Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model
Abstract
1. Introduction
- (1)
- Visualize the deposition distribution of intranasally administered sprays and subsequent liquid film translocation in the nasal cavity using different angles of administration, head positions, number of spray applications, and inhalation flow rates.
- (2)
- Visualize the effect of formulation viscosity on the dosimetry of intranasal sprays.
- (3)
- Quantify the deposition of intranasal sprays in the olfactory cleft, the turbinate region, the front nose, as well as the nasopharynx.
- (4)
- Examine the results and compare the performance between the different test cases to determine the optimal combination of factors that lead to maximum bioavailability in the olfactory region (i.e., delivery of clinically significant doses).
2. Materials and Methods
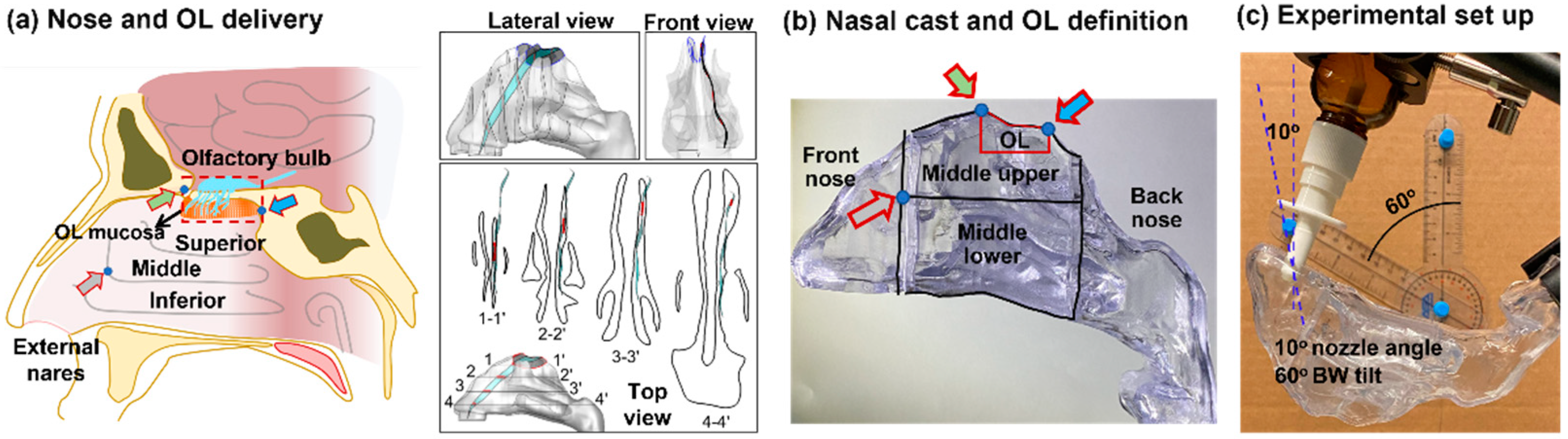
2.1. Nasal Cast Model
2.2. Study Design
2.3. Inhaler and Spray Solutions
2.4. Protocol for Nasal Spray Delivery
2.5. Image-Based Estimation of Olfactory Dosimetry
