Abstract
Cancer is a leading cause of death worldwide, and the main treatment methods for this condition are surgery, chemotherapy, and radiotherapy. These treatment methods are invasive and can cause severe adverse reactions among organisms, so nanomaterials are increasingly used as structures for anticancer therapies. Dendrimers are a type of nanomaterial with unique properties, and their production can be controlled to obtain compounds with the desired characteristics. These polymeric molecules are used in cancer diagnosis and treatment through the targeted distribution of some pharmacological substances. Dendrimers have the ability to fulfill several objectives in anticancer therapy simultaneously, such as targeting tumor cells so that healthy tissue is not affected, controlling the release of anticancer agents in the tumor microenvironment, and combining anticancer strategies based on the administration of anticancer molecules to potentiate their effect through photothermal therapy or photodynamic therapy. The purpose of this review is to summarize and highlight the possible uses of dendrimers regarding the diagnosis and treatment of oncological conditions.
1. Introduction
1.1. Generalities
Dendrimers are nanomaterials with unique properties used in cancer diagnosis and treatment, targeting tumor cells, controlling the release of anticancer agents, and combining anticancer strategies [1]. Cancer is a malignant condition characterized by the uncontrolled proliferation of atypical cells. Cancer progression is supported by the imbalance or damage of proto-oncogenes that encode proteins involved in the development and differentiation of tumor cells, but also tumor suppressor genes that encode proteins that produce inhibitory signals for cells in need and produce apoptosis [2]. According to the World Health Organization, cancer is the leading cause of death globally, and lung cancer is the most common cause of cancer-related death [3,4]. As the mortality rate associated with cancer is increasing, research on cancer therapies is growing, and identifying the most effective treatment method is the goal of most scientists [5,6]. The main treatment methods for this group of pathologies include surgical excision, chemotherapy, radiotherapy, and immunotherapy. The effectiveness of these therapies depends on the severity of the disease and the particular reaction of each organism. However, a major problem encountered in patients undergoing the above-mentioned therapies is that these therapies cannot distinguish between tumor cells and healthy cells in the body, resulting in the occurrence of serious adverse reactions [5,7,8,9]. These adverse reactions are most often represented by alopecia, decreased immunity through the suppression of bone marrow function, fever, nausea, vomiting, hepatotoxicity, cardiotoxicity, neurotoxicity, electrolyte imbalances, and decreased muscle tone [10,11]. One way to avoid these adverse reactions is to use targeted therapies, where drugs or mechanisms for destroying tumor cells are specifically directed to the tumor [8,12,13]. Targeted therapy is a new-generation chemotherapy that seeks to target certain proteins or genes specifically linked to a particular cancer or tumor vasculature that promotes its growth [2]. Nanotechnology is an option for such therapies and is based on the synthesis of “tools” that act as transporters of drugs to a specific tumor target [14,15,16,17]. This review aims to give an overview of the potential diagnostic and therapeutic applications of dendrimers for oncological conditions.
1.2. Methods
The process of research for this review involved a thorough and precise search for relevant bibliographic sources across several databases, such as PubMed, Google Scholar, Cochrane, and Embase. The objective was to identify potential sources that contain information on the utilization of nanoparticles and dendrimers in cancer diagnosis and treatment. To conduct the search, the authors used a combination of appropriate keywords, including nanoparticles, dendrimers, cancer diagnosis, cancer treatment, and targeting. The search process was comprehensive, and the authors considered all relevant sources that appeared during the search. Initially, the authors identified over 227 bibliographic sources, which they evaluated carefully to determine their relevance to the study’s focus. Only sources whose information was pertinent to the research question and whose results were consistent with similar studies were included. As a result, the authors narrowed down the pool to 217 sources, which were considered relevant and included in the final review. The methodology used by the authors was designed to ensure a rigorous and comprehensive analysis of the available literature on the use of dendrimers in cancer diagnosis and treatment. The inclusion criteria were strict to ensure that only high-quality and relevant sources were included in the review. By doing so, the authors increased the reliability and validity of the study’s findings.
1.3. Nanoparticles: Classification and Characterization
Nanotechnology is extremely important in medicine in developing systems whose shape and size can be controlled to improve and individualize their physicochemical and pharmacological properties, and the systems obtained can be used for various purposes for numerous diseases, including cancer [18,19,20].
Nanoparticles are tiny materials having size ranges from 1 to 100 nm that can be obtained naturally or synthesized artificially, and research has shown that they are more effective than chemotherapy or radiotherapy used independently, producing minor adverse reactions [14,21,22,23]. Nanomaterials are used as biological markers, contrast agents for imaging, medical care products, pharmaceutical products, and drug delivery systems, as well as in the detection, diagnosis, and treatment of various types of diseases, including cancer [18,24,25,26].
The use of nano systems in medicine has numerous advantages, which are presented in Figure 1. In the case of oncological pathologies, the main advantage of using nanoparticles is that they can be directed to the sites of tumors by conjugating them with different monoclonal antibodies or peptide ligands that exhibit specificity for the receptors of the cells that these systems are intended to reach [27,28].
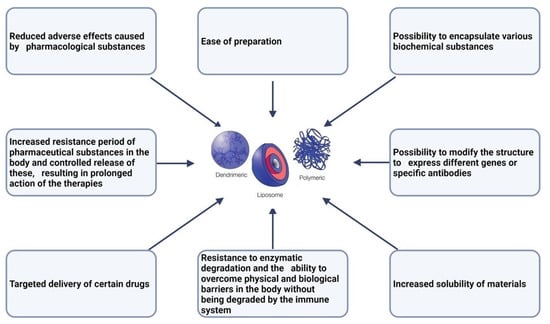
Figure 1.
Advantages of nano systems in medicine [18,29,30,31].
Nanoparticles can be classified based on several characteristics: size, charge, chemical properties, morphology, state(s) [32]. The main nano systems used in biomedicine are presented in Table 1.

Table 1.
Nanoparticles in drug delivery: a comparative analysis of polymeric nanoparticles, carbon nanotubes, liposomes, gold nanoparticles, micelles, solid lipid nanoparticles, and dendrimers.
Among the nanoparticles mentioned in Table 1, in the following, we will present dendrimers in more detail as nanoparticles with multiple applications in the oncological field.
2. Dendrimers: Polymer-Based Nanoparticles
Our planet is full of various structures that have a dendritic, branched architecture, from neuronal dendritic branches to the rich branching of tree roots. Some researchers believe that these dendritic structures have evolved over time due to the need for highly complex and efficient surfaces that allow for the processes of absorption, extraction, or distribution of different substances in the living world [50]. Inspired by his pastime as a horticulturist, Donald Tomalia, along with his colleagues, discovered hyper-branched molecules called dendrimers in the early 1980s [50,51]. The first dendrimers obtained were polyamidoamines (PAMAM) and are called starburst dendrimers. They are composed of a core of ethylenediamine or ammonia, which is surrounded by amino groups on the outside [51,52,53]. Unlike linear polymers, dendrimers are highly branched molecules that can be synthesized at the desired size and molecular weight, and whose monomeric units have the ability to self-organize [14,51,54,55]. Some of the existing types of dendrimers are presented throughout the following Table 2.

Table 2.
Types of dendrimers [2,56].
As previously noted, the structure of dendrimers is represented by a central core, surrounded by branches, and the outermost layer of their structure is represented by a multivalent surface. The synthesis of these molecules can be done divergently or convergently [14,57,58,59]. The two synthesis methods differ in the growth direction of the dendrimer. In the case of divergent synthesis, the synthesis begins with the formation of the central molecule, the core of the dendrimer, and then this base molecule interacts with monomers, causing the structure to grow outward [60,61]. The addition of monomers to the outside of the molecule occurs for several generations in a row, adding one layer of the first-generation dendrimer. Each layer of monomers represents a generation [51,57,61,62]. Dendrimers with few layers, from generations 0, 1, or 2, have open, asymmetric structures, and as the branches become larger (dendrimers of generation 4 or higher), the dendrimers become compactly packed, taking on a globular shape. However, the synthesis of these polymer molecules cannot be infinite due to the lack of space [51,63,64,65,66]. A problem that may arise in the synthesis of dendrimers by the divergent method is the appearance of incomplete terminal groups, and this problem can be avoided by synthesis through the convergent method, in which the dendrimer is synthesized starting from the final branches. When a structure of the desired size, formed from branched monomers, is reached, it attaches to a core molecule. This method does not carry the risk of defects appearing in the final structure of the dendrimer, and purification is easy to achieve, but it cannot be used for the synthesis of high-generation dendrimers [51,67,68]. After the synthesis of dendrimers, both in their core and between the branches formed by the monomers, cavities and channels will form. The interiors of dendrimers, as well as the groups on the surfaces of these structures, can be loaded with various medicinal substances [14,51,62,69,70,71,72]. In addition, the free branches of dendrimers can be linked to other molecules in the class of nanomaterials, radioligands, or other functional molecules that reduce cytotoxicity and increase the biocompatibility of the polymer in the body. In this way, by attaching different ligand molecules, dendrimers can be targeted to different tissues [50,73]. Due to the numerous advantages of dendrimers, these polymeric molecules are increasingly being used in medicine, providing utility in the diagnosis and treatment of various conditions, directing drug substances in the body, or increasing the efficacy of other therapies [74,75,76,77,78,79,80].
