNAT2 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minakova, E.; Warner, B.B. Maternal Immune Activation, Central Nervous System Development and Behavioral Phenotypes. Birth Defects Res. 2018, 110, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Atladóttir, H.Ó.; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Careaga, M.; Murai, T.; Bauman, M.D. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol. Psychiatry 2017, 81, 391–401. [Google Scholar] [CrossRef]
- Haddad, F.L.; Patel, S.V.; Schmid, S. Maternal Immune Activation by Poly I:C as a Preclinical Model for Neurodevelopmental Disorders: A Focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020, 113, 546–567. [Google Scholar] [CrossRef]
- Cleal, J.K.; Lofthouse, E.M.; Sengers, B.G.; Lewis, R.M. A Systems Perspective on Placental Amino Acid Transport. J. Physiol. 2018, 596, 5511–5522. [Google Scholar] [CrossRef]
- Dumolt, J.H.; Powell, T.L.; Jansson, T. Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction. Obstet. Gynecol. Clin. N. Am. 2021, 48, 247–266. [Google Scholar] [CrossRef]
- McDonald, J.W.; Johnston, M.V. Physiological and Pathophysiological Roles of Excitatory Amino Acids during Central Nervous System Development. Brain Res. Rev. 1990, 15, 41–70. [Google Scholar] [CrossRef]
- Tabatabaie, L.; Klomp, L.W.; Berger, R.; de Koning, T.J. L-Serine Synthesis in the Central Nervous System: A Review on Serine Deficiency Disorders. Mol. Genet. Metab. 2010, 99, 256–262. [Google Scholar] [CrossRef]
- Kurbat, M.N.; Lelevich, V.V. Metabolism of Amino Acids in the Brain. Neurochem. J. 2009, 3, 23–28. [Google Scholar] [CrossRef]
- Zaragozá, R. Transport of Amino Acids Across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Gether, U.; Andersen, P.H.; Larsson, O.M.; Schousboe, A. Neurotransmitter Transporters: Molecular Function of Important Drug Targets. Trends Pharmacol. Sci. 2006, 27, 375–383. [Google Scholar] [CrossRef]
- McColl, E.R.; Piquette-Miller, M. Poly(I:C) Alters Placental and Fetal Brain Amino Acid Transport in a Rat Model of Maternal Immune Activation. Am. J. Reprod. Immunol. N. Y. 1989 2019, 81, e13115. [Google Scholar] [CrossRef]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting Viral Sensing Mediated by Toll-like Receptors to Design Innovative Vaccines. npj Vaccines 2021, 6, 127. [Google Scholar] [CrossRef]
- Missig, G.; Robbins, J.O.; Mokler, E.L.; McCullough, K.M.; Bilbo, S.D.; McDougle, C.J.; Carlezon, W.A. Sex-Dependent Neurobiological Features of Prenatal Immune Activation via TLR7. Mol. Psychiatry 2020, 25, 2330–2341. [Google Scholar] [CrossRef]
- Chatterjee, P.; Weaver, L.E.; Doersch, K.M.; Kopriva, S.E.; Chiasson, V.L. Placental Toll-Like Receptor 3 and Toll-Like Receptor 7/8 Activation Contributes to Preeclampsia in Humans and Mice. PLoS ONE 2012, 7, 41884. [Google Scholar] [CrossRef]
- Damm, J.; Wiegand, F.; Harden, L.M.; Gerstberger, R.; Rummel, C.; Roth, J. Fever, Sickness Behavior, and Expression of Inflammatory Genes in the Hypothalamus after Systemic and Localized Subcutaneous Stimulation of Rats with the Toll-like Receptor 7 Agonist Imiquimod. Neuroscience 2012, 201, 166–183. [Google Scholar] [CrossRef]
- Miyajima, A.; Sunouchi, M.; Mitsunaga, K.; Yamakoshi, Y.; Nakazawa, K.; Usami, M. Sexing of Postimplantation Rat Embryos in Stored Two-Dimensional Electrophoresis (2-DE) Samples by Polymerase Chain Reaction (PCR) of an Sry Sequence. J. Toxicol. Sci. 2009, 34, 681–685. [Google Scholar] [CrossRef]
- Amino Acid Analysis: Methods. Available online: https://lab.research.sickkids.ca/sparc-molecular-analysis/services/amino-acid-analysis/amino-acid-analysis-methods/ (accessed on 14 June 2023).