2.6. Statistical Analysis
3. Results
3.1. Characterization of Spray Viscosity and Aerosol Generation
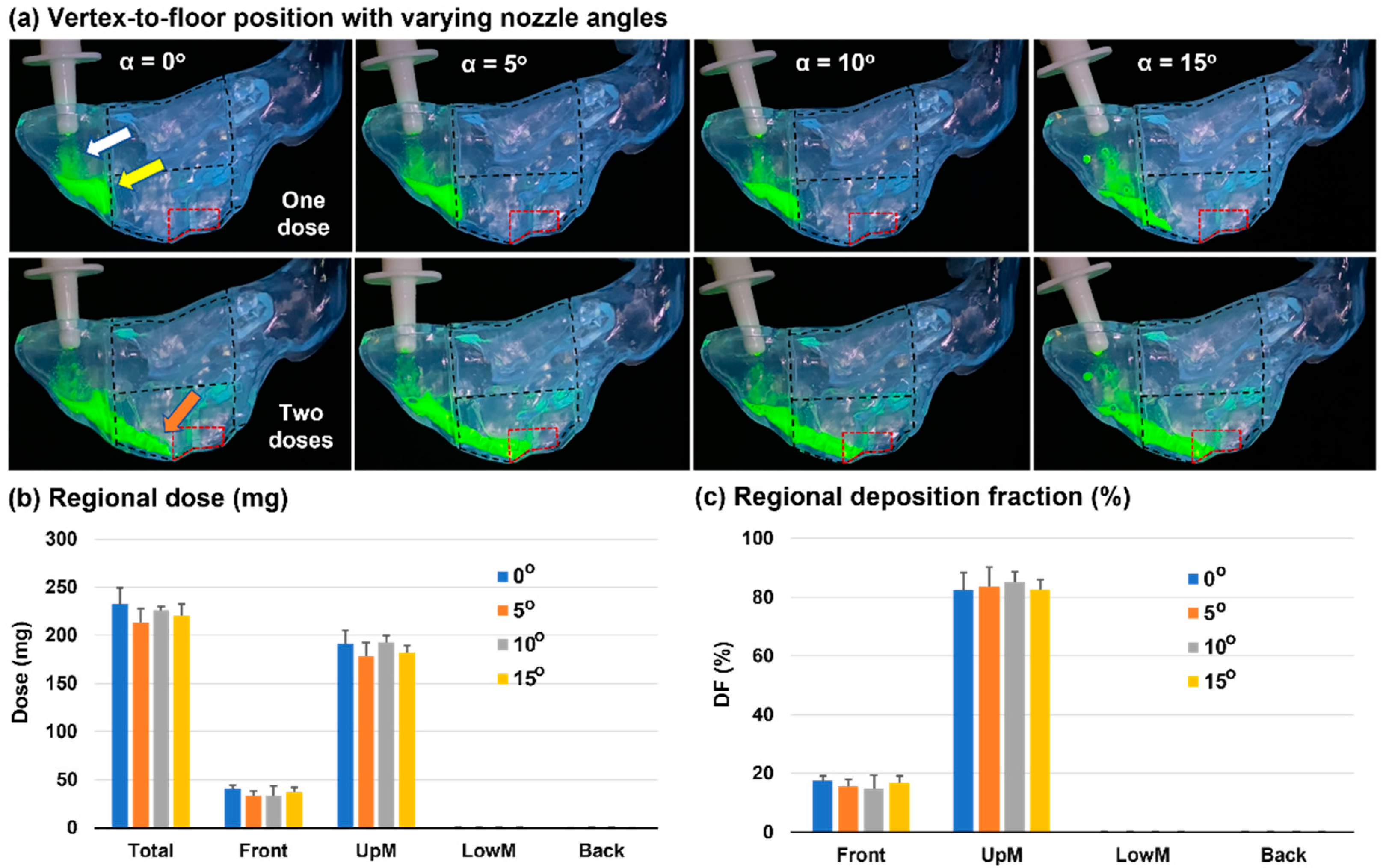
3.2. Vertex-To-Floor Head Position for Olfactory Delivery
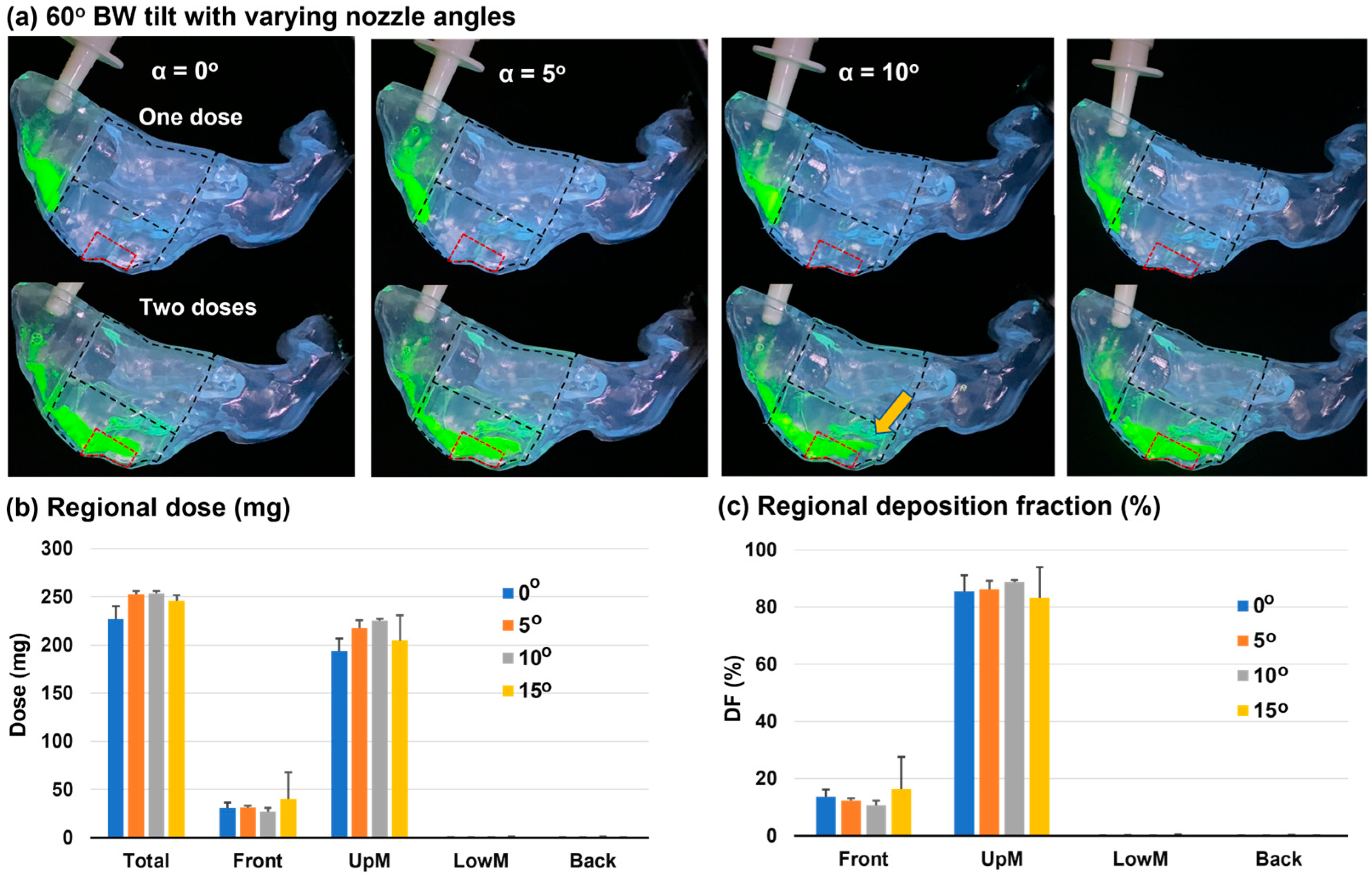
3.3. Head Position: 60° Backward Tilt from the Supine Position
3.3.1. Deposition Distribution
3.3.2. Dynamic Formation and Translocation of Liquid Film
3.4. Head Positions of 45° and 30° Backward Tilt from the Supine Position
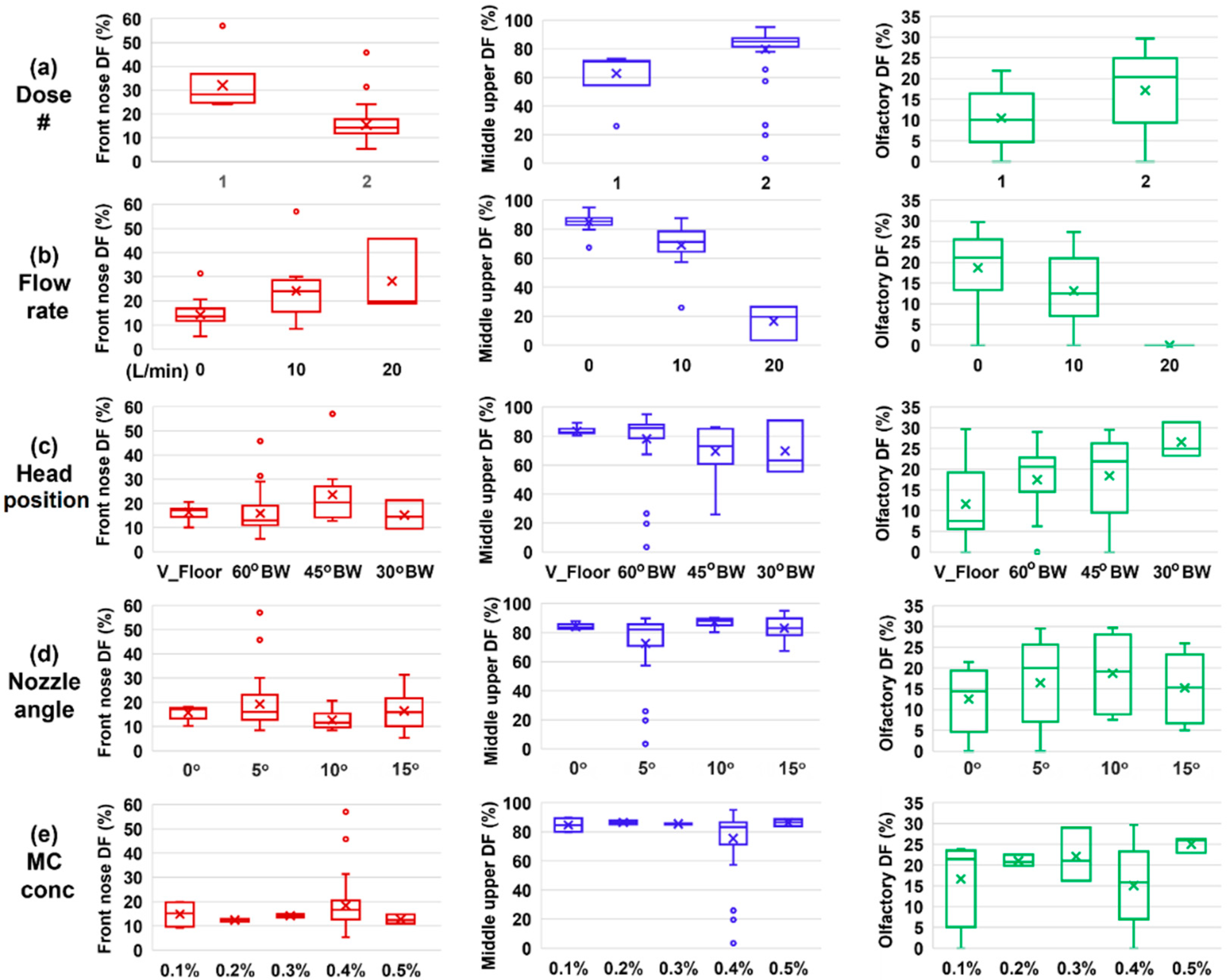
3.5. Effect of Inhalation Flow Rate
