Abstract
Analytical method validation ensures that a method provides trustworthy information about a particular sample when applied in accordance with the predefined protocol. According to regulatory standards, the rheological characteristics of topically applied semisolid formulations are one of the key elements involved in microstructure equivalence documentation. Therefore, for generic drug product manufacturers, it is a dire need to take a step forward in rheology method development and validation procedures. This paper aims to apply Analytical Quality by Design (AQbD) principles towards the development and validation of rheology methods for topical creams, as complex semisolid formulations. Risk assessment was carried out through an Ishikawa diagram and an estimate failure mode, effects, and criticality analysis (FMECA). Sample application, peltier temperature control, and sample rest time were identified as critical method variables (CMVs), and a 23 full factorial design was applied to understand their impact on rotational, creep recovery and, oscillatory measurements. The development of the method was carried out as per the ICH Q8-Q10, and Q14 guidelines and validated according to ICH Q2 (R2) guideline. The method demonstrated adequate precision (RSD < 15%), as well as selectivity. AQbD provided a comprehensive framework for developing a reliable and effective rheology method for this type of formulation.
1. Introduction
Topical products, commonly developed to exert a local action, have been used throughout history for cosmetic and therapeutic purposes [1]. Semisolid dosage forms aiming toward medical application, such as ointments, pastes, gels, rigid foams, and creams, display a complex multiphasic structure, which is deeply characterized by pseudoplastic behavior [2,3]. Semisolid topical formulations display interdependent relationships between their structure, physical properties, manufacturing process, and performance when compared to other dosage forms [4]. From a thermodynamic point of view, cream formulations are inherently unstable systems and, therefore, tend to break down over time due to the contribution of several physicochemical mechanisms, including gravitational separation, flocculation, coalescence, particle coalescence, Ostwald ripening, and phase separation [5]. As a result of these processes, changes in pH, viscosity, and color are frequently observed, which may compromise their stability and performance [6].
Clobetasol propionate (CP) is a prednisolone derivative that is commercially available in a wide range of topically applied dosage forms such as creams, ointments, solutions, foams, and gels [7]. CP applicability is closely related to its potency, being useful in a variety of skin disorders, ranging from itching, redness, dryness, crushing, scaling, inflammation, and discomfort of various scalp and skin conditions, including eczema and psoriasis [8,9,10,11,12,13]. Nevertheless, clinically, CP exhibits poor skin permeability, which leads to a reduction in the therapeutic efficacy at the target site. However, this drawback can be overcome by a proper selection of the right formulation, where the assessment of the microstructure presents itself as one of the features with paramount relevance in formulation performance. Taking this information into account, in the present study, a clobetasol propionate formulation was used.
The monitoring of rheological properties, by establishing the correlation between viscosity and shear stress, regards an important tool during the development of semisolid dosage forms since it sheds light on why some formulations flow, while others retain structure under shear. This behavior is of paramount importance from a patient compliance perspective [14,15]. On the other hand, the importance of these relationships is also crucial during the production stage, where rheological properties need to be assessed after manufacture and during shelf life in order to ensure that the formulation is physically stable [16]. The time- and temperature-dependent change in viscosity provides pharmaceutical formulations with rheological flexibility. This can subsequently affect the release profile of the active pharmaceutical ingredient from the semisolid matrix [2,17] and impact their permeation behavior [18].
Recently, the Food and Drug Administration (FDA) introduced the draft guideline on Physicochemical and Structure (Q3) Characterization of Topical Drug Products Submitted in ANDAs (2022) [19]. Furthermore, the European Medicine Agency (EMA) has also been vocal on this subject, through the release of the draft guideline on quality and equivalence of topical products (2018) [20,21]. In these documents, the characterization of rheological behavior is actively highlighted, as the applicants are highly encouraged to submit a complete rheological profile, addressing rotational and oscillatory measurements. For all the appointed reasons, the establishment of a well-defined and robust framework applied to rheology method development and validation is of outmost importance [15].
The Analytical Quality by Design (AQbD) concept, introduced in 2018, regards the translation of Quality by Design (QbD) principles to analytical method development [22]. The main rationale of AQbD relies on the continuous effort to improve analytical method selectivity and robustness, through a thoughtful identification and control of the critical method variables (CMVs) of the selected method [23,24]. The application of design of experiments (DoE) tools within this scope enables the attainment of mathematical relationships describing the impact of the CMVs on critical analytical attributes (CAAs) [25]. The interpretation of these relationships is crucial to define the optimal method conditions [26,27,28,29].
Taking into account the updated regulatory background, the specific objective of this work is to propose a workflow, based on AQbD principles, towards the development and validation of a rheology method applied to semisolid formulations. To the extent of our knowledge, this is the first literature report addressing this framework.
The following stages were considered within this scope:
- Definition of the analytical target profile (ATP): type of sample, type of the product, method application, type of analytical method, and instrument desirability;
- Risk assessment performance: made through an Ishikawa diagram and a failure mode, effects, and criticality (FMECA) analysis, in order to clearly define the selection of both CMVs and CAAs;
- Design of experiments (DoE): resorting to a 2k full factorial design to identify the parameters that have a more preponderant role in the method ATP, estimated through the desirability function;
- The last step comprised the performance of validation studies, a crucial part in every AQbD application. The optimized rheological settings were carefully validated in terms of precision and selectivity, in line with the existing guidelines, as well as other scientific reports [15,30,31,32,33].
In an attempt to summarize the main objectives of the present work, as well as to pinpoint the key concepts supporting an AQbD-based development and validation approach addressing rheology methods, Figure 1 is introduced.
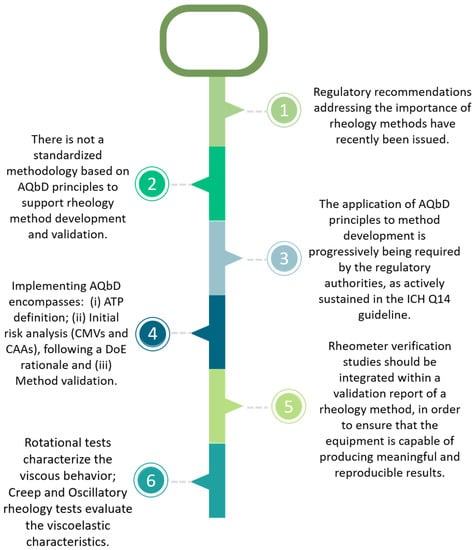
Figure 1.
AQbD key concepts applied to a rheology method.
2. Materials and Methods
2.1. Materials
Clobetasol propionate, chlorocresol, glyceryl stearate, cetostearyl alcohol, citric acid, sodium citrate, propylene glycol, beeswax, and purified water were provided by Laboratórios Basi Indústria Farmacêutica S.A. (Mortágua, Portugal). Three batches of a commercially available clobetasol propionate 0.525 mg/g cream were used during rheology method applicability studies.
Viscosity reference standard RT5000 (Fungilab, Barcelona, Spain) was used for rheometer equipment verification studies.
2.2. Methods
The rheological analysis was carried out in a HAAKETM MARSTM 60 Rheometer (ThermoFisher Scientific, Karlsruhe, Germany) with controlled temperature maintained by a thermostatic circulator and peltier temperature module (TM-PE-P) for cones and plates. All data were analyzed with HAAKE Rheowin® Data Manager v.4.82.0002 software (ThermoFisher Scientific, Karlsruhe, Germany). Statistical analysis was performed using JMP v.17 software (Cary, IL, USA).
Viscosity measurements were also performed using a Rotavisc Lo-vi viscosimeter (IKA®, Werke GmnH & Co. KG, Mindelheim, Germany) with SP12 spindle at 1 rpm. These measurements were performed at 20 °C.
2.2.1. Preparation of Clobetasol Propionate Cream Formulations
Clobetasol propionate o/w cream formulations were conventionally prepared using Ultra-Turrax X 10/25 (Ystral GmbH, Dottingen, Germany) equipment (Table 1). Both continuous and dispersed phases were separately prepared and heated to 60 °C. Afterward, the active pharmaceutical ingredient was solubilized in the dispersed phase. The produced cream formulations were stored at 20–25 °C. Batches of 0.5 kg were considered.