- The Galaxy Community. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2022 Update. Nucleic Acids Res. 2022, 50, W345–W351. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Banerjee-Basu, S.; Packer, A. SFARI Gene: An Evolving Database for the Autism Research Community. Dis. Model. Mech. 2010, 3, 133–135. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. Neurobiol. Dis. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Wu, W.-L.; Hsiao, E.Y.; Yan, Z.; Mazmanian, S.K.; Patterson, P.H. The Placental Interleukin-6 Signaling Controls Fetal Brain Development and Behavior. Brain. Behav. Immun. 2017, 62, 11–23. [Google Scholar] [CrossRef]
- Goines, P.E.; Croen, L.A.; Braunschweig, D.; Yoshida, C.K.; Grether, J.; Hansen, R.; Kharrazi, M.; Ashwood, P.; Van de Water, J. Increased Midgestational IFN-γ, IL-4 and IL-5 in Women Bearing a Child with Autism: A Case-Control Study. Mol. Autism 2011, 2, 13. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet Lond. Engl. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Moreno-Eutimio, M.A.; López-Macías, C.; Pastelin-Palacios, R. Bioinformatic Analysis and Identification of Single-Stranded RNA Sequences Recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV Genomes. Microbes Infect. 2020, 22, 226. [Google Scholar] [CrossRef]
- Kaplan, E.; Zubedat, S.; Radzishevsky, I.; Valenta, A.C.; Rechnitz, O.; Sason, H.; Sajrawi, C.; Bodner, O.; Konno, K.; Esaki, K.; et al. ASCT1 (Slc1a4) Transporter Is a Physiologic Regulator of Brain D-Serine and Neurodevelopment. Proc. Natl. Acad. Sci. USA 2018, 115, 9628–9633. [Google Scholar] [CrossRef]
- Heimer, G.; Marek-Yagel, D.; Eyal, E.; Barel, O.; Oz Levi, D.; Hoffmann, C.; Ruzzo, E.K.; Ganelin-Cohen, E.; Lancet, D.; Pras, E.; et al. SLC1A4 Mutations Cause a Novel Disorder of Intellectual Disability, Progressive Microcephaly, Spasticity and Thin Corpus Callosum. Clin. Genet. 2015, 88, 327–335. [Google Scholar] [CrossRef]
- Burnet, P.W.J.; Hutchinson, L.; von Hesling, M.; Gilbert, E.-J.; Brandon, N.J.; Rutter, A.R.; Hutson, P.H.; Harrison, P.J. Expression of D-Serine and Glycine Transporters in the Prefrontal Cortex and Cerebellum in Schizophrenia. Schizophr. Res. 2008, 102, 283–294. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony, R.V.; Brown, T.L.; Hillman, S.L.; Spencer, R.; David, A.L.; Rosario, F.J.; et al. Placenta-Specific Slc38a2/SNAT2 Knockdown Causes Fetal Growth Restriction in Mice. Clin. Sci. Lond. Engl. 1979 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
- Mandò, C.; Tabano, S.; Pileri, P.; Colapietro, P.; Marino, M.A.; Avagliano, L.; Doi, P.; Bulfamante, G.; Miozzo, M.; Cetin, I. SNAT2 Expression and Regulation in Human Growth-Restricted Placentas. Pediatr. Res. 2013, 74, 104–110. [Google Scholar] [CrossRef]