Some of the strengths of the use of dendrimers have already been noted, and Table 3 summarizes the advantages of these polymer-based nanoparticles and the advantages of using dendrimers in cancer treatment.

Table 3.
Advantages of dendrimers.
Although dendrimers have multiple advantages for use in biomedicine, these structures also present some disadvantages. One of these is the toxicity that dendrimers have in biological systems. Due to the terminal NH2 groups and the cationic charge on the dendrimer surface, they can interact with the negatively charged cell membranes, producing cell lysis and implicitly cytotoxicity [82,83]. It seems that PAMAM dendrimers are among the most toxic, and their toxicity depends on the dendrimer generation, the lower-generation ones being less toxic. PAMAM dendrimers whose surface is modified with anionic molecules such as carboxyl groups or with PEG polymers are less or not at all toxic. It was also proven that the modification of the surface of the dendrimer so that the amino groups are replaced with aldehyde groups led to lower toxicity. Similar to PAMAM dendrimers, PPI dendrimers also show toxicity [82,83].
The toxicity of dendrimers is generally characterized, in addition to cytotoxicity, by hemolytic toxicity and hematological toxicity. The hemolytic toxicity is caused by the interaction of the free cationic terminal groups of the dendrimers with the red blood cells (RBC), an interaction that determines hemolysis [82]. For instance, Bhadra et al. developed fourth-generation PAMAM dendrimers for the release of an anticancer drug and found that the hemolytic toxicity of these dendrimers was around 15.3–17.3% [82,84]. Subsequently, Asthana et al. had similar results when they evaluated the same dendrimers for hemolytic toxicity and observed up to 18% toxicity [82,85]. Analyzing different generations of PPI dendrimers, a value of 35.7% hemolysis was observed for the fourth-generation dendrimers and 49.2% hemolysis for the fifth-generation dendrimers [82].
Hemolysis caused by cationic dendrimers directly influences the hematological parameters—in this case, it is a hematological toxicity. Analyzing the effect of the dendrimer PPI on blood parameters such as the number of white blood cells, the number of red blood cells, the concentration of hemoglobin, the hematocrit, and the mean corpuscular hemoglobin, a significant decrease in the number of red cells and a significant increase in the number of white blood cells were observed. Moreover, the concentration of hemoglobin, the average corpuscular hemoglobin, and the hematocrit decreased dramatically according to Agashe et al. [82,86].
Dendrimer toxicity in vivo is rarely studied, but Roberts et al. studied the in vivo toxicity of third-, fifth-, and seventh-generation PAMAM dendrimers in Swiss Webster mice and observed that only seventh-generation PAMAM dendrimers produced biological complications. The authors concluded that dendrimers do not present properties that prevent their use in biological applications. However, in order to avoid any of the possible adverse effects, it is necessary to modify the surfaces of the dendrimers so that they are more biocompatible [82,87].
Besides the disadvantage of the toxicity of dendrimers, the possibility of these structures being immunogenic was also investigated, but it seems that immunogenicity was not identified—it was very weak. Agashe et al. investigated the immunogenicity of 5.0 G PPI dendrimers in Balb/C mice using ELISA to monitor the antibody titer and concluded that the dendrimers did not elicit any detectable humoral immune response under the experimental conditions. Thus, dendrimers are treated by the host’s immune system as “native” and this is an advantage for their use in drug delivery [82,86].
Due to their properties of encapsulating and transporting different molecules, but also the possibility of modifying their surface so as to present a high degree of biocompatibility, dendrimers can be used in cancer diagnosis.
3. The Use of Dendrimers in Cancer Diagnosis
Cancer encompasses a group of invasive diseases, which is why the early diagnosis of these diseases is extremely important in order to initiate effective treatment as early as possible. Cancer diagnosis can be established through many methods, two of which are magnetic resonance imaging (MRI) and computed tomography (CT) [88,89,90,91]. MRI is a technique used to obtain anatomical images of the internal organs and the vascular tree [51,92,93]. The use of contrast agents to obtain these images significantly improves their quality, and the administration of contrast agents can be done using dendrimers [1,56,88,94]. Contrast agents commonly used in MRI are paramagnetic metal cations of gadolinium, such as Gd(III)-N,N′,N″,N‴-tetracarboxymethyl-1,4,7,10-tetraazacyclododecane (Gd(III)-DOTA) and Gd(III)-diethylenetriamine pentaacetic acid (Gd(III)-DTPA), or other derivatives thereof. However, due to their low molecular weight, these agents can diffuse from blood vessels and have a short circulation time in the body, and differentiated targeting towards diseased and normal tissues is inefficient. This disadvantage can be minimized by attaching gadolinium cations to the surfaces of dendrimers [51,56,63,70,88,95,96].
The efficiency of using dendrimers conjugated with contrast agents has been demonstrated by Bourne et al., who tested the efficiency of gadolinium-conjugated dendrimers in imaging the pelvic blood vessels of rabbits. They obtained much clearer images and much better contrast between blood vessels and the soft tissues around them when using gadolinium dendrimers [51,97]. Furthermore, PAMAM dendrimers labelled with Gd have been used to evaluate the development of lymphatic vessels in tumors [88,98,99].
Other authors have noted that these dendrimer systems could be used as molecular probes to amplify the signals of contrast agents when they reach a tumor microenvironment. This is achievable due to the ability to modify the surface properties and compositions of dendrimers by attaching specific antibodies or ligands targeting tumor receptors [95,100,101,102]. Dendrimers can also be covered with a transducer film functionalized with receptors for certain biological analytes. When the analytes bind to specific receptors, the transducer film will be mechanically stimulated and will produce a signal. In this way, cancer biomarkers can be detected [103,104,105,106].
PEG-covered PAMAM dendrimers have been used for subsequent conjugation with antibodies, folic acid, or biotin molecules, serving for specific capture of circulating tumor cells [103,107,108]. Additionally, the surface of the dendrimer can be covered with DNA/RNA or biotinylated antibodies to detect cancer antigens [103,109,110].
Thus, dendrimers can be used in many ways for the diagnosis of cancer, with the possibility of combining multiple utilities of these macromolecules for a single investigation, such as dendrimers used both in targeting the tumor and improving the images obtained by MRI.
In addition to the uses mentioned so far in cancer diagnosis, dendrimers can also be used for immunodiagnosis. Immunodiagnosis is based on the generation of signals that can be easily visualized when there is an antigen–antibody interaction between certain target molecules. The use of antibodies in immunodiagnostics allows the attachment of a single group that emits a fluorescent signal when the antigen–antibody complex has been formed, but, using dendrimers, a large number of signal molecules can be attached (for example, fluorescein) and the fluorescence signals are greatly improved; thus, immunodiagnosis is influenced by the density of molecules capable of emitting light signals [111].
A study analyzed the fluorescent signals emitted by antibody–fluorescein conjugates compared to the fluorescent signals emitted by antibody–dendrimer–fluorescein complexes and it was proven that the intensity and clarity of the fluorescence signals was significantly stronger when dendrimers were used [112].
The aim of another study was to synthesize a complex immunodiagnostic device based on dendrimers; PAMAM dendrimers with a ferrocene core were used, i.e., dendrimers that were coated with cysteamine-modified gold electrorides. The molecules obtained had the function of providing the analytical redox signal generated by the ferrocene fragments and immobilizing the prostate-specific antibody (PSA) with the help of the primary amino group on the surface of the dendrimer. The best results were obtained using first-generation dendrimers, with these complexes detecting PSA from 10 pg/mL to 100 ng/mL [113].
As mentioned previously, one of the greatest advances in the field of cancer is the use of molecules that can precisely target the tumor microenvironment, and dendrimers, in addition to their use in diagnosis, can also be used in the targeting and treatment of tumors.
4. Targeting and Treatment
One advantage of dendrimers is their ability to be synthesized with specific characteristics depending on the intended application. This is especially important for obtaining dendrimers that can be distributed to the sites of tumors and can transport anticancer drugs for treatment. Dendrimers can target anticancer drugs through encapsulation, covalent bonding, or electrostatic interactions, and drugs can be stored either inside the dendrimers or on their surface functional groups [114,115,116].
