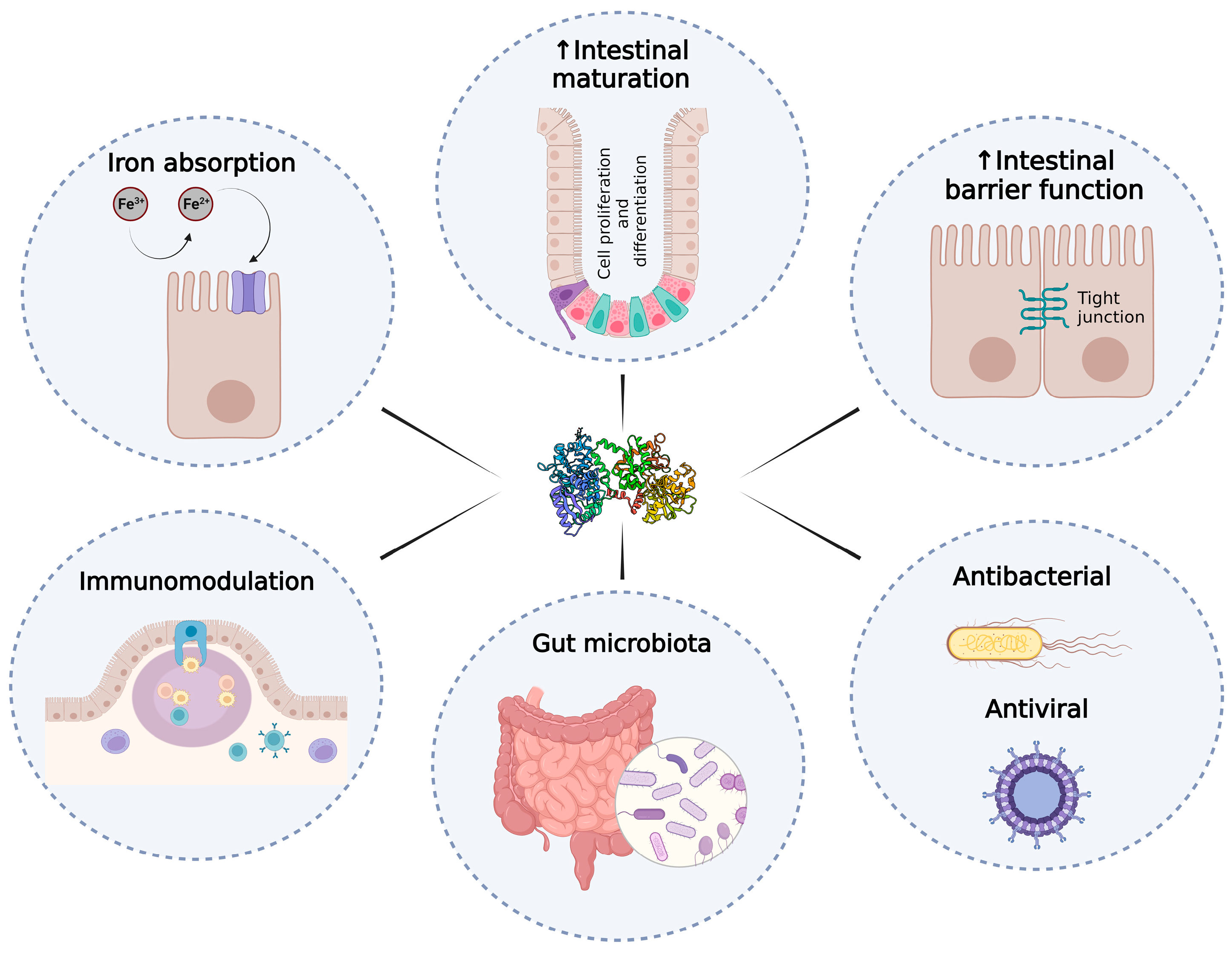
The Role of Lactoferrin in Intestinal Health
Abstract
1. Introduction
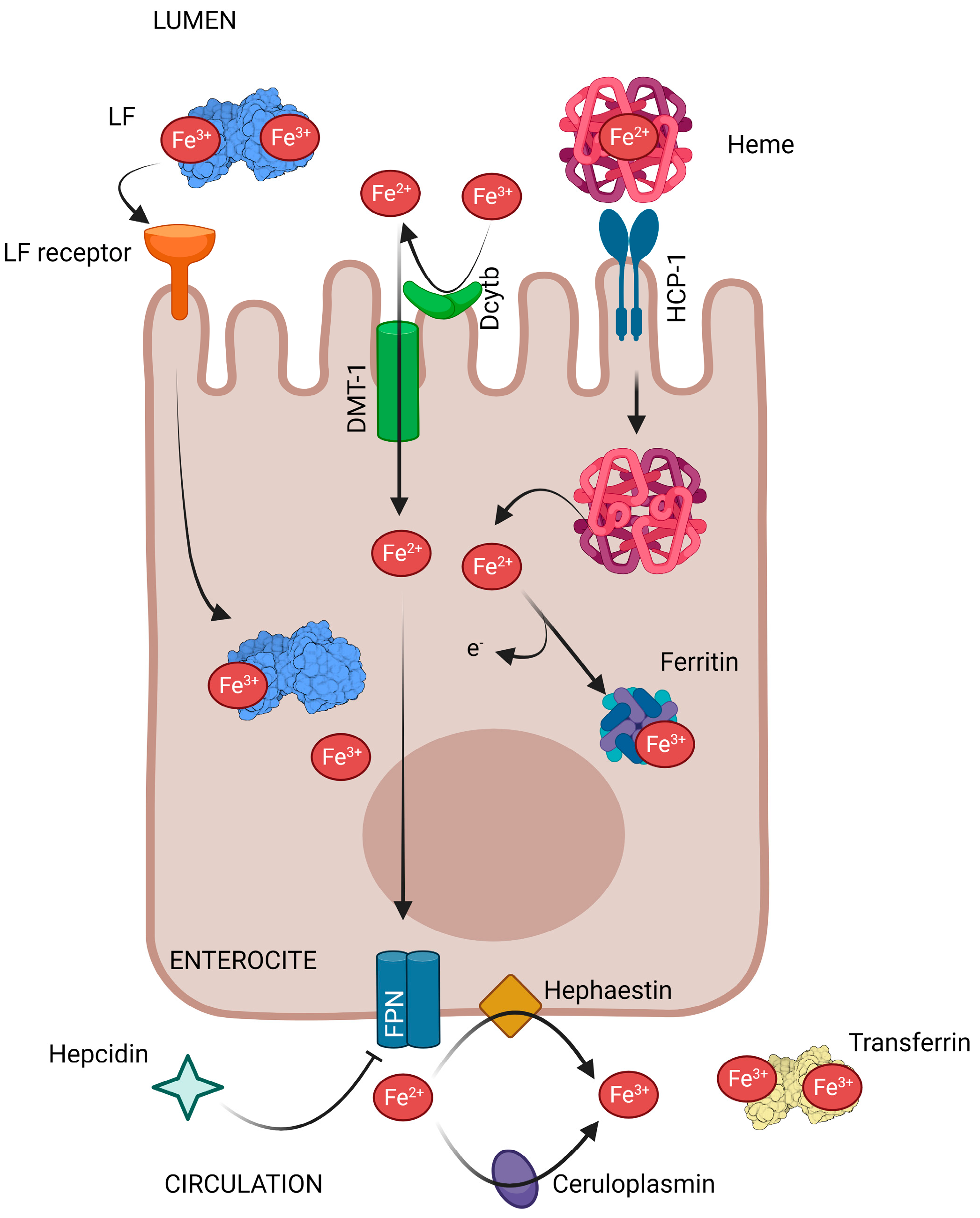
2. Lactoferrin and Intestinal Iron Absorption
3. Lactoferrin and Intestinal Growth, Maturation and Damage Repair
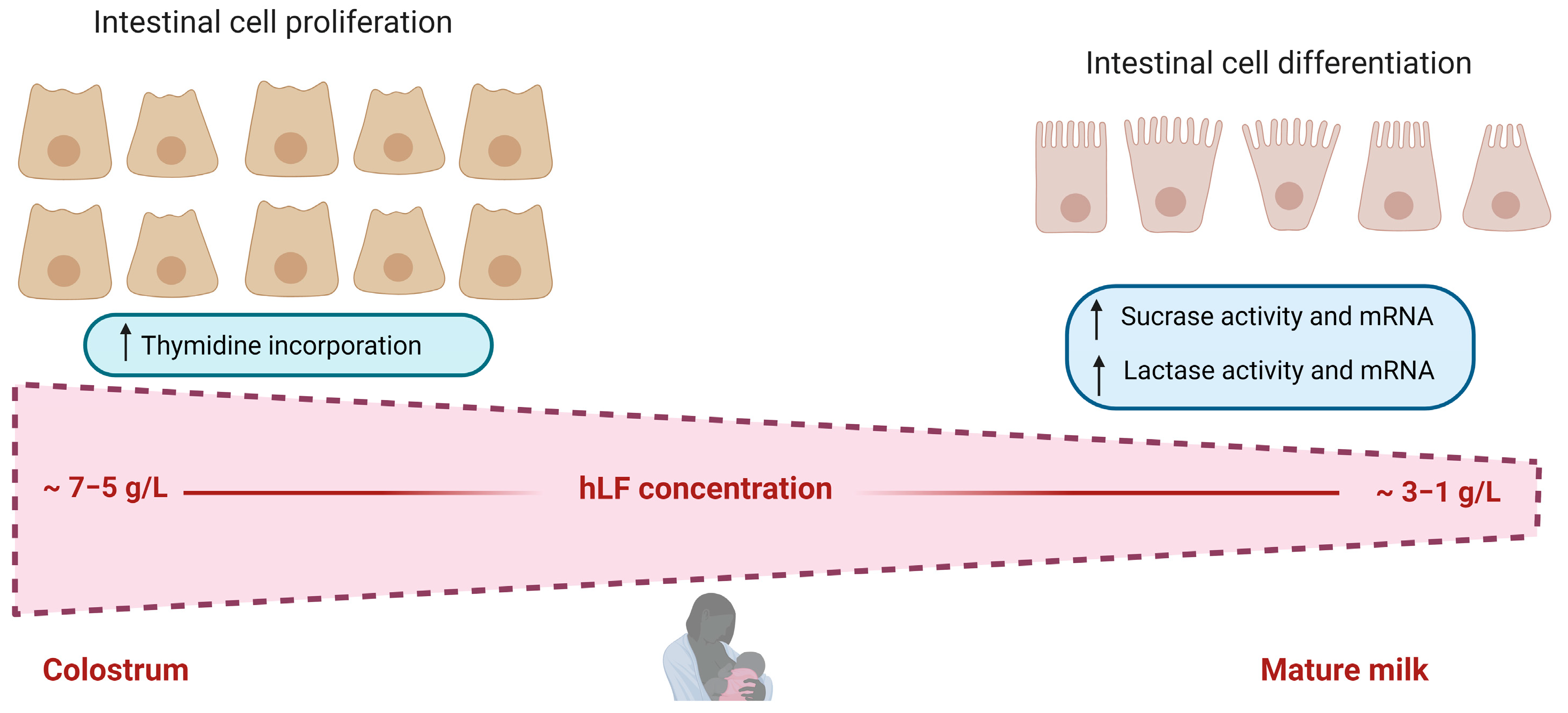
3.1. Lactoferrin and Intestinal Growth and Maturation
3.2. Lactoferrin and Intestinal Damage Repair
4. Lactoferrin and the Intestinal Immune System
4.1. Regulation of the Innate Immune System
4.2. Regulation of the Adaptive Immune System
| In Vivo Models | LF Source/Dose/Time | LF Activity | Reference |
|---|---|---|---|
| Patients with adenomatous colorectal polyps | 1.5 or 3 g bLF daily for 1 year | ↑ IFN-α in the colon ↑ NK cell activity and CD4(+) cells in the polyps | [69,97] |
| Healthy humans | 100 mg bLF for 7 days, followed by 200 mg bLF for 7 days | ↑ CD4(+) and CD8(+) T cells in peripheral blood lymphocytes | [98] |
| Very-low-birth-weight neonates | 200 mg bLF daily throughout hospitalization | ↑ Treg levels | [99] |
| BALB/c mice with implanted tumours | 1000 mg/kg talactoferrin (TLF) daily for 3 weeks | ↑ IFN-Y production in intestinal mucosa ↑ CD8(+) T lymphocytes and NK cells in Peyer´s patch | [70] |
| Healthy C57BL/6 mice | 0.2% and 2% bLF for 4 consecutive days | ↑ Apoptotic CD4 and ↓ TNF-α expression in intestinal lymphocytes | [100] |
| Healthy BALB/c mice | 30 mg/kg/day of bLF for 7 days or a single administration at 300 mg/kg on day 7 | ↑ Mature IL-18 in the mucosa of the small intestine | [101] |
| BALB/c mice stressed by immobilization | bLF (50, 500, and 5000 μg) for 7 days | ↑ Total IgA, secretory IgA, IL-4, and IL-6 (500 µg) in proximal intestine ↑ Total IgA, IL-4 (5000 µg) in distal intestine | [102] |
| BALB/c mice infected with a lethal or sublethal dose of S. typhimurium | 5 or 100 mg of bLF for 7 days | ↑ Total and S. typhimurium-specific IgG, and IgM in serum and IgA in intestinal secretions | [95] |
| Healthy BALB/c mice | 5 mg of bLF for 7, 14, 21, or 28 days | ↑ IgA and IgM antibodies, IgA+ and IgM+ plasma cells, total B cells, CD4(+) and CD8(+) T lymphocytes either in Peyer’s patches or lamina propria of the distal small intestine | [89] |
| Healthy BALB/c mice | 0.05 mg/g or 1 mg/g body weight of bLF for 4 weeks | ↑ Total immunoglobulins, IgA, and IgG in the intestinal fluid and in Peyer’s patches | [94] |
| BALB/c mice infected with an avirulent strain of S. typhimurium | bLF (5 mg/Kg) three times a week for 3 weeks | ↑ S. typhimurium-specific IgA, IgG2b, and IgG1 in fecal pellets and sera | [96] |
| Healthy BALB/c mice | bLF (50, 500, or 5000 μg) for 7 days | ↑ Total and specific IgA in intestinal secretions ↑ IL-2 and IL-5 in intestinal mucosa | [103] |
| Vitamin D-deficient C57BL/6J mice | 100 mg/kg and 1000 mg/kg of bLF for 24 weeks | ↓ Serum inflammatory cytokines (TNF-α, IL-6, and TGF-β) ↓ TLR-4 expression and NF-κB P65 activity in the colon | [104] |