Table 1.
Qualitative composition of the clobetasol propionate cream formulation.
To document the discriminatory power of the proposed method, during validation studies, three formulations (Table 2) were manufactured:

Table 2.
Formulations addressed for DoE and method validation studies.
- (i)
- Formulation F1, considered as the main formulation and also used for DoE studies;
- (ii)
- Formulation F2, containing a different glycerol monostearate content, while formulation variable. This excipient was selected due to its thickening role;
- (iii)
- Formulation F3, which was produced using a different homogenization rate, while process variable. Product development studies revealed that a change in this operational setting highly impacted the rheological characteristics of the product.
Note that the latter two formulations (F2 and F3) were considered to establish the rheology method selectivity.
2.2.2. Equipment Verification
A viscosity curve was traced using the reference standard to verify the rheometer. Two temperatures were considered, 25 °C to mimic standard manufacturing specifications and 32 °C to mimic skin application. Triplicate measurements were performed.
2.2.3. AQbD Rheology Method Development
AQbD is rooted in ICH guidelines Q8 and Q9, which have been translated into the analytical domain through several white papers as well as the USP <1220> [34], Simões and coworkers [15], and forthcoming ICH Q14 [22]. The philosophy behind and strategies for the implementation of AQbD and the associated life cycle management of an analytical method have been combined according to the recently issued FDA draft guideline on Physicochemical and Structure (Q3) Characterization of Topical Drug Products Submitted in ANDAs (2022) [19] and the EMA draft guideline on quality and equivalence of topical products (2018) [20,21].
Analytical Target Profile
ATP refers to a prospective summary of the quality characteristics intended for the analytical method. In other words, it describes the appropriate attributes to be measured and relevant performance characteristics for a specific analytical method [27,35]. In this context, a comprehensive review of the ATP points to the selection of the critical method variables, method design, and development activities [36].
The ATP, described in Section 3.2, was tailored taking into account the Product Quality Target Profile (QTPP) and Critical Quality Attributes (CQA). Furthermore, regulatory requirements as well as relevant guidelines were likewise considered within this scope [22].
Initial Risk Assessment
Risk assessment regards the identification of the analytical parameters that could negatively impact CAAs. This assessment helps to identify the inherent risks and, at the same time, provide measures, processes, and controls to reduce their impact [37,38]. An Ishikawa diagram was traced to identify the risks, in order to provide a basis for risk evaluation and decisions on risk control. Afterward, the risk evaluation was performed by means of a failure mode, effects, and criticality analysis (FMECA), with the sole purpose to increase the knowledge of risk and to prevent failure. The output of an FMECA regards a relative risk “score” for each failure mode, which is then used to rank the modes on a relative basis. Risk quantification is to be performed by considering the severity (S), probability of occurrence (O), and detectability (D) of each parameter using a numerical scale 1–5, with 1 being the lowest severity, probability, and undetectability, and 5 the highest. For each factor, the rank and prioritization of the risk were conducted according to the risk priority number (RPN) given by RPN = S × O × D. The factors presenting higher RPN values were subjected to a further optimization analytical process [39,40,41].
Method Optimization
After risk assessment, design of experiments (DoE) should be conducted for method development, in order to screen or optimize method conditions as highlighted per Fukuda and collaborators (2018) [25].
The choice of suitable CMVs is extremely important, as it conditions the experimental results and respective interpretation. These were considered for rotational, creep recovery, and oscillatory measurements. This approach aimed at assessing the impact of different rheological critical method variables. The selected CMVs included the sample application mode, peltier temperature control, and sample rest time.
For DoE studies, a two-level full factorial design, 2k, with three variables was used. K factors were considered, each at 2 levels, including low and high levels. These levels are numerically expressed as −1, and +1, respectively. By applying a 23 full factorial design, a total of eight autonomous experiments (three replicates per experiment) were conducted to determine the impact of the selected CMVs on the responses.
To evaluate the DoE responses, both Student’s t-test and ANOVA were conducted to assess the statistical significance of the experimental parameters in the regression model. Please note the formulation F1 was used for DoE studies.
- Rotational measurements
Rotational measurements were performed using a cone (P35 2°/Ti, 35 mm diameter, 2° angle)-and-plate (TMP 35) geometry configuration. The measurements were carried out using a gap distance of 1 mm.
Viscosity curve
The viscosity curve (η = f(γ)) exhibits a dependence of both shear stress (τ) and apparent viscosity on the shear rate (ẏ). Furthermore, the viscosity curve is also time-dependent when considering a controlled-rate mode [42,43]. To trace the viscosity curve, the shear rate was linearly (CS mode) increased from 5 to 500 Pa for DoE studies, whilst for method validation, a 10 to 900 Pa range was considered. Both methods regarded a run time of 300 s and the collection of 30 data points.
The following CAAs were regarded for this test: zero-shear viscosity (η0), yield point (τ0.ROT), and infinite-shear viscosity (η∞).
Thixotropic profile
Flow curves (τ = f(γ)) were attained by the shear rate ramp-up from 0.1 to 300 s−1 (ascendant curve) and ramp-down from 300 to 0.1 s−1 (descendent curve). The thixotropic behavior was estimated by considering the hysteresis loop area (SR).
- 2.
- Creep recovery
The creep recovery test aims to describe the slow steady flow of a material under low-stress conditions [44]. More specifically, it evaluates the elastic and viscous components of the samples and their recovery profile, after being subjected to a shear stress. The test must be performed within the viscoelastic region, where the microstructure remains undisturbed. The measured response in a creep test is usually presented in terms of creep equilibrium compliance (Je, Pa−1), which corresponds to the ratio of the measured strain to the applied stress, or inverse modulus and the response elastic reformation (γe, %) [45,46,47]. The creep recovery test was performed using a cone (P35 2°/Ti, 35 mm diameter, 2° angle)-and-plate (TMP 35) geometry configuration. The measurements were carried out using a gap distance of 1 mm, with a shear stress of 50 Pa (within the LVR), over 200 s on the sample, followed by a recovery phase where the stress was suddenly removed, and the sample was allowed 200 s to recover the elastic part of the deformation.
- 3.
- Oscillatory measurements
Oscillatory measurements were performed using a plate (P35/Ti, 35 mm diameter)–plate (TMP 35) geometry configuration. The measurements were carried out using a gap distance of 1 mm.
Amplitude sweep
The oscillatory stress sweep test was performed at a constant frequency of 1 Hz from 0.5 to 1500 Pa. The following CAAs were regarded for this test: linear viscoelastic region (LVR), yield point (τ0.OSC), and flow point (τf).
Frequency sweep
The frequency sweep was conducted from 70 to 0.1 Hz, at a constant shear stress of 5.0 Pa. The following CAAs were regarded for this test: elastic modulus (G′) and viscous modulus (G″).
Definition of the Optimal Operational Settings
In an attempt to determine the optimal operational settings, the responses were ranked according to their impact on the rheology behavior, as well as meeting the ATP criteria for the analytical procedure [48].
2.2.4. Method Validation
After DoE experiments, the best conditions proceeded for validation studies. Since there are no specific guidelines for rheology method validation, the ICH Q2 (R2) guideline as well as the rheology tutorial proposed by Simões et al. (2020) were regarded as directives for addressing method precision and selectivity [15,22,49].
Precision
The precision of an analytical method procedure expresses the closeness of agreement between a series of measurements obtained from multiple samplings of the homogeneous sample, applied under the prescribed conditions [49]. Rheology method precision was determined by assessing the method repeatability and intermediate precision by F1 formulation. The acceptance criterion was set to an % RSD less than 15% [50]. A minimum of twelve determinations for each measurement were considered.
Selectivity
The selectivity of the rheology method refers to the ability of the method to detect changes in product performance, generally demonstrated by determining the effect of deliberate meaningful changes in the formulations [26]. In other words, the selectivity regards the ability of the method to provide a different response to a different formulation. To achieve this, two different formulations with changes in critical manufacturing variables and quantitative excipient composition (F2 and F3, previously detailed in Section 2.2.1) were specifically manufactured and cross-compared with the nominal formulation (F1). Then, the method selectivity was documented statistically by ANOVA with a Tukey multiple comparison test. Pairwise comparisons between the nominal formulation (F1) and the specifically manufactured formulations F2 and F3 were conducted. The differences between the means were considered significant at a value of p < 0.05.
2.2.5. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 software (San Diego, CA, USA) by applying a one-way ANOVA with a Tukey multiple comparison test. JMP v.17 software (Cary, IL, USA) was used for statistical analysis of the fitted models, including Student’s t-test, in order to test whether the terms were statistically significant in the regression model. The statistical analysis was considered significant when the regression Prob > F and t-test Prob > |t| were less than 0.05. The maximum squared regression coefficient (R2) indicated how well the model fitted to the experimental data, and the closer the value is to 1, the better the fit.
3. Results and Discussion
As described in the introduction section, several steps were considered to reach the ultimate goal of the present work: the application of an AQbD framework to the development and validation of a rheology method.
The initial and more theoretical components of the present work contemplated the definition of the ATP, as well as a complete risk assessment analysis resorting to Ishikawa and FMECA tools. DoE studies were then performed for all rheology methods considered, taking into account the CMVs chosen—sample application mode, peltier temperature control, and sample rest time. A total of eight autonomous experiences with three replicates were considered for each method. These results were analyzed using Student’s t-test, ANOVA, and the desirability function to determine the optimal conditions for each method. In the last stage of the work, method validation and method applicability studies were conducted. Figure 2 summarizes the workflow followed in the present work.
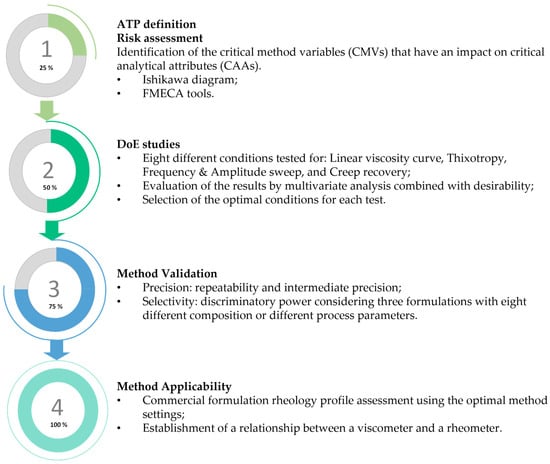
Figure 2.
Workflow followed for the development, validation, and application of the rheology method.
3.1. Equipment Verification
In order to comply with good manufacturing practices (GMPs), manufacturers should have a rigorous verification or qualification policy for all software systems as well as equipment used during production and quality control operations. Equipment verification was performed with a peltier controlled temperature at 32 °C. This temperature was chosen to mimic the physiological skin temperature [33,51,52]. Environmental factors such as a suitable working area, workbench levelness, and a satisfactory compressor system were ensured.
The acceptance criterium (RSD < 15%), which is in agreement with FDA guidelines, was considered.
Table 3 depicts the viscosity results retrieved from the Newtonian reference standard.

