The Protective Effect of Mangiferin on Formaldehyde-Induced HT22 Cell Damage and Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Cells
2.3. Reagents
2.4. Antibodies
2.5. Cell Culture
2.6. Experimental Design
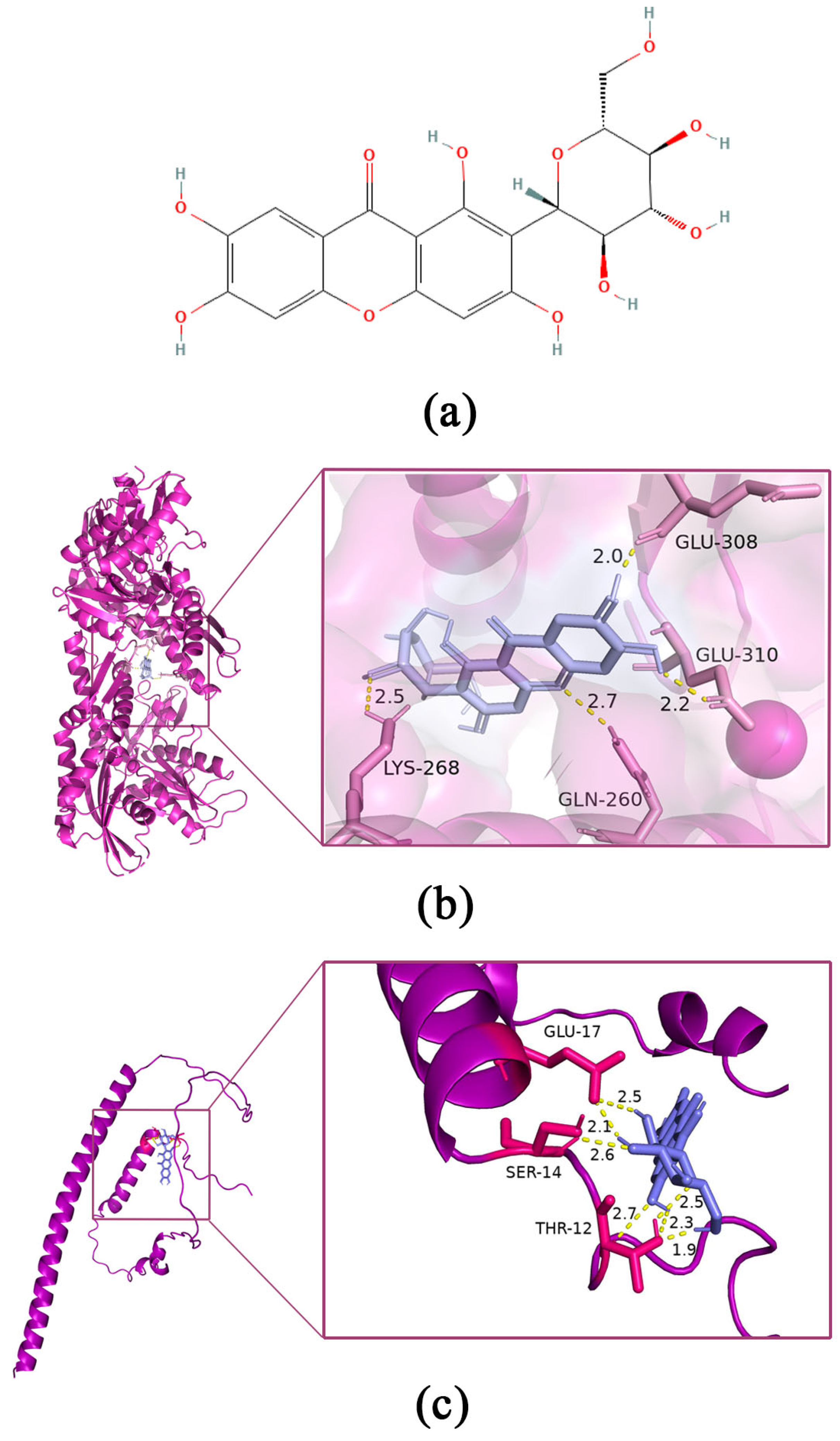
2.7. Molecular Docking
2.8. Cytotoxicity Assay
2.9. Ca2+ Concentration Determination
2.10. Determination of Oxidative Damage
2.11. Western Blotting Assays
2.12. Y-maze Test
2.13. Novel Object Recognition Test
2.14. Immunohistochemistry
2.15. Statistical Analysis
3. Results
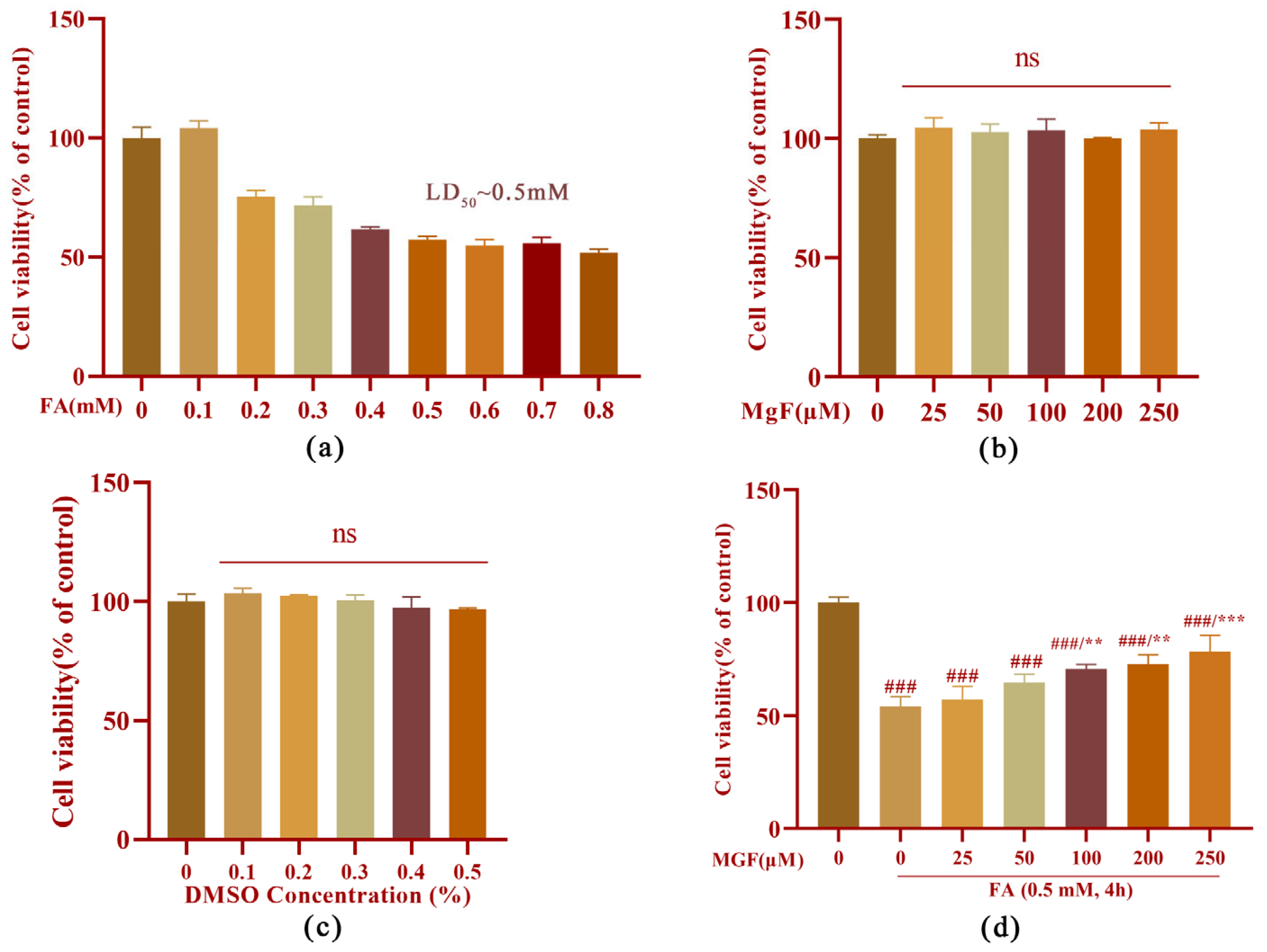
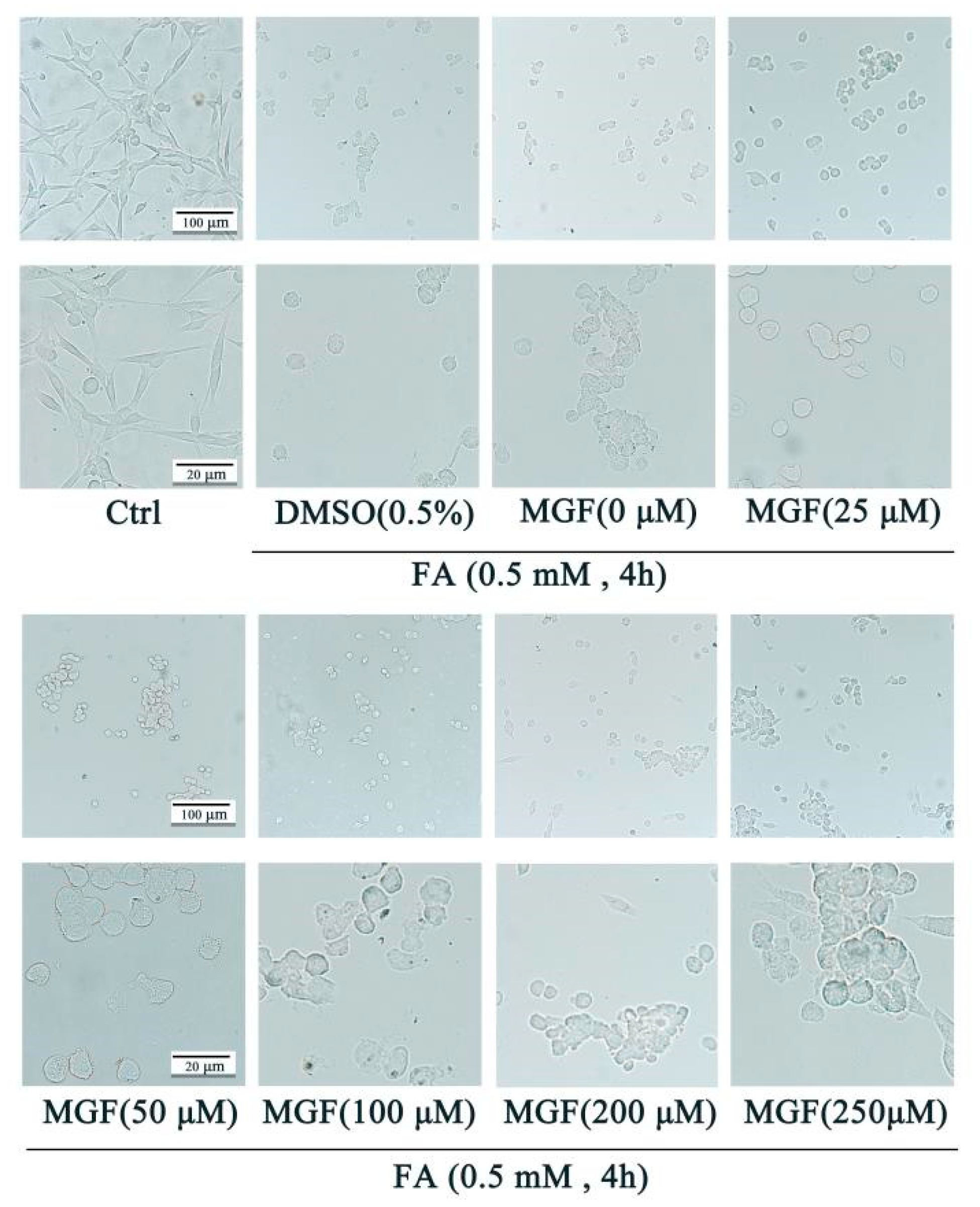
3.1. MGF Protects HT22 Cells from FA-Induced Neurotoxicity
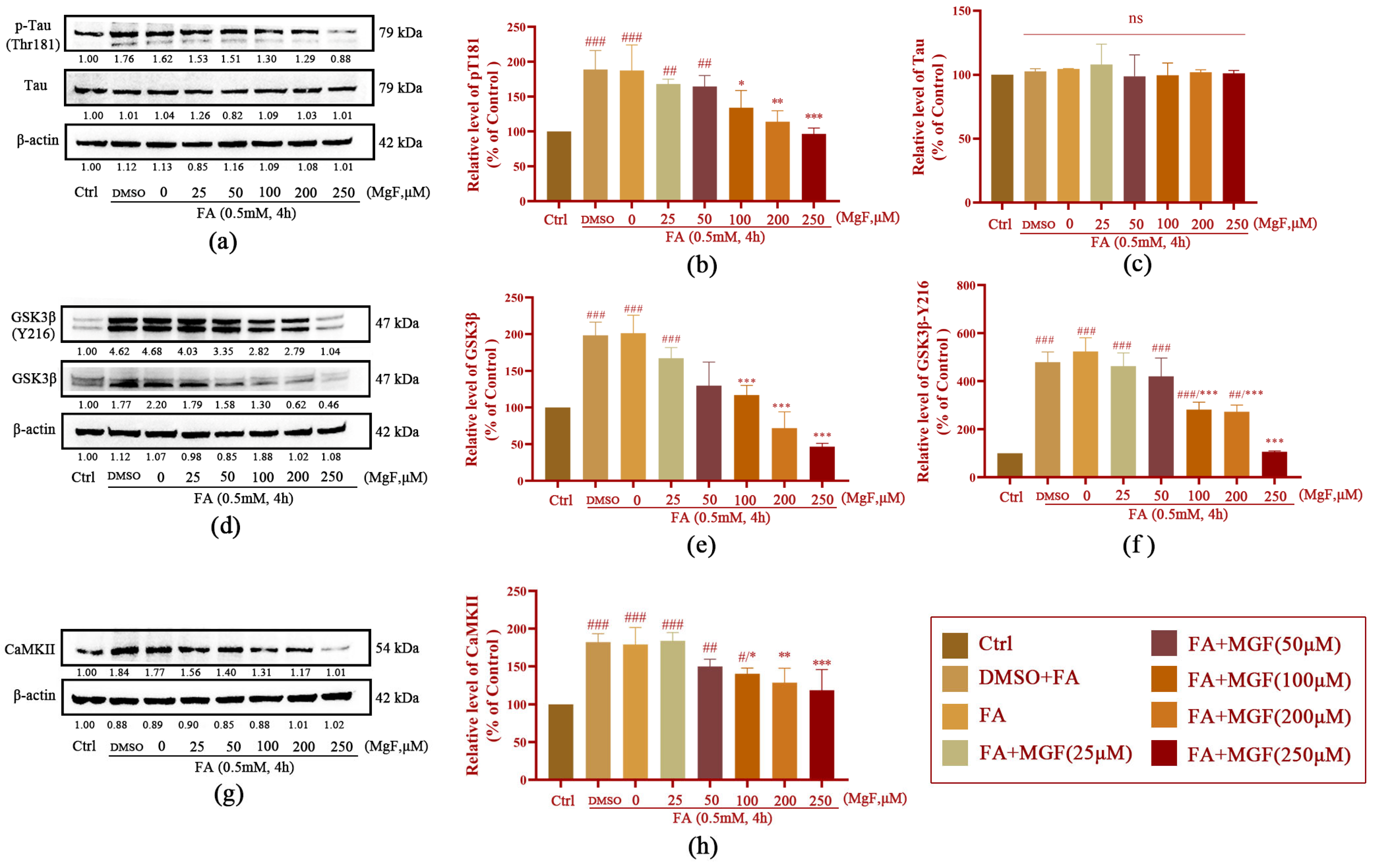
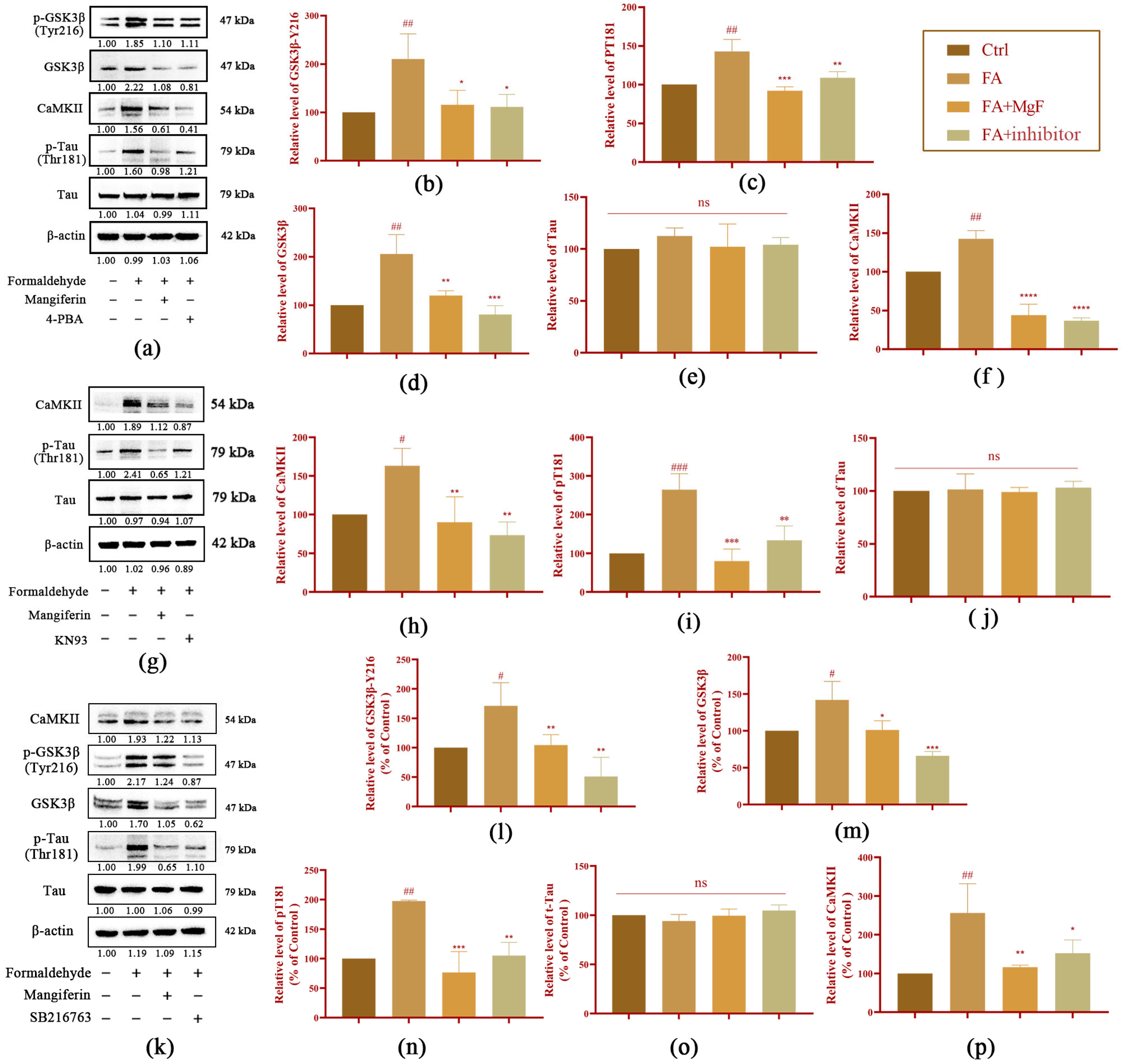
3.2. MGF Decreases FA-Induced Tau Hyperphosphorylation by Inhibiting GSK3β and CaMKII
3.3. MGF Ameliorates FA-Induced ERS in HT22 cells
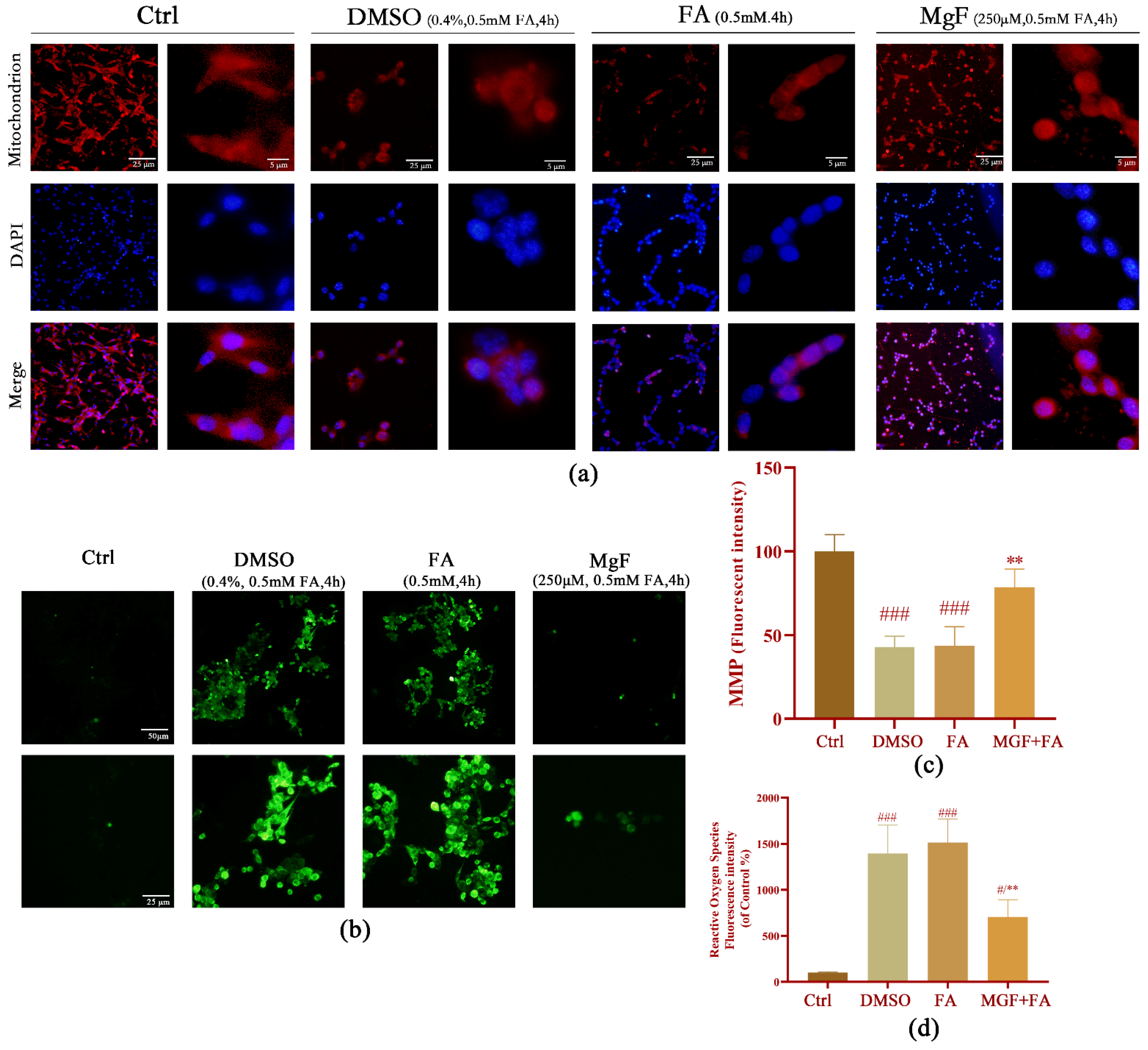
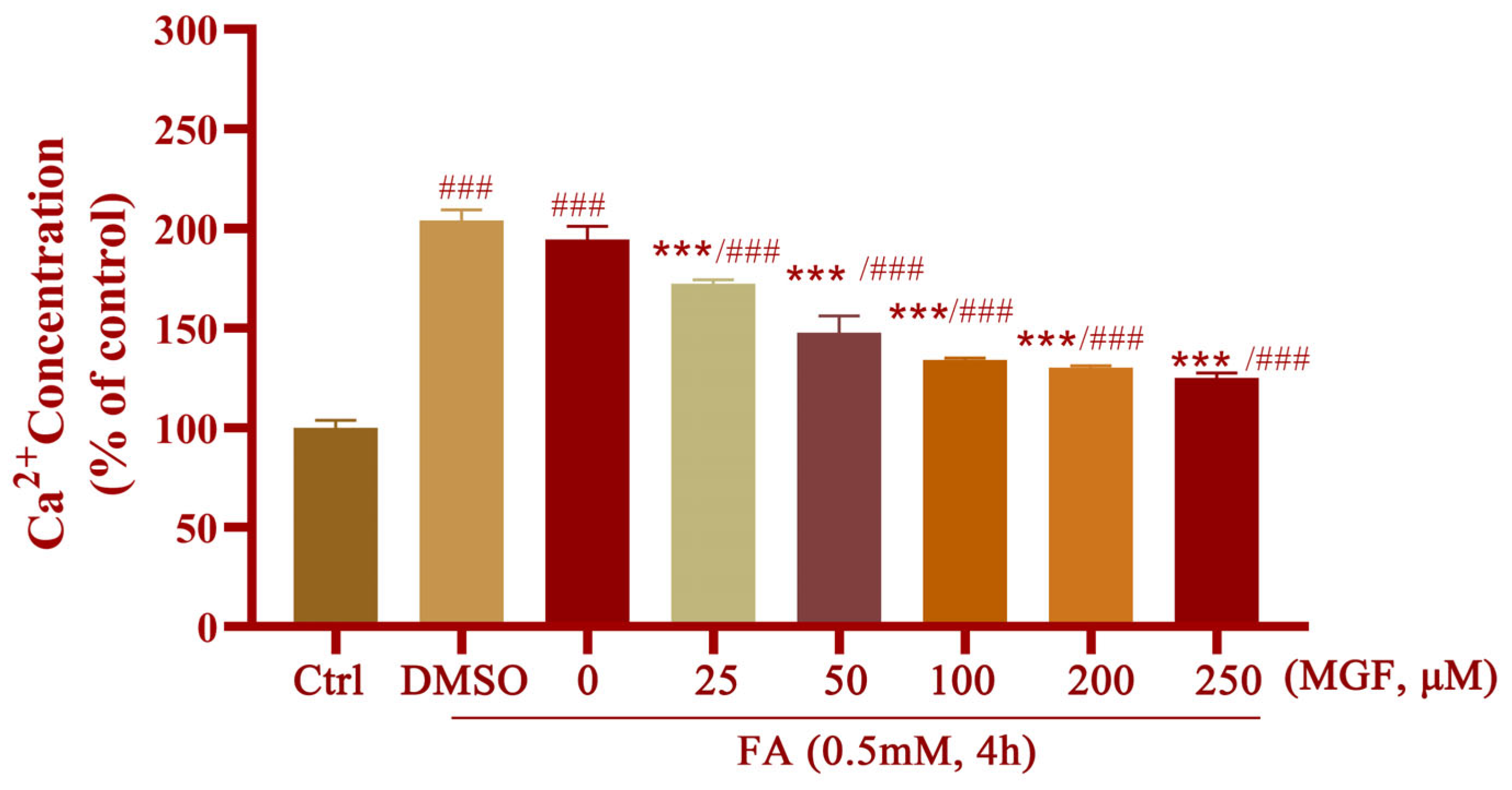
3.4. MGF Ameliorates FA-Induced Oxidative Stress and Ca2+ Overload in HT22 Cells
3.5. MGF Improves FA-Induced Spatial Memory and Cognitive Impairment in Mice
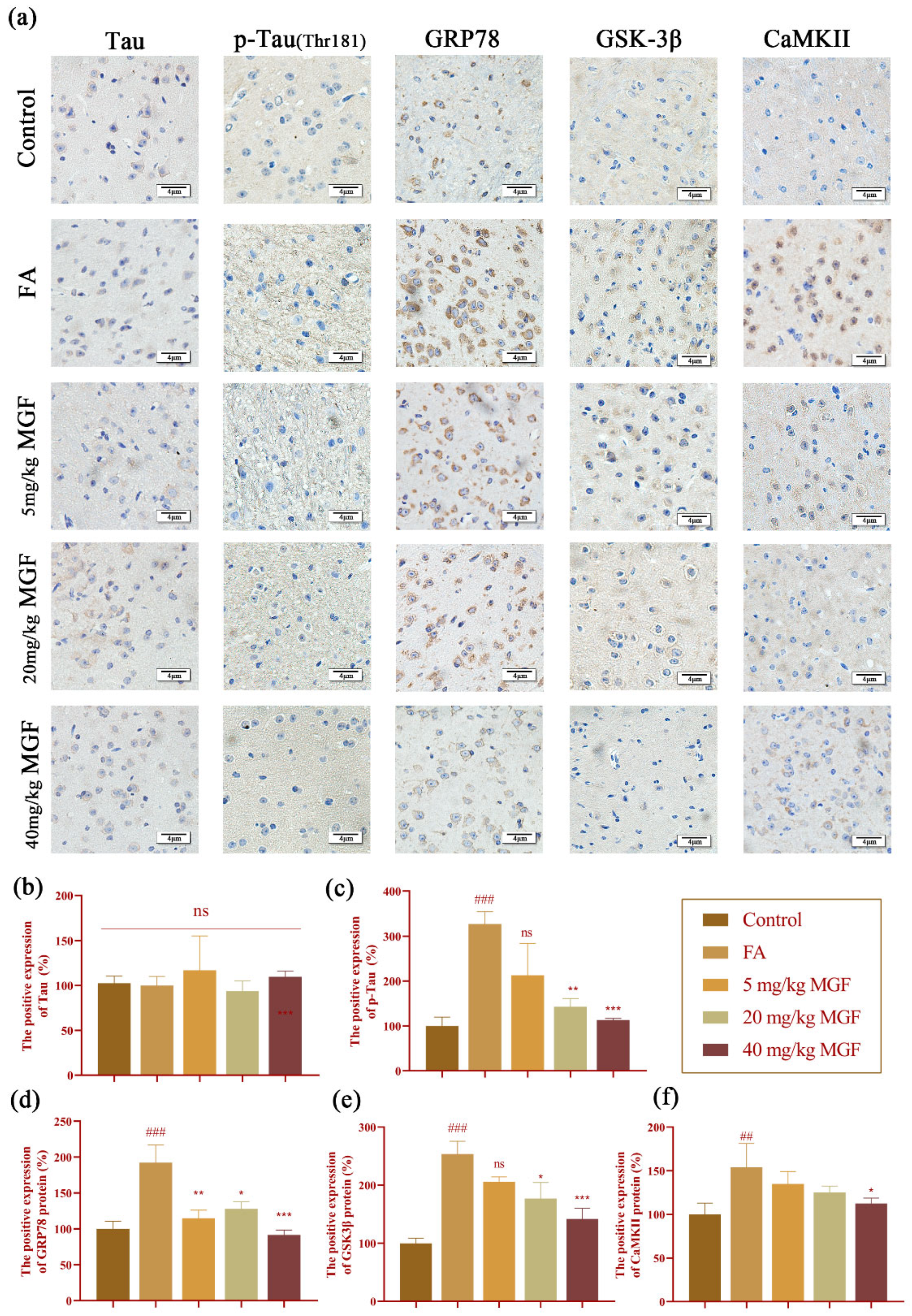
3.6. MGF Reduced FA-Induced Tau Hyperphosphorylation and the Expression of GRP78, GSK-3β, and CaMKII in the Brain of Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, H.; Jiang, S.; Wang, A.; Zou, X.; Wang, Y.; Li, W.; Zhang, Y.; Sun, M.; Ren, Q.; et al. Nutrition in Alzheimer’s disease: A review of an underappreciated pathophysiological mechanism. Sci. China Life Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Twarowski, B.; Herbet, M. Inflammatory processes in Alzheimer’s disease-pathomechanism, diagnosis and treatment: A review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Monte, W.C.; Hu, X.; He, Y.; He, R. Formaldehyde as a trigger for protein aggregation and potential target for mitigation of age-related, progressive cognitive impairment. Curr. Top. Med. Chem. 2016, 16, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wu, R.; Ye, M.; Zhao, Y.; Kang, J.; Ma, P.; Li, J.; Yang, X. Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Toxicol. Mech. Methods 2018, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Miao, J.; Rizak, J.; Zhai, R.; Wang, Z.; Huma, T.; Li, T.; Zheng, N.; Wu, S.; Zheng, Y.; et al. Alzheimer’s disease and methanol toxicity (part 2): Lessons from four rhesus macaques (Macaca mulatta) chronically fed methanol. J. Alzheimer’s Dis. 2014, 41, 1131–1147. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Miao, J.; Rizak, J.; Yang, J.; Zhai, R.; Zhou, J.; Qu, J.; Wang, J.; Yang, S.; et al. Alzheimer’s disease and methanol toxicity (part 1): Chronic methanol feeding led to memory impairments and tau hyperphosphorylation in mice. J. Alzheimer’s Dis. 2014, 41, 1117–1129. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Liu, S.; Fan, L.; Yang, Y.; Huang, Y.; Song, J. Small interfering rna targeting of peroxiredoxinⅱ gene enhances formaldehyde-induced toxicity in bone marrow cells isolated from balb/c mice. Ecotoxicol. Environ. Saf. 2019, 181, 89–95. [Google Scholar] [CrossRef]
- Li, L.; Hua, L.; He, Y.; Bao, Y. Differential effects of formaldehyde exposure on airway inflammation and bronchial hyperresponsiveness in balb/c and c57bl/6 mice. PLoS ONE 2017, 12, e0179231. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, S.M.; Choi, K.C. Treatment of human placental choriocarcinoma cells with formaldehyde and benzene induced growth and epithelial mesenchymal transition via induction of an antioxidant effect. Int. J. Environ. Res. Public Health 2017, 14, 854. [Google Scholar] [CrossRef]
- Nadalutti, C.A.; Prasad, R.; Wilson, S.H. Perspectives on formaldehyde dysregulation: Mitochondrial DNA damage and repair in mammalian cells. DNA Repair 2021, 105, 103134. [Google Scholar] [CrossRef] [PubMed]
- Kiviluoto, S.; Vervliet, T.; Ivanova, H.; Decuypere, J.P.; De Smedt, H.; Missiaen, L.; Bultynck, G.; Parys, J.B. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim. Biophys. Acta 2013, 1833, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.C.; Zhou, J.; Lv, T.; Qi, L.; Wang, S.D.; Nakamura, H.; Yodoi, J.; Bai, J. Induction of endoplasmic reticulum stress and the modulation of thioredoxin-1 in formaldehyde-induced neurotoxicity. Neurotoxicology 2012, 33, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Kim, C.-W.; Hwang, K.-A.; Choi, D.-W.; Choi, K.-C. Three components of cigarette smoke altered the growth and apoptosis of metastatic colon cancer cells via inducing the synthesis of reactive oxygen species and endoplasmic reticulum stress. Environ. Toxicol. Pharmacol. 2016, 45, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Choi, H.; Min, J.P.; Dong, I.K.; Park, S.H. The er stress-mediated decrease in ddah1 expression is involved in formaldehyde-induced apoptosis in lung epithelial cells. Food Chem. Toxicol. 2013, 62, 763–769. [Google Scholar] [CrossRef]