| Specific-pathogen-free BALB/cByJ Jcl mice | bLF (2.5 g/kg of body weight) for 1 day | ↓ TLR3 gene, IFN-γ and IL-10 and ↑ NOD2 gene, IFN-β, and IL-12p40 in the small intestine | [105] |
| Specific pathogen-free C57BL/6 mice | bLF (30, 100, 300, 1000 mg/kg body weight) for 7 days | ↑ IL-18, IFN-α, IFN-β, and NK cell activity in Peyer’s patches and mesenteric lymph nodes | [106] |
| C57BL/6 mice with intestinal dysbiosis induced by clindamycin | 35 mg of native or iron-saturated bLF for 10 days | Saturated bLF reverted the decrease in the expression of TLR2, TLR8, and TLR9 induced by clindamycin in the colon | [107] |
| Healthy C57BL/6 mice | 500 mg/kg bLF for 3 days | ↑ IFN-Y and IL-10 production by intestinal intraepithelial lymphocytes (IEL) and mesenteric lymph-node (MLN) cells | [108] |
| DSS murine colitis model and TNFΔARE/+ murine model of ileitis | 500 mg/kg/day of a rhLF (VEN-120) for 7 or 14 days | ↑ Treg levels in the intestinal lamina propria ↓ IL-17 and IFNγ, and ↑ IL-10 produced by CD4+ T cells of the lamina propria | [109] |
| C57BL/6 mice with DSS-induced colitis | 2 mg/mouse of hLF twice a day during DSS exposure | ↓ Serum IL-1β levels and IL-12 ↓ CD4 cells, F4/80+macrophages, and TNF-α producing cells in the distal colon | [110] |
| Mouse model with intestinal damage induced by LPS | 100 mg/kg/day of holo-rhLF or apo-rhLFfor 14 days | Apo-rhLF ↓ TNF-α, IL-1β, and IL-6 in the colon and ↑ Expression of anti-inflammatory factor, IFN-γ | [73] |
| BALB/c mice with DSS-induced colitis | Diet containing 1.25% (wt/vol) of bovine iron-saturated LF (Fe-bLF) | Fe-bLF ↓ Myeloperoxidase activity (neutrophil infiltration) ↓ DSS-induced expression of IFN-γ, TNF-α, IL-4, IL-5, GM-CSF, and NO in the colon | [111] |
| Mouse model of DSS-induced colitis | 100 mg/kg of bLF for 14 days | ↓ IL-1β, IL-6, and TNF-α in the colon ↑ IL-10 and TGF-β in the colon | [76] |
| C57BL/6 mice with necrotizing enterocolitis | rhLF (0.3 g/kg/day) for 3 days | ↓ IL-6 and TNF-α expression, and ↑ intestinal stem cell marker Lgr5 | [112] |
| Rats with DSS-induced colitis | 200 mg/kg/day of bLF starting 3 days before beginning DSS administration, until death. | ↓ Pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in the colon ↓ Myeloperoxidase activity ↑ Anti-inflammatory cytokines IL-4 and IL-10 in the colon | [75] |
| A preterm pig model | 10 g/L of bLF-enriched formula | bLF ↓ IL-1β in the proximal small intestine | [113] |
| Healthy piglets | 2, 11, or 20 mg/g of rhLF for 30 days | Enhanced Th1 (↑ IL-12) and Th2 (↑ IL-10) cell responses ↑ TLR2 and NF-κB P65 in the intestine ↑ Total IgG and IgA in the plasma and ↑ IgG in the colon | [114] |
| In Vitro Models | LF Source/Dose/Time | LF Activity | Reference |
|---|---|---|---|
| Caco-2 cells | 50 mg/mL of TLF, bLF or hLF for 72 h | hLF ↑ expression of TGF-β1 | [115] |