Table 3.
Viscosity values from a Newtonian reference standard. Results report to n = 3.
3.2. AQbD Rheology Method Development
3.2.1. Definition of ATP
The ATP of the rheology method applied to the clobetasol propionate cream formulations is depicted in Table 4. An effort was made to standardize the rheological methodology to comprehensively address the characterization of all operational parameters in a robust and efficient manner.

Table 4.
Analytical target profile elements considered for the optimization of the rheology method for a 0.5 mg/g clobetasol propionate cream formulation.
3.2.2. Initial Risk Assessment
In order to mitigate the initial risk assessment, an Ishikawa diagram and FMECA were carried out to identify and quantify all possible causes of disruption during rotational, creep recovery, and oscillatory measurements.
An Ishikawa diagram (depicted in Figure 3) dissects the method development process into various fractions such as analyst, environment, equipment, method, measurement, and data [53]. Each fraction provides an insight into factors that can affect the CAAs. As shown in Figure 3, according to previous knowledge, it was possible to identify several analytical settings which may have a direct repercussion on the rheological output. Based on this analysis, FMECA was carried out in order to rank the risk.
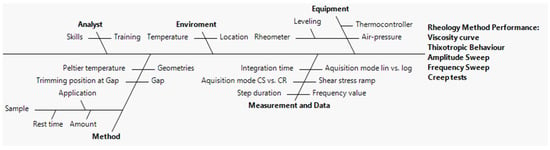
Figure 3.
Ishikawa diagram depicting the cause-and-effect relationship on the selected CAAs of the rheology method.
FMECA is a tool to identify potential problems during method development. It is an inductive method used for identification of hazards of a system with single-point failure. Table 5 shows the criteria used to assess FMECA scores. Risk acceptance is achieved by comparing the RPN score with defined acceptance levels. FMECA aids to rank and prioritize these factors into low, medium, and high risks based on analytical method hazards, as well as the probability that it will occur [54,55,56,57,58].

Table 5.
FMECA criteria to set up analysis scores.
As previously mentioned, several CAAs were retrieved from each rheological test, according to Table 6. These are described below.

Table 6.
Critical analytical attributes considered for the rheological tests.
For rotational measurements, two tests were considered: viscosity curve and thixotropy tests. The following responses were studied for the viscosity curve:
- Zero-shear viscosity is the limiting value of the shear rate-dependent viscosity function at an “infinitely low” shear rate, meaning the first Newtonian range with the plateau value;
- Infinite-shear viscosity is the limiting value of the shear rate-dependent viscosity function at an “infinitely high” shear rate, meaning the last Newtonian range with the plateau value;
- Yield point (also called yield stress) is the lowest shear stress value above which a material behaves as a fluid, and below which the material acts as a solid; in other words, it is the minimum shear stress required to initiate flow [59].
The time-dependent behavior, also known as thixotropic behavior, refers to the reduction in structural strength during a shear load phase and a more or less rapid but complete structural regeneration during the subsequent period of rest. The area between the upward and downward curves is called the “hysteresis area”; if the value is positive, the sample shows structural breakdown and if the value is negative, the sample shows structural build-up upon shearing. The hysteresis area indicates how fast the sample structure recovers after the load is removed [59].
Oscillatory tests were likewise performed. These were divided into two main parts: amplitude and frequency sweep experiments. The responses for amplitude sweep were:
- Linear viscoelastic range (LVR region), which indicates the range in which the test can be carried out without destroying the structure of the sample and represents a material’s ability in preserving its microstructure when exposed to rising shear stress;
- Yield point, which stands for the stress value at which the curve begins to deviate noticeably from the LVR plateau or from the corresponding fitted straight line used for analysis;
- Flow point, representing the shear stress value where the moduli cross over (G′ = G″) [41].
Regarding frequency sweep, the storage modulus (G′) represents the magnitude of energy stored in a material, whereas the loss modulus (G″) represents the energy loss due to viscous dissipation. Therefore, a material presents elastic properties when G′ < G″ and viscous properties when G′ > G″ [60].
Because of the relatively low consistency of many pharmaceutical formulations, it is often difficult to apply small enough stresses within the linear viscoelastic region for an oscillation test. In this context, the evaluation of the creep recovery test is an alternative for determining the relaxation time and viscoelastic properties of a material. A constant stress below yield stress is applied to the material and the deformation is monitored with time. Compliance (J) is defined as the reciprocal of the modulus, J = 1/G = γ/τ, where G is the modulus and γ is the strain. Creep recovery behavior aids in understanding the deformation mechanisms of the sample. Creep testing delivers strain or compliance measurements as a function of time under very slow stresses. High values of creep equilibrium compliance are characteristic of weaker internal structures [45,61,62]. Equilibrium compliance is the elastic response to strain of a viscoelastic material [63]. The elastic reformation value indicates the elastic portion of the viscoelastic behavior [59].
Table 7 shows the failure mode (the way in which a failure is observed), failure cause (the determination of causes of the failure mode), failure effect (the immediate consequences of a failure on the operation, function, or functionality), risk priority number (RPN), and recommended actions.

Table 7.
Failure modes studied in FMECA for rheology method.
The effects can be further classified with the calculation of theRPN, which is based on three categories (RPN interval for each category: category 1: low risk, value < 20; category 2: medium risk, value between 20–30; category 3: high risk, value > 30). Acceptance levels must be defined on a case-by-case basis, always focused on method quality. According to prior knowledge, the following CMVs were considered and may pose a direct repercussion on rheological endpoints: sample application, sample rest time, and peltier temperature.
3.2.3. Optimization of the Rheological Measurements
The optimal conditions for the rheological measurements were selected using a two-level, three-factor, 2k full factorial planning resorting to JMP 17.0 software (Cary, IL, USA). Most CAAs were identified based on the initial risk assessment analysis (Figure 3 and Table 7). Eight autonomous analyses, with three replicates each, were conducted to determine the effect of the three factors of each rheology endpoint (Table 8 and Table 9).

Table 8.
Critical method variables (CMVs) for experimental design and respective codification to assess the behavior of each condition on rotational and oscillatory measurements.