3.6. Effects of Solution Viscosity
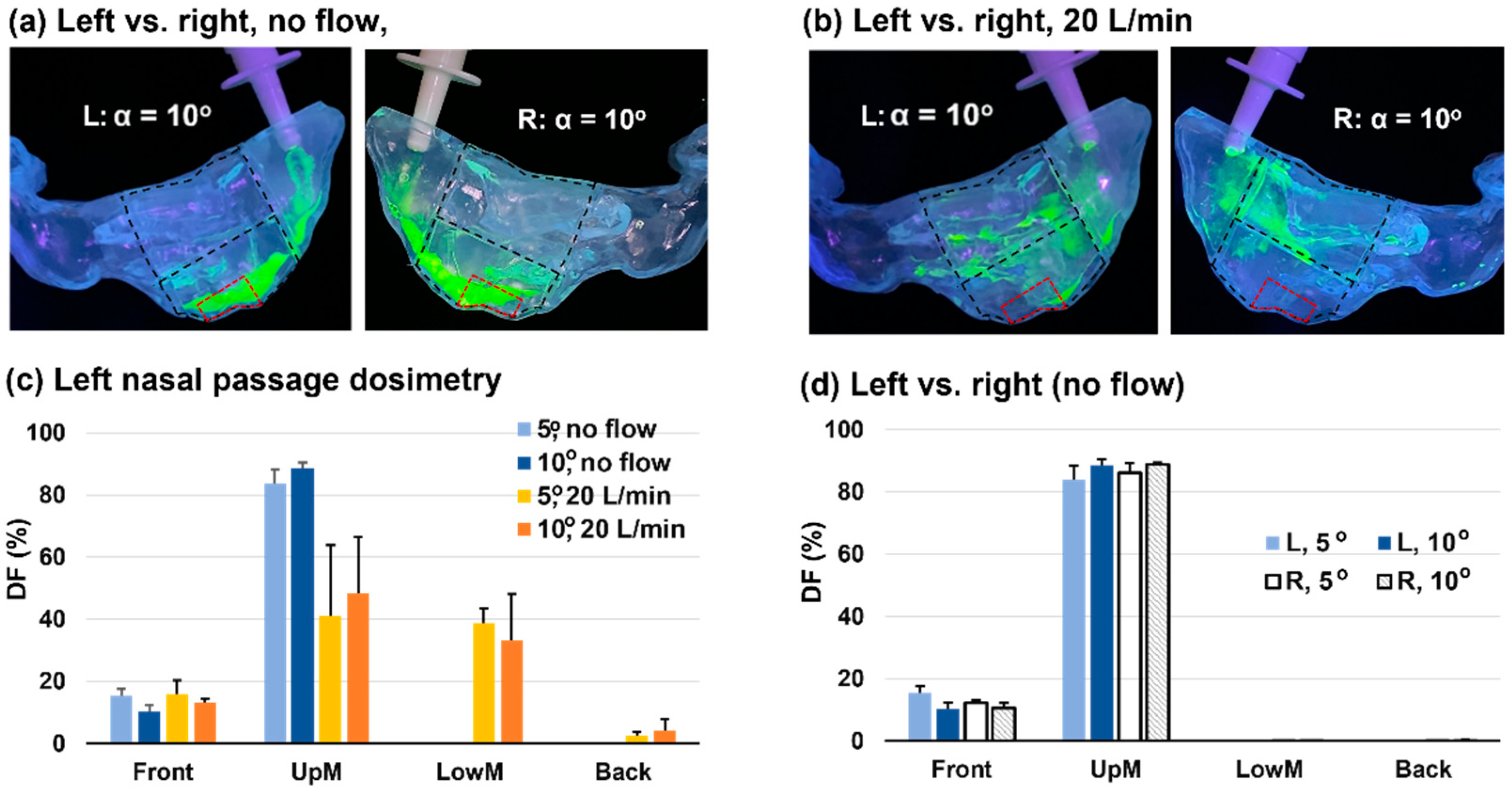
3.7. Left-Right Discrepancy in Dosimetry
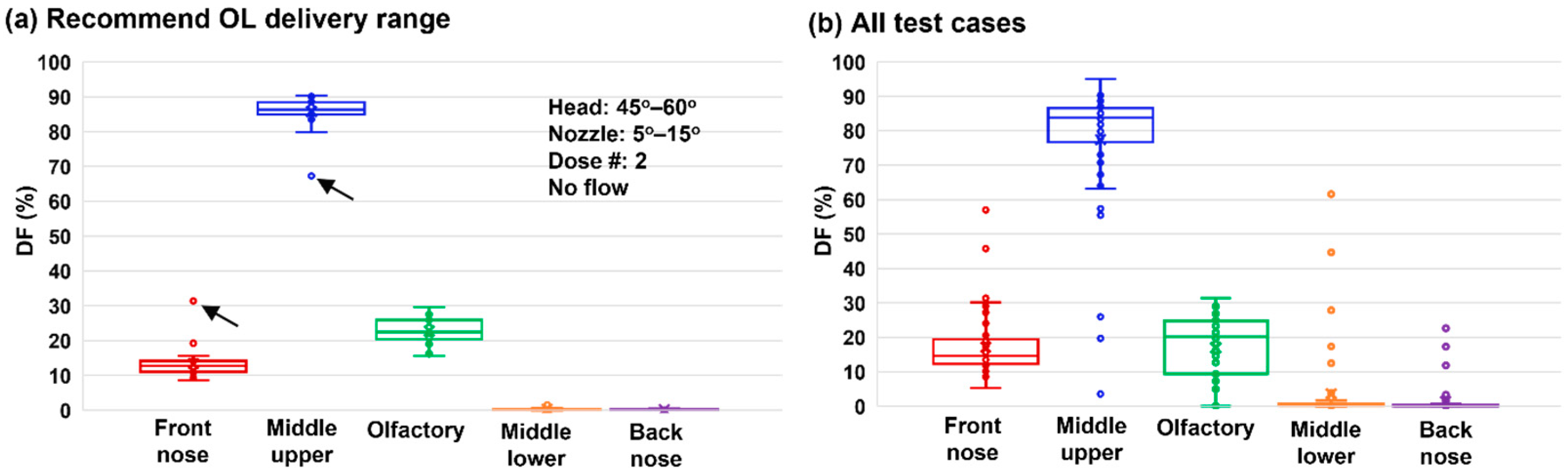
3.8. Image-Based Olfactory Dosimetry Estimation
3.9. Deposition Sensitivity Analysis to Delivery Variables
4. Discussion
5. Conclusions
- (1)
- The OL dosimetry depended not only on the initial deposition of spray droplets but also on the liquid film translocation.
- (2)
- A two-dose application from the soft-mist inhaler was needed to mobilize the liquid film and enable it to move to the olfactory region.
- (3)
- Recommended OL delivery parameters included: a head position tilting 45–60° backward from the supine position, a nozzle angle ranging 5–10° counterclockwise from the nostril normal, two doses, and no inhalation flow.
- (4)
- With the recommended protocol, a delivery efficiency of 22.7 ± 3.7% was achieved in the strictly defined OL region.
- (5)
- The presence of inhalation flow reduced the liquid film translocation to the OL region, with negligible OL doses at 20 L/min.