| Caco-2 cells | Native and Fe-saturated bLF and hLF (400 mg/mL or 50 mg/mL) for 72 h | Native forms ↑ TGF-β1 Holo-forms ↑ IL-18 secretion | [12] |
| Caco-2/TC7 cells | bLF (0.5, 1, 2, 5 or 10 mg/mL) for 24 h | ↓ Expression levels of TLR4 with 10 mg/mL | [116] |
| Caco-2/TC7 cells | 100 μg/mL bLF for 3 h | ↑ IFN-α, IFN-β, TLR-3, TLR-7, IRF3, and IRF7 | [117] |
| SARS-CoV-2 infected-Caco-2 cells | LF pre-infection treatment ↓ IL-1β, IL-6, and IL-10, and ↑ TGF-β1 | ||
| Caco-2 and RAW 246.7 cell lines treated with LPS | 1, 1.5 and 2 mg/mL of rhLF | ↓ IL-8 and ROS production | [77] |
| Caco-2 cells and organ cultures infected with E. coli strain LF82 | 1 mg/mL bLF | ↓ TNF-α, IL-8, and IL-6 | [78] |
| Rat intestinal epithelial cells IEC-18 and Caco-2 cells treated with H2O2 | 0.01, 0.1 and 1 g/L of rhLF for 24 h | ↓ IL-6 and ↑ gene expression of Lgr5 and Wnt/β-catenin | [118] |
| Peritoneal macrophage culture | 1000 mg/mL of bLF for 24 h | ↑ Mature IL-18, IFN-Y and IL-15 and ↓ expression of IFN-α | [101] |
| RPMI 8226 B cells | 0.5–4 µmol/L apo-LF for 1 h before stimulation with CpG for 16 h | ↓ NF-kB activation ↓ IL-8 and IL-12p40 expression induced by CpG | [84] |
| Porcine intestinal epithelial cells (PsIc1) treated with LPS | bLF (0–10 g/L) for 24 h | Low doses (0.1–1 g/L) ↓ IL-8, and NF-κB and hypoxia-inducible factor-1α (HIF-1α) activation | [113] |
| Intestinal epithelial cell line HT-29 | bLF (0.002, 0.02, 0.2, or 2 mg/mL) for 1 h prior to the addition of poly I:C | ↑ IFN-λ1 in the culture supernatant and ↑ IFN-λ1 and IFN-λ2 mRNA levels | [119] |
| LPS-challenged THP-1 cells | 0.125–2 mg/mL bLF | ↓ TNF-α release from LPS-activated THP-1 cells | [120] |
4.3. Lactoferrin as Fecal Biomarker of Inflammation
5. Lactoferrin in Some Intestinal Disorders Caused by Bacteria
5.1. Lactoferrin’s Effect on Late-Onset Sepsis and Necrotizing Enterocolitis
5.2. Lactoferrin against Some Pathogenic Bacteria Causing Intestinal Diseases
5.3. Lactoferrin against Some Viruses Causing Intestinal Disorders
6. Modulatory Effects of Lactoferrin on Gut Microbiota
6.1. Mouse Models
6.2. Piglet Models
6.3. Human Studies
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent advances and prospects in purification and heterologous expression of lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Collard, K.J. Iron homeostasis in the neonate. Pediatrics 2009, 123, 1208–1216. [Google Scholar] [CrossRef]
- Frazer, D.M.; Darshan, D.; Anderson, G.J. Intestinal iron absorption during suckling in mammals. BioMetals 2011, 24, 567–574. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Lactoferrin for Prevention and Treatment of Anemia and Inflammation in Pregnant Women: A Comprehensive Review. Biomedicines 2021, 9, 898. [Google Scholar] [CrossRef]
- EMA.EU. ICH Guideline M9 on Biopharmaceutics Classification System Based Biowaivers. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 26 September 2022).
