3.1. Radiolysis of Protein Solutions
To better understand the mechanism of protein aggregation induced by
•OH or
radicals, we conducted pulse radiolysis experiments and spectroscopic measurements for solutions of peptide and proteins, including papain, ovalbumin, insulin, calf thymus histone, β-lactoglobulin or lysozyme. The HSA oxidation process related to electron transfer has been investigated by pulse radiolysis and photochemical methods and has recently been described by us [
4]. The aim of our study was an attempt to answer the question of whether it would be possible to observe the formation of DT both by pulse radiolysis and spectroscopic techniques. In living organisms, there are two major reactive oxygen species (ROS), superoxide and hydroxyl radicals that are being continuously formed in a process of the reduction in oxygen to water. The consequence of an excess of ROS in organisms, especially
•OH radicals, is many diseases, such as atherosclerosis, cancer and neurological disorders. The hydroxyl radical is a very reactive species. For this reason, the reactions of
•OH radicals with various compounds are not selective. The primary mechanisms of the reactions of an
•OH radical with organic compounds involve OH addition and H abstraction and, to lesser extent, electron transfer reactions (preferentially on the surface of albumin molecules). The azide radical is one of the most important one-electron oxidants commonly used in radiation chemistry studies involving molecules of biological significance. In contrast to the
•OH radical,
radical appears to react primarily via one-electron transfer and it is much more selective in its reaction than is the hydroxyl radical. However, there are several reports indicating the possibility of azide radical addition to organic compounds containing double bonds in aqueous solutions [
9,
10]. One of the methods of protein cross-linking is the formation of dityrosine bridges, which are formed as a result of the recombination of long-lived tyrosyl radicals (TyrO
•) generated under oxidative conditions. Our own study [
4] and data presented in the literature [
11,
12] indicate that in aqueous solutions of tyrosine, TyrO
• radicals are formed as one of the products of reaction with hydroxyl radicals. As a result of the reaction of
•OH or
radicals with HSA molecules, stable protein aggregates of low and high molecular weight are formed. Covalent bonds are detected between biomolecules, and at low radiation doses, mainly dimers are observed. In the case of mild oxidants as azide radicals, HSA aggregates are characterized by classic DT fluorescence. Our measurements clearly revealed that
penetrates to the HSA interior [
4]. However, in the reaction of albumin with
•OH radicals, essentially, we did not observe DT. The high reactivity of