- Liu, X.J.; Wei, J.; Shang, Y.H.; Huang, H.C.; Lao, F.X. Modulation of aβpp and gsk3β by endoplasmic reticulum stress and involvement in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Veeresh, P.; Kaur, H.; Sarmah, D.; Mounica, L.; Verma, G.; Kotian, V.; Kesharwani, R.; Kalia, K.; Borah, A.; Wang, X.; et al. Endoplasmic reticulum-mitochondria crosstalk: From junction to function across neurological disorders. Ann. N. Y. Acad. Sci. 2019, 1457, 41–60. [Google Scholar] [CrossRef]
- Pan, B.; Sun, J.; Liu, Z.; Wang, L.; Huo, H.; Zhao, Y.; Tu, P.; Xiao, W.; Zheng, J.; Li, J. Longxuetongluo capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and mapk-mediated mechanisms. J. Adv. Res. 2021, 33, 215–225. [Google Scholar] [CrossRef]
- Cai, Y.; Arikkath, J.; Yang, L.; Guo, M.-L.; Periyasamy, P.; Buch, S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 2016, 12, 225–244. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Y.; Zhang, H.; Han, F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Fewell, S.W.; Travers, K.J.; Weissman, J.S.; Brodsky, J.L. The action of molecular chaperones in the early secretory pathway. Annu. Rev. Genet. 2001, 35, 149–191. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; Molinari, M.; Helenius, A. Setting the standards: Quality control in the secretory pathway. Science 1999, 286, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Li, M.J.; Shang, Y.H.; Liu, Y.R.; Han-Chang, H.; Lao, F.X. Zeaxanthin attenuates the vicious circle between endoplasmic reticulum stress and tau phosphorylation: Involvement of gsk-3beta activation. J. Alzheimer’s Dis. 2022, 86, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Gartner, U.; Janke, C.; Holzer, M.; Vanmechelen, E.; Arendt, T. Postmortem changes in the phosphorylation state of tau-protein in the rat brain. Neurobiol. Aging 1998, 19, 535–543. [Google Scholar] [CrossRef]
- Mair, W.; Muntel, J.; Tepper, K.; Tang, S.; Biernat, J.; Seeley, W.W.; Kosik, K.S.; Mandelkow, E.; Steen, H.; Steen, J.A. Flexitau: Quantifying post-translational modifications of tau protein in vitro and in human disease. Anal. Chem. 2016, 88, 3704–3714. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on tau protein phosphorylation in Alzheimer’s disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Boufidou, F. Plasma p-tau181 for the discrimination of Alzheimer’s disease from other primary dementing and/or movement disorders. Biomolecules 2022, 12, 1099. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in n2a cells. Front. Neurosci. 2016, 10, 598. [Google Scholar] [CrossRef]