- FDA.GOV. M9 Biopharmaceutics Classification System-Based Biowaivers. Available online: https://www.fda.gov/media/117974/download (accessed on 26 September 2022).
- Conesa, C.; Pocoví, C.; Pérez, M.-D.; Calvo, M.; Sánchez, L. Recombinant Human Lactoferrin and Iron Transport Across Caco-2 Monolayers: Effect of Heat Treatment on the Binding to Cells. J. Agric. Food Chem. 2008, 56, 2831–2837. [Google Scholar] [CrossRef]
- Conesa, C.; PocovÍ, C.; PÉRez, M.-D.; Calvo, M.; SÁNchez, L. Transport of Iron Bound to Recombinant Human Lactoferrin from Rice and Iron Citrate Across Caco-2 Cell Monolayers. Biosci. Biotechnol. Biochem. 2009, 73, 2615–2620. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Jiang, R.; Du, X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular Cloning and Functional Expression of a Human Intestinal Lactoferrin Receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef]
- Wrackmeyer, U.; Hansen, G.H.; Seya, T.; Danielsen, E.M. Intelectin: A novel lipid raft-associated protein in the enterocyte brush border. Biochemistry 2006, 45, 9188–9197. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol. Pharm. Bull. 2008, 31, 1605–1608. [Google Scholar] [CrossRef]
- Mikogami, T.; Marianne, T.; Spik, G. Effect of intracellular iron depletion by picolinic acid on expression of the lactoferrin receptor in the human colon carcinoma cell subclone HT29-18-C1. Biochem. J. 1995, 308, 391–397. [Google Scholar] [CrossRef]
- Lopez, V.; Suzuki, Y.A.; Lönnerdal, B. Ontogenic changes in lactoferrin receptor and DMT1 in mouse small intestine: Implications for iron absorption during early life. Biochem. Cell Biol. 2006, 84, 337–344. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.-X.; Liu, Y.; Xi, E.-Z.; An, J.-J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef]
- Ward, P.P.; Mendoza-Meneses, M.; Cunningham, G.A.; Conneely, O.M. Iron Status in Mice Carrying a Targeted Disruption of Lactoferrin. Mol. Cell. Biol. 2003, 23, 178–185. [Google Scholar] [CrossRef]
- Davidsson, L.; Kastenmayer, P.; Yuen, M.; Lönnerdal, B.; Hurrell, R.F. Influence of lactoferrin on iron absorption from human milk in infants. Pediatr. Res. 1994, 35, 117–124. [Google Scholar] [CrossRef]
- Rosa, G.; Trugo, N.M. Iron uptake from lactoferrin by intestinal brush-border membrane vesicles of human neonates. Braz. J. Med. Biol. Res. 1994, 27, 1527–1531. [Google Scholar]
- Lönnerdal, B.; Bryant, A. Absorption of iron from recombinant human lactoferrin in young US women. Am. J. Clin. Nutr. 2006, 83, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Xu, T.; Luo, J.; Luo, Y.; An, P. Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials. Nutrients 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Anaemia Reduction Efforts among Women of Reproductive Age: Impact, Achievement of Targets and the Way forward for Optimizing Efforts; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pietropaoli, M.; Gessani, S.; Valenti, P. The influence of lactoferrin, orally administered, on systemic iron homeostasis in pregnant women suffering of iron deficiency and iron deficiency anaemia. Biochimie 2009, 91, 44–51. [Google Scholar] [CrossRef]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Schümann, K.; Ettle, T.; Szegner, B.; Elsenhans, B.; Solomons, N.W. On risks and benefits of iron supplementation recommendations for iron intake revisited. J. Trace Elem. Med. Biol. 2007, 21, 147–168. [Google Scholar] [CrossRef]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron Absorption is Greater from Apo-Lactoferrin and is Similar Between Holo-Lactoferrin and Ferrous Sulfate: Stable Iron Isotope Studies in Kenyan Infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, N.a.A.N. Scientific Opinion on bovine lactoferrin. EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- FDA. GRN No. 77. Milk-Derived Lactoferrin. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=77&sort=GRN_No&order=DESC&startrow=1&type=basic&search=lactoferrin (accessed on 27 September 2022).
- Uchida, T.; Oda, T.; Sato, K.; Kawakami, H. Availability of lactoferrin as a natural solubilizer of iron for food products. Int. Dairy J. 2006, 16, 95–101. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Sizonenko, S.V. Lactoferrin and prematurity: A promising milk protein? Biochem. Cell Biol. 2016, 95, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, Z.; Wang, X.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin, a Critical Player in Neonate Intestinal Development: RHLF may be a Good Choice in Formula. J. Agric. Food Chem. 2021, 69, 8726–8736. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, R.; Lönnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem. Cell Biol. 2012, 90, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Bornhorst, G.M.; Henrick, B.M.; German, J.B. Protein Digestion of Baby Foods: Study Approaches and Implications for Infant Health. Mol. Nutr. Food Res. 2018, 62, 1700231. [Google Scholar] [CrossRef]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk during the First Twelve Weeks of Lactation. In Bioactive Components of Human Milk; Newburg, D.S., Ed.; Springer US: Boston, MA, USA, 2001; Volume 501, pp. 241–247. [Google Scholar]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tomé, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. BioMetals 2014, 27, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Transcriptomic profiling of intestinal epithelial cells in response to human, bovine and commercial bovine lactoferrins. Biometals 2014, 27, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, S.; Walker, W.A.; Sanderson, I.R. Iron Saturation Alters the Effect of Lactoferrin on the Proliferation and Differentiation of Human Enterocytes (Caco-2 Cells). Biol. Neonate 1995, 67, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.J.; Wong, W.W.; Smith, E.O. Influence of changes in lactase activity and small-intestinal mucosal growth on lactose digestion and absorption in preterm infants. Am. J. Clin. Nutr. 2005, 81, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sawicki, V.; Lewis, A.; Hanson, L.; Nuijens, J.H.; Neville, M.C. Human Lactoferrin in the Milk of Transgenic Mice Increases Intestinal Growth in Ten-Day-Old Suckling Neonates. In Bioactive Components of Human Milk; Newburg, D.S., Ed.; Springer: Boston, MA, USA, 2001; Volume 501, pp. 107–113. [Google Scholar]
- Wang, Y.; Shan, T.; Xu, Z.; Liu, J.; Feng, J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J. Anim. Sci. 2006, 84, 2636–2641. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Comstock, S.S.; Yi, C.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin increases intestinal cell proliferation in neonatal piglets. J. Nutr. 2014, 144, 1401–1418. [Google Scholar] [CrossRef]
- Miyakawa, M.; Oda, H.; Tanaka, M. Clinical research review: Usefulness of bovine lactoferrin in child health. BioMetals 2022, 36, 473–489. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Du, X.; Jiang, R. Biological activities of commercial bovine lactoferrin sources. Biochem. Cell Biol. 2021, 99, 35–46. [Google Scholar] [CrossRef]
- FDA. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&sort=GRN_No&order=DESC&startrow=1&type=basic&search=lactoferrin (accessed on 8 October 2022).