•OH radicals with protein amino acids results in a greater probability of reaction with non-aromatic protein residues, and thus a lower efficiency of dityrosine bridge formation. For this reason, in our experiments, we also used the azide radical to study the process of one-electron oxidation of proteins.
Many reactions important from a medical point of view of proteins involve charge transfer, including those initiated by ionizing radiation. Kinetic experiments have conclusively shown that electron transfer from a tyrosine residue (donor of electron) to the tryptophanyl radical (acceptor of electron) can take place over large distances through protein interiors. The tryptophan radical (reduction potential about 1V at pH = 7) within protein shell oxidizes tyrosine residue to the phenoxyl radical (TyrO
•). Both radicals absorb light in different spectral regions, which is an advantage in radiolysis measurements to track electron transfer. The long-range intramolecular electron transfer (LRET) process is much more complicated than was previously thought. For example, the LRET in the hen egg-white lysozyme accompanying radical transformation Trp
• → TyrO
• was observed [
13,
14,
15]. However, some tryptophan and tyrosine residues are not involved in the long-range intramolecular electron transfer process, or LRET does not always take place in the protein structure. In the case of lysozyme and β-lactoglobulin, our pulse radiolysis results provide helpful information with regard to electron transfer in protein solutions. In both cases, electron transfer accompanying radical transformation Trp
• → TyrO
• was observed. This process is easy to observe for relatively low molecular weight proteins (~20 kDa). When
radicals react with proteins containing Trp and Tyr residue, one-electron oxidation of the TrpH residue is followed by an efficient, rapid intramolecular process, which could be regarded as hydrogen atom transfer from TyrOH to Trp
•. In the case of a reaction of azide radicals with lysozyme (
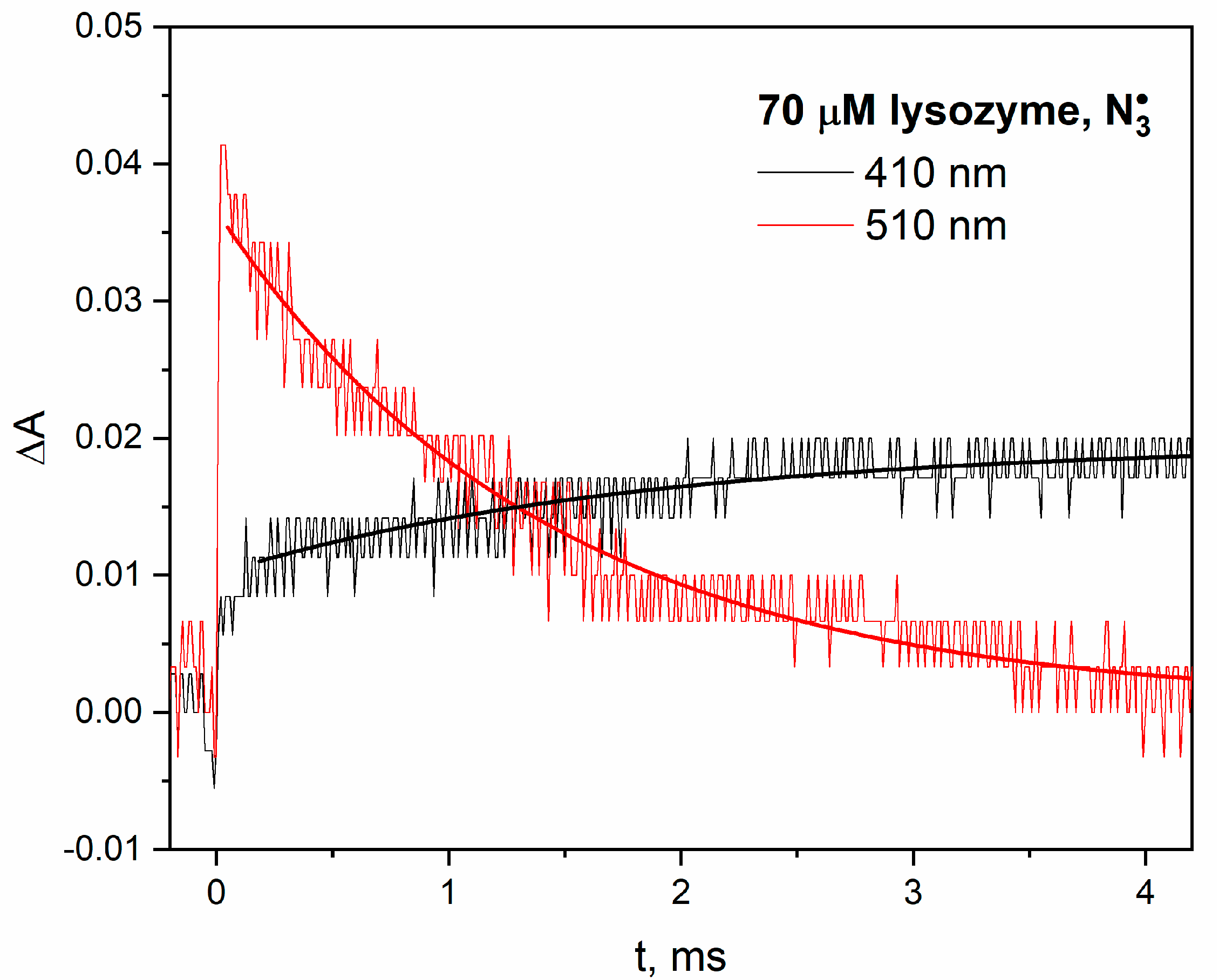
Figure 1) or β-lactoglobulin (see
Figure S3 in
Supplementary Materials), kinetic analysis indicates that the decay of Trp
• is correlated with the formation of TyrO
• (the trace at 410 nm evolves with the same kinetics as Trp
• decays). The decay of Trp
• and formation of TyrO
• within β-lactoglobulin structure takes place on a time scale of microseconds. For β-lactoglobulin, which has been studied in detail, the first-order transformation Trp
• → TyrO
• is independent of protein concentration, showing that the charge transfer is intramolecular rather than intermolecular. Our measurements are in agreement with the literature [
16,
17,
18,
19] (Figure 1A,C of Ref. [
17]).
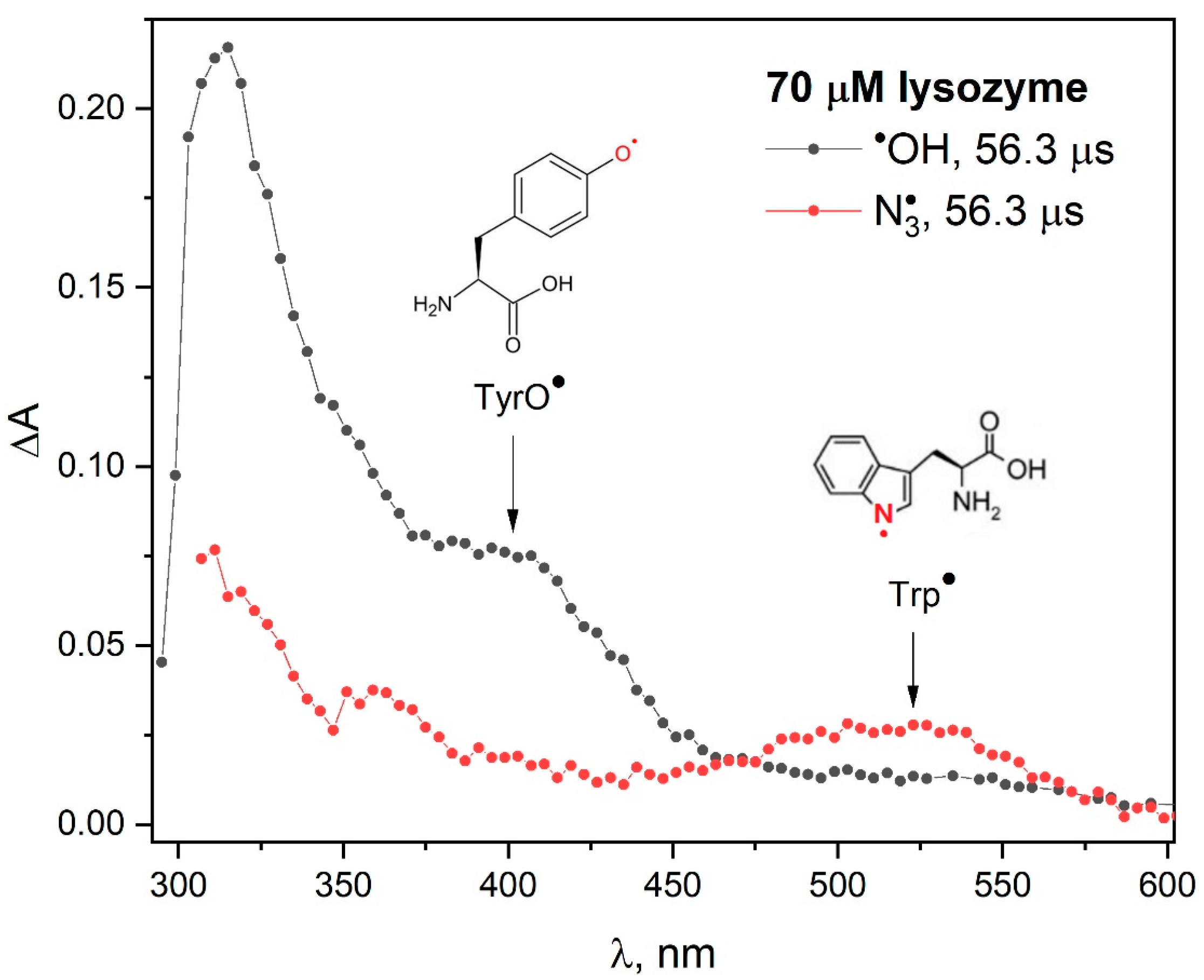
Our pulse radiolysis experiments confirmed that the reaction mechanism of azide radicals with lysozyme is significantly different from the processes associated with the attack of hydroxyl radicals on this protein. The attack of
•OH radicals on lysozyme mainly induces the formation of tyrosyl radicals (