- Meares, G.P.; Mines, M.A.; Beurel, E.; Eom, T.Y.; Song, L.; Zmijewska, A.A.; Jope, R.S. Glycogen synthase kinase-3 regulates endoplasmic reticulum (er) stress-induced chop expression in neuronal cells. Exp. Cell Res. 2011, 317, 1621–1628. [Google Scholar] [CrossRef]
- Tscheschner, H.; Meinhardt, E.; Schlegel, P.; Jungmann, A.; Lehmann, L.H.; Müller, O.J.; Most, P.; Katus, H.A.; Raake, P.W. Camkii activation participates in doxorubicin cardiotoxicity and is attenuated by moderate grp78 overexpression. PLoS ONE 2019, 14, e0215992. [Google Scholar] [CrossRef] [PubMed]
- Walia, V.; Chaudhary, S.K.; Kumar Sethiya, N. Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochem. Int. 2021, 143, 104939. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, R.; Hering, A.; Stefanowicz-Hajduk, J.; Cal, K.; Barańska, H. The effect of mangiferin on skin: Penetration, permeation and inhibition of ecm enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Fanshi, F.; Lai, Y.H.; Chen, J.R.; Hao, E.; Deng, J.; Hsiao, C.D. Mechanism of anti-dementia effects of mangiferin in a senescence accelerated mouse (samp8) model. Biosci. Rep. 2019, 39, BSR20190488. [Google Scholar] [CrossRef]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Delgado-Olmos, I.; Gamero-Carrasco, C.; Fernandez-Ponce, M.T.; Casas, L.; Mantell, C.; Garcia-Alloza, M. Long-term mangiferin extract treatment improves central pathology and cognitive deficits in app/ps1 mice. Mol. Neurobiol. 2017, 54, 4696–4704. [Google Scholar] [CrossRef]
- Arora, M.K.; Kisku, A.; Jangra, A. Mangiferin ameliorates intracerebroventricular-quinolinic acid-induced cognitive deficits, oxidative stress, and neuroinflammation in wistar rats. Indian J. Pharmacol. 2020, 52, 296–305. [Google Scholar]
- Campos-Esparza, M.R.; Sánchez-Gómez, M.V.; Matute, C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 2009, 45, 358–368. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.Y.; Tang, X.C. Cholinergic deficiency involved in vascular dementia: Possible mechanism and strategy of treatment. Acta Pharmacol. Sin. 2009, 30, 879–888. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Effect of n-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 98–104. [Google Scholar] [CrossRef]