- Matthews, J.C.; Beveridge, M.J.; Malandro, M.S.; Rothstein, J.D.; Campbell-Thompson, M.; Verlander, J.W.; Kilberg, M.S.; Novak, D.A. Activity and Protein Localization of Multiple Glutamate Transporters in Gestation Day 14 vs. Day 20 Rat Placenta. Am. J. Physiol. 1998, 274, C603–C614. [Google Scholar] [CrossRef]
- Wu, X.; Xie, C.; Zhang, Y.; Fan, Z.; Yin, Y.; Blachier, F. Glutamate-Glutamine Cycle and Exchange in the Placenta-Fetus Unit during Late Pregnancy. Amino Acids 2015, 47, 45–53. [Google Scholar] [CrossRef]
- McColl, E.R.; Piquette-Miller, M. Viral Model of Maternal Immune Activation Alters Placental AMPK and MTORC1 Signaling in Rats. Placenta 2021, 112, 36–44. [Google Scholar] [CrossRef]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Mammalian Target of Rapamycin Signalling Modulates Amino Acid Uptake by Regulating Transporter Cell Surface Abundance in Primary Human Trophoblast Cells. J. Physiol. 2013, 591, 609–625. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; Patterson, P.H. Activation of the Maternal Immune System Induces Endocrine Changes in the Placenta via IL-6. Brain Behav. Immun. 2011, 25, 604–615. [Google Scholar] [CrossRef]
- Aban, M.; Cinel, L.; Arslan, M.; Dilek, U.; Kaplanoglu, M.; Arpaci, R.; Dilek, S. Expression of Nuclear Factor-Kappa B and Placental Apoptosis in Pregnancies Complicated with Intrauterine Growth Restriction and Preeclampsia: An Immunohistochemical Study. Tohoku J. Exp. Med. 2004, 204, 195–202. [Google Scholar] [CrossRef]
- Keleher, M.R.; Erickson, K.; Kechris, K.; Yang, I.V.; Dabelea, D.; Friedman, J.E.; Boyle, K.E.; Jansson, T. Associations between the Activity of Placental Nutrient-Sensing Pathways and Neonatal and Postnatal Metabolic Health: The ECHO Healthy Start Cohort. Int. J. Obes. 2020, 44, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.-R. The Role of NFκB in Healthy and Preeclamptic Placenta: Trophoblasts in the Spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef] [PubMed]
- Papuchova, H.; Latos, P.A. Transcription Factor Networks in Trophoblast Development. Cell. Mol. Life Sci. 2022, 79, 337. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Groudine, M. A New Member of the Cationic Amino Acid Transporter Family Is Preferentially Expressed in Adult Mouse Brain. J. Biol. Chem. 1997, 272, 26780–26786. [Google Scholar] [CrossRef]
- Richards, L.A.; Schonhoff, C.M. Nitric Oxide and Sex Differences in Dendritic Branching and Arborization. J. Neurosci. Res. 2021, 99, 1390–1400. [Google Scholar] [CrossRef]
- Kudo, Y.; Boyd, C.A.R. Characterisation of L-tryptophan Transporters in Human Placenta: A Comparison of Brush Border and Basal Membrane Vesicles. J. Physiol. 2001, 531, 405–416. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gomez-Fernández, A.; de la Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Gil-Campos, M. Metabolic Profiling in Children with Autism Spectrum Disorder with and without Mental Regression: Preliminary Results from a Cross-Sectional Case–Control Study. Metabolomics 2019, 15, 99. [Google Scholar] [CrossRef]