- Liu, L.; Jiang, R.; Lönnerdal, B. Assessment of bioactivities of the human milk lactoferrin–osteopontin complex in vitro. J. Nutr. Biochem. 2019, 69, 10–18. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, L.; Du, X.; Lönnerdal, B. Evaluation of Bioactivities of the Bovine Milk Lactoferrin–Osteopontin Complex in Infant Formulas. J. Agric. Food Chem. 2020, 68, 6104–6111. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, R.; Liu, J.; Lönnerdal, B. The bovine Lactoferrin-Osteopontin complex increases proliferation of human intestinal epithelial cells by activating the PI3K/Akt signaling pathway. Food Chem. 2020, 310, 125919. [Google Scholar] [CrossRef]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Garas, L.C.; Feltrin, C.; Kristina Hamilton, M.; Hagey, J.V.; Murray, J.D.; Bertolini, L.R.; Bertolini, M.; Raybould, H.E.; Maga, E.A. Milk with and without lactoferrin can influence intestinal damage in a pig model of malnutrition. Food Funct. 2016, 7, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-N.; Li, S.-L.; Yang, X.; Wang, J.-Q.; Zheng, N. The Protective Effects of Lactoferrin on Aflatoxin M1-Induced Compromised Intestinal Integrity. Int. J. Mol. Sci. 2022, 23, 289. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; Van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunctionin vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, M.; Guo, J.; Feng, Z. Effects of Bovine Lactoferrin on Rat Intestinal Epithelial Cells. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 645–651. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Xu, J.-Y.; Zhang, R.; Zhang, Z.; Zhao, L.; Qin, L.-Q. Effects of lactoferrin on X-ray-induced intestinal injury in Balb/C mice. Appl. Radiat. Isot. 2019, 146, 72–77. [Google Scholar] [CrossRef]
- Brandt, A.; Nier, A.; Jin, C.J.; Baumann, A.; Jung, F.; Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C.; Bergheim, I. Consumption of decaffeinated coffee protects against the development of early non-alcoholic steatohepatitis: Role of intestinal barrier function. Redox Biol. 2019, 21, 101092. [Google Scholar] [CrossRef]
- Qin, T.; Yang, J.; Huang, D.; Zhang, Z.; Huang, Y.; Chen, H.; Xu, G. DOCK4 stimulates MUC2 production through its effect on goblet cell differentiation. J. Cell Physiol. 2021, 236, 6507–6519. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Drago-Serrano, M.E.; León-Sicairos, N.; de la Garza, M. Lactoferrin: A Nutraceutical with Activity against Colorectal Cancer. Front. Pharmacol. 2022, 13, 855852. [Google Scholar] [CrossRef]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, M.; Curcio, C.; Varadhachary, A.; Cavallo, F.; Engelmayer, J.; Blezinger, P.; Pericle, F.; Forni, G. Requirement for IFN-gamma, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Cancer Res. 2007, 67, 6425–6432. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef]
- Fan, L.L.; Yao, Q.Q.; Wu, H.M.; Wen, F.; Wang, J.Q.; Li, H.Y.; Zheng, N. Protective effects of recombinant lactoferrin with different iron saturations on enteritis injury in young mice. J. Dairy Sci. 2022, 105, 4791–4803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hua, R.; Zhang, B.; Guan, Q.; Zeng, J.; Wang, X.; Wang, B. Oral Administration of Bovine Lactoferrin-Derived Lactoferricin (Lfcin) B Could Attenuate Enterohemorrhagic Escherichia coli O157:H7 Induced Intestinal Disease through Improving Intestinal Barrier Function and Microbiota. J. Agric. Food Chem. 2019, 67, 3932–3945. [Google Scholar] [CrossRef]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Nakajima, A.; Ueno, N.; Saito, T.; Sekihara, H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 2002, 17, 1291–1298. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Xiao, D.; Shu, G.; Gu, L. Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites. Front. Nutr. 2021, 8, 660598. [Google Scholar] [CrossRef]
- Aly, E.; López-Nicolás, R.; Darwish, A.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In vitro effectiveness of recombinant human lactoferrin and its hydrolysate in alleviating LPS-induced inflammatory response. Food Res. Int. 2019, 118, 101–107. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin prevents invasion and inflammatory response following E. coli strain LF82 infection in experimental model of Crohn’s disease. Dig. Liver. Dis. 2014, 46, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Shen, C.J.; Hsu, W.H.; Chang, Y.H.; Lin, H.T.; Chen, H.L.; Chen, C.M. Lactoferrin: An iron-binding antimicrobial protein against Escherichia coli infection. Biometals 2011, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Brock, J. Lactoferrin: A multifunctional immunoregulatory protein? Immunol. Today 1995, 16, 417–419. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef]
- Mulligan, P.; White, N.R.; Monteleone, G.; Wang, P.; Wilson, J.W.; Ohtsuka, Y.; Sanderson, I.R. Breast milk lactoferrin regulates gene expression by binding bacterial DNA CpG motifs but not genomic DNA promoters in model intestinal cells. Pediatr. Res. 2006, 59, 656–661. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046–1060. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Cooper, C.; Nonnecke, E.; Lönnerdal, B.; Murray, J. The lactoferrin receptor may mediate the reduction of eosinophils in the duodenum of pigs consuming milk containing recombinant human lactoferrin. Biometals 2014, 27, 1031–1038. [Google Scholar] [CrossRef]
- Cutone, A.; Ianiro, G.; Lepanto, M.S.; Rosa, L.; Valenti, P.; Bonaccorsi di Patti, M.C.; Musci, G. Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers 2020, 12, 3806. [Google Scholar] [CrossRef]
- Arciniega-Martínez, I.M.; Campos-Rodríguez, R.; Drago-Serrano, M.E.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Reséndiz-Albor, A.A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. 2016, 64, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef]
- Zong, X.; Cao, X.; Wang, H.; Zhao, J.; Lu, Z.; Wang, F.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 modulates immune homoeostasis to defend lipopolysaccharide-triggered intestinal inflammation in mice. Br. J. Nutr. 2019, 121, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tomé, D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem. Cell Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef]
- Cruz-Hernández, T.R.; Gómez-Jiménez, D.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. Analysis of the intestinal IgA response in mice exposed to chronic stress and treated with bovine lactoferrin. Mol. Med. Rep. 2021, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, H.; Dubarry, M.; Rautureau, M.; Tomé, D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J. Dairy Res. 1998, 65, 283–293. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Rivera-Aguilar, V.; Reséndiz-Albor, A.A.; Campos-Rodríguez, R. Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immunol. Lett. 2010, 134, 35–46. [Google Scholar] [CrossRef]