- Lakshmi, B.V.; Sudhakar, M.; Prakash, K.S. Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: Behavioral and biochemical alterations in rats. Biol. Trace Elem. Res. 2015, 165, 67–74. [Google Scholar] [CrossRef]
- Jung, K.; Lee, B.; Han, S.J.; Ryu, J.H.; Kim, D.H. Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol. Pharm. Bull. 2009, 32, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kasbe, P.; Jangra, A.; Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ormö, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal structure of the Aequorea victoria green fluorescent protein. Science 1996, 273, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Olson, A.J. Automated docking of substrates to proteins by simulated annealing. Proteins 1990, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Miao, J.; Su, T.; Liu, Y.; He, R. Formaldehyde induces hyperphosphorylation and polymerization of tau protein both in vitro and in vivo. Biochim. Biophys. Acta 2013, 1830, 4102–4116. [Google Scholar] [CrossRef] [PubMed]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Dellu, F.; Contarino, A.; Simon, H.; Koob, G.F.; Gold, L.H. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol. Learn. Mem. 2000, 73, 31–48. [Google Scholar] [CrossRef]
- Tian, S.W.; Yu, X.D.; Cen, L.; Xiao, Z.Y. Glutamate transporter glt1 inhibitor dihydrokainic acid impairs novel object recognition memory performance in mice. Physiol. Behav. 2019, 199, 28–32. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. JoVE J. Vis. Exp. 2017, 126, e55718. [Google Scholar]
- Song, Y.; Wang, J. Overview of chinese research on senile dementia in mainland china. Ageing Res. Rev. 2010, 9 (Suppl. 1), S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Rizak, J.D.; Ma, Y.; Hu, X. Is formaldehyde the missing link in ad pathology? The differential aggregation of amyloid-beta with apoe isoforms in vitro. Curr. Alzheimer Res. 2014, 11, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, X.; Tong, Z. Formaldehyde-crosslinked nontoxic aβ monomers to form toxic aβ dimers and aggregates: Pathogenicity and therapeutic perspectives. ChemMedChem 2021, 16, 3376–3390. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Han, C.; Qiang, M.; Wang, W.; Lv, J.; Zhang, S.; Luo, W.; Li, H.; Luo, H.; Zhou, J.; et al. Age-related formaldehyde interferes with DNA methyltransferase function, causing memory loss in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 100–110. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, J.; Luo, W.; Wang, W.; Li, F.; Li, H.; Luo, H.; Lu, J.; Zhou, J.; Wan, Y.; et al. Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiol. Aging 2011, 32, 31–41. [Google Scholar] [CrossRef]