In the case of cancer, the targeted delivery of dendrimers is crucial to reduce side effects on healthy tissues such as internal organs and bone marrow, which can occur when chemotherapy drugs are administered freely [56,88]. Dendrimers can be conjugated with both drugs and targeting molecules, such as monoclonal antibodies, folic acid, or various peptides, to achieve more specific targeting [56,103,117,118,119]. Additionally, poli (ether hydroxylamine) dendrimers have been used to improve the water solubility of poorly soluble anticancer drugs [76,103,120].
Dendrimers can be distributed to specific targets via passive or active pathways [14,56,88,121,122,123]. The passive pathway is based on the accumulation of PEGylated dendrimers in tumor tissues due to the permeability and retention effects of tumors [124]. Tumors have irregular vascularization formed through tumor angiogenesis, and lymphatic drainage is inefficient, which leads to the retention of dendrimer macromolecules in the tumor microenvironment [14,27,56,88,125,126,127]. Active targeting is achieved by conjugating dendrimers loaded with drugs with different specific targeting molecules, which mediate interactions with specific cell receptors [14,56,88,123,126].
Dendrimers are used to transport a variety of pharmaceutical substances through encapsulation [128]. PAMAM dendrimers in which cisplatin, an anticancer drug, was encapsulated showed slower release of the drug compared to its free administration. Additionally, it was observed that they accumulated in solid tumors and produced lower toxicity [70,129,130]. The slower release of encapsulated substances was also observed in the case of PAMAM dendrimers with silver [70,131,132].
A study that examined the encapsulation behaviour of adriamycin and methotrexate in third- and fourth-generation dendrimers conjugated with monomethyl ether poly(ethylene glycol) chains showed that the most efficient encapsulation (6.5 molecules of adriamycin and 26 molecules of methotrexate per dendrimer) occurred in G4-PAMAM dendrimers with PEG2000 chains. It was also observed that the drug was released slowly when the medium had low ionic strength but rapidly in an isotonic medium [70,133].
The conjugation of nanoparticles with polyethylene glycol (PEG) is important to prolong their circulation time and prevent their destruction by the host’s immune system [134,135,136,137]. When the anticancer drug 5-fluorouracil was encapsulated in PEGylated dendrimers, namely G4-PAMAM conjugated to carboxymethyl PEG5000, it was released more slowly and had lower hemolytic toxicity compared to encapsulation in dendrimers without PEG on their surfaces [84].
The conjugation of dendrimers with folic acid is a good targeting method for tumors, as numerous tumor cells overexpress folic acid receptors [138]. The rapid divisions that occur in cancer cells require increased amounts of folic acid, which is a source of carbon necessary for DNA synthesis. Therefore, as many types of cancer overexpress folic acid receptors, the folic acid molecules on the surfaces of dendrimers can bind to these receptors and be internalized into the cell [56,139,140]. G5-PAMAM dendrimers conjugated with folic acid, whose free amine groups were covered with glycidol, which subsequently reacted with methotrexate, resulted in the much slower release of the drug compared to the release produced by the free administration of the drug. These dendrimers showed high specificity for human epithelial carcinoma cells [88,141,142].
Dendrimers that encapsulate certain peptides can be used as cancer treatment vaccines. The administration of dendrimers conjugated with Epitope Pan DR-binding (PADRE) peptides and ovalbumin (OVA) plasmids as a vaccine inhibited tumor growth. Double vaccination with this complex in C57BL mice resulted in the inhibition of tumor growth in 100% of cases, while animals that received plasmids encoding OVA by electroporation only showed a slowdown in tumor growth in 60% of cases [103,143,144].
The use of monoclonal antibodies in dendrimer synthesis has the role of specifically targeting tumor cells that express certain antigens [145,146]. For example, G5-PAMAM dendrimers on the surfaces of which a specific antibody to PSMA antigens—which are overexpressed in prostate cancer—was inserted specifically bound to PSMA-positive cells, and the conjugate was internalized into these cells [88,147].
Another category of molecules that can be conjugated with dendrimers to target tumor cells is aptamers. Aptamers are single-stranded oligonucleotides known for their increased ability to bind to target cells. They are known for being resistant to a wide range of temperatures and to different pH values, and they are extremely stable and non-immunogenic and have a low production cost, which is why they are preferred instead of antibodies. Although they are not considered by the body as a foreign structure, aptamers cannot undergo endonuclease degradation, which is why the conjugation of aptamers with dendrimers is an effective therapeutic method [148,149].
We previously predicted that dendrimers are structures that cause toxicity, and coating them with aptamers reduces dendrimer toxicity. Practically speaking, the aptamer–dendrimer complex is an advantage for each of these two separate structures [148].
Taghdisi et al. analyzed the therapeutic action of dendrimers based on aptamers with a double targeting strategy. The dendrimers were conjugated with MUC1 and AS1411 aptamers, but also encapsulated epirubicin, an anticancer drug. The MUC1 aptamer selectively binds to transmembrane glycosylated mucin-1 (glycoprotein), which is overexpressed in many tumors, while the single-chain AS1411 aptamer specifically binds to the nuclear membrane protein nucleolin, which is overexpressed on the plasma membranes of tumor cells. The obtained dendrimer–aptamer conjugate delivered epirubicin to MCF-7 breast cancer cells and C26 colon carcinoma cells. Using flow cytometry, it was shown that the dendrimer–aptamer complex was specifically internalized into tumor cells (MCF-7 and C26) by receptor-mediated endocytosis using the MUC1 aptamer and by using the non-standard mechanism of the AS1411 aptamer, leading to the accumulation of the complex in the nucleus. No internalization was observed in Chinese Hamster Ovary (CHO) cells, for which the aptamers had no specificity. In addition, the internalization of the complex was better using both aptamers than in the case of using each aptamer separately. Furthermore, the antitumor action was better when the dendrimer–aptamer complex was administered than when epirubicin was administered alone [150,151].
As previously mentioned, in the case of oncological pathologies, targeted administration of anticancer molecules is extremely important [13,152]. In addition to precisely targeting anticancer substances, their slow release, and their long-term retention in tumors, dendrimers can be involved in cancer treatment through other mechanisms, such as the use of polyphenol dendrimers with antioxidant action. These can be targeted towards tumors to reduce oxidative stress, which is involved in the apoptotic pathway [103,153,154].
Another way in which dendrimers can be used in cancer treatment is based on neutron capture [155]. Some dendrimers (G6-G8 PAMAM, G5 PPI) have been conjugated with DTPA or DOTA chelating agents, as well as with neutron capture elements based on gadolinium, and it has been observed that the administration of these complexes to laboratory animals carrying tumors resulted in efficient anticancer activity [103,156,157]. Neutron capture agents interact with thermal neutrons and cause nucleus destruction and DNA strand breaks [158]. These agents have small sizes and can easily diffuse from tissues, but their use through conjugation with dendrimers results in the accumulation of larger amounts of Gd in tumors, which leads to increased anticancer effects [103,159,160].
The use of dendrimers in the treatment of cancer can also consider the direction to tumors of some molecules that absorb light radiation and whose action is toxic for tumor cells. Such use of dendrimers applies in the case of photothermal or photodynamic therapy.
5. Photothermal Therapy
It is known that a high body temperature is dangerous because it causes protein denaturation and cellular damage [161]. This phenomenon also applies to cancer cells, as many studies have shown that photothermal therapy based on hyperthermia can be used in anticancer therapies [77,162,163,164]. The tumor microenvironment is characterized by low oxygen pressure and a low pH due to inefficient circulation at this level. Maintaining high temperatures between 41 and 48 °C in these areas is cytotoxic to cells and will cause cell death [163,165,166]. Due to inefficient circulation, anticancer agents reach the tumor in small amounts, and, in this case, hyperthermia can be used concomitantly with chemotherapy or radiation therapy to achieve better treatment results [77,162,163,167,168].
Photothermal therapy involves the destruction of cancer cells by increasing the temperature of the tumor tissue following exposure to infrared light. In these cancer treatment methods, nano systems play an important role as they can be used to absorb infrared rays, thus increasing the efficiency of heat production in tumors [14,169,170,171,172].
Nano systems can be released into the tumor environment either by direct injection or by targeted delivery, and the molecules that reach the tumor environment will be used as photo-absorbents that will produce thermal energy when illuminated with near-infrared light [33,77,173,174].