Table 9.
Design matrix used for optimization of rotational and oscillatory measurements.
To assess the influence of each factor and their respective combination on the responses, the polynomial coefficients were determined for each rotational, creep recovery, and oscillatory measurement’s response. A higher coefficient magnitude indicates a stronger main effect on the system. Additionally, if the coefficient has a positive sign, an increase in its level leads to an increase in the response. If the sign is negative, an increase in the independent variable level leads to a decrease in the response [64,65]. The integrated analysis of these responses yielded distinct models, whose coefficient values are presented in Figure 4 and Tables S1 and S2. Further, these results are discussed in the following sections.
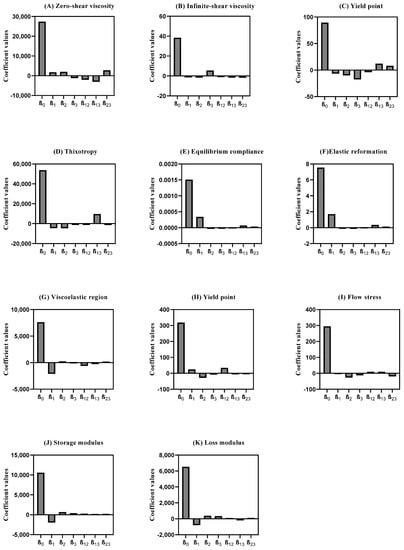
Figure 4.
Coefficient values of rheology measurements were extracted from mathematical models obtained from DoE. (A) Zero-shear viscosity; (B) Infinite-shear viscosity; (C) Yield point (rot); (D) Thixotropy; (E) Equilibrium compliance; (F) Elastic Reformation; (G) Viscoelastic region; (H) Yield point (osc); (I) Flow stress; (J) Storage modulus; (K) Loss modulus. Results report to n = 3.
The main goal supporting method optimization relied on the maximization of most of the rheology outputs, thus enabling a more comprehensive documentation of the rheology behavior, without compromising the discriminatory capacity of the method [66].
Actual by predicted plots of rotational and creep responses (CAAs) presenting a better goodness of fit from DoE experiments are shown in Figure S1 and S2. The diagonal line corresponds to the Y = X line. For a theoretical perfect fit, all the points would be on this diagonal. These curves provide a visual indication of significance at the 5% level.
The desirability (D) function is described as the weighted geometric mean for several responses or, alternatively, a value between 0 and 1 per response. A value of D different from zero indicates that all responses are in a desirable range, whilst a value close to 1 is pointed out as the combination of the different criteria considered optimal (Figures S3 and S4). As such, when D = 1, it means that the response values are close to the target ones [65]. In addition to that, Tables S3 and S4 depict the evaluation of ANOVA also performed for model fitness.
Interaction plots (Figure 5) display means for the levels of one factor on the x axis and a separate line for each level of another factor. Interaction effects were also analyzed by regression analysis and ANOVA for the responses in rotational, creep recovery, and oscillatory measurements. When the effects were significant, the results were interpreted considering the interaction effects. Note that parallel lines indicate no interaction and intersecting lines indicate possible interactions.
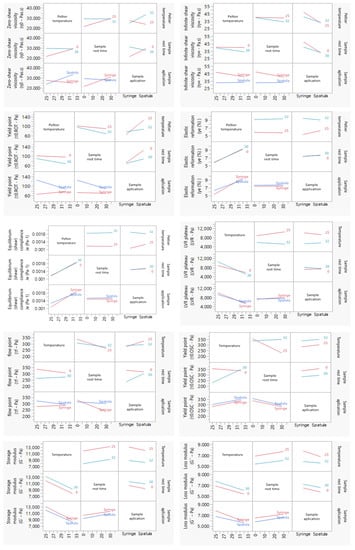
Figure 5.
Interaction plots of the DoE rheology responses. A line segment is represented per level of the row effect, and response values predicted by the model are summarized by line segments. Note that non-parallel line segments give visual indication of possible interactions. However, the p-value for such a suggested interaction should be verified to consubstantiate that it exists; please report to Figure 5. Results report to n = 3.
After conducting the DoE experiments and analyzing data, with the response of the desirability function, it was possible to establish the final rotational, creep recovery, and oscillatory measurement conditions, which were then applied during method validation studies.
Rotational Measurements
Viscosity curve
As displayed in the viscosity curve (Figure 6A), all experiments exhibited a non-Newtonian behavior, since the viscosity decreased with an increase in the shear rate, which classifies the system as pseudoplastic or shear-thinning [67]. The zero-shear viscosity depicts the strain response in the low-stress region and yields a high-viscosity plateau labeled as the zero-shear viscosity (η0) [18,68]. This endpoint was determined at approximately 22.0 Pa and is useful, as it reflects product viscosity in the steady state or, in other words, the product’s state within the container. This rheological response is mostly affected by the interaction between the peltier temperature and sample application (β13). Another major effect of this specific rheological response relies on the synergy effect between the sample rest time and sample application (β23). This may occur because the syringe causes major extrusion in the sample prior to the analysis, since as the stress increases, plastic flow occurs at critical stress.
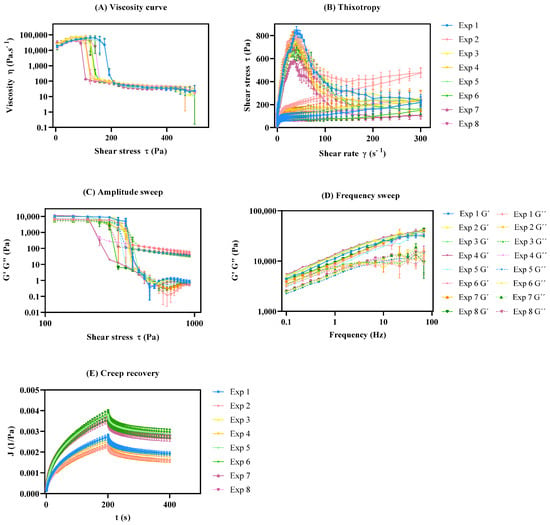
Figure 6.
Rheology profiles of DoE. All results report to n = 3 ± SEM. (A) Viscosity curve; (B) Thixotropic behavior; (C) Amplitude sweep; (D) Frequency sweep; (E) Creep recovery.
On the other hand, the infinite-shear viscosity (η∞) presented a less expressive coefficient magnitude, when compared to the η0 (Figure 5B). This endpoint refers to the second constant viscosity plateau and can be several orders of magnitude lower than η0 depending on the degree of shear thinning [69,70,71]. The results demonstrated that the sample application had a positive impact on the response (β3), whilst sample application by means of a syringe tended to increase this response. Once again, the interaction between peltier temperature and sample application (β13) proved to have a significant, but negative, impact on this CAA.
Another rotational endpoint regards the yield point. This is the minimum force that must be applied to start sample flow, and was calculated from the “steady stress sweep” method [72]. DoE results show that the sample application is of outmost importance, with the syringe application yielding lower results. This rheological endpoint was also negatively affected by the sample rest time, with lower rest times leading to lower yield point values. The interaction between the peltier temperature and the sample application (β13) is also considerable, however it has the opposite trend [73].
The desirability profiler for the viscosity curve suggests performing the analysis at 32 °C for peltier temperature, zero minutes for sample rest time, and sample application using a spatula (Figure S2).
Rheological modeling
To obtain details of DoE rheological parametric evaluation, viscosity and shear rate rheograms were analyzed by fitting results with various models, such as Ostwald–de Waele, Cross, Herschel–Buckley, Bingham, and Casson models (Table 10).