- Mei, Y.; Jiang, C.; Wan, Y.; Lv, J.; Jia, J.; Wang, X.; Yang, X.; Tong, Z. Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell 2015, 14, 659–668. [Google Scholar] [CrossRef]
- Song, Y.X.; Miao, J.Y.; Qiang, M.; He, R.Q.; Wang, X.M.; Li, W.W. Icariin protects sh-sy5y cells from formaldehyde-induced injury through suppression of tau phosphorylation. Chin. J. Integr. Med. 2016, 22, 430–437. [Google Scholar] [CrossRef]
- Sun, P.; Chen, J.Y.; Li, J.; Sun, M.R.; Mo, W.C.; Liu, K.L.; Meng, Y.Y.; Liu, Y.; Wang, F.; He, R.Q.; et al. The protective effect of geniposide on human neuroblastoma cells in the presence of formaldehyde. BMC Complement. Altern. Med. 2013, 13, 152. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Ferrari, C.; Sorbi, S. The complexity of Alzheimer’s disease: An evolving puzzle. Physiol. Rev. 2021, 101, 1047–1081. [Google Scholar] [CrossRef]
- Llorens-Martin, M.; Jurado, J.; Hernandez, F.; Avila, J. Gsk-3beta, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [PubMed]
- Ghosh, A.; Giese, K.P. Calcium/calmodulin-dependent kinase ii and Alzheimer’s disease. Mol. Brain 2015, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; Wootz, H.; Korhonen, L. Er stress and neurodegenerative diseases. Cell Death Differ. 2006, 13, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Tydlacka, S.; Wang, C.E.; Wang, X.; Li, S.; Li, X.J. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J. Neurosci. 2008, 28, 13285–13295. [Google Scholar] [CrossRef]
- Han, K.S.; Li, N.; Raven, P.A.; Fazli, L.; Frees, S.; Ettinger, S.; Park, K.C.; Hong, S.J.; Gleave, M.E.; So, A.I. Inhibition of endoplasmic reticulum chaperone protein glucose-regulated protein 78 potentiates anti-angiogenic therapy in renal cell carcinoma through inactivation of the perk/eif2α pathway. Oncotarget 2015, 6, 34818–34830. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Martínez, G.; Hetz, C. Memory loss in Alzheimer’s disease: Are the alterations in the upr network involved in the cognitive impairment? Front. Aging Neurosci. 2014, 6, 8. [Google Scholar] [CrossRef]