Photothermal therapies are based on the use of a laser, whose power, exposure time, and wavelength may vary depending on the properties of the tumor tissue on which it will act [33,175,176]. In the case of this anticancer therapy, dendrimers can be used as devices to absorb light radiation [103,177]. Li et al. used PEG-PAMAM dendrimers, which they hybridized for the absorption of infrared rays with a gold nanorod (GNR). Single irradiation as well as administration of the dendrimers mentioned above without irradiation did not cause damage to HeLA cells derived from human cervical cancer. However, when these dendrimers with a GNR core were subjected to irradiation, the generated heat caused an increase in temperature in the irradiated tumor tissue with an NIR laser and a decrease in tumor volume [178]. Due to the frequent use of dendrimers to transport medicinal substances, they can be used for combined therapy to deliver both chemotherapeutic and photothermal therapy molecules to tumor cells [178].
Another complex study, conducted by Grześkowiak et al., showed the effects of G3-PAMAM dendrimers conjugated with polydopamine (PDA) molecules. Polydopamine has high efficiency in the process of transforming near-infrared light into thermal energy [179]. The study results showed that coating PDA spheres with PAMAM dendrimers reduced the viability of cancer cells compared to the administration of pure PDA spheres. The WST-1 test and the staining of live/dead cells showed that cancer cells still had a high survival rate following the administration of G3-PAMAM-PDA, but their viability was significantly reduced after laser irradiation. After 48 h, complete cell death was observed in irradiated cancer cells containing dendrimers in a concentration of at least 10 µg/mL [180]. The main mechanism used in photothermal therapy is presented in Figure 2.
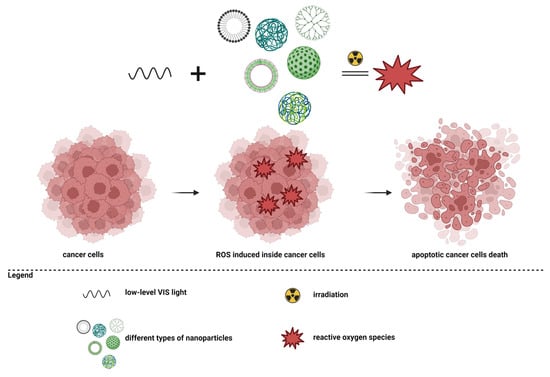
Figure 2.
Photothermal therapy in cancer.
6. Photodynamic Therapy
Photodynamic therapy (PDT) is a therapeutic strategy based on the production of reactive oxygen species, which subsequently cause cell death [181]. For this mechanism, a photosensitizer, oxygen molecules, and light are necessary. Essentially, a photosensitizer must reach the level of the tumor tissue, and then the area is stimulated with an appropriate wavelength, and the photosensitizer will form singlet oxygen species. Intracellularly, reactive oxygen species will accumulate, and increased oxidative stress will determine cell death through apoptosis or necrosis [33,56,181,182,183,184,185,186]. The principle of photodynamic therapy is presented in Figure 3.
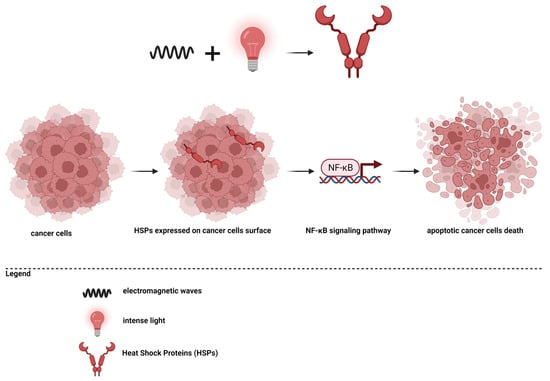
Figure 3.
Photodynamic therapy in cancer.
PDT can be used to destroy tumor cells, for tumor neovascularization, or to increase the inflammatory response and attract immune cells to the tumor environment [56,187,188,189]. Since it is difficult to obtain deep light penetration, this therapy is greatly improved by using targeted delivery systems. Due to retention processes, the molecular weight, and hydrophilicity, dendrimers used to deliver photosensitizers have yielded promising results for photodynamic therapy [56,190,191].
One photosensitizer agent is phthalocyanine. PAMAM dendrimers that have encapsulated phthalocyanine in the central core have produced cell death to a high percentage following irradiation with halogen light for 10 min. The administration of PAMAM dendrimers with phthalocyanine without irradiation did not affect the viability of cancer cells [103,192].
Photodynamic therapy is non-invasive, does not produce lesions or scars at the site of application, and can be used multiple times, but it is not effective for deeply developed cancers that have metastasized in multiple areas of the body [33,103,181,193].
Kojima et al. compared PEG-PAMAM dendrimers to PEG-PPI dendrimers, both loaded with protoporphyrin IX (PpIX). Their results showed that PEG-PPI dendrimers produced greater toxicity after irradiation compared to PEG-PAMAM dendrimers. The PEG-PPI-PpIX complex was more stable and generated the release of singlet oxygen, and PpIX molecules reached the mitochondria, generating increased phototoxicity [194].
Another means of using dendrimers in anticancer therapies is represented by their conjugation with molecules of genetic material, an upcoming aspect that will be presented.
7. Gene Transfection
Dendrimers can be used as delivery vectors for genetic material (Figure 4). They can transfer DNA or RNA molecules for cancer treatment [195,196]. PAMAM dendrimers with amino end groups can interact with the phosphate groups in nucleic acid molecules, forming complexes that will be directed to tumors and allow the transfer of genetic material through endocytosis into cancer cells and then into their nuclei [51,56,101,197,198,199].
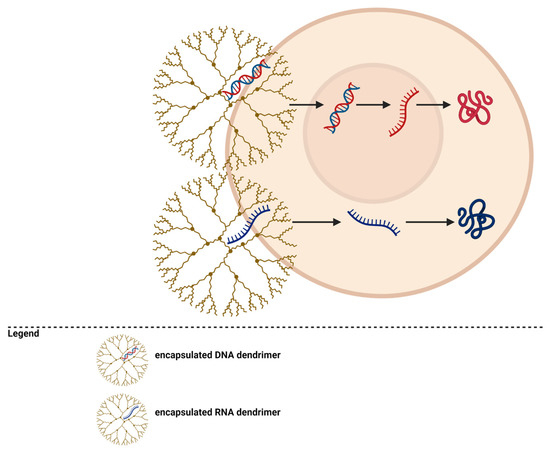
Figure 4.
Dendrimers for gene transfection.
A transfection system based on dendrimers is commercially available under the name SuperFectTM. These dendrimers are stable and can transport a larger amount of genetic material than viral vectors, and the release into the nucleus has been shown to be more efficient than when liposomes are used [51,200,201].
It seems that for gene transfection, dendrimers with an excess of amino groups compared to the phosphate groups of the genetic material are more efficient [56,202].
A therapeutic strategy to stop the progression of malignant tumors can target angiogenesis [203,204,205,206,207]. For this therapeutic strategy, dendrimers associated with anionic oligomers were used to release angiotensin and genes that determine the production of tissue inhibitor of metalloproteinase—TIMP-2. Gene transfer to breast cancer tissue significantly reduced the proliferation of endothelial cells [204,208,209].
PAMAM G1-G4 Tomalia-type dendrimers with a di-n-dodecylamine molecule as the core were synthesized. They formed complexes with DNA, and dendrimers from G2-G4 were able to cross cell membranes and deliver DNA efficiently [56,210,211].
In addition to PAMAM dendrimers, PPI dendrimers have also been used in gene transfection. G1-G5-PPI dendrimers conjugated with PEG molecules and containing cationic ammonium interior groups were used to transfect ss-DNAzyme oligomers into ovarian carcinoma cells. The use of this complex resulted in efficient and stable gene delivery [103,212].
As noted earlier, dendrimers are suitable for combining different therapeutic strategies. When dendrimers are synthesized to contain different genetic material fragments, their interiors can also include pharmaceutical substances such as doxorubicin. In this way, dendrimers will target tumors and allow for both chemotherapy administration and the delivery of genetic material to suppress tumor function [1,103,207,213].
The mechanism of gene therapy using dendrimers is presented in Figure 5.
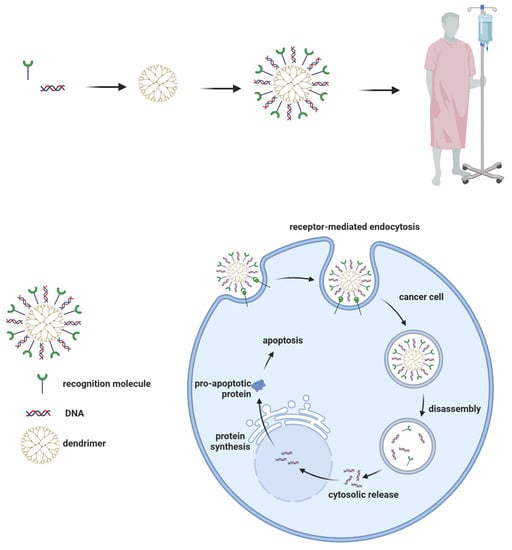
Figure 5.