- Jang, Y.S.; Seo, G.Y.; Lee, J.M.; Seo, H.Y.; Han, H.J.; Kim, S.J.; Jin, B.R.; Kim, H.J.; Park, S.R.; Rhee, K.J.; et al. Lactoferrin causes IgA and IgG2b isotype switching through betaglycan binding and activation of canonical TGF-β signaling. Mucosal Immunol. 2015, 8, 906–917. [Google Scholar] [CrossRef]
- Alexander, D.B.; Iigo, M.; Hamano, H.; Kozu, T.; Saito, Y.; Saito, D.; Kakizoe, T.; Xu, J.; Yamauchi, K.; Takase, M.; et al. An ancillary study of participants in a randomized, placebo-controlled trial suggests that ingestion of bovine lactoferrin promotes expression of interferon alpha in the human colon. J. Funct. Foods 2014, 10, 305–317. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Akin, I.M.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.D.; Ikinciogullari, A.; Turmen, T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef]
- Spagnuolo, P.A.; Bird, R.P.; Hoffman-Goetz, L. Effect of short-term dietary intake of bovine lactoferrin on intestinal lymphocyte apoptosis in healthy mice. Nutrition 2007, 23, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.B.; Long, N.; Xu, J.; Fukamachi, K.; Futakuchi, M.; Takase, M.; Tsuda, H. Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 2009, 91, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Peña-Juárez, M.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Effect of Bovine Lactoferrin Treatment Followed by Acute Stress on the IgA-Response in Small Intestine of BALB/c Mice. Immunol. Investig. 2016, 45, 652–667. [Google Scholar] [CrossRef]
- Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sánchez-Gómez, M.C.; Campos-Rodríguez, R. Modulation by bovine lactoferrin of parameters associated with the IgA response in the proximal and distal small intestine of BALB/c mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, Y.X.; Zhao, L.; Ren, F.Z.; Guo, H.Y. Lactoferrin stimulates the expression of vitamin D receptor in vitamin D deficient mice. J. Funct. Foods 2019, 55, 48–56. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takakura, N.; Yamauchi, K.; Tamura, Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin. Vaccine Immunol. 2006, 13, 239–245. [Google Scholar] [CrossRef]
- Kuhara, T.; Yamauchi, K.; Tamura, Y.; Okamura, H. Oral administration of lactoferrin increases NK cell activity in mice via increased production of IL-18 and type I IFN in the small intestine. J. Interferon Cytokine Res. 2006, 26, 489–499. [Google Scholar] [CrossRef]
- Bellés, A.; Aguirre-Ramírez, D.; Abad, I.; Parras-Moltó, M.; Sánchez, L.; Grasa, L. Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct. 2022, 13, 5854–5869. [Google Scholar] [CrossRef]
- Takakura, N.; Wakabayashi, H.; Yamauchi, K.; Takase, M. Influences of orally administered lactoferrin on IFN-gamma and IL-10 production by intestinal intraepithelial lymphocytes and mesenteric lymph-node cells. Biochem. Cell Biol. 2006, 84, 363–368. [Google Scholar] [CrossRef] [PubMed]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Haversen, L.A.; Baltzer, L.; Dolphin, G.; Hanson, L.A.; Mattsby-Baltzer, I. Anti-Inflammatory Activities of Human Lactoferrin in Acute Dextran Sulphate-Induced Colitis in Mice. Scand. J. Immunol. 2003, 57, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Stathopoulos, S.; Haggarty, N.W.; MacGibbon, A.K.H.; Palmano, K.P.; Roy, K.; Rowan, A.; Krissansen, G.W. Comparative activities of milk components in reversing chronic colitis. J. Dairy Sci. 2016, 99, 2488–2501. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Li, B.; Robinson, S.C.; Lee, C.; O’Connell, J.S.; Bindi, E.; Zheng, S.; Sherman, P.M.; Pierro, A. Lactoferrin Reduces Necrotizing Enterocolitis Severity by Upregulating Intestinal Epithelial Proliferation. Eur. J. Pediatr. Surg. 2020, 30, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Li, Y.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E.W. Effects of bovine lactoferrin on the immature porcine intestine. Br. J. Nutr. 2014, 111, 321–331. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Zhao, J.; Wang, J.; Dai, Y.; Zhao, Y.; Meng, Q.; Li, N. Supplementation transgenic cow’s milk containing recombinant human lactoferrin enhances systematic and intestinal immune responses in piglets. Mol. Biol. Rep. 2014, 41, 2119–2128. [Google Scholar] [CrossRef]
- Jiang, R.; Du, X.; Lönnerdal, B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 642–652. [Google Scholar] [CrossRef]
- Buey, B.; Bellés, A.; Latorre, E.; Abad, I.; Pérez, M.D.; Grasa, L.; Mesonero, J.E.; Sánchez, L. Comparative effect of bovine buttermilk, whey, and lactoferrin on the innate immunity receptors and oxidative status of intestinal epithelial cells. Biochem. Cell Biol. 2021, 99, 54–60. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Lee, C.; Zhu, H.; Zheng, S.; Pierro, A. Protective effects of lactoferrin on injured intestinal epithelial cells. J. Pediatr. Surg. 2019, 54, 2509–2513. [Google Scholar] [CrossRef]
- Shin, K.; Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Effects of lactoferrin on the production of interferon-λ by the human intestinal epithelial cell line HT-29. Biochem. Cell Biol. 2017, 95, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Maddox, I.S.; Ferguson, L.R.; Shu, Q. Evaluation of the cytoprotective effects of bovine lactoferrin against intestinal toxins using cellular model systems. Biometals 2010, 23, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.C.; Chaze, T.; Coïc, Y.-M.; Injarabian, L.; Jonsson, F.; Lombion, N.; Selimoglu-Buet, D.; Souphron, J.; Ridley, C.; Vonaesch, P.; et al. MUB40 Binds to Lactoferrin and Stands as a Specific Neutrophil Marker. Cell Chem. Biol. 2018, 25, 483–493.e9. [Google Scholar] [CrossRef] [PubMed]
- Caccaro, R.; D’Incá, R.; Sturniolo, G.C. Clinical utility of calprotectin and lactoferrin as markers of inflammation in patients with inflammatory bowel disease. Expert Rev. Clin. Immunol. 2010, 6, 551–558. [Google Scholar] [CrossRef]
- Angriman, I.; Scarpa, M.; D’Incà, R.; Basso, D.; Ruffolo, C.; Polese, L.; Sturniolo, G.C.; D’Amico, D.F.; Plebani, M. Enzymes in feces: Useful markers of chronic inflammatory bowel disease. Clin. Chim. Acta 2007, 381, 63–68. [Google Scholar] [CrossRef]
- Lopez, R.N.; Leach, S.T.; Lemberg, D.A.; Duvoisin, G.; Gearry, R.B.; Day, A.S. Fecal biomarkers in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 577–582. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Marano, C.; O’Brien, C.; Szapary, P.; Zhang, H.; Johanns, J.; Leong, R.W.; Hisamatsu, T.; Van Assche, G.; et al. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2244–2255.e9. [Google Scholar] [CrossRef]
- Johnson, L.M.; White, S.K.; Schmidt, R.L. Are calprotectin and lactoferrin equivalent screening tests for inflammatory bowel disease? Clin. Chim. Acta 2020, 510, 191–195. [Google Scholar] [CrossRef]
- Prata, M.M.; Havt, A.; Bolick, D.T.; Pinkerton, R.; Lima, A.; Guerrant, R.L. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J. Transl. Sci. 2016, 2, 134–139. [Google Scholar] [CrossRef]