Table 10.
Regression parameters resulting from the different rheological models fitting to the acquired rheological data (experiment 5).
The flow behavior of samples without yield stress (τ0) can be described using the Ostwald–de Waele Equation (1):
where η is the viscosity (Pa·s−1), k is the flow coefficient, and exponent n refers to the flow index. It indicates the following: n < 1 for shear-thinning, n > 1 for shear-thickening, and n = 1 for ideally viscous flow behavior. Fitting to the Ostwald–de Waele model is appropriate where the measurement data are entirely within the shear-thinning regime across all the shear rates tested.
Cross model fluids behave similarly to those described by the Ostwald–de Waele model over a range of shear rates, but transition to regions of constant viscosity above and below this range. This model can be described according to Equation (2):
where η0 is the zero-shear viscosity, η∞ is the infinite-shear viscosity, and ẏ and n are fluid-specific parameters.
Herschel–Bulkley fluid relates the shear stress to the strain rate and can be described mathematically as described in Equation (3):
where τ0 is the yield stress, k is the consistency factor, and n is the flow index.
The material follows a shear-thinning flow behavior. The Bingham plastic model can best reflect such flow and the model can be described according to Equation (4) [14].
where ηρ is the plastic viscosity and τ0 stands for the yield stress.
Finally, the Casson model is also used to model flow curves showing a yield point, reported as Equation (5):
where τ0 is Casson yield point and ηρ is the Casson viscosity [41,59,74,75,76].
Regarding Experiment 5 from DoE, as suggested per the desirability results, and considering the R2 values, the Herschel–Bulkley R2 = 0.9534) model provided the best ability for predicting the shear flow behavior (Table 10). The data retrieved from the model are consistent with the shear-thinning flow behavior exhibited when a stress larger than the yield stress is reached. Note that the accurate determination of τ0 is dependent on both the rheological method and the model function used, being a parameter that highly impacts the spreadability of topical dosage forms and sensory attributes [41].
Thixotropic profile
Pseudoplastic systems can present a phenomenon called thixotropy, as is the case obtained in this work (Figure 6B), because even with the shear velocity removed, the system tends to regain the initial structure in such a way that the ascending and descending curves of the rheogram are displaced, resulting in a hysteresis area. The thixotropy is directly proportional to the hysteresis area; therefore, the larger the hysteresis area, the greater the formulation thixotropy [66,77]. The goal for the thixotropic method was to minimize the relative thixotropic area response (SR—Pa·s). In other words, the main target was to develop a method that enabled a more rapid and complete regeneration of the formulation [59].
Figure 4D displays the coefficient values attained for the SR. The high-magnitude interaction of coefficient β13 (peltier temperature and sample application) suggests an increase in this CAA. Experiment 1 shows a larger area of the thixotropic loop. Therefore, it can be expected that the formulation in these experimental conditions slowly recovers after the removal of the shear rate or stress [78,79]. Regarding the peltier temperature at 25 °C, the viscosity rises and slows extrusion. However, since all chemical processes slow at low temperatures, thixotropic recovery also slows. The desirability profiler suggests performing the thixotropic analysis at 32 °C for peltier temperature, thirty minutes for sample rest time, and with a spatula as the sample application mode (Figure S2).
Creep Recovery
Figure 6E illustrates the typical creep recovery behavior of a viscoelastic material. The creep curve with an upward curvature indicates that the structure breaks down quickly under the influence of the shear stress and a viscosity reduction should occur [14,80]. The creep recovery test is used to analyze the viscoelastic behavior by performing two shear stress steps. The goal for the creep recovery test was to maximize the responses creep equilibrium compliance (Je, Pa−1) and the elastic reformation (γe, %). The shear compliance is the reciprocal value of the shear modulus which can be foreseen as “rigidity” [81,82]. The elastic reformation indicates the elastic portion of the viscoelastic behavior. Creep tests essentially provide information at low stress over long periods of time (equivalent to low frequencies), but at high frequencies, it could be unavoidably inaccurate [14]. The peltier temperature (β1) regards the main CMV that statistically impacts both equilibrium compliance and elastic reformation (Figure 5E,F). The desirability profiler suggests performing the creep recovery analysis at 32 °C for peltier temperature, thirty minutes for sample rest time, and sample application using a syringe (Figure S2).
Oscillatory Measurements
The oscillatory measurements are given by the elastic distribution, termed as the storage modulus (G′), since it represents the storage of energy and the viscous contribution, termed as the loss modulus (G″), since it represents energy loss. Oscillatory method DoE studies aimed to maximize the responses for amplitude (LVR region, yield point, flow point) and frequency sweep (storage modulus (G′—Pa), loss modulus (G″—Pa). These tests can be further divided into amplitude sweep tests and frequency sweep tests.
Amplitude sweep
Amplitude sweep is an important test to determine the linear viscoelastic region (LVR). The LVR regards the plateau in which the microstructure of the sample remains undisrupted. In other words, it represents the plateau where the sample maintains both G′ and G″ despite changes in the shear stress (Figure 6C) [59,83]. This is a critical input parameter for subsequent frequency-based measurements. Considering the coefficient magnitude, the main method parameter impacting the LVR response regards the peltier temperature. At lower temperatures, most of the LVR response is higher. In line with this observation, the interaction between the temperature and the sample rest time also proved to have a statistically significant effect on this CAA, with lower temperatures and lower rest times, yielding higher LVR values [84,85,86]. At higher temperatures, material components have more thermal energy and, hence, a lower stress input is required to initiate flow. Magnitude of the interaction coefficient β12 suggests that the existence of interactions between the factors for yield point response (Figure 5H). Consequently, yield point (τ0.OSC) tends to decrease with increasing temperature so long as there is no thermally induced structural enhancement at elevated temperature. For the flow stress, the β2 coefficient indicates a negative impact of sample rest time on this response.
The desirability profiler suggests performing the amplitude sweep analysis at 25 °C for peltier temperature, zero minutes for sample rest time, and sample application using a spatula (Figure S2).
Frequency sweep
Frequency sweep measurements enable the determination of the viscoelastic properties of a sample as a function of timescale [59,84]. After the LVR has been defined by amplitude sweep, its structure can be further characterized using frequency sweep analysis. The considered outputs in this test were the storage modulus (G′—Pa) and the loss modulus (G″—Pa) [87].
Factorial design results showed that a decrease in the peltier temperature increased both CAAs. However, the sample rest time and the application method also proved to be significant. The response is directly proportional to an increase in rest time and spatula application, enabling higher G′ and G″ values (Figure 4J,K). Figure 6C,D shows what could be classified as a well-structured system. In this case, particles are strongly associated; the G′ is greater than the G″ and both are almost independent of frequency (Figure 6D). The frequency sweep curve gives a good rheological description of how the product behaves during storage and application [88].
The desirability profiler suggests performing the frequency sweep analysis at 25 °C for peltier temperature, thirty minutes for sample rest time, and sample application using a syringe (Figure S2).
3.2.4. Risk Assessment Update
After performing the optimization studies, the risk assessment analysis was updated in order to capture the reduced level of risk, based on our improved method understanding (please see Table 11). The updated analysis was used to assess high risk items and the activities underway in order to provide corrective actions. According to the results, the main failure modes, including sample application mode, sample rest time, and peltier temperature control, are still at the top of the ranking, and could be the main critical method variables that lead to incorrect data analysis.

Table 11.
Updated FMECA after rheology method optimization.
FMECA was also most useful to define job considerations, quality data points, preventive method actions, and activities necessary to minimize failure risk. The updated levels demonstrate that these specific method variables should be carefully selected due to their significant impact on rheology CAAs.
The details of the risk assessment, considering how method failure might be detected, must be performed in the experiment. This approach may seem costly with light benefit. However, once the work is completed, the ongoing management of the risk-based FMECA is much simpler and the benefits include the development of stronger compliance defense in the method, both in terms of justification of the potential impact of an instrument failure on results and reduction of risks because the possibility of an undetected method failure has been significantly reduced [89,90].
3.2.5. Optimal Operational Settings
The optimal operational settings (Table 12) were established following a multidimensional approach considering the relative impact of each CMV per rheology measurements based on method factors and settings.

Table 12.
CMVs’ effect on the meaningful rheology method performance CAAs.
3.3. Method Validation
According to the analytical guidelines, analytical method validation is an essential requirement to perform numerous assessments designed to verify that an analytical test system is suitable for its intended reason and is capable of providing robust and legitimate analytical data [49,91].
3.3.1. Precision
Repeatability and intermediate precision of twelve replicates showed relative standard deviation values less than 15% for all measurements. The results are displayed in Table 13.

Table 13.
Repeatability and intermediate precision results from rotational and oscillatory measurements. Results report to a n = 12 ± SEM.
3.3.2. Selectivity
To evaluate selectivity, i.e., the ability of the methods to accurately identify distinct formulations, three pairwise statistical comparisons were performed: (i) F1 vs. F2; (ii) F1 vs. F3; and (iii) F2 vs. F3. If the CAAs of each formulation present significant differences, the method is considered to be selective. The results summarized in Table 14 show that low p-values are obtained for most of the comparisons, indicating that there are significant differences among the formulations.

Table 14.
Selectivity results from rotational and oscillatory measurements. Results report to n = 12 ± SEM.
There were, however, no statistically significant differences found for zero-shear viscosity and yield point between F2 vs. F3 (p-value = 0.9944 and 0.1535, respectively). Nevertheless, these two CAAs display significant differences between F1 vs. F2 and F1 vs. F3; therefore, punctual results do not undermine the overall selectivity results.
From the data analysis (Figure 7), the formulation with distinct glycerol monostearate content (F2) and distinct manufacturing conditions (F3) displays significant differences in the cream microstructure, when compared to the nominal formulation (F1).
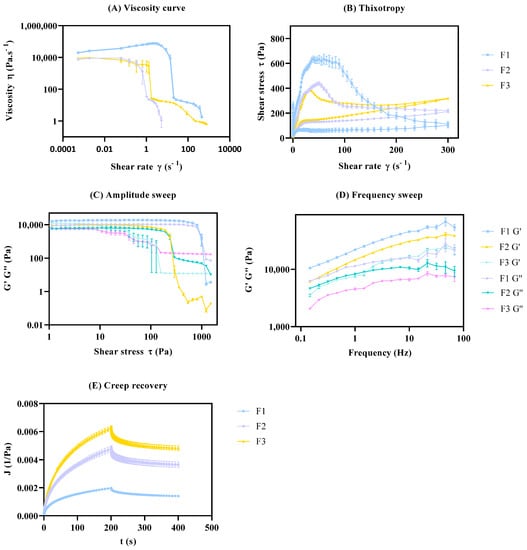
Figure 7.
Rheology validation studies. Effect of glycerol monostearate (F2) and homogenization rate (F3) on rheology profile. Results report to n = 12 ± SEM. (A) Viscosity curve; (B) Thixotropic; (C) Amplitude sweep; (D) Frequency sweep; (E) Creep recovery.
Please note that the evaluation of the method robustness could also have been performed; however, change in these CMVs cannot be equated due to their significant impact over the selected CAAs.
3.4. Summing-Up
❖ A detailed rheological characterization of a clobetasol propionate 0.5 mg/g cream formulation provided information on the product’s aesthetic properties, patient compliance, and overall quality profile;
❖ The AQbD concept, including a risk assessment to rank the impact of CMVs over CAAs, was considered in experimental design and the rheology measurement methods for the acquisition of a suitable rheology profile;
❖ A broad range of endpoints was considered throughout a rheology profile analysis, namely zero-shear viscosity, infinite-shear viscosity, rotational yield point, relative thixotropic area, creep equilibrium compliance, response elastic reformation, LVR plateau, oscillatory yield point, flow point, storage modulus, and loss modulus;
❖ Overall procedures and respective acceptance criteria regarding regulatory validation components such as equipment verification, precision, and discriminatory power were herein proposed;
❖ In view of the applicability and overall importance of rheology, this paper summarizes a practical standardization of procedures for the foundational development and validation of this method.
4. Bridging Compendial Testing with Rheometry-Based Approach
After rheology method development using an AQbD approach and validation, the rheology method’s applicability was determined by investigating the rheology profile of three batches of a commercially available clobetasol propionate 0.5 mg/g cream. The main objective of this analysis was to establish a quality control (QC) method for the rheological assessment of this specific topical product.
In addition to the RSD % (RSD < 15%) evaluation for each CAA, between the replicates, a range for acceptance criteria was established using the precision results to calculate the upper and lower control limits for each CAA. The low and high limits were estimated as follows: CAA average ± (standard deviation × 3) [92]. According to Table 15, the majority of the CAAs displayed compliant results concerning rotational, creep recovery, and oscillatory measurements, thus reinforcing the suitability of the proposed methods. Nevertheless, two samples (2 and 3) presented non-compliant results. Even though all the CAAs proved compliant with the previously established criteria (RSD < 15%), which are in agreement with FDA guidelines, a critical evaluation should be made on the specification range, bearing in mind the intrinsic variability associated with these dosage forms.