Dendrimers’ mechanism for gene therapy.
8. Conclusions
Dendrimers are nanoscale drug delivery systems for anticancer drugs that can target the tumor site. These biocompatible nano systems have properties that can be used for diagnostic purposes, transdermal drug delivery, and medication conveyance in cancer.
The majority of current anticancer drugs do not differentiate cancerous cells from normal cells, and they lead to systemic toxicity and side effects. Dendrimers can be successfully used for gene therapy or for delivering the antineoplastic agent in cancerous cells, by active targeting, without causing any toxicity. Dendrimers can be directed specifically toward cancer cells (e.g., by antibodies specific for tumor-associated antigens) and the ingestion into the cell can be receptor-mediated. Therefore, they will ensure selective intratumoral accumulation and reduced systemic toxicity.
Including dyes or other materials (genetic materials, targeting agents) within the dendrimer structure (by encapsulation, complexation, or conjugation) can make dendrimers useful as diagnostic tools or for tumor localization and therapy monitoring.
Dendrimers have the potential to be used as theragnostic particles, with both diagnostic and therapeutic functions at the same time (drug delivery and therapy monitoring for optimum drug dosage and tumor growth).
9. Future Perspectives
Dendrimers’ approach of DNA-based nanomaterial delivery in cancer therapy could significantly improve cancer diagnosis and therapy.
Dendrimers can represent part of a multimodal nanoparticle that could ensure the precise delivery of antitumor drugs and could double the efficiency of diagnosis and therapy.
Manipulating the architecture of the dendrimers, their properties (efficiency of delivery and biocompatibility) can be increased to a more efficient level, ensuring the enhancement of the bioavailability for problematic drugs.
Continuous research in nano-oncology can lead dendrimers to become the newest class of curative anticancer therapeutic agents.
Author Contributions
Conceptualization, A.C. and A.G.D.; methodology, R.C.; software, A.-M.C.; validation, A.S.Ș., A.C.M. and A.C.; formal analysis, A.S.Ș.; investigation, C.-B.C.; resources, A.G.D.; data curation, A.C.; writing—original draft preparation, A.C.M.; writing—review and editing, R.C.; visualization, A.S.Ș.; supervision, A.G.D.; project administration, A.S.Ș.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was granted by project PDI-PFE-CDI 2021, entitled Increasing the Performance of Scientific Research, Supporting Excellence in Medical Research and Innovation, PROGRES, no. 40PFE/30.12.2021 of Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules 2022, 27, 3237. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Md, S.; Kesharwani, P. RGD Engineered Dendrimer Nanotherapeutic as an Emerging Targeted Approach in Cancer Therapy. J. Controll. Release 2021, 340, 221–242. [Google Scholar] [CrossRef] [PubMed]
- What Is Cancer? NIH 2021. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 28 April 2023).
- Cancer. World Health Organization 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 28 April 2023).
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034370. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M. Why We Do What We Do. A Brief Analysis of Cancer Therapies. EXCLI J. 2020, 19, 1401. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, V. Chemotherapy: Managing Side Effects and Safe Handling. Can. Vet. J. 2009, 50, 665. [Google Scholar]
- Turvey, B.E.; Crowder, S. Anabolic Steroid Abuse in Public Safety Personnel: A Forensic Manual; Academic Press: Cambridge, MA, USA, 2015; ISBN 0-12-802877-7. [Google Scholar]
- Sawyers, C. Targeted Cancer Therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef]
- Wilkes, G.M. Targeted Therapy: Attacking Cancer with Molecular and Immunological Targeted Agents. Asia-Pac. J. Oncol. Nurs. 2018, 5, 137–155. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Samasca, G.; Florian, I.A.; Lupan, I.; Craciun, A.M. The Nanosystems Involved in Treating Lung Cancer. Life 2021, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy-a Mini-Review. Int. J. Med. Sci. 2020, 17, 2964. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467. [Google Scholar]
- Mosleh-Shirazi, S.; Abbasi, M.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Kumar, B.V.; Patra, C.N.; Panda, J.R.; Mohanta, B.C.; Palei, N.N. Nanotechnology: A Novel Approach for Drug Development in Health Care System. Curr. Nanomater. 2020, 5, 12–25. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and Perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gautam, A.; van Veggel, F.C. Synthesis of Nanoparticles, Their Biocompatibility, and Toxicity Behavior for Biomedical Applications. J. Mater. Chem. B 2013, 1, 5186–5200. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Demetzos, C.; Pippa, N. Advanced Drug Delivery Nanosystems (ADDnSs): A Mini-Review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery. Biomed. Rep. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Dobrovolskaia, M.; Farahani, K.; Goodwin, A.; Joshi, A.; Lee, H.; Meade, T.; Pomper, M.; Ptak, K.; Rao, J. Nanoparticles for Cancer Imaging: The Good, the Bad, and the Promise. Nano Today 2013, 8, 454–460. [Google Scholar] [CrossRef]
- Ross, J.S. Targeted Therapy for Cancer: Integrating Diagnostics and Therapeutics. Am. J. Cancer 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Patras, L.; Banciu, M. Intercellular Crosstalk via Extracellular Vesicles in Tumor Milieu as Emerging Therapies for Cancer Progression. Curr. Pharm. Des. 2019, 25, 1980–2006. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Vader, P.; van Dommelen, S.M.; van Solinge, W.W.; Schiffelers, R.M. Exosome Mimetics: A Novel Class of Drug Delivery Systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering Nano-Drug Biointerface to Overcome Biological Barriers toward Precision Drug Delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Mishra, S.; Banode, K.; Belgamwar, V. Nanotechnology: A Tool for Targeted Drug Delivery. In Nanotechnology Applied to Pharmaceutical Technology; Springer International Publishing: Cham, Switzerland, 2017; pp. 116–123. [Google Scholar]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Abdelkader, H.; Alani, A.W.; Alany, R.G. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Self-Assembly, Fabrication, Characterization, Drug Delivery Applications and Limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Dutu, A.G.; Sovrea, A.; Constantin, A.-M.; Samasca, G.; Masalar, A.L.; Ifju, B.; Linga, E.; Neamti, L.; Tranca, R.A. Nanocarriers for Drug Delivery: An Overview with Emphasis on Vitamin D and K Transportation. Nanomaterials 2022, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.M.; Lee, J.H. Electrical Conductivity and Compressive Strength of Cement Paste with Multiwalled Carbon Nanotubes and Graphene Nanoplatelets. Appl. Sci. 2022, 12, 1160. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 748. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Al-Tikriti, Y.; Hansson, P. Drug-Induced Phase Separation in Polyelectrolyte Microgels. Gels 2021, 8, 4. [Google Scholar] [CrossRef]
- Hanafy, N.A.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Khatak, S.; Sara, U.S. Solid Lipid Nanoparticles-a Review. Int. J. Appl. Pharm 2013, 5, 8–18. [Google Scholar]
- Tomalia, D.A.; Fréchet, J.M. Discovery of Dendrimers and Dendritic Polymers: A Brief Historical Perspective. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and Applications. Acta Biochim. Pol. 2001, 48, 199–208. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly (Amidoamine), Polypropylenimine, and Related Dendrimers and Dendrons Possessing Different 1→ 2 Branching Motifs: An Overview of the Divergent Procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Munavalli, B.B.; Naik, S.R.; Torvi, A.I.; Kariduraganavar, M.Y. Dendrimers. In Functional Polymers; Springer: Berlin/Heidelberg, Germany, 2019; pp. 289–345. [Google Scholar]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, V. Dendrimers: A Class of Polymer in the Nanotechnology for Drug Delivery. In Nanomedicine for Drug Delivery and Therapeutics; Scrivener Publishing: Beverly, MA, USA, 2013; pp. 373–405. [Google Scholar]
- Boas, U.; Heegaard, P.M. Dendrimers in Drug Research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Khalil, M.; Gurbuz, M.; Simone, T.M.; Mousa, S.A. Nanoparticles and Cancer Therapy: A Concise Review with Emphasis on Dendrimers. Int. J. Nanomed. 2009, 4, 1–7. [Google Scholar]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, M.; Rahman, M. Dendrimers: A New Race of Pharmaceutical Nanocarriers. BioMed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst Pamam Dendrimers: Synthetic Approaches, Surface Modifications, and Biomedical Applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Gillani, S.S.; Munawar, M.A.; Khan, K.M.; Chaudhary, J.A. Synthesis, Characterization and Applications of Poly-Aliphatic Amine Dendrimers and Dendrons. J. Iran. Chem. Soc. 2020, 17, 2717–2736. [Google Scholar] [CrossRef]
- Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef]
- Fischer, M.; Vögtle, F. Dendrimers: From Design to Application—A Progress Report. Angew. Chem. Int. Ed. 1999, 38, 884–905. [Google Scholar] [CrossRef]
- Caminati, G.; Turro, N.J.; Tomalia, D.A. Photophysical Investigation of Starburst Dendrimers and Their Interactions with Anionic and Cationic Surfactants. J. Am. Chem. Soc. 1990, 112, 8515–8522. [Google Scholar] [CrossRef]
- Sato, K.; Anzai, J. Dendrimers in Layer-by-Layer Assemblies: Synthesis and Applications. Molecules 2013, 18, 8440–8460. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Huang, A.Y.-T.; Kao, C.-L.; Selvaraj, A.; Peng, L. Solid-Phase Dendrimer Synthesis: A Promising Approach to Transform Dendrimer Construction. Mater. Today Chem. 2023, 27, 101285. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Karimi, M.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahrami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged Platforms: Modification, Drug Delivery and Nanotoxicity. Opening Synthetic Cages to Release the Tiger. Nanoscale 2017, 9, 1356–1392. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications—Reflections on the Field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Shende, P.; Govardhane, S. Strategies-Based Intrathecal Targeted Drug Delivery System for Effective Therapy, Modeling, and Controlled Release Action. In Modeling and Control of Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 213–225. [Google Scholar]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular Modeling to Study Dendrimers for Biomedical Applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef] [PubMed]
- Ardabevskaia, S.; Milenin, S. From Dendrimers to Megamers: The State-of-the-Art. INEOS Open 2021, 4, 176–188. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139. [Google Scholar]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Glazer, E.S.; Curley, S.A. Non-Invasive Radiofrequency Ablation of Malignancies Mediated by Quantum Dots, Gold Nanoparticles and Carbon Nanotubes. Ther. Deliv. 2011, 2, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Curto, S.; Taj-Eldin, M.; Fairchild, D.; Prakash, P. Microwave Ablation at 915 MHz vs 2.45 GHz: A Theoretical and Experimental Investigation. Med. Phys. 2015, 42, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven Challenges for Nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Sefah, K.; Bamrungsap, S.; Chang, H.-T.; Tan, W. Selective Photothermal Therapy for Mixed Cancer Cells Using Aptamer-Conjugated Nanorods. Langmuir 2008, 24, 11860–11865. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N. Dendrimer Toxicity: Let’s Meet the Challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Hamidi, A.; Sharifi, S.; Davara, S.; Ghasemi, S.; Omidi, Y.; Rashidi, M.-R. Novel Aldehyde-Terminated Dendrimers; Synthesis and Cytotoxicity Assay. Bioimpacts 2012, 2, 97–103. [Google Scholar]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N. A PEGylated Dendritic Nanoparticulate Carrier of Fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Asthana, A.; Chauhan, A.S.; Diwan, P.V.; Jain, N.K. Poly (Amidoamine)(PAMAM) Dendritic Nanostructures for Controlled Sitespecific Delivery of Acidic Anti-Inflammatory Active Ingredient. Aaps Pharmscitech 2005, 6, E536–E542. [Google Scholar] [CrossRef]
- Agashe, H.B.; Dutta, T.; Garg, M.; Jain, N. Investigations on the Toxicological Profile of Functionalized Fifth-generation Poly (Propylene Imine) Dendrimer. J. Pharm. Pharmacol. 2006, 58, 1491–1498. [Google Scholar] [CrossRef]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary Biological Evaluation of Polyamidoamine (PAMAM) StarburstTM Dendrimers. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Grinstaff, M.W. Therapeutic and Diagnostic Applications of Dendrimers for Cancer Treatment. Adv. Drug Deliv. Rev. 2008, 60, 1037–1055. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and Cancer: A Review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef]
- Hussain, T.; Nguyen, Q.T. Molecular Imaging for Cancer Diagnosis and Surgery. Adv. Drug Deliv. Rev. 2014, 66, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. New Technologies for Human Cancer Imaging. J. Clin. Oncol. 2008, 26, 4012. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Aghayev, A.; Steigner, M. Vascular CT and MRI: A Practical Guide to Imaging Protocols. Insights Imaging 2018, 9, 215–236. [Google Scholar] [CrossRef]
- Carnevale, L.; Lembo, G. Innovative MRI Techniques in Neuroimaging Approaches for Cerebrovascular Diseases and Vascular Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 2656. [Google Scholar] [CrossRef]
- Longmire, M.R.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Dendrimers as High Relaxivity MR Contrast Agents. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2014, 6, 155–162. [Google Scholar] [CrossRef]
- Sampathkumar, S.; Yarema, K.J. Dendrimers in Cancer Treatment and Diagnosis. Nanotechnol. Life Sci. Online 2007, 7, 1–43. [Google Scholar]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef]
- Bourne, M.W.; Margerun, L.; Hylton, N.; Campion, B.; Lai, J.; Derugin, N.; Higgins, C.B. Evaluation of the Effects of Intravascular MR Contrast Media (Gadolinium Dendrimer) on 3D Time of Flight Magnetic Resonance Angiography of the Body. J. Magn. Reson. Imaging 1996, 6, 305–310. [Google Scholar] [CrossRef]
- Kobayashi, H.; Reijnders, K.; English, S.; Yordanov, A.T.; Milenic, D.E.; Sowers, A.L.; Citrin, D.; Krishna, M.C.; Waldmann, T.A.; Mitchell, J.B. Application of a Macromolecular Contrast Agent for Detection of Alterations of Tumor Vessel Permeability Induced by Radiation. Clin. Cancer Res. 2004, 10, 7712–7720. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Majeti, B.K.; Thallapally, P.K.; Forrest, M.L. Advances in Lymphatic Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Brechbiel, M.W. Dendrimer-Based Macromolecular MRI Contrast Agents: Characteristics and Application. Mol. Imaging 2003, 2, 15353500200303100. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent Advances in Dendrimer-Based Nanovectors for Tumor-Targeted Drug and Gene Delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef]
- Cai, X.; Hu, J.; Xiao, J.; Cheng, Y. Dendrimer and Cancer: A Patent Review (2006–Present). Expert Opin. Ther. Pat. 2013, 23, 515–529. [Google Scholar] [CrossRef]
- Palena, P.D.; Barto, R.R.; Borders, T.L.; Stuart, J.A. Nano-Getter Device. U.S. Patent No. 8,236,243, 2012. [Google Scholar]
- Lohcharoenkal, W.; Abbas, Z.; Rojanasakul, Y. Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors 2021, 11, 364. [Google Scholar] [CrossRef]
- Jain, K.K.; Jain, K.K. Nanomolecular Diagnostics. In The Handbook of Nanomedicine; Spinger: Berlin/Heidelberg, Germany, 2017; pp. 133–200. [Google Scholar]
- Hong, S.; Eddington, D.; Myung, J.H.; Launiere, C. Methods and Devices for Capturing Circulating Tumor Cells. U.S. Patent Application No. 13/265,916, 2012. [Google Scholar]
- Zhao, Y.; Liu, S.; Li, Y.; Jiang, W.; Chang, Y.; Pan, S.; Fang, X.; Wang, Y.A.; Wang, J. Synthesis and Grafting of Folate–PEG–PAMAM Conjugates onto Quantum Dots for Selective Targeting of Folate-Receptor-Positive Tumor Cells. J. Colloid Interface Sci. 2010, 350, 44–50. [Google Scholar] [CrossRef]
- Wei, F.; Liao, W. Probe Immobilization and Signal Amplification for Polymer-Based Biosensor. U.S. Patent No. 9,127,304, 8 September 2015. [Google Scholar]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Ong, K.; Jenkins, A.; Cheng, R.; Tomalia, D.; Durst, H.; Jensen, J.; Emanuel, P.; Swim, C.; Yin, R. Dendrimer Enhanced Immunosensors for Biological Detection. Anal. Chim. Acta 2001, 444, 143–148. [Google Scholar] [CrossRef]
- Sánchez, A.; Villalonga, A.; Martínez-García, G.; Parrado, C.; Villalonga, R. Dendrimers as Soft Nanomaterials for Electrochemical Immunosensors. Nanomaterials 2019, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Çevik, E.; Bahar, Ö.; Şenel, M.; Abasıyanık, M.F. Construction of Novel Electrochemical Immunosensor for Detection of Prostate Specific Antigen Using Ferrocene-PAMAM Dendrimers. Biosens. Bioelectron. 2016, 86, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N. Dendrimers in Anticancer Drug Delivery: Mechanism of Interaction of Drug and Dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Majhi, S.R.; Behera, D.J.; Kar, R.K. Dendrimers: A Potential Carrier for Cancer Therapy. High Technol. Lett. 2022, 28, 11. [Google Scholar]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Baker, J.R., Jr.; Shukla, R.; Thomas, T.P. Dendrimer Based Compositions and Methods of Using the Same. Patent No.:US-20090088376-A1/2009-04-02, 2009. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?CC=US&NR=2009088376&KC=&FT=E&locale=en_EP (accessed on 28 April 2023).