- Kuwabara, H.; Yamasue, H.; Koike, S.; Inoue, H.; Kawakubo, Y.; Kuroda, M.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; et al. Altered Metabolites in the Plasma of Autism Spectrum Disorder: A Capillary Electrophoresis Time-of-Flight Mass Spectroscopy Study. PLoS ONE 2013, 8, e73814. [Google Scholar] [CrossRef]
- Carl, G.F.; Brogan, M.P.; Young, B.K. Is Plasma Serine a Marker for Psychosis? Biol. Psychiatry 1992, 31, 1130–1135. [Google Scholar] [CrossRef]
- Goff, D.C.; Wine, L. Glutamate in Schizophrenia: Clinical and Research Implications. Schizophr. Res. 1997, 27, 157–168. [Google Scholar] [CrossRef]
- Cao, B.; Wang, D.; Brietzke, E.; McIntyre, R.S.; Pan, Z.; Cha, D.; Rosenblat, J.D.; Zuckerman, H.; Liu, Y.; Xie, Q.; et al. Characterizing Amino-Acid Biosignatures amongst Individuals with Schizophrenia: A Case-Control Study. Amino Acids 2018, 50, 1013–1023. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, R.; Xu, Y.; Zhou, Z.; Guan, P.; Wu, Y.; Zhou, J.; Cheng, Z.; Zhang, L. Altered Metabolic Characteristics in Plasma of Young Boys with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 4897–4907. [Google Scholar] [CrossRef]
- Raghavan, R.; Anand, N.S.; Wang, G.; Hong, X.; Pearson, C.; Zuckerman, B.; Xie, H.; Wang, X. Association between Cord Blood Metabolites in Tryptophan Pathway and Childhood Risk of Autism Spectrum Disorder and Attention-Deficit Hyperactivity Disorder. Transl. Psychiatry 2022, 12, 270. [Google Scholar] [CrossRef]
- Carvajal-Oliveros, A.; Campusano, J.M. Studying the Contribution of Serotonin to Neurodevelopmental Disorders. Can This Fly? Front. Behav. Neurosci. 2021, 14, 601449. [Google Scholar] [CrossRef]
- Tamiji, J.; Crawford, D.A. The Neurobiology of Lipid Metabolism in Autism Spectrum Disorders. Neurosignals 2010, 18, 98–112. [Google Scholar] [CrossRef]
- Zhou, X.; Long, T.; Haas, G.L.; Cai, H.; Yao, J.K. Reduced Levels and Disrupted Biosynthesis Pathways of Plasma Free Fatty Acids in First-Episode Antipsychotic-Naïve Schizophrenia Patients. Front. Neurosci. 2020, 14, 784. [Google Scholar] [CrossRef]
- Casquero-Veiga, M.; Romero-Miguel, D.; MacDowell, K.S.; Torres-Sanchez, S.; Garcia-Partida, J.A.; Lamanna-Rama, N.; Gómez-Rangel, V.; Romero-Miranda, A.; Berrocoso, E.; Leza, J.C.; et al. Omega-3 Fatty Acids during Adolescence Prevent Schizophrenia-Related Behavioural Deficits: Neurophysiological Evidences from the Prenatal Viral Infection with PolyI:C. Eur. Neuropsychopharmacol. 2021, 46, 14–27. [Google Scholar] [CrossRef]
- Weiser, M.J.; Mucha, B.; Denheyer, H.; Atkinson, D.; Schanz, N.; Vassiliou, E.; Benno, R.H. Dietary Docosahexaenoic Acid Alleviates Autistic-like Behaviors Resulting from Maternal Immune Activation in Mice. Prostaglandins Leukot. Essent. Fatty Acids 2016, 106, 27–37. [Google Scholar] [CrossRef]
- Stra6l STRA6-like [Rattus norvegicus (Norway Rat)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/298077 (accessed on 10 January 2023).