- (6)
- The vertex-to-floor head position was not optimal for OL delivery, with lower OL delivery efficiency and higher variability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badhan, R.K.; Kaur, M.; Lungare, S.; Obuobi, S. Improving brain drug targeting through exploitation of the nose-to-brain route: A physiological and pharmacokinetic perspective. Curr. Drug Deliv. 2014, 11, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.H.; Liu, J.; Chen, X.; Thorne, R.G.; Fawcett, J.R.; Ala, T.A.; Rahman, Y.-E. Delivery of 125I-NGF to the Brain via the Olfactory Route. Drug Deliv. 1997, 4, 87–92. [Google Scholar] [CrossRef]
- Jin, K.; Xie, L.; Childs, J.; Sun, Y.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann. Neurol. 2003, 53, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert. Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Yuan, J.E.; Zhang, Y.; Nevorski, D.; Wang, Z.; Zhou, Y. Visualization and Quantification of Nasal and Olfactory Deposition in a Sectional Adult Nasal Airway Cast. Pharm. Res. 2016, 33, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., 2nd. Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J. Neuroimmune Pharmacol. 2007, 2, 81–86. [Google Scholar] [CrossRef]
- Illum, L. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef]
- Misra, A.; Kher, G. Drug delivery systems from nose to brain. Curr. Pharm. Biotechnol. 2012, 13, 2355–2379. [Google Scholar] [CrossRef]
- Xi, J.; Lei, L.R.; Zouzas, W.; April Si, X. Nasally inhaled therapeutics and vaccination for COVID-19: Developments and challenges. MedComm (2020) 2021, 2, 569–586. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W. Characterization of submicrometer aerosol deposition in extrathoracic airways during nasal exhalation. Aerosol Sci. Technol. 2009, 43, 808–827. [Google Scholar] [CrossRef]
- Si, X.A.; Sami, M.; Xi, J. Liquid Film Translocation Significantly Enhances Nasal Spray Delivery to Olfactory Region: A Numerical Simulation Study. Pharmaceutics 2021, 13, 903. [Google Scholar] [CrossRef]
- Garcia, G.J.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- González-Botas, J.H.; Seara, A.P. Nasal gel and olfactory cleft. Acta Otorrinolaringol. Esp. 2012, 63, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Computational characterization of inhaled droplet transport to the nasopharynx. Sci. Rep. 2021, 11, 6652. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Akash, M.M.H.; Lao, Y.; Balivada, P.A.; Ato, P.; Ka, N.K.; Mituniewicz, A.; Silfen, Z.; Suman, J.; Chakravarty, A.; et al. A model-based approach to improve intranasal sprays for respiratory viral infections. medRxiv 2022. [Google Scholar] [CrossRef]
- Basu, S.; Holbrook, L.T.; Kudlaty, K.; Fasanmade, O.; Wu, J.; Burke, A.; Langworthy, B.W.; Farzal, Z.; Mamdani, M.; Bennett, W.D.; et al. Numerical evaluation of spray position for improved nasal drug delivery. Sci. Rep. 2020, 10, 10568. [Google Scholar] [CrossRef]
- Valentine, R.; Athanasiadis, T.; Thwin, M.; Singhal, D.; Weitzel, E.K.; Wormald, P.J. A prospective controlled trial of pulsed nasal nebulizer in maximally dissected cadavers. Am. J. Rhinol. 2008, 22, 390–394. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Skretting, A.; Winderen, M.; Holand, T. Breath actuated device improves delivery to target sites beyond the nasal valve. Laryngoscope 2006, 116, 466–472. [Google Scholar] [CrossRef]
- Möller, W.; Schuschnig, U.; Celik, G.; Münzing, W.; Bartenstein, P.; Häussinger, K.; Kreyling, W.G.; Knoch, M.; Canis, M.; Becker, S. Topical drug delivery in chronic rhinosinusitis patients before and after sinus surgery using pulsating aerosols. PLoS ONE 2013, 8, e74991. [Google Scholar] [CrossRef] [PubMed]
- Washington, N.; McGlashan, J.A.; Jackson, S.J.; Bush, D.; Pitt, K.G.; Rawlins, D.A.; Gill, D.A. The effect of nasal patency on the clearance of radiolabeled saline in healthy volunteers. Pharm. Res. 2000, 17, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Laube, B.; Dalby, R. The Effect of Formulation Variables and Breathing Patterns on the Site of Nasal Deposition in an Anatomically Correct Model. Pharm. Res. 2005, 22, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L.; Sharpless, G.; Shermer, C.; Nasir, O.; Sullivan, V.; Powell, K. Deposition of albuterol aerosol generated by pneumatic nebulizer in the Sophia Anatomical Infant Nose-Throat (SAINT) model. Pharm. Res. 2010, 27, 1722–1729. [Google Scholar] [CrossRef]