- Amjad, M.W. Dendrimers in Anticancer Targeted Drug Delivery: Accomplishments, Challenges and Directions for Future. Pharm. Pharmacol. 2021, 9, 4–16. [Google Scholar] [CrossRef]
- Svenson, S.; Chauhan, A.S. Peham Dendrimers as Excipients. Patent No.:WO-2008030591-A2/2008-03-13, 2009. Available online: https://patents.google.com/patent/WO2008030591A2/en?oq=WO-2008030591-A2+%2f+2008-03-13 (accessed on 28 April 2023).
- Rizvi, S.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Vieira Gonzaga, R.; da Silva Santos, S.; Da Silva, J.V.; Campos Prieto, D.; Feliciano Savino, D.; Giarolla, J.; Igne Ferreira, E. Targeting Groups Employed in Selective Dendrons and Dendrimers. Pharmaceutics 2018, 10, 219. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-Cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M. Site-Specific Drug Delivery, Targeting, and Gene Therapy. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 473–505. [Google Scholar]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as Drug Carriers: Applications in Different Routes of Drug Administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-Platinate: A Novel Approach to Cancer Chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Hoang Thi, T.T. Modified Carboxyl-Terminated PAMAM Dendrimers as Great Cytocompatible Nano-Based Drug Delivery System. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef]
- Balogh, L.; Swanson, D.R.; Tomalia, D.A.; Hagnauer, G.L.; McManus, A.T. Dendrimer− Silver Complexes and Nanocomposites as Antimicrobial Agents. Nano Lett. 2001, 1, 18–21. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer-Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of Polyamidoamine Dendrimers Having Poly (Ethylene Glycol) Grafts and Their Ability to Encapsulate Anticancer Drugs. Bioconjugate Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular Vesicle-Based Therapeutics: Natural versus Engineered Targeting and Trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-PEG Immunity: Emergence, Characteristics, and Unaddressed Questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2015, 7, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Gao, Y.; Tang, Y.; He, R.-R.; Liu, T.-L.; He, Y.; Sun, S.; Li, B.-Y.; Li, Y.-B.; Liu, G. PEG-Conjugated PAMAM Dendrimers Mediate Efficient Intramuscular Gene Expression. AAPS J. 2009, 11, 395–405. [Google Scholar] [CrossRef] [PubMed]
- WIENER, E.C.; KONDA, S.; SHADRON, A.; BRECHBIEL, M.; Gansow, O. Targeting Dendrimer-Chelates to Tumors and Tumor Cells Expressing the High-Affinity Folate Receptor. Investig. Radiol. 1997, 32, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Baker, J.R., Jr. Targeting Cancer Cells with DNA-Assembled Dendrimers: A Mix-and-Match Strategy for Cancer. Cell Cycle 2005, 4, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the Folate Receptor for Active Targeting of Cancer Nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R., Jr. Targeted Drug Delivery with Dendrimers: Comparison of the Release Kinetics of Covalently Conjugated Drug and Non-Covalent Drug Inclusion Complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef]
- Majoros, I.J.; Williams, C.R.; Becker, A.; Baker Jr, J.R. Methotrexate Delivery via Folate Targeted Dendrimer-based Nanotherapeutic Platform. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2009, 1, 502–510. [Google Scholar] [CrossRef]
- Daftarian, P.; Kaifer, A.E.; Li, W.; Blomberg, B.B.; Frasca, D.; Roth, F.; Chowdhury, R.; Berg, E.A.; Fishman, J.B.; Al Sayegh, H.A. Peptide-Conjugated PAMAM Dendrimer as a Universal DNA Vaccine Platform to Target Antigen-Presenting CellsPPD as a Universal Platform for Genetic Vaccine. Cancer Res. 2011, 71, 7452–7462. [Google Scholar] [CrossRef]
- Graham-Gurysh, E.G.; Carpenter, B.W.; Beck, W.A.; Varma, D.M.; Vincent, B.G.; Bachelder, E.M.; Ainslie, K.M. Delivery Strategies for Cancer Vaccines and Immunoadjuvants. In Systemic Drug Delivery Strategies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 359–408. [Google Scholar]
- Yang, H. Targeted Nanosystems: Advances in Targeted Dendrimers for Cancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 309–316. [Google Scholar] [CrossRef]
- Quinteros, D.A.; Bermúdez, J.M.; Ravetti, S.; Cid, A.; Allemandi, D.A.; Palma, S.D. Therapeutic Use of Monoclonal Antibodies: General Aspects and Challenges for Drug Delivery. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 807–833. [Google Scholar]
- Patri, A.K.; Myc, A.; Beals, J.; Thomas, T.P.; Bander, N.H.; Baker, J.R. Synthesis and in Vitro Testing of J591 Antibody− Dendrimer Conjugates for Targeted Prostate Cancer Therapy. Bioconjugate Chem. 2004, 15, 1174–1181. [Google Scholar] [CrossRef]
- Sheikh, A.; Kesharwani, P. An Insight into Aptamer Engineered Dendrimer for Cancer Therapy. Eur. Polym. J. 2021, 159, 110746. [Google Scholar] [CrossRef]
- Sheikh, A.; Kaur, H.; Abourehab, M.A.; Alam, M.S.; Kesharwani, P. Aptamer-Functionalized Dendrimers for Targeted Cancer Therapy. Aptamers Eng. Nanocarriers Cancer Ther. 2023, 2023, 255–275. [Google Scholar]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Lavaee, P.; Jalalian, S.H.; Robati, R.Y.; Abnous, K. Double Targeting and Aptamer-Assisted Controlled Release Delivery of Epirubicin to Cancer Cells by Aptamers-Based Dendrimer in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2016, 102, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Shi, X.; Ceña, V.; Majoral, J.-P. Dendrimer–and Polymeric Nanoparticle–Aptamer Bioconjugates as Nonviral Delivery Systems: A New Approach in Medicine. Drug Discov. Today 2020, 25, 1065–1073. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Faijanur-Rob-Siddiquee, M.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention, and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Lee, C.Y. Dendritic Nano-Antioxidants. Patent No.: US 8,895,032 B2, 2014. Available online: https://patents.google.com/patent/US8895032B2/en (accessed on 28 April 2023).