Figure 2). When lysozyme reacts with
radical, the absorption spectrum shows a distinct band in the spectral range of 450–600 nm. The analysis of pulse radiolysis measurements clearly indicates that the main reaction product of the
radical with lysozyme are the Trp
• radicals. However, the concentration of TyrO
• radicals in lysozyme (absorption band with a maximum at 410 nm) was lower compared to other pulsed irradiated proteins [
13,
20,
21]. Electron transfer over long distances (though not as effective as in the case of lysozyme) was also observed in irradiated HSA solutions [
22]. The authors estimated that in the case of HSA, the yield of charge transfer Trp
• → TyrO
• is about 20%. The disappearance of the Trp
• radical concentration (determined in radiolysis measurements while taking into account the molar Trp
• coefficient: 1750 dm
3 mol
−1 cm
−1 at 520 nm) and the increase in the TyrO
• radical concentration (molar absorbance coefficient 2700 dm
3 mol
−1 cm
−1 at 410 nm) in the case of HSA are not identical. The publication [
22] showed, by measuring the maximum concentration of the TyrO
• radical (buildup absorbance at 410 nm), that the efficiency of electron transfer over long distances is only 20%. Analysis of the kinetics of Trp
• radical decay shows that almost 75% of these radicals have disappeared over the time scale of TyrO
• formation. The disappearance of the TyrO
• radical within seconds has been linked to the formation of DT. The presence of one tryptophan group in the HSA structure makes this reaction channel less important in our case.
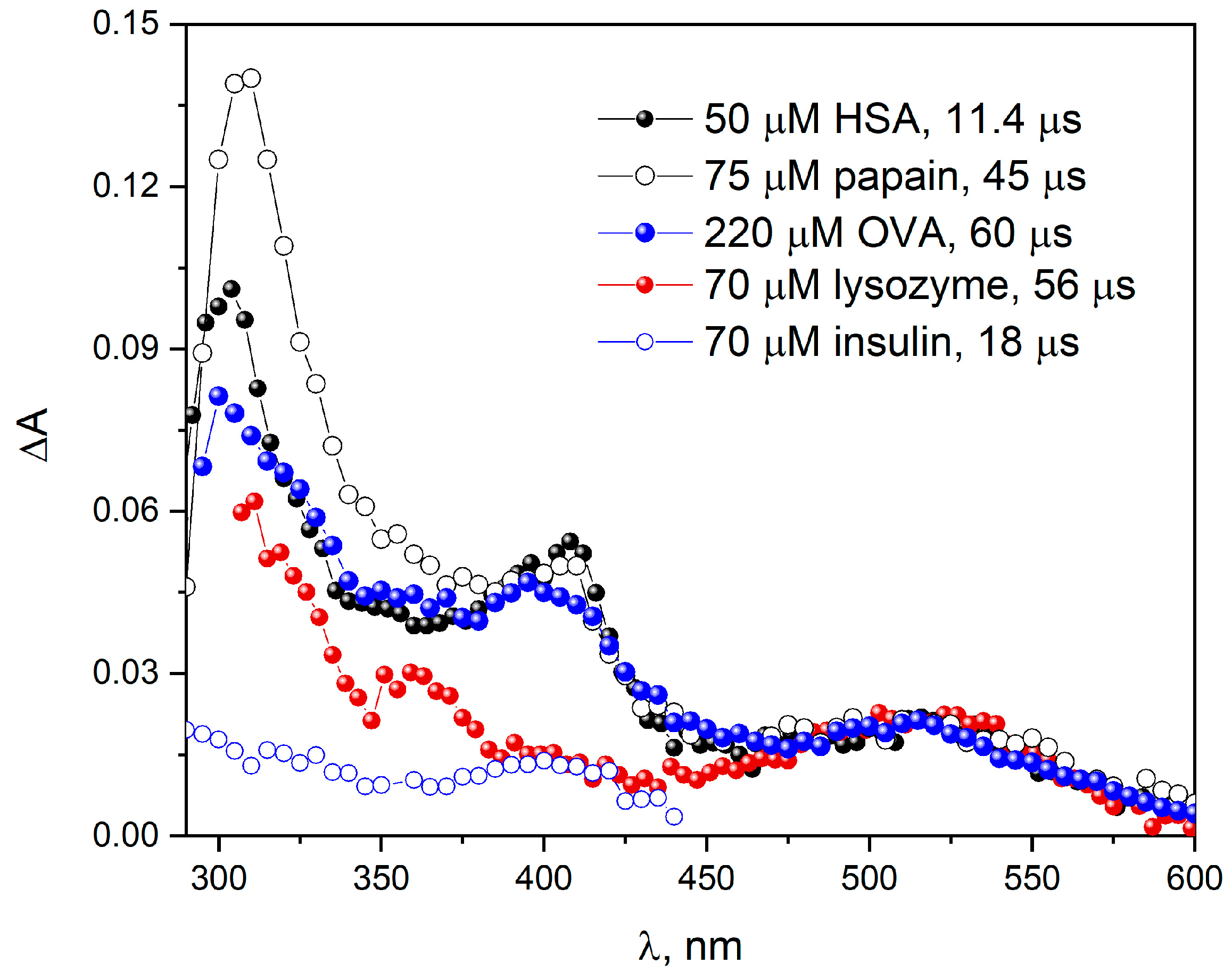
When papain or OVA reacts with
radicals (
Figure 3), similar transient absorption spectra were obtained as in the case of the reaction of the azide radical with HSA [
4]. Pulse radiolysis measurements of the N
2O-saturated buffer solutions (pH 7.2; 0.1 M NaN
3) of all studied proteins and the enzyme were performed (in the separated experiments). The reaction of
with HSA, OVA and papain [
23] leads to the formation of the Tyr meta isomer (band centered at ~305 nm), phenoxyl radicals TyrO
• (band with a maximum near 410 nm) and Tyr
• radicals (band with a maximum at 520 nm). In the spectral range 300–400 nm, the light Trp
• and cation radical TrpH
•+ are also absorbed. In contrast to the abovementioned proteins, the main process of the reaction of
radicals with insulin is the formation of TyrO
• (band with a maximum at 410 nm). Significant differences were observed in the 300–400 nm region, where most of the aromatic radicals absorb light [
10]. Both Trp
• and its proton adduct also absorb in this region. For this reason, changes in spectral range 300–400 nm may result from different degrees of protonation of aromatic radicals. The pH effects on the transient absorption spectra of Trp in the presence of azide radicals suggest that several transients contribute to the observed absorption spectra in this region [
10].