- Han, F.; Zhang, R.; Yang, X.; Ma, S.; Hu, S.; Li, B. Neuroprotective Effect of Aldose Reductase Knockout in a Mouse Model of Spinal Cord Injury Involves NF-ΚB Pathway. Exp. Brain Res. 2022, 240, 853–859. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Li, X.; Jiang, Y.; Wang, C.; Zhang, Y.; Kong, Q.; Tian, C.; Dai, Y.; Zhao, W.; et al. Excess Salt Intake Promotes M1 Microglia Polarization via a P38/MAPK/AR-Dependent Pathway after Cerebral Ischemia in Mice. Int. Immunopharmacol. 2020, 81, 106176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.-W.; Li, J.; Dong, X.; Wang, Y.-H.; Ma, Z.-Z.; Jiang, Y.; Jin, H.-W.; Tu, P.-F. Anti-Neuroinflammatory Efficacy of the Aldose Reductase Inhibitor FMHM via Phospholipase C/Protein Kinase C-Dependent NF-ΚB and MAPK Pathways. Toxicol. Appl. Pharmacol. 2013, 273, 159–171. [Google Scholar] [CrossRef] [PubMed]
- SFARI Gene: RAB2A. Available online: https://gene.sfari.org/database/human-gene/RAB2A (accessed on 10 January 2023).
- SFARI Gene: GPR85. Available online: https://gene.sfari.org/database/human-gene/GPR85 (accessed on 10 January 2023).
- SFARI Gene: GUCY1A2. Available online: https://gene.sfari.org/database/human-gene/GUCY1A2 (accessed on 10 January 2023).
- SFARI Gene: IL1RAPL1. Available online: https://gene.sfari.org/database/human-gene/IL1RAPL1 (accessed on 10 January 2023).
- SFARI Gene: TRIM23. Available online: https://gene.sfari.org/database/human-gene/TRIM23 (accessed on 10 January 2023).
- Matsumoto, M.; Straub, R.E.; Marenco, S.; Nicodemus, K.K.; Matsumoto, S.; Fujikawa, A.; Miyoshi, S.; Shobo, M.; Takahashi, S.; Yarimizu, J.; et al. The Evolutionarily Conserved G Protein-Coupled Receptor SREB2/GPR85 Influences Brain Size, Behavior, and Vulnerability to Schizophrenia. Proc. Natl. Acad. Sci. USA 2008, 105, 6133–6138. [Google Scholar] [CrossRef] [PubMed]