- Ho, Y.S.; Yang, X.; Lau, J.C.; Hung, C.H.; Wuwongse, S.; Zhang, Q.; Wang, J.; Baum, L.; So, K.F.; Chang, R.C. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: Implication in Alzheimer’s disease pathogenesis. J. Alzheimer’s Dis. 2012, 28, 839–854. [Google Scholar] [CrossRef]
- Liu, Z.C.; Chu, J.; Lin, L.; Song, J.; Ning, L.N.; Luo, H.B.; Yang, S.S.; Shi, Y.; Wang, Q.; Qu, N.; et al. Sil1 rescued bip elevation-related tau hyperphosphorylation in er stress. Mol. Neurobiol. 2016, 53, 983–994. [Google Scholar] [CrossRef]
- Wu, W.; Huang, S.; Luo, X. The role of activation of perk in activating glycogen synthase kinase 3(gsk-3) by oleic acid(oa) in type 2 diabetes. Int. J. Pediatr. Endocrinol. 2013, 2013, O35. [Google Scholar] [CrossRef]
- Gomez, L.; Thiebaut, P.A.; Paillard, M.; Ducreux, S.; Abrial, M.; Crola Da Silva, C.; Durand, A.; Alam, M.R.; Van Coppenolle, F.; Sheu, S.S.; et al. The sr/er-mitochondria calcium crosstalk is regulated by gsk3β during reperfusion injury. Cell Death Differ. 2016, 23, 313–322. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Alzheimer’s disease: A short introduction to the calmodulin hypothesis. AIMS Neurosci. 2019, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Wu, Q.; Smith, A.; Grundke-Iqbal, I.; Iqbal, K. Tau is phosphorylated by gsk-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by a-kinase. FEBS Lett. 1998, 436, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Lai, B.; Zheng, Z.; Zhang, Y.; Luo, J.; Wang, C.; Chen, Y.; Woodgett, J.R.; Li, M. Inhibitory phosphorylation of gsk-3 by camkii couples depolarization to neuronal survival. J. Biol. Chem. 2010, 285, 41122–41134. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef]
- Gao, T.; Gao, C.; Liu, Z.; Wang, Y.; Jia, X.; Tian, H.; Lu, Q.; Guo, L. Inhibition of noncanonical ca(2+) oscillation/calcineurin/gsk-3β pathway contributes to anti-inflammatory effect of sigma-1 receptor activation. Neurochem. Res. 2022, 47, 264–278. [Google Scholar] [CrossRef]
- Umansky, C.; Morellato, A.E.; Rieckher, M.; Scheidegger, M.A.; Martinefski, M.R.; Fernández, G.A.; Pak, O.; Kolesnikova, K.; Reingruber, H.; Bollini, M.; et al. Endogenous formaldehyde scavenges cellular glutathione resulting in redox disruption and cytotoxicity. Nat. Commun. 2022, 13, 745. [Google Scholar] [CrossRef]
- Zerin, T.; Kim, J.S.; Gil, H.W.; Song, H.Y.; Hong, S.Y. Effects of formaldehyde on mitochondrial dysfunction and apoptosis in sk-n-sh neuroblastoma cells. Cell Biol. Toxicol. 2015, 31, 261–272. [Google Scholar] [CrossRef]