Table 15.
Commercial clobetasol propionate 0.5 mg/g cream results. Results report to n = 6 ± SD.
In quality control routine, viscometers are commonly employed to measure the viscosity of semisolid formulations during manufacture. Furthermore, the viscosity determination, by means of a rotational viscometer, is one of the analyses that are commonly presented in a Certificate of Analysis.
Typically, a viscometer employs a mechanical bearing that limits the speed and torque capabilities of the instrument, whereas a rheometer uses a low-friction air bearing. This means a viscometer can be a solution for material process or production tests that require simple flow measurements on Newtonian materials (where viscosity is independent of shear rate). However, the acquisition of a complete rheology profile warrants the determination of a better characterization of flow and deformation, as it measures the viscosity of the sample over a predetermined shear rate/shear stress range.
In this context, the viscosity of the same commercial samples was also analyzed by a conventional viscometer, in order to establish a relationship with the viscosity results obtained with the rheometer. Six measurements were performed, and average viscosity values were calculated. The viscosity results for samples 1, 2, and 3 were 309 Pa·s−1 (SD 0.73), 300 Pa·s−1 (SD 1.11), and 274 Pa·s−1 (SD 0.88), respectively. Figure 8 depicts the viscosity curve from the rheometer and the relative viscosity from the viscometer. As it can be seen, the relative viscosity measurements comprise the shear-thinning range of the viscosity curve, which does not resemble the viscosity of the product in the container nor the viscosity of the sample upon application. This highlights the relevance of rheology method development, which provides a comprehensive knowledge on the viscosity, flow behavior, and yield point of the product under development, ultimately yielding a more efficient optimization of the product manufacturing process.
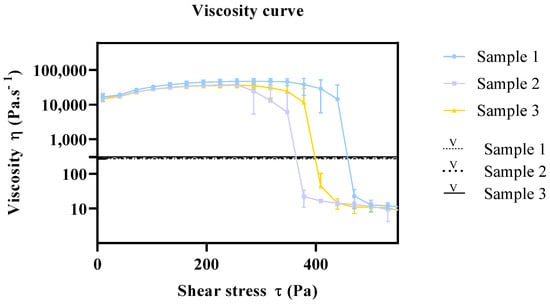
Figure 8.
Viscosity relationship of rheometry and viscometer. Results report to n = 6 ± SEM. Key: V: viscometer samples.
5. Conclusions
An AQbD-based rheology method for rotational, creep recovery, and oscillatory measurements was timely developed and validated under the umbrella of a comprehensive regulatory framework. Following risk assessment and factorial design tools, the impact of peltier temperature, sample rest time, and sample application on the responses stemming from rotational, creep recovery, and oscillatory measurements was estimated. DoE findings led to the definition of the best settings to describe the precision and selectivity of the rheological method, underlying “the right conditions at the first time” approach. Ultimately, the method was successfully applied to the analysis of the commercial products and could be used for routine quality control in pharmaceutical environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15071810/s1, Figure S1. Actual plots displaying predictions of rotational and creep recovery measurement responses (CAAs) presenting a better goodness of fit from DoE. Results report to n = 3; Figure S2. Overall desirability for rotational measurement optimization, according to the target imposed per CMV from DoE. Results report to n = 3; Figure S3. Actual plots displaying predictions of oscillatory measurement responses (CAAs) presenting a better goodness of fit from DoE. Results report to n = 3; Figure S4. Overall desirability for oscillatory measurement optimization, according to the target imposed per CMV from DoE. Results report to n = 3; Table S1. DoE coefficient values for critical analytical attributes of rotational measurements and respective summary of fit of the selected critical method variable. * Statistically significant coefficients, as extracted from Student’s t-test. Results report to n = 3; Table S2. DoE coefficient values for critical analytical attributes of oscillatory measurements and respective summary of fit of the selected critical method variable. * Statistically significant coefficients, as extracted from Student’s t-test. Results report to n = 3; Table S3. ANOVA parameters for the characterization of the model fitting per CAA from DoE. Results report to n = 3.
Author Contributions
Conceptualization, L.C. and M.M.; methodology L.C. and M.M.; software, L.C. and M.M.; formal analysis L.C. and M.M.; investigation, L.C. and M.M.; resources, C.V.; data curation, C.V.; writing—original draft preparation, L.C. and M.M.; writing—review and editing, C.V.; visualization, C.V.; supervision, C.C. and C.V.; funding acquisition, C.C. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
Lucas Chiarentin acknowledges the research grant PD/BDE/150717/2020 assigned by FCT (Fundação para a Ciência e a Tecnologia) and Laboratórios Basi from the Drugs R&D Doctoral Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available upon request.
Acknowledgments
The authors acknowledge Laboratórios Basi—Indústria Farmacêutica S.A. for the kind donation of all formulation components. The authors also thank UCQFarma for making available the Haake MARS 60 Rheometers (Thermo Scientific, Karlsruhe, Germany). Moreover, we also acknowledge Coimbra Chemistry Centre (CQC), supported by FCT, through the Project UID/QUI/00313/2020.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AQbD | Analytical Quality by Design |
| FMECA | Failure mode, effects, and criticality analysis |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| DoE | Design of experiments |
| CP | Clobetasol propionate |
| ATP | Analytical target profile |
| QTPP | Quality target product profile |
| CQA | Critical quality attribute |
| CAA | Critical analytical attribute |
| CMV | Critical method variable |
| RPN | Risk priority number |
| ANOVA | One-way analysis of variance |
| o/w | Oil-in-water |
| F1 | Clobetasol propionate 0.5 mg/g cream formulation |
| F2 | Clobetasol propionate 0.5 mg/g cream formulation manufactured with 5% w/w of glyceryl monostearate amount |
| F3 | Clobetasol propionate 0.5 mg/g cream formulation with different manufactured process |
| CR | Controlled-rate |
| CS | Controlled-stress |
| γ | Shear rate |
| τ | Shear stress |
| Je | Creep compliance |
| γe | Elastic reformation |
| LVR | Linear viscoelastic region |
| G″ | Loss modulus |
| G′ | Storage modulus |
| RSD | Relative standard deviation |
| SR | Thixotropic relative area |
| τf | Flow point |
| τ0.OSC | Oscillatory yield point |
| τ0.ROT | Rotational yield point |
| ƞ∞ | Infinite-shear viscosity |
| ƞ0 | Zero-shear viscosity |
| CP | Cone–plate |
| PP | Plate–plate |
References
- Folzer, E.; Gonzalez, D.; Singh, R.; Derendorf, H. Comparison of Skin Permeability for Three Diclofenac Topical Formulations: An in Vitro Study. Pharmazie 2014, 69, 27–31. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Brites, G.; Basso, J.; Miranda, M.; Miguel Neves, B.; Vitorino, C.; Cruz, M.T. Development of a New Hydrogel for the Prevention of Allergic Contact Dermatitis. Int. J. Pharm. 2022, 628, 122265. [Google Scholar] [CrossRef]
- SUN, C.C. Materials Science Tetrahedron—A Useful Tool for Pharmaceutical Research and Development. J. Pharm. Sci. 2009, 98, 1671–1687. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Developing Cream Formulations: Renewed Interest in an Old Problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Yusop, S.M. Determination of Stability of Cosmetic Formulations Incorporated with Water-Soluble Elastin Isolated from Poultry. J. King Saud Univ. Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, M.; Rao, R. Topical Delivery of Clobetasol Propionate Loaded Nanosponge Hydrogel for Effective Treatment of Psoriasis: Formulation, Physicochemical Characterization, Antipsoriatic Potential and Biochemical Estimation. Mater. Sci. Eng. C 2021, 119, 111605. [Google Scholar] [CrossRef]