- Montani, C.; Ramos-Brossier, M.; Ponzoni, L.; Gritti, L.; Cwetsch, A.W.; Braida, D.; Saillour, Y.; Terragni, B.; Mantegazza, M.; Sala, M.; et al. The X-Linked Intellectual Disability Protein IL1RAPL1 Regulates Dendrite Complexity. J. Neurosci. 2017, 37, 6606–6627. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal Immune Activation: Reporting Guidelines to Improve the Rigor, Reproducibility, and Transparency of the Model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]


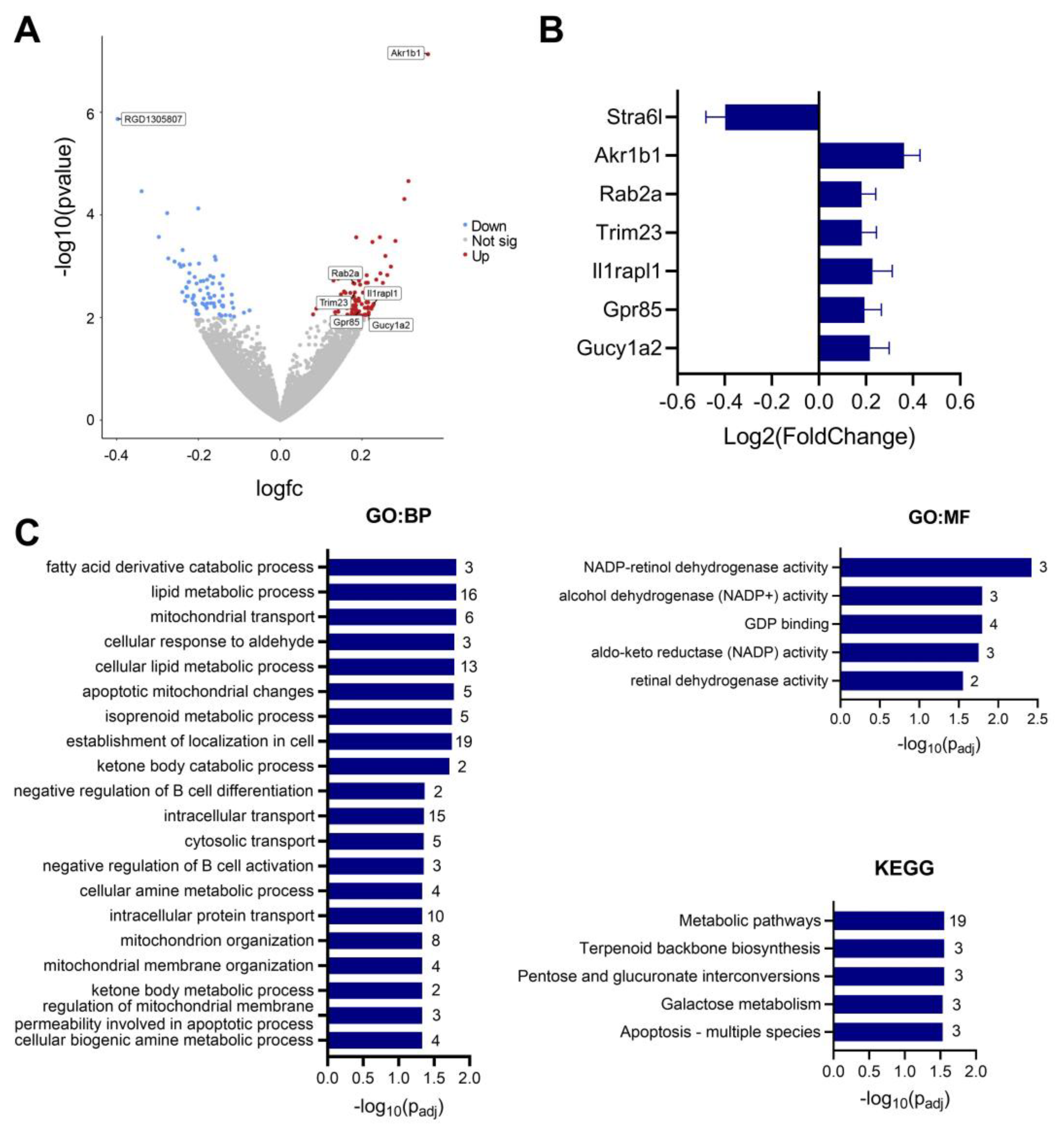
| CTL (pg/mL) | IMQ (pg/mL) | |
|---|---|---|
| IL-6 | 46 ± 12 | 585 ± 49 *** |
| IL-1β | 56 ± 22 | 57 ± 10 |
| TNF-α | 2 ± 0.5 | 5.5 ± 3.5 |
| IL-17a | 0.8 ± 0.1 | 0.9 ± 0.2 |
| IFN-γ | 80 ± 27 | 361 ± 85 * |
| IP-10 | 258 ± 87 | 1020 ± 220 * |
| MCP-1 | 337 ± 36 | 1850 ± 373 ** |
| MIP-1α | 19 ± 1 | 44 ± 5 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McColl, E.R.; Henderson, J.T.; Piquette-Miller, M. Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation. Pharmaceutics 2023, 15, 1857. https://doi.org/10.3390/pharmaceutics15071857
McColl ER, Henderson JT, Piquette-Miller M. Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation. Pharmaceutics. 2023; 15(7):1857. https://doi.org/10.3390/pharmaceutics15071857
Chicago/Turabian StyleMcColl, Eliza R., Jeffrey T. Henderson, and Micheline Piquette-Miller. 2023. "Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation" Pharmaceutics 15, no. 7: 1857. https://doi.org/10.3390/pharmaceutics15071857
APA StyleMcColl, E. R., Henderson, J. T., & Piquette-Miller, M. (2023). Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation. Pharmaceutics, 15(7), 1857. https://doi.org/10.3390/pharmaceutics15071857