- Nadalutti, C.A.; Stefanick, D.F.; Zhao, M.L.; Horton, J.K.; Prasad, R.; Brooks, A.M.; Griffith, J.D.; Wilson, S.H. Mitochondrial dysfunction and DNA damage accompany enhanced levels of formaldehyde in cultured primary human fibroblasts. Sci. Rep. 2020, 10, 5575. [Google Scholar] [CrossRef]
- Li, Y.-J.; Sui, Y.-J.; Dai, Y.-H.; Deng, Y.-L. Lc determination and pharmacokinetics study of mangiferin in rat plasma and tissues. Chromatographia 2008, 67, 957–960. [Google Scholar] [CrossRef]
- Martínez Sánchez, G.; Candelario-Jalil, E.; Giuliani, A.; León, O.S.; Sam, S.; Delgado, R.; Núñez Sellés, A.J. Mangifera indica L. Extract (qf808) reduces ischaemia-induced neuronal loss and oxidative damage in the gerbil brain. Free Radic. Res. 2001, 35, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.X.; Chen, Y.M.; He, J.; Zeng, T.; Wang, J.H. Effects of morphine and its withdrawal on y-maze spatial recognition memory in mice. Neuroscience 2007, 147, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.T.; Sekar, M.; Gan, S.H.; Pandy, V.; Bonam, S.R. Protective effect of mangiferin on memory impairment: A systematic review. Saudi J. Biol. Sci. 2021, 28, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/nlrp3 inflammasome activation with regulation of ampk in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, N.; Tian, X.; Su, J.; Qin, Y.; He, R.; He, X. The Protective Effect of Mangiferin on Formaldehyde-Induced HT22 Cell Damage and Cognitive Impairment. Pharmaceutics 2023, 15, 1568. https://doi.org/10.3390/pharmaceutics15061568
Chen F, Wang N, Tian X, Su J, Qin Y, He R, He X. The Protective Effect of Mangiferin on Formaldehyde-Induced HT22 Cell Damage and Cognitive Impairment. Pharmaceutics. 2023; 15(6):1568. https://doi.org/10.3390/pharmaceutics15061568
Chicago/Turabian StyleChen, Fan, Na Wang, Xinyan Tian, Juan Su, Yan Qin, Rongqiao He, and Xiaping He. 2023. "The Protective Effect of Mangiferin on Formaldehyde-Induced HT22 Cell Damage and Cognitive Impairment" Pharmaceutics 15, no. 6: 1568. https://doi.org/10.3390/pharmaceutics15061568
APA StyleChen, F., Wang, N., Tian, X., Su, J., Qin, Y., He, R., & He, X. (2023). The Protective Effect of Mangiferin on Formaldehyde-Induced HT22 Cell Damage and Cognitive Impairment. Pharmaceutics, 15(6), 1568. https://doi.org/10.3390/pharmaceutics15061568