- Suman, J.D.; Laube, B.L.; Dalby, R. Validity of in vitro tests on aqueous spray pumps as surrogates for nasal deposition, absorption, and biologic response. J. Aerosol Med. 2006, 19, 510–521. [Google Scholar] [CrossRef]
- Fleming, J.S.; Conway, J.H.; Bolt, L.; Holgate, S.T. A comparison of planar scintigraphy and SPECT measurement of total lung deposition of inhaled aerosol. J. Aerosol Med. 2003, 16, 9–19. [Google Scholar] [CrossRef]
- Bondesson, E.; Bengtsson, T.; Borgström, L.; Nilsson, L.E.; Norrgren, K.; Trofast, E.; Wollmer, P. Planar gamma scintigraphy--points to consider when quantifying pulmonary dry powder aerosol deposition. Int. J. Pharm. 2003, 258, 227–240. [Google Scholar] [CrossRef]
- Kundoor, V.; Dalby, R.N. Assessment of nasal spray deposition pattern in a silicone human nose model using a color-based method. Pharm. Res. 2010, 27, 30–36. [Google Scholar] [CrossRef]
- Kundoor, V.; Dalby, R.N. Effect of formulation- and administration-related variables on deposition pattern of nasal spray pumps evaluated using a nasal cast. Pharm. Res. 2011, 28, 1895–1904. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Holmes, T.D.; Gao, J.; Guilmette, R.A.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Gizurarson, S. Method for Administration of Active Substances to the Olfactory Region. Patents CA 2298596, 2 February 2000. [Google Scholar]
- Wang, J.; Bentz, J.; Anderson, R. Nasal Device for Delivery to the Olfactory Region. U.S. Patent 20070119451A1, 31 May 2007. [Google Scholar]
- Milk, D.G.; Khong, G.C.; Çam, O.H.; Alfaro-Iraheta, F.; Tierney, C.; Kassem, F.; Leong, S.C. A Comparison between Mygind and Kaiteki positions in administration of drops to the olfactory cleft. Clin. Otolaryngol. 2021, 46, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Merkus, P.; Ebbens, F.A.; Muller, B.; Fokkens, W.J. The ‘best method’ of topical nasal drug delivery: Comparison of seven techniques. Rhinology 2006, 44, 102–107. [Google Scholar]
- Cannady, S.B.; Batra, P.S.; Citardi, M.J.; Lanza, D.C. Comparison of delivery of topical medications to the paranasal sinuses via “vertex-to-floor” position and atomizer spray after FESS. Otolaryngol. Head Neck Surg. 2005, 133, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Merkonidis, C.; Cuevas, M.; Gudziol, V.; Matsuwaki, Y.; Hummel, T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: A pilot study in healthy subjects. Eur. Arch. Otorhinolaryngol. 2016, 273, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Bäck, L.J.; Hytönen, M.L.; Malmberg, H.O.; Ylikoski, J.S. Submucosal bipolar radiofrequency thermal ablation of inferior turbinates: A long-term follow-up with subjective and objective assessment. Laryngoscope 2002, 112, 1806–1812. [Google Scholar] [PubMed]
- Seifelnasr, A.; Talaat, M.; Ramaswamy, P.; Si, X.A.; Xi, J. A supine position and dual-dose applications enhance spray dosing to the posterior nose: Paving the way for mucosal immunization. Pharmaceutics 2023, 15, 359. [Google Scholar] [CrossRef]
- Xi, J.; Kim, J.; Si, X.; Corley, R.A.; Zhou, Y. Modeling of inertial depositions in scaled models of rat and human nasal airways: Towards in vitro regional dosimetry in small animals. J. Aerosol Sci. 2016, 99, 78–93. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Cheng, Y.-S.; Yeh, H.-C.; Swift, D.L. Deposition of ultrafine aerosols in the head airways during natural breathing and during simulated breath holding using replicate human upper airway casts. Aerosol Sci. Technol. 1995, 23, 465–474. [Google Scholar] [CrossRef]
- Kelly, J.T.; Asgharian, B.; Kimbell, J.S.; Wong, B. Particle depositon in human nasal airway replicas manufactured by different methds. Part II: Ultrafine particles. Aerosol Sci. Technol. 2004, 38, 1072–1079. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Si, X.A.; Zhou, Y. Nasal dilation effects on olfactory deposition in unilateral and bi-directional deliveries: In vitro tests and numerical modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Nevorski, D.; White, T.; Zhou, Y. Nasal and olfactory deposition with normal and bidirectional intranasal delivery techniques: In vitro tests and numerical simulations. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 118–131. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Xi, J.; Irshad, H.; Cheng, Y.-S. Nasal deposition in infants and children. J. Aerosol Med. 2014, 26, 110–116. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.E.; Alshaiba, M.; Cheng, D.; Firlit, Z.; Johnson, A.; Nolan, A.; Su, W.C. Design and testing of electric-guided delivery of charged particles to the olfactory region: Experimental and numerical Studies. Curr. Drug Deliv. 