- Carneiro, S.P. Desenvolvimento e Caracterização de Nanocápsulas de Propionato de Clobetasol Obtidas Por Polimerização in Situ Para Tratamento de Psoríase. Master’s Thesis, Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2013. [Google Scholar]
- Kasongo, K.W. Development and in Vitro Evaluation of a Clobetasol 17-Propionate Topical Cream Formulation. Master’s Thesis, Faculty of Pharmacy, Rhodes University, Grahamstown, South Africa, 2007. [Google Scholar]
- Jacob, S.E.; Steele, T. Corticosteroid Classes: A Quick Reference Guide Including Patch Test Substances and Cross-Reactivity. J. Am. Acad. Dermatol. 2006, 54, 723–727. [Google Scholar] [CrossRef]
- Marks, J.G.; Elsner, P.; DeLeo, V.A. Contact and Occupational Dermatology; Mosby: New York, NY, USA, 2002; ISBN 9780323014731. [Google Scholar]
- Gordon, M.L. The Role of Clobetasol Propionate Emollient 0.05% in the Treatment of Patients with Dry, Scaly, Corticosteroid-Responsive Dermatoses. Clin. Ther. 1998, 20, 26–39. [Google Scholar] [CrossRef]
- Hajare, A.A.; Velapure, P.D.; Rathod, P.N.; Patil, K.S.; Chopade, S.S. Formulation and Evaluation of Solid Lipid Nanoparticle Gel for Topical Delivery of Clobetasol Propionate To Enhance Its Permeation Using Silk Sericin As Permeation Enhancer. Int. J. Pharm. Sci. Res. 2020, 11, 2356–2365. [Google Scholar] [CrossRef]
- Langley, N.; Michniak-Kohn, B.; Osborne, D.W. The Role of Microstructure in Topical Drug Product Development; Springer: Westlake Village, CA, USA, 2019; ISBN 9783030173548. [Google Scholar]
- Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- aylor, K.; Aulton, M. Aulton Pharmaceutics, The Design and Manufacture of Medicines, 5th ed.Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780702070051. [Google Scholar]
- J Mastropietro, D. Rheology in Pharmaceutical Formulations-A Perspective. J. Dev. Drugs 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Dabbaghi, M.; Namjoshi, S.; Panchal, B.; Grice, J.E.; Prakash, S.; Roberts, M.S.; Mohammed, Y. Viscoelastic and Deformation Characteristics of Structurally Different Commercial Topical Systems. Pharmaceutics 2021, 13, 1351. [Google Scholar] [CrossRef]
- US Food and Drug Administration Guidance for Industry—Physicochemical and Structural (Q3) Characterization of Topical Drug Products Submitted in ANDAs. Available online: https://www.fda.gov/media/162471/download (accessed on 4 March 2023).
- Navarro-Pujol, F.; Bulut, S.; Hessman, C.; Karabelas, K.; Nieto, C.; Fernandez-Campos, F. Pilot Equivalence Study Comparing Different Batches of Topical 0.025% Capsaicin Emulsion: Product Microstructure, Release, and Permeation Evaluation. Pharmaceutics 2021, 13, 83. [Google Scholar] [CrossRef]
- Draft Guideline on Quality and Equivalence of Topical Products—CHMP/QWP/708282/2018; European Medicines Agency: London, UK, 2018; pp. 1–36.
- ICH Analytical Procedure Development—ICH (Q14); European Medicines Agency: Amsterdam, The Netherlands, 2022; pp. 1–61.
- Grangeia, H.B.; Silva, C.; Simões, S.P.; Reis, M.S. Quality by Design in Pharmaceutical Manufacturing: A Systematic Review of Current Status, Challenges and Future Perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef]
- Raman, N.V.V.S.S.; Mallu, U.R.; Bapatu, H.R. Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.D.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) Applied to Pharmaceutical and Analytical Quality by Design (QbD). Brazilian J. Pharm. Sci. 2018, 54, 1–16. [Google Scholar] [CrossRef]
- Miranda, M.; Cardoso, C.; Vitorino, C. Quality and Equivalence of Topical Products: A Critical Appraisal. Eur. J. Pharm. Sci. 2020, 148, 105082. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. AQbD as a Platform for IVRT Method Development—A Regulatory Oriented Approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef]
- Verch, T.; Campa, C.; Chéry, C.C.; Frenkel, R.; Graul, T.; Jaya, N.; Nakhle, B.; Springall, J.; Starkey, J.; Wypych, J.; et al. Analytical Quality by Design, Life Cycle Management, and Method Control. AAPS J. 2022, 24, 34. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Review Article Understanding Pharmaceutical Quality by Design. Am. Assoc. Pharm. Sci. 2014, 16, 771–783. [Google Scholar]
- Pharmaceutical Development—ICH Q8(R2); European Medicines Agency: Amsterdam, The Netherlands, 2009.
- European Medicines Agency Guideline on Quality of Oral Modified Release Products; European Medicines Agency: Amsterdam, The Netherlands, 2014; pp. 1–17.
- Food and Drug Administration Draft Guidance on Acyclovir Cream; Food and Drug Administration: Silver Spring, MD, USA, 2016; pp. 1–26.
- European Medicines Agency Guideline on Quality and Equivalence of Topical Products; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 1–36.
- United States Pharmacopeia. Analytical Procedure Lifecycle, General Chapter <1220>; United States Pharmacopeia: Rockville, MD, USA, 2022; pp. 1–18. [Google Scholar]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Almalki, W. Handbook of Analytical Quality by Design, 1st ed.; Elsevier: Chennai, India, 2021; ISBN 978-0-12-820332-3. [Google Scholar]
- Carriço, C.; Pinto, P.; Gonçalves, M.; Ribeiro, H.M.; Marto, J. Design and Characterization of a New Quercus Suber-Based Pickering Emulsion for Topical Application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef]
- Andrade, C.L.; De La O Herrera, M.A.; Lemes, E.M.B. A Model of Risk Analysis in Analytical Methodology for Biopharmaceutical Quality Control. PDA J. Pharm. Sci. Technol. 2018, 72, 317–331. [Google Scholar] [CrossRef]
- Sumit, K.; Shikha, T.; Deepika, T.; Ashish, B. A Quantitative Approach for Pharmaceutical Quality by Design Patterns. Inventi Rapid: Pharm Anal. Qual. Assur. 2012, 2012, 1–8. [Google Scholar]
- Vukašinović, M.; Savić, S.; Cekić, N.; Ilić, T.; Pantelić, I.; Savić, S.D. Efficient Development of Green Emulsifier/Emollient-Based Emulsion Vehicles: From RSM Optimal Experimental Design to Abridged In Vivo Assessment. Pharmaceutics 2023, 15, 486. [Google Scholar] [CrossRef]
- Quality Risk Management ICH (Q9). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-3.pdf (accessed on 21 January 2023).
- Simões, A.; Veiga, F.; Vitorino, C. Progressing towards the Sustainable Development of Cream Formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef]
- Yamamoto, R.; Onuki, A. Nonlinear Rheology of a Highly Supercooled Liquid. Europhys. Lett. 1997, 40, 61–66. [Google Scholar] [CrossRef]
- Helleloid, G.T. On the Computation of Viscosity-Shear Rate Temperature Master Curves for Polymeric Liquids. Morehead Electron. J. Appl. Math. 2001, 1, 1–11. [Google Scholar]
- Hardt, N.A.; Boom, R.M.; van der Goot, A.J. Wheat Dough Rheology at Low Water Contents and the Influence of Xylanases. Food Res. Int. 2014, 66, 478–484. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Rheology Basic: Creep-Recovery and Stress Relaxation. In Dynamic Mechanical Analysis; CRC Press: Boca Raton, FL, USA, 2020; pp. 45–68. [Google Scholar]
- Figueroa, J.D.C.; Hernández, Z.J.E.; Rayas-Duarte, P.; Peña, R.J. Stress Relaxation and Creep Recovery Tests Performed on Wheat Kernels versus Doughs: Influence of Glutenins on Rheological and Quality Properties. Cereal Foods World 2013, 58, 139–144. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometer, 2nd ed.; Thermo Electron (Karlsruhe): Karlsruhe, Germany, 2004. [Google Scholar]
- Kochling, J.; Wu, W.; Hua, Y.; Guan, Q.; Castaneda-Merced, J. A Platform Analytical Quality by Design (AQbD) Approach for Multiple UHPLC-UV and UHPLC-MS Methods Development for Protein Analysis. J. Pharm. Biomed. Anal. 2016, 125, 130–139. [Google Scholar] [CrossRef]
- Harmonisation, I.C. ICH Guideline Q2(R2) on Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 20 January 2023).