Recently, we have shown that in the reaction of azide radicals with BSA, the intensity of the absorption band originating from Trp residues is twice as intense as that recorded for the HSA solution. This indicates that both tryptophan residues in BSA are accessible to azide radicals in a similar way. After normalizing the spectra obtained in the initial stage of oxidation of the examined objects (
Figure 3), three main bands (with maxima 305, 410 and 520 nm) are observed, but the spectra are not identical. The correlation of the shape of the spectrum of the primary oxidized samples with the ratio of the number of tryptophan and tyrosine residues is clearly observed (see
Table S1 in
Supplementary Materials). The intensity of the band (spectrum normalized to the radical band of the tryptophan residue) associated with the Tyr
• radical is the lowest for lysozyme (smallest tyrosine residue/tryptophan residue ratio: 0.5 (3/6)). For papain and OVA, the pulse radiolysis transient spectra are similar and the Tyr/Trp ratio values are similar: 3.8 for papain (19/5) and 3.33 (10/3) for OVA.
The literature data show that ovalbumin agglomerates are linked by covalent bonds [
24]. Pulse radiolysis measurements indicate that OVA aggregation is not a cross-linking process. There is no recombination of OVA radicals formed as a result of the reaction with hydroxyl radicals.
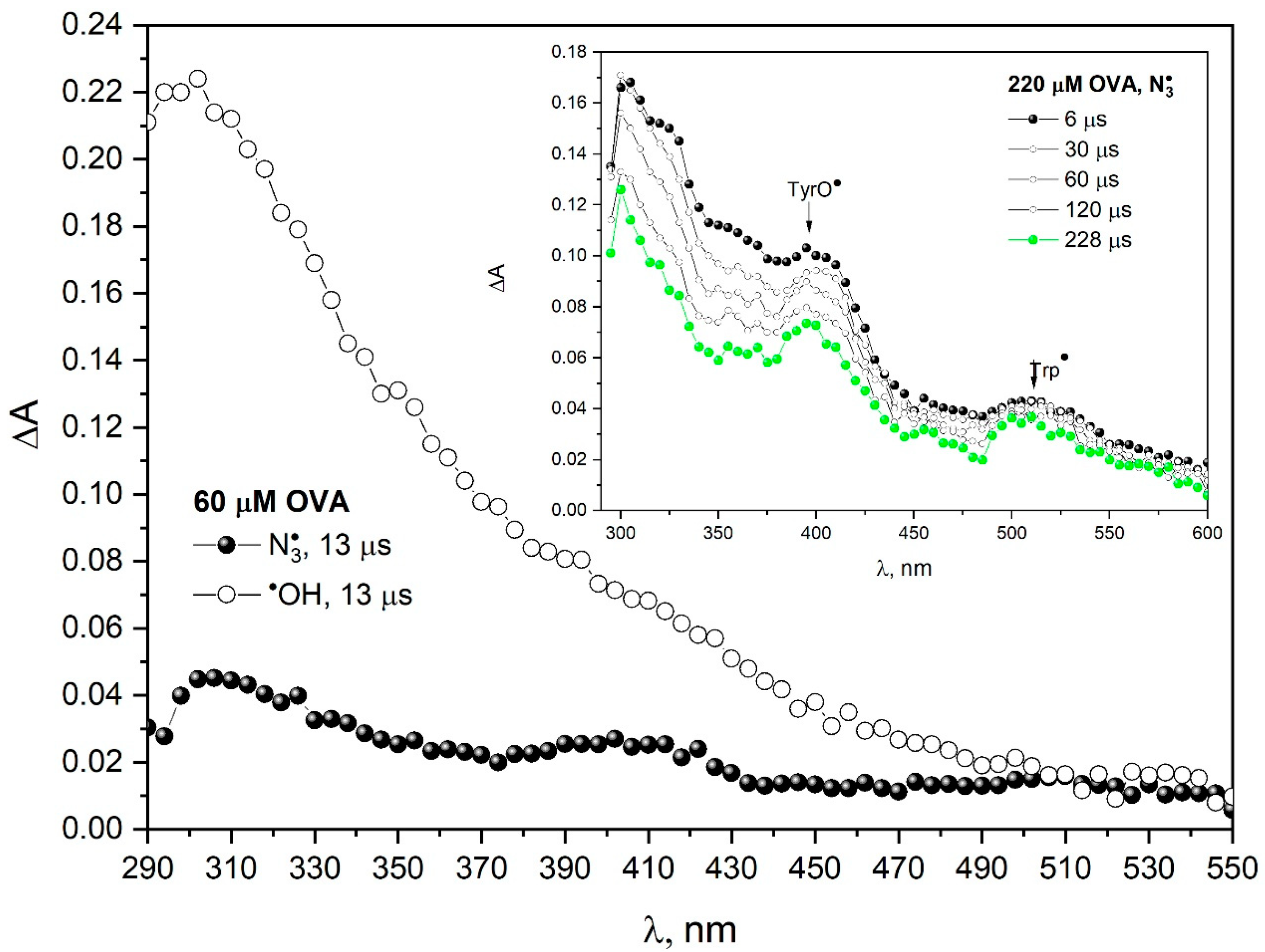
Figure 4 presents the transient absorption spectra of primary products of OVA one-electron oxidation by azide radicals (N
2O-saturated 60 µM OVA solution containing 0.1 M NaN
3). These spectra are compared with the spectra obtained after oxidation of OVA by
•OH radicals (N
2O-saturated 60 µM OVA solution). The transient absorption spectrum of the oxidation products of OVA by
•OH radicals can be considered structureless except for the maximum at 300 nm, shoulder at 350 and 410 nm and weak absorbance at 530 nm [
24]. The absorption spectra of the protein after the reaction with
•OH radicals were difficult to analyze due to the lack of selectivity of the hydroxyl radical, which resulted in the formation of many different radicals. The hydroxyl radical can react with all the amino acids in proteins (preferentially on the protein surface), but the azide radical reacts mostly with Tyr and Trp. For this reason, the OVA spectrum after reaction with hydroxyl radicals is less resolved than for the azide radical. The insert in
Figure 4 shows transient absorption spectra recorded at various times after the electron pulse irradiation (200 Gy) of N
2O-saturated aqueous solution containing 220 µM OVA and 0.1 M NaN
3.