- Borkowska, A.; Liberek, A.; Łuczak, G.; Jankowska, A.; Plata-Nazar, K.; Korzon, M.; Kamińska, B. Fecal lactoferrin, a marker of intestinal inflammation in children with inflammatory bowel disease. Acta Biochim. Pol. 2015, 62, 541–545. [Google Scholar] [CrossRef]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Loftus, E.V.; Panaccione, R.; Chen, L.S.; Peterson, S.; McConnell, J.; Baudhuin, L.; Hanson, K.; Feagan, B.G.; Harmsen, S.W.; et al. Relationships Between Disease Activity and Serum and Fecal Biomarkers in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2008, 6, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, K.; Lykowska-Szuber, L.; Eder, P.; Krela-Kazmierczak, I.; Stawczyk-Eder, K.; Szymczak, A.; Michalak, M.; Studniarek, A.; Linke, K. The diagnostic usefulness of fecal lactoferrin in the assessment of Crohn’s disease activity. Eur. J. Intern. Med. 2015, 26, 623–627. [Google Scholar] [CrossRef]
- Bushen, O.Y.; Kohli, A.; Pinkerton, R.C.; Dupnik, K.; Newman, R.D.; Sears, C.L.; Fayer, R.; Lima, A.A.M.; Guerrant, R.L. Heavy cryptosporidial infections in children in northeast Brazil: Comparison of Cryptosporidium hominis and Cryptosporidium parvum. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 378–384. [Google Scholar] [CrossRef]
- Kohli, A.; Bushen, O.Y.; Pinkerton, R.C.; Houpt, E.; Newman, R.D.; Sears, C.L.; Lima, A.A.M.; Guerrant, R.L. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, C.J.; Lin, T.Y.; Lai, M.W.; Chao, H.C.; Kong, M.S. Usefulness of fecal lactoferrin in predicting and monitoring the clinical severity of infectious diarrhea. World J. Gastroenterol. 2011, 17, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Mϋller, M.J.; Paul, T.; Seeliger, S. Necrotizing enterocolitis in premature infants and newborns. J. Neonatal Perinat. Med. 2016, 9, 233–242. [Google Scholar] [CrossRef]
- Sherman, M.P. Lactoferrin and Necrotizing Enterocolitis. Clin. Perinatol. 2013, 40, 79–91. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates: A Randomized Trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- Turin, C.G.; Zea-Vera, A.; Pezo, A.; Cruz, K.; Zegarra, J.; Bellomo, S.; Cam, L.; Llanos, R.; Castañeda, A.; Tucto, L.; et al. Lactoferrin for prevention of neonatal sepsis. BioMetals 2014, 27, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Zegarra, J.; Cam, L.; Llanos, R.; Pezo, A.; Cruz, K.; Zea-Vera, A.; Cárcamo, C.; Campos, M.; Bellomo, S. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr. Infect. Dis. J. 2015, 34, 571–576. [Google Scholar] [CrossRef]
- Pammi, M.; Gautham, K.S. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, Cd007137. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, L.; Lu, C.; Wang, Q.; Pan, B.; Wang, Q.; Tian, J.; Ge, L. Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1186. [Google Scholar] [CrossRef]
- The ELFIN Trial Investigators Group. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Cadwell, K.H.; Colombel, J.F.; Shah, S.C. The role of gastrointestinal pathogens in inflammatory bowel disease: A systematic review. Therap. Adv. Gastroenterol. 2021, 14, 17562848211004493. [Google Scholar] [CrossRef]
- Azimi, T.; Nasiri, M.J.; Chirani, A.S.; Pouriran, R.; Dabiri, H. The role of bacteria in the inflammatory bowel disease development: A narrative review. APMIS 2018, 126, 275–283. [Google Scholar] [CrossRef]
- Barnich, N.; Darfeuille-Michaud, A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 5571–5576. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Scotti, M.J.; Conte, A.L.; Marazzato, M.; Zagaglia, C.; Longhi, C.; Berlutti, F.; Musci, G.; et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. Int. J. Mol. Sci. 2019, 20, 5666. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Brown, E.L.; Guion, C.E.; Chen, J.Z.; McMahon, R.J.; Cleary, T.G. Effect of lactoferrin on Enteroaggregative E. coli (EAEC). Biochem. Cell Biol. 2006, 84, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villaseñor, H.; Canizalez-Román, A.; Velazquez-Roman, J.; Nazmi, K.; Bolscher, J.G.M.; Leon-Sicairos, N. Protective effects of lactoferrin chimera and bovine lactoferrin in a mouse model of enterohaemorrhagic Escherichia coli O157:H7 infection. Biochem. Cell Biol. 2012, 90, 405–411. [Google Scholar] [CrossRef]
- Rybarczyk, J.; Kieckens, E.; Vanrompay, D.; Cox, E. In vitro and in vivo studies on the antimicrobial effect of lactoferrin against Escherichia coli O157:H7. Vet. Microbiol. 2017, 202, 23–28. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, A.N.; Giugliano, L.G. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 2001, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Tazume, S.; Shimizu, K.; Matsuzawa, H.; Dosako, S.i.; Isoda, H.; Tsukiji, M.; Fujimura, R.; Muranaka, Y.; Isihida, H. Inhibitory Effects of Bovine Lactoferrin on the Adherence of Enterotoxigenic Escherichia coli to Host Cells. Biosci. Biotechnol. Biochem. 2000, 64, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef]
- Chilton, C.H.; Crowther, G.S.; Śpiewak, K.; Brindell, M.; Singh, G.; Wilcox, M.H.; Monaghan, T.M. Potential of lactoferrin to prevent antibiotic-induced Clostridium difficile infection. J. Antimicrob. Chemother. 2016, 71, 975–985. [Google Scholar] [CrossRef]
- Otake, K.; Sato, N.; Kitaguchi, A.; Irahara, T.; Murata, S.; Shiraga, K.; Ogawa, Y.; Fujiwara, T.K.; Koike, K.; Yokota, H. The Effect of Lactoferrin and Pepsin-Treated Lactoferrin on IEC-6 Cell Damage Induced by Clostridium Difficile Toxin B. Shock 2018, 50, 119–125. [Google Scholar] [CrossRef]
- Braim, S.; Śpiewak, K.; Brindell, M.; Heeg, D.; Alexander, C.; Monaghan, T. Lactoferrin-Loaded Alginate Microparticles to Target Clostridioides difficile Infection. J. Pharm. Sci. 2019, 108, 2438–2446. [Google Scholar] [CrossRef]
- Anikst, V.E.; Gaur, R.L.; Schroeder, L.F.; Banaei, N. Organism burden, toxin concentration, and lactoferrin concentration do not distinguish between clinically significant and nonsignificant diarrhea in patients with Clostridium difficile. Diagn. Microbiol. Infect. Dis. 2016, 84, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Dian, Z.; Sun, Y.; Zhang, G.; Xu, Y.; Fan, X.; Yang, X.; Pan, Q.; Peppelenbosch, M.; Miao, Z. Rotavirus-related systemic diseases: Clinical manifestation, evidence and pathogenesis. Crit. Rev. Microbiol. 2021, 47, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. Norovirus Vaccine: Priorities for Future Research and Development. Front. Immunol. 2020, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Siciliano, R.; Rega, B.; Giansanti, F.; Valenti, P.; Antonini, G. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Biophys. Acta 2001, 1528, 107–115. [Google Scholar] [CrossRef]
- Parrón, J.A.; Ripollés, D.; Ramos, S.J.; Pérez, M.D.; Semen, Z.; Rubio, P.; Calvo, M.; Sánchez, L. Antirotaviral potential of lactoferrin from different origin: Effect of thermal and high pressure treatments. BioMetals 2018, 31, 343–355. [Google Scholar] [CrossRef]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sánchez, J.; Alfaro, S.; Lönnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef]