- Food and Drug Administration—FDA Bioanalytical Method Validation—Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 5 February 2023).
- Rath, S.; Kanfer, I. A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics 2020, 12, 119. [Google Scholar] [CrossRef]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.R.C.; Rytting, H.J.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Pharmacop. Forum 2009, 35, 750–764. [Google Scholar] [CrossRef]
- Varzakas, T. HACCP and ISO22000: Risk Assessment in Conjunction with Other Food Safety Tools Such as FMEA, Ishikawa Diagrams and Pareto. In Encyclopedia of Food and Health; Elsevier: Kalamata, Germany, 2015; pp. 295–302. ISBN 9780123849533. [Google Scholar]
- Pinthong, T.; Yooyod, M.; Daengmankhong, J.; Tuancharoensri, N.; Mahasaranon, S.; Viyoch, J.; Jongjitwimol, J.; Ross, S.; Ross, G.M. Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings. Gels 2023, 9, 459. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, E.P.; Reis, R.L.; Oliveira, J.M. Development of Conjugated Kefiran-Chondroitin Sulphate Cryogels with Enhanced Properties for Biomedical Applications. Pharmaceutics 2023, 15, 1662. [Google Scholar] [CrossRef]
- Wang, X.; Rong, L.; Shen, M.; Yu, Q.; Chen, Y.; Li, J.; Xie, J. Rheology, Texture and Swallowing Characteristics of a Texture-Modified Dysphagia Food Prepared Using Common Supplementary Materials. Foods 2023, 12, 2287. [Google Scholar] [CrossRef]
- Evans, P.E. Failure Mode Effects and Criticality Analysis. Conf. Rec. Midcon 1987, 11, 168–171. [Google Scholar] [CrossRef]
- Ford Motor Company Failure Mode and Effects Analysis, FMEA Handbook (with Robustness Linkages). Ford Mot. Co. 2011, 13, 286.
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Network: Hanover, Germany, 2014; ISBN 9783866306509. [Google Scholar]
- Pisal, P.B.; Patil, S.S.; Pokharkar, V.B. Rheological Investigation and Its Correlation with Permeability Coefficient of Drug Loaded Carbopol Gel: Influence of Absorption Enhancers. Drug Dev. Ind. Pharm. 2013, 39, 593–599. [Google Scholar] [CrossRef]
- Talens, P.; Castells, M.L.; Verdú, S.; Barat, J.M.; Grau, R. Flow, Viscoelastic and Masticatory Properties of Tailor Made Thickened Pea Cream for People with Swallowing Problems. J. Food Eng. 2021, 292, 110265. [Google Scholar] [CrossRef]
- Karaman, S.; Yilmaz, M.T.; Cankurt, H.; Kayacier, A.; Sagdic, O. Linear Creep and Recovery Analysis of Ketchup-Processed Cheese Mixtures Using Mechanical Simulation Models as a Function of Temperature and Concentration. Food Res. Int. 2012, 48, 507–519. [Google Scholar] [CrossRef]
- Maurya, C.S.; Sarkar, C. Rheological and Creep and Recovery Behavior of Carbonyl Iron Water-Based Magnetorheological Gel Using Laponite as an Additive and Oleic Acid as a Surfactant. Rheol. Acta 2022, 61, 99–110. [Google Scholar] [CrossRef]
- Vitorino, C.; Carvalho, F.A.; Almeida, A.J.; Sousa, J.J.; Pais, A.A.C.C. The Size of Solid Lipid Nanoparticles: An Interpretation from Experimental Design. Colloids Surf. B Biointerfaces 2011, 84, 117–130. [Google Scholar] [CrossRef]
- Vitorino, C.; Silva, S.; Gouveia, F.; Bicker, J.; Falcão, A.; Fortuna, A. QbD-Driven Development of Intranasal Lipid Nanoparticles for Depression Treatment. Eur. J. Pharm. Biopharm. 2020, 153, 106–120. [Google Scholar] [CrossRef]
- Herh, P.; Tkachuk, J.; Wu, S.; Bernzen, M.; Rudolph, B. Rheology of Pharmaceutical and Cosmetic Semisolids. Am. Lab. 1998, 30, 12–14. [Google Scholar]
- Salas-Bringas, C. Development and Verification of Methods for the Rheological Characterization of Materials for the Process Industry. Ph.D. Thesis, Norwegian University of Life Sciences, Ås, Norway, 2011. [Google Scholar]
- Stokes, J.R.; Telford, J.H. Measuring the Yield Behaviour of Structured Fluids. J. Nonnewton. Fluid Mech. 2004, 124, 137–146. [Google Scholar] [CrossRef]
- Dakhil, H.; Auhl, D.; Wierschem, A. Infinite-Shear Viscosity Plateau of Salt-Free Aqueous Xanthan Solutions. J. Rheol. 2019, 63, 63–69. [Google Scholar] [CrossRef]
- Instruments, M. A Basic Introduction to Rheology. Available online: https://cdn.technologynetworks.com/TN/Resources/PDF/WP160620BasicIntroRheology.pdf (accessed on 2 March 2023).
- Escudier, M.P.; Gouldson, I.W.; Pereira, A.S.; Pinho, F.T.; Poole, R.J. On the Reproducibility of the Rheology of Shear-Thinning Liquids. J. Nonnewton. Fluid Mech. 2001, 97, 99–124. [Google Scholar] [CrossRef]
- Chen, T. Rheological Techniques for Yield Stress Analysis. Available online: https://www.tainstruments.com/pdf/literature/RH025.pdf (accessed on 2 March 2023).
- Viscosity Testing Lab—The Centre for Industrial Rheology. Available online: https://www.rheologylab.com/services/viscosity-testing-lab/?campaign=15568012273&content=569564286655&keyword=viscosity%20measurements&gclid=Cj0KCQjw8qmhBhClARIsANAtbodGDikwos4vK5Pcned5OrWd3uXRL14PktKOinE9SM0yr66E9-f8y78aAmJ4EALw_wcB (accessed on 3 April 2023).
- Reid, E. Rheology Model Fitting and Corrections. Available online: https://blog.rheosense.com/rheology-model-fitting-and-corrections (accessed on 3 March 2023).
- Stanciu, I.; Ouerfelli, N. An Extended Casson Equation for Rheological Properties of Soybean Oil at Different Temperatures and Atmospheric Pressure. J Biochem Tech 2020, 11, 52–57. [Google Scholar]
- Mukhopadhyay, S.; De, P.R.; Bhattacharyya, K.; Layek, G.C. Casson Fluid Flow over an Unsteady Stretching Surface. Ain Shams Eng. J. 2013, 4, 933–938. [Google Scholar] [CrossRef]
- TA Instruments Rheology Aplications Note: Rheology Software Models (Flow). Available online: http://www.tainstruments.com/pdf/literature/RN9.pdf (accessed on 4 March 2023).
- Meyer, F. Investigating the Thixotropic and Shear Recovery Behaviour of Paints and Coatings. PPCJ Polym. Paint Colour J. 2014, 204, 26–29. [Google Scholar]
- Liu, J.; Wang, R.; Gao, F.; Zhou, J.; Cen, K. Rheology and Thixotropic Properties of Slurry Fuel Prepared Using Municipal Wastewater Sludge and Coal. Chem. Eng. Sci. 2012, 76, 1–8. [Google Scholar] [CrossRef]
- Daver, F.; Kajtaz, M.; Brandt, M.; Shanks, R.A. Creep and Recovery Behaviour of Polyolefin-Rubber Nanocomposites Developed for Additive Manufacturing. Polymers 2016, 8, 437. [Google Scholar] [CrossRef]
- Netzsch Creep (Rheology). Available online: https://analyzing-testing.netzsch.com/en/training-know-how/glossary/creep-rheology (accessed on 20 April 2023).
- Rheologylab Creep Test for Liquids and Semisolids. Available online: https://www.rheologylab.com/articles/creep-test/ (accessed on 20 April 2023).
- Van Vliet, T.; Lyklema, H. Rheology. Fundam. Interface Colloid Sci. 2005, 4, 1–14. [Google Scholar]
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Petit, J.Y.; Wirquin, E.; Khayat, K.H. Effect of Temperature on the Rheology of Flowable Mortars. Cem. Concr. Compos. 2010, 32, 43–53. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Rheological Analysis of Dispersions by Frequency Sweep Testing. Available online: https://www.azom.com/article.aspx?ArticleID=2884# (accessed on 27 April 2023).
- Mcgregor, P.L.; Hong, P.; Pham, T. Mitigating Risk of Validated Analytical Procedure Failures When Upgrading or Replacing LC Assets: Harnessing the Power of Quality by Design (QbD) Principles; Waters Corporation: Milford, MA, USA, 2021; pp. 1–10. [Google Scholar]
- Life Cycle Risk Assessment of HPLC Instruments. Available online: https://www.chromatographyonline.com/view/life-cycle-risk-assessment-hplc-instruments (accessed on 3 May 2023).
- Bonfilio, R.; Cazedey, E.C.L.; de Araújo, M.B.; Salgado, H.R.N. Analytical Validation of Quantitative High-Performance Liquid Chromatographic Methods in Pharmaceutical Analysis: A Practical Approach. Crit. Rev. Anal. Chem. 2012, 42, 87–100. [Google Scholar] [CrossRef]
- A Guide to Control Charts. Available online: https://www.isixsigma.com/control-charts/a-guide-to-control-charts/ (accessed on 27 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).