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Barrett, T.; Ravizzini, G.; Choyke, P.L.; Kobayashi, H. Dendrimers Application Related to Bioimaging. IEEE Eng. Med. Biol. Mag. Q. Mag. Eng. Med. Biol. Soc. 2009, 28, 12. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P. Methods for Tumor Treatment Using Dendrimer Conjugates. U.S. Patent Application No. 11/371,780, 2006. [Google Scholar]
- Opina, A.C.; Wong, K.J.; Griffiths, G.L.; Turkbey, B.I.; Bernardo, M.; Nakajima, T.; Kobayashi, H.; Choyke, P.L.; Vasalatiy, O. Preparation and Long-Term Biodistribution Studies of a PAMAM Dendrimer G5–Gd-BnDOTA Conjugate for Lymphatic Imaging. Nanomedicine 2015, 10, 1423–1437. [Google Scholar] [CrossRef]
- Jamsranjav, E.; Ito, A.; Kato, Y.; Tatebe, Y.; Takase, N.; Yoshida, S. DNA Strand Breaks Induced by Fast and Thermal Neutrons from YAYOI Research Reactor in the Presence and Absence of Boric Acid. Radiat. Res. 2019, 191, 483–489. [Google Scholar] [CrossRef]
- Tjarks, W.; Tiwari, R.; Byun, Y.; Narayanasamy, S.; Barth, R.F. Carboranyl Thymidine Analogues for Neutron Capture Therapy. Chem. Commun. 2007, 47, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Yue, H.; Tegafaw, T.; Ahmad, M.Y.; Liu, S.; Nam, S.-W.; Chang, Y.; Lee, G.H. Gadolinium Neutron Capture Therapy (GdNCT) Agents from Molecular to Nano: Current Status and Perspectives. ACS Omega 2022, 7, 2533–2553. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R. Cellular Effects of Hyperthermia: Relevance to the Minimum Dose for Thermal Damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in Combined Treatment of Cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J. Heating the Patient: A Promising Approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cao, F.; Ma, W.; Tang, Y.; Aljahdali, B.; Alasir, M.; Dibart, S. Cancer Cells Resist Hyperthermia Due to Its Obstructed Activation of Caspase 3. Rep. Pract. Oncol. Radiother. 2020, 25, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Sapareto, S.A.; Dewey, W.C. Thermal Dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kumaravel, S.; Sharma, A.; Duran, C.L.; Bayless, K.J.; Chakraborty, S. Hypoxic Tumor Microenvironment: Implications for Cancer Therapy. Exp. Biol. Med. 2020, 245, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Horsman, M.R. Modulation of the Tumor Vasculature and Oxygenation to Improve Therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Mosca, S.; Gardner, B.; Palombo, F.; Matousek, P.; Stone, N. Nanoparticle-Mediated Photothermal Therapy Limitation in Clinical Applications Regarding Pain Management. Nanomaterials 2022, 12, 922. [Google Scholar] [CrossRef] [PubMed]
- Sagar, V.; Nair, M. Near-Infrared Biophotonics-Based Nanodrug Release Systems and Their Potential Application for Neuro-Disorders. Expert Opin. Drug Deliv. 2018, 15, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Aliannezhadi, M.; Minbashi, M.; Tuchin, V.V. Effect of Laser Intensity and Exposure Time on Photothermal Therapy with Nanoparticles Heated by a 793-Nm Diode Laser and Tissue Optical Clearing. Quantum Electron. 2018, 48, 559. [Google Scholar] [CrossRef]
- Sheng, W.; He, S.; Seare, W.J.; Almutairi, A. Review of the Progress toward Achieving Heat Confinement—The Holy Grail of Photothermal Therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar] [CrossRef]
- B Chithrani, D. Nanoparticles for Improved Therapeutics and Imaging in Cancer Therapy. Recent Pat. Nanotechnol. 2010, 4, 171–180. [Google Scholar] [CrossRef]
- Li, X.; Takeda, K.; Yuba, E.; Harada, A.; Kono, K. Preparation of PEG-Modified PAMAM Dendrimers Having a Gold Nanorod Core and Their Application to Photothermal Therapy. J. Mater. Chem. B 2014, 2, 4167–4176. [Google Scholar] [CrossRef]
- Mei, S.; Xu, X.; Priestley, R.D.; Lu, Y. Polydopamine-Based Nanoreactors: Synthesis and Applications in Bioscience and Energy Materials. Chem. Sci. 2020, 11, 12269–12281. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, B.F.; Maziukiewicz, D.; Kozłowska, A.; Kertmen, A.; Coy, E.; Mrówczyński, R. Polyamidoamine Dendrimers Decorated Multifunctional Polydopamine Nanoparticles for Targeted Chemo-and Photothermal Therapy of Liver Cancer Model. Int. J. Mol. Sci. 2021, 22, 738. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The Cancer Genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J. Cytotoxic Effects of Gold Nanoparticles: A Multiparametric Study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Wang, K.-P.; Mo, J.-G.; Xiong, L.; Wen, Y. Photodynamic Therapy Regulates Fate of Cancer Stem Cells through Reactive Oxygen Species. World J. Stem Cells 2020, 12, 562. [Google Scholar] [CrossRef]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The Potential of Photodynamic Therapy in Current Breast Cancer Treatment Methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Tan, L.; Shen, X.; He, Z.; Lu, Y. The Role of Photodynamic Therapy in Triggering Cell Death and Facilitating Antitumor Immunology. Front. Oncol. 2022, 12, 863107. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mroz, P.; Dai, T.; Huang, Y.; Denis, T.G.S.; Hamblin, M.R. Photodynamic Therapy for Cancer and for Infections: What Is the Difference? Isr. J. Chem. 2012, 52, 691–705. [Google Scholar] [CrossRef]
- Avci, P.; Erdem, S.S.; Hamblin, M.R. Photodynamic Therapy: One Step Ahead with Self-Assembled Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Nakagishi, Y.; Morimoto, Y.; Lai, P.-S.; Miyazaki, K.; Urano, K.; Horie, S.; Kumagai, M.; Fukushima, S.; Cheng, Y. Enhanced Photodynamic Cancer Treatment by Supramolecular Nanocarriers Charged with Dendrimer Phthalocyanine. J. Controll. Release 2009, 133, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-L.; Syu, W.-J.; Nishiyama, N.; Kataoka, K.; Lai, P.-S. Dendrimer Phthalocyanine-Encapsulated Polymeric Micelle-Mediated Photochemical Internalization Extends the Efficacy of Photodynamic Therapy and Overcomes Drug-Resistance in Vivo. J. Controll. Release 2011, 155, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of Poly (Ethylene Glycol)-Attached Dendrimers Encapsulating Photosensitizers for Application to Photodynamic Therapy. Bioconjugate Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.M.; Rus, L.-L.; Frum, A.; Morgovan, C.; Butuca, A.; Totan, M.; Juncan, A.M.; Gligor, F.G.; Arseniu, A.M. Dendrimers as Non-Viral Vectors in Gene-Directed Enzyme Prodrug Therapy. Molecules 2021, 26, 5976. [Google Scholar] [CrossRef]
- Kanvinde, S.; Kulkarni, T.; Deodhar, S.; Bhattacharya, D.; Dasgupta, A. Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer. BioTech 2022, 11, 6. [Google Scholar] [CrossRef]
- Shah, D.S.; Sakthivel, T.; Toth, I.; Florence, A.T.; Wilderspin, A.F. DNA Transfection and Transfected Cell Viability Using Amphipathic Asymmetric Dendrimers. Int. J. Pharm. 2000, 208, 41–48. [Google Scholar] [CrossRef]
- Richardson, S.C.; Pattrick, N.G.; Stella Man, Y.; Ferruti, P.; Duncan, R. Poly (Amidoamine) s as Potential Nonviral Vectors: Ability to Form Interpolyelectrolyte Complexes and to Mediate Transfection in Vitro. Biomacromolecules 2001, 2, 1023–1028. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V.P. Dendrimers for SiRNA Delivery. Pharmaceuticals 2013, 6, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Rehni, A.; Kalra, R.; Joshi, G.; Kumar, M. Dendrimers and Their Pharmaceutical Applications–a Review. Die Pharm. Int. J. Pharm. Sci. 2008, 63, 491–496. [Google Scholar]
- Tripathy, S.; Das, M.K. Dendrimers and Their Applications as Novel Drug Delivery Carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker Jr, J. Efficient Transfer of Genetic Material into Mammalian Cells Using Starburst Polyamidoamine Dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Shcharbin, D.; Klajnert, B.; Bryszewska, M. Dendrimers in Gene Transfection. Biochemistry 2009, 74, 1070–1079. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis Inhibitors in Cancer Therapy: Mechanistic Perspective on Classification and Treatment Rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef]
- Comunanza, V.; Bussolino, F. Therapy for Cancer: Strategy of Combining Anti-Angiogenic and Target Therapies. Front. Cell Dev. Biol. 2017, 5, 101. [Google Scholar] [CrossRef]
- Vincent, L.; Varet, J.; Pille, J.; Bompais, H.; Opolon, P.; Maksimenko, A.; Malvy, C.; Mirshahi, M.; Lu, H.; Vannier, J. Efficacy of Dendrimer-mediated Angiostatin and TIMP-2 Gene Delivery on Inhibition of Tumor Growth and Angiogenesis: In Vitro and in Vivo Studies. Int. J. Cancer 2003, 105, 419–429. [Google Scholar] [CrossRef]
- Arenas, I.A.; Xu, Y.; Lopez-Jaramillo, P.; Davidge, S.T. Angiotensin II-Induced MMP-2 Release from Endothelial Cells Is Mediated by TNF-α. Am. J. Physiol. Cell Physiol. 2004, 286, C779–C784. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Kihara, F.; Hirayama, F.; Uekama, K. Enhancement of Gene Expression by Polyamidoamine Dendrimer Conjugates with α-, β-, and γ-Cyclodextrins. Bioconjugate Chem. 2001, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mahato, R.I.; Sung, Y.K.; Kim, S.W. Development of Biomaterials for Gene Therapy. Mol. Ther. 2000, 2, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Tack, F.; Janssen, H.M.; Meijer, E.W.; Janicot, M.M.F.; Brewster, M.E. Modified Poly (Propylene-Imine) Dendrimers and Their Use as Transfection Agents for Anionic Bioactive Factors. U.S. Pat. Appl. 2010, 11, 576. [Google Scholar]
- Luo, D.; Li, Y. Dendrimer-like Modular Delivery Vector. U.S. Pat. Appl. 2012, 13, 95. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).