2016, 13, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Kimbell, J.S.; Overton, J.H.; Subramaniam, R.P.; Schlosser, P.M.; Morgan, K.T.; Conolly, R.B.; Miller, F.J. Dosimetry modeling of inhaled formaldehyde: Binning nasal flux predictions for quantitative risk assessment. Toxicol. Sci. 2001, 64, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.D.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.D.; Kimbell, J.S.; Asgharian, B. Analysis of particle deposition in the turbinate and olfactory regions using a human nasal computational fluid dynamics model. J. Aerosol Med. 2006, 19, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Si, X.A.; Peters, S.; Nevorski, D.; Wen, T.; Lehman, M. Understanding the mechanisms underlying pulsating aerosol delivery to the maxillary sinus: In vitro tests and computational simulations. Int. J. Pharm. 2017, 520, 254–266. [Google Scholar] [CrossRef]
- Si, X.; Xi, J.; Kim, J. Effect of laryngopharyngeal anatomy on expiratory airflow and submicrometer particle deposition in human extrathoracic airways. Open J. Fluid. Dyn. 2013, 3, 286–301. [Google Scholar] [CrossRef]
- Masiuk, T.; Kadakia, P.; Wang, Z. Development of a physiologically relevant dripping analytical method using simulated nasal mucus for nasal spray formulation analysis. J. Pharm. Anal. 2016, 6, 283–291. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef]
- Landis, M.S.; Boyden, T.; Pegg, S. Nasal-to-CNS drug delivery: Where are we now and where are we heading? An industrial perspective. Ther. Deliv. 2012, 3, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Si, X.A.; Xi, J.; Kim, J.; Zhou, Y.; Zhong, H. Modeling of release position and ventilation effects on olfactory aerosol drug delivery. Respir. Physiol. Neurobiol. 2013, 186, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Laminar airflow and nanoparticle or vapor deposition in a human nasal cavity model. J. Biomech. Eng. 2006, 128, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Sengoku, R.; Matsushima, S.; Bono, K.; Sakuta, K.; Yamazaki, M.; Miyagawa, S.; Komatsu, T.; Mitsumura, H.; Kono, Y.; Kamiyama, T.; et al. Olfactory function combined with morphology distinguishes Parkinson’s disease. Park. Relat. Disord. 2015, 21, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Shiga, H.; Taki, J.; Okuda, K.; Watanabe, N.; Tonami, H.; Nakagawa, H.; Kinuya, S.; Miwa, T. Prognostic value of olfactory nerve damage measured with thallium-based olfactory imaging in patients with idiopathic olfactory dysfunction. Sci. Rep. 2017, 7, 3581. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Baba, T.; Hasegawa, T.; Sugeno, N.; Konno, M.; Takeda, A. Differentiating Parkinson’s disease from multiple system atrophy by [123I] meta-iodobenzylguanidine myocardial scintigraphy and olfactory test. Park. Relat. Disord. 2011, 17, 698–700. [Google Scholar] [CrossRef]
- Prado, G.L.; Itabashi, Y.; Noda, H.; Miura, H.; Mariya, Y.; Abe, Y. Olfactory neuroblastoma visualized by Technetium-99m-ECD SPECT. Radiat. Med. 2001, 19, 267–270. [Google Scholar]
- Kolanjiyil, A.V.; Alfaifi, A.; Aladwani, G.; Golshahi, L.; Longest, W. Importance of spray-wall interaction and post-deposition liquid motion in the transport and delivery of pharmaceutical nasal sprays. Pharmaceutics 2022, 14, 956. [Google Scholar] [CrossRef]
- Perkins, E.L.; Basu, S.; Garcia, G.J.M.; Buckmire, R.A.; Shah, R.N.; Kimbell, J.S. Ideal particle sizes for inhaled steroids targeting vocal granulomas: Preliminary study using computational fluid dynamics. Otolaryngol. Head Neck Surg. 2018, 158, 511–519. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifelnasr, A.; Si, X.A.; Xi, J. Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model. Pharmaceutics 2023, 15, 1657. https://doi.org/10.3390/pharmaceutics15061657
Seifelnasr A, Si XA, Xi J. Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model. Pharmaceutics. 2023; 15(6):1657. https://doi.org/10.3390/pharmaceutics15061657
Chicago/Turabian StyleSeifelnasr, Amr, Xiuhua April Si, and Jinxiang Xi. 2023. "Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model" Pharmaceutics 15, no. 6: 1657. https://doi.org/10.3390/pharmaceutics15061657
APA StyleSeifelnasr, A., Si, X. A., & Xi, J. (2023). Visualization and Estimation of Nasal Spray Delivery to Olfactory Mucosa in an Image-Based Transparent Nasal Model. Pharmaceutics, 15(6), 1657. https://doi.org/10.3390/pharmaceutics15061657