In the case of ovalbumin, we do not observe the Trp• → TyrO• transformation (we did not observe a build-up of absorption at 410 nm). The decay of absorbance at 410 and 510 nm within OVA structure takes place on a time scale of milliseconds in contrast to HSA, where this process takes place on a time scale of seconds. In the reaction of papain with azide radicals, charge transfer between tryptophan and tyrosine was also not observed.
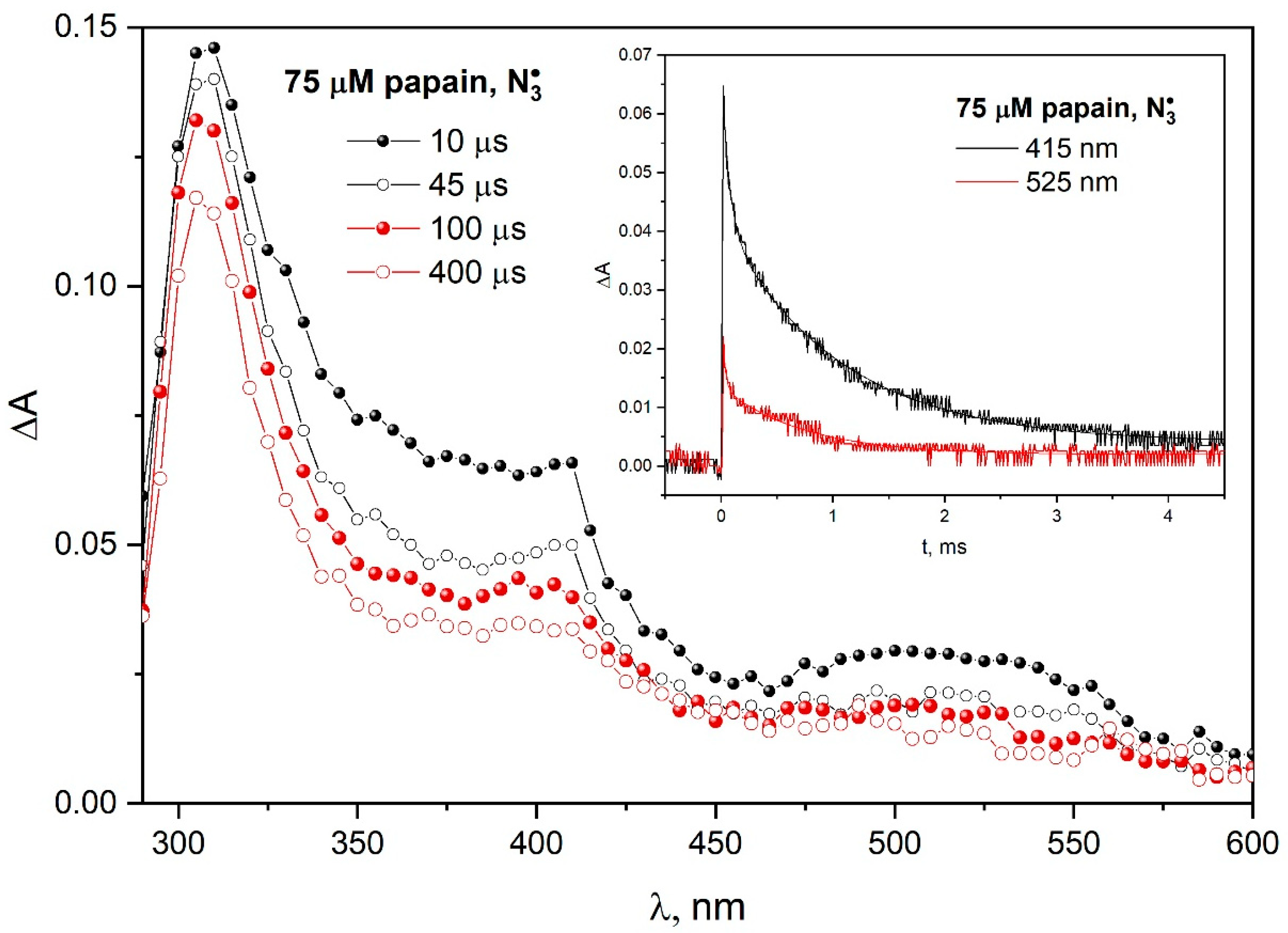
Figure 5 shows transient absorption spectra recorded at various times after the electron pulse irradiation of N
2O-saturated aqueous solution 75 µM papain and 0.1 M NaN
3. The insert in
Figure 5 shows the kinetics pattern of transient absorption decay recorded for 415 and 525 nm for both Trp
• and TyrO
• radical decays according to complex kinetics. The absence of an increase in absorbance due to the oxidation of TyrOH to TyrO
• occurring simultaneously with the reduction in Trp
• to Trp may indicate a lack of electron transfer from the tyrosine residue to the tryptophanyl radical in the papain structure. Such intramolecular electron transfer is effective, for example, in β-lactoglobulins (see
Figure S3 in
Supplementary Materials), but in the case of albumins, it is ineffective and slow, taking place in the seconds. In some cases, the charge transfer is essentially stoichiometric or does not take place in the protein structure. The complex kinetics of the decay of both bands associated with TyrO
• and Trp
• radicals and the large measurement noise do not allow an attempt to extract information about a possible partial long-distance transfer from the decay of the 410 nm band in our case. For this reason, we do not exclude a transfer Trp
• → TyrO
• in the case of papain.