- Ishikawa, H.; Awano, N.; Fukui, T.; Sasaki, H.; Kyuwa, S. The protective effects of lactoferrin against murine norovirus infection through inhibition of both viral attachment and replication. Biochem. Biophys. Res. Commun. 2013, 434, 791–796. [Google Scholar] [CrossRef]
- Oda, H.; Kolawole, A.O.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral effects of bovine lactoferrin on human norovirus. Biochem. Cell Biol. 2020, 99, 166–172. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Mondot, S.; Kang, S.; Furet, J.P.; Aguirre de Carcer, D.; McSweeney, C.; Morrison, M.; Marteau, P.; Dore, J.; Leclerc, M. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm. Bowel Dis. 2011, 17, 185–192. [Google Scholar] [CrossRef]
- Chen, L.; Wilson, J.E.; Koenigsknecht, M.J.; Chou, W.C.; Montgomery, S.A.; Truax, A.D.; Brickey, W.J.; Packey, C.D.; Maharshak, N.; Matsushima, G.K.; et al. Corrigendum: NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 2017, 18, 1270. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, S.Y.; Bae, E.A.; Huh, C.S.; Ahn, Y.T.; Lee, J.H.; Kim, D.H. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int. Immunopharmacol. 2008, 8, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Su, Y.W.; Ong, W.K.; Cheng, T.H.; Tsai, Y.C. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 2011, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, F.Z.; Xiong, L.; Zhao, L.; Guo, H.Y. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Cano, P.G.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef]
- Di Gioia, D.; Aloisio, I.; Mazzola, G.; Biavati, B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biot. 2014, 98, 563–577. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, G.; Yao, Y.; Guo, S.; Lu, K.; Sheng, Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J. Trauma 2006, 61, 650–657. [Google Scholar] [CrossRef]
- Li, D.M.; He, Q.; Yang, H.H.; Du, Y.F.; Yu, K.Q.; Yang, J.; Tong, X.; Guo, Y.X.; Xu, J.Y.; Qin, L.Q. Daily Dose of Bovine Lactoferrin Prevents Ethanol-Induced Liver Injury and Death in Male Mice by Regulating Hepatic Alcohol Metabolism and Modulating Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, e2100253. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Wang, Y.; Kirpich, I.; Liu, Y.; Ma, Z.; Barve, S.; McClain, C.J.; Feng, W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 2011, 179, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Bjorck, I.; Backhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen 2019, 8, e00825. [Google Scholar] [CrossRef]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef]
- Li, L.; Ma, C.; Hurilebagen; Yuan, H.; Hu, R.; Wang, W.; Weilisi. Effects of lactoferrin on intestinal flora of metabolic disorder mice. BMC Microbiol. 2022, 22, 181. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodriguez-Nogales, A.; Hidalgo-Garcia, L.; Garrido-Mesa, J.; Ruiz-Malagon, A.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Leyva-Jimenez, F.J.; et al. The Beneficial Effects of Lippia Citriodora Extract on Diet-Induced Obesity in Mice Are Associated with Modulation in the Gut Microbiota Composition. Mol. Nutr. Food Res. 2020, 64, e2000005. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Rodriguez-Daza, M.C.; Roquim, M.; Dudonne, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hao, Y.; Mao, R.; Yang, N.; Zheng, X.; Li, B.; Wang, Z.; Zhang, Q.; Teng, D.; Wang, J. Effects of dietary supplementation of bovine lactoferrin on growth performance, immune function and intestinal health in weaning piglets. Biometals 2022, 36, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, J.; Teng, D.; Wang, X.; Mao, R.; Yang, N.; Ma, X. A prospective on multiple biological activities of lactoferrin contributing to piglet welfare. Biochem. Cell Biol. 2021, 99, 66–72. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, F.; Zhu, W.; Wang, J. Effects of early-life lactoferrin intervention on growth performance, small intestinal function and gut microbiota in suckling piglets. Food Funct. 2019, 10, 5361–5373. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Early-life lactoferrin intervention modulates the colonic microbiota, colonic microbial metabolites and intestinal function in suckling piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6185–6197. [Google Scholar] [CrossRef]
- Peran, L.; Sierra, S.; Comalada, M.; Lara-Villoslada, F.; Bailon, E.; Nieto, A.; Concha, A.; Olivares, M.; Zarzuelo, A.; Xaus, J.; et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br. J. Nutr. 2007, 97, 96–103. [Google Scholar] [CrossRef]
- Fernandes, A.; Jobby, R. Bacteriocins from lactic acid bacteria and their potential clinical applications. Appl. Biochem. Biotech. 2022, 194, 4377–4399. [Google Scholar] [CrossRef]
- Chen, J.S.; Kang, B.J.; Jiang, Q.; Han, M.M.; Zhao, Y.R.; Long, L.N.; Fu, C.X.; Yao, K. Alpha-Ketoglutarate in Low-Protein Diets for Growing Pigs: Effects on Cecal Microbial Communities and Parameters of Microbial Metabolism. Front. Microbiol. 2018, 9, 1057. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.Y.; Yu, H.; Wang, J.; Zhu, W.Y. Response of Colonic Mucosa-Associated Microbiota Composition, Mucosal Immune Homeostasis, and Barrier Function to Early Life Galactooligosaccharides Intervention in Suckling Piglets. J. Agr. Food Chem. 2019, 67, 578–588. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The Maturing Development of Gut Microbiota in Commercial Piglets during the Weaning Transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- De Witte, C.; Flahou, B.; Ducatelle, R.; Smet, A.; De Bruyne, E.; Cnockaert, M.; Taminiau, B.; Daube, G.; Vandamme, P.; Haesebrouck, F. Detection, isolation and characterization of Fusobacterium gastrosuis sp. nov. colonizing the stomach of pigs. Syst. Appl. Microbiol. 2017, 40, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J. Pediatr. 2016, 173, S37–S42. [Google Scholar] [CrossRef]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Sortino, O.; Hullsiek, K.H.; Richards, E.; Rupert, A.; Schminke, A.; Tetekpor, N.; Quinones, M.; Prosser, R.; Schacker, T.; Sereti, I.; et al. The Effects of Recombinant Human Lactoferrin on Immune Activation and the Intestinal Microbiome Among Persons Living with Human Immunodeficiency Virus and Receiving Antiretroviral Therapy. J. Infect. Dis. 2019, 219, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. https://doi.org/10.3390/pharmaceutics15061569
Conesa C, Bellés A, Grasa L, Sánchez L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics. 2023; 15(6):1569. https://doi.org/10.3390/pharmaceutics15061569
Chicago/Turabian StyleConesa, Celia, Andrea Bellés, Laura Grasa, and Lourdes Sánchez. 2023. "The Role of Lactoferrin in Intestinal Health" Pharmaceutics 15, no. 6: 1569. https://doi.org/10.3390/pharmaceutics15061569
APA StyleConesa, C., Bellés, A., Grasa, L., & Sánchez, L. (2023). The Role of Lactoferrin in Intestinal Health. Pharmaceutics, 15(6), 1569. https://doi.org/10.3390/pharmaceutics15061569