Histone is a protein with a relatively low molecular weight (less than 23 kDa) that is characterized by a high content of basic amino acids, mainly lysine and arginine. There are only two cysteine residues within a histone structure; no cystine and tryptophan residues are in the polypeptide chain. It is known from the literature that the rate constant of the reaction of histones with
•OH radicals change significantly with the ionic strength and pH of the solution, in contrast to BSA, where no influence of the ionic strength on the reaction with the hydroxyl radical was found [
25]. An acidic environment inhibits the process of histone aggregation, and high pH values favor this process. We conducted pulse radiolysis experiments for His in a buffer solution of pH 7.2, because below pH 6, the aggregated form of His is unstable.
Figure S4 (see in the
Supplementary Materials) shows transient absorption spectra recorded at various times after 17 ns electron pulse irradiation (dose 55 Gy) of N
2O-saturated aqueous solutions containing 1.4 mg/mL histone and 0.1 M NaN
3. The selected absorption spectra indicate that the reactions of the used oxidizing species (
•OH or
) with the histone lead to the formation of a limited number of products. In the case of azide radicals, new absorption in the visible region with a maximum near 400 nm is due to the phenoxyl radical of tyrosine, TyrO
•. Our study confirms that hydroxyl radicals are not effective (essentially, there is no signal of TyrO
•, see
Figure S4 in
Supplementary Materials) in generating TyrO
• radicals and, consequently, DT formation in histone solutions, which is consistent with previous studies by other researchers. The transient absorption spectrum of N
2O-saturated buffer solutions containing histone is in agreement with the absorption spectrum of histone solution after irradiation described in the literature [
25,
26]. Histones do not contain tryptophan residues in their structure and have a smaller number of aromatic amino acids compared to human or bovine albumin; therefore, the transient absorption spectrum recorded in pulse radiolysis is not intense, as it was for HSA or OVA.
We are aware that the accessibility of radicals to individual amino acids in the polypeptide chain of various proteins is important in the analysis of the albumin oxidation process. Pulse radiolysis measurements combined with enzyme activity tests allowed to determine which amino acid residues in biomolecules are modified as a result of reactions with free radicals [
27,
28].
3.2. Spectroscopic Analysis of Protein Aggregation Process Induced by Ionizing Radiation
In this paragraph, we focused on the photochemical characterization of protein dimers and aggregates generated by irradiation under oxidative conditions (
•OH or
radical). Our DLS measurements suggest that many proteins tend to dimerize prior to irradiation. The formation of protein dimers is accompanied by the appearance of a new “blue” emission in the spectrum. Fortunately, the luminescence of light by radiation-induced protein aggregates differs from the emission of self-aggregates (mainly dimers) [
4]. In most cases, as a result of the irradiation of protein solutions during oxidative stress, the formation of dimers/aggregates leads to an emission band with a maximum of about 400 nm. In the same spectral region, a band characteristic of dityrosine is observed. Steady-state pulse radiolysis measurements can be divided into two groups: (a) measurements for protein solutions with a concentration below 100 µM and (b) measurements for concentrated protein solutions (above 300 µM). The vast majority of measurements were made for solutions with a low albumin concentration (low albumin concentration allows minimizing emissions from high-molecular aggregates). The consequence of the formation of protein dimers in neat solutions is a difficult analysis of the size of aggregates generated by ionizing radiation. As it turned out, as a result of irradiation of protein solutions, in most cases, spontaneous (non-covalent) dimers are replaced with dimers stabilized by a cross-link. Moreover, the presence of dimers in neat protein solutions make it difficult to distinguish spontaneous aggregates from those generated by radiation which apply the DT identification procedure proposed by Davies et al. [
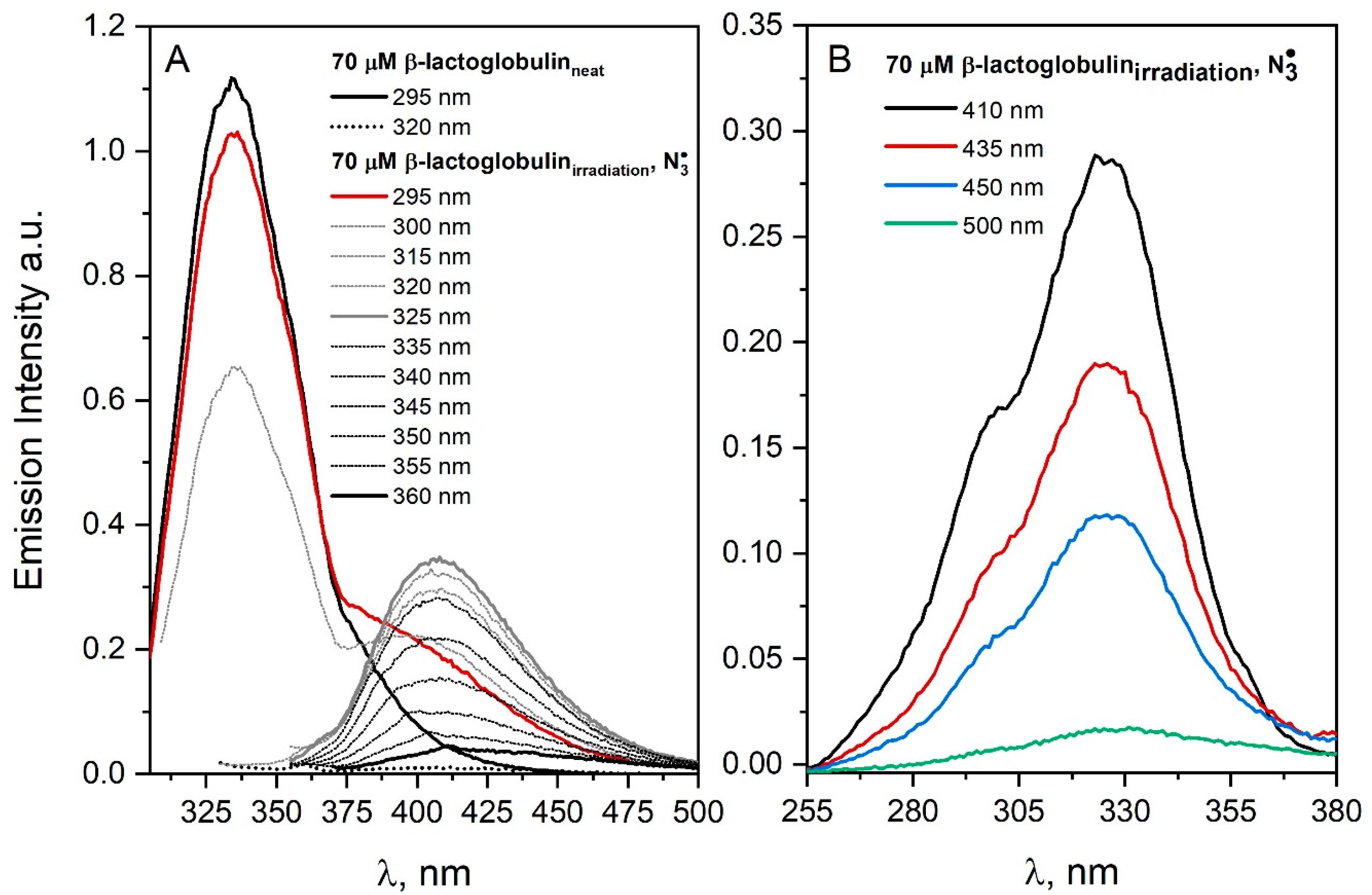
20]. This is due to the fact that the peak of the DT fluorescence band is in the same region in relation to the peak of the emission band of the spontaneous dimers or aggregates. The difficulties described above will be shown on the example of experiments carried out for lactoglobulin solutions. This protein molecule contains two tryptophan and 4 tyrosine residues. Excitation of N
2O-saturated and irradiated aqueous β-lactoglobulin (70 μM) solution containing NaN
3 (0.1 M) with the 320 nm light leads to the emission corresponding to the emission of DT centered at near 400 nm, as shown in
Figure 6A. This indicates the presence of dimers stabilized by a covalent bond between the tyrosine residues after irradiation. Excitation of irradiated β-lactoglobulin solution (
Figure 6A) with light above 320 nm does not lead to the appearance of new emission bands in the spectrum of protein. The band observed above 370 nm after excitation with a 295 nm light of irradiated N
2O-saturated β–lactoglobulin solution indicates energy transfer from *Trp214 to protein aggregates (FRET—Förster resonance energy transfer) (
Figure 6). This is evidenced by the detection of the emission of both
*Trp and protein aggregates after the excitation of the irradiated β-lactoglobulin solution with 295 nm light absorbed only by Trp residue (red line,
Figure 6A). In the case of irradiated histone solution (in the case of
•OH and
radicals) or lysozyme (
) under oxidative conditions, energy transfer was less efficient. Tyrosine molecule excited in neutral aqueous solutions shows the fluorescence of both the acidic and alkaline form of Tyr (shoulder around 295 nm as shown in
Figure 6B) [
4].
When β-lactoglobulin reacts with
•OH radicals, species other than DT are formed. Two populations of protein aggregates are observed after irradiation of N
2O-saturated β-lactoglobulin solution (70 µM, 5600 Gy). The strong scattering of light due to aggregate formation is particularly well-visible in the case of concentrated solutions of β-lactoglobulin after reaction with the
•OH radical (see
Figure S5 in
Supplementary Materials).
Analysis of the dynamic light scattering measurement (DLS) after irradiation of N
2O-saturated solution containing β-lactoglobulin (70 μM) confirmed the formation of protein aggregates. In the native solution, β-lactoglobulin forms dimers with mean particles of about 4 nm in size. [
29]. It is important to note that β-lactoglobulin exists as a stable dimer (36.7 kDa) at pH values near its isoelectric point (i.e., pH 5.2) at room temperature [
30,
31,
32]. The formation of dimers, trimers and higher oligomers was reported to be more extensive at pH 7.5. The presence of dimers in a neat solution before irradiation can significantly affect the process of protein aggregation. Moreover, β-lactoglobulin solution irradiated in a lower protein concentration (3 mg/mL) showed more aggregates than in higher concentration at the same radiation dose [
32,
33]. After irradiation of the N
2O-saturated β-lactoglobulin solution with dose 5600 Gy, two populations of protein aggregates are observed (size of generated nanoparticles was about 20 nm and 52 nm). The reaction of the azide radical with β-lactoglobulin leads to the formation of mainly protein dimers (the size of generated species was around 8 nm).
The important observation from DLS measurements was the presence of high molecular aggregates in the N2O-saturated and irradiated β-lactoglobulin solution. The •OH radical can react with all the amino acids in the protein structure (preferentially on the protein surface), but reacts mostly with Tyr and Trp. The high reactivity of hydroxyl radicals with protein amino acids results in a lower relative yield of oxidation of aromatic residues and a lower probability of DT formation. In the case of non-selective, highly reactive species such as hydroxyl radicals, there is a high probability that a TyrO• radical from one oxidized β-lactoglobulin molecule will react with a non-TyrO• radical formed on another modified albumin molecule. The radical leads to the formation of only radicals located on aromatic amino acid residues, mainly on or near the surface of proteins. Thus, the probability of the recombination of TyrO• radicals generated on the surfaces of two different protein molecules increases, which leads to the formation of intermolecular DT.
The time-resolved fluorescence measurements for β-lactoglobulin solution before and after irradiation revealed that there is an increase in the life-time of β-lactoglobulin aggregates generated by ionizing radiation. Decay at 410 nm is characterized by the fluorescence lifetime 3.55 ns before irradiation and the lifetime is about 3.76 ns after irradiation of β-lactoglobulin solution (see
Figure S6 in
Supplementary Materials). The determined emission lifetime values made it possible to distinguish self-aggregates of β-lactoglobulin from aggregates induced by ionizing radiation on the basis of time-resolved fluorescence measurements. In our opinion, the use of a more accurate measurement technique based on single photon-counting technique should help distinguish protein aggregates obtained by the oxidation of albumin molecules with azide or hydroxyl radicals.
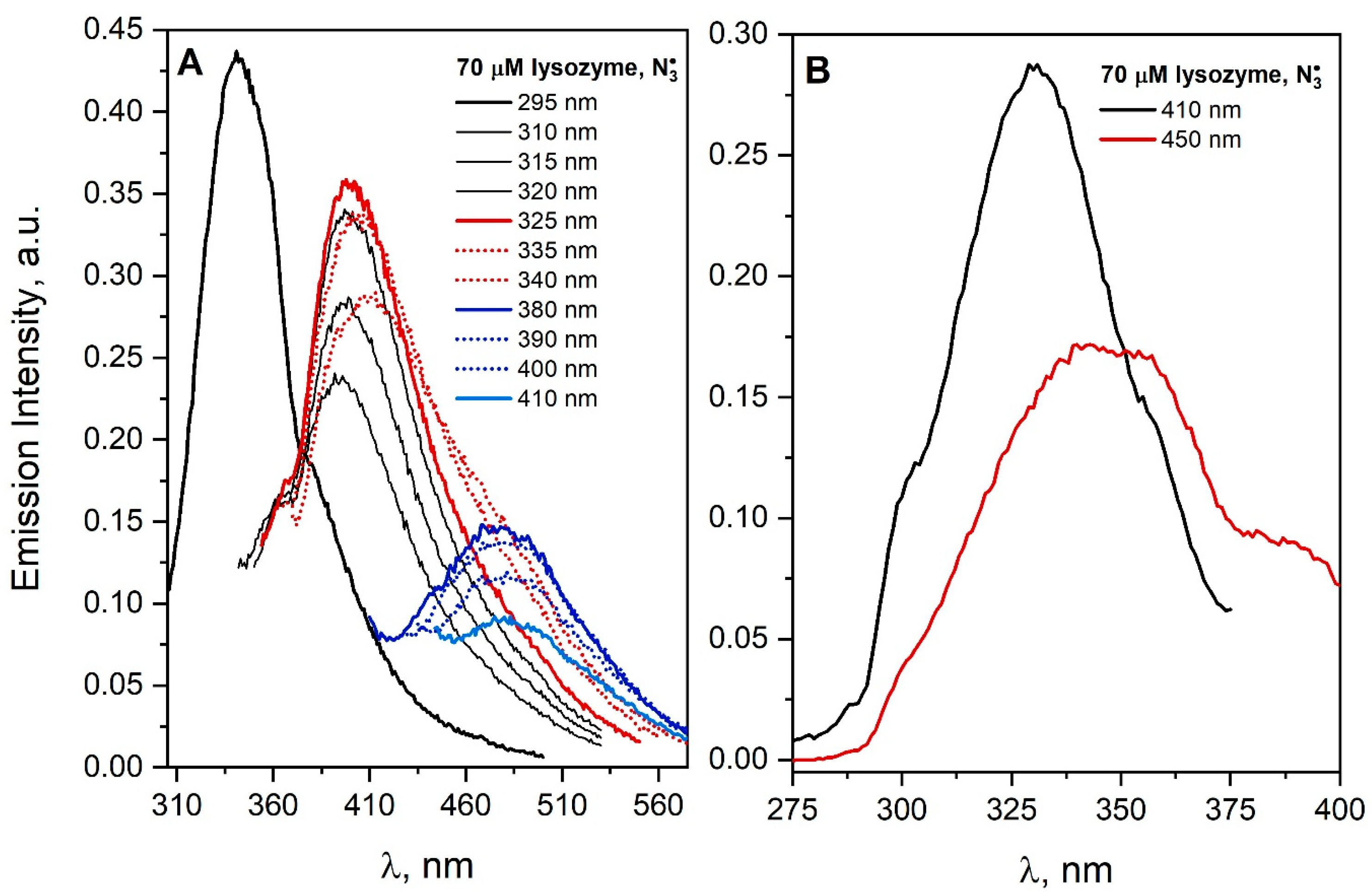
The emission spectra of a saturated N
2O aqueous solution containing 70 µM lysozyme and 0.1 M NaN
3 were recorded after irradiation with dose 3200 Gy and shown in
Figure 7. The specific DT fluorescence band dominates in the lysozyme spectrum. A similar conclusion regarding the formation of DT was reached in the case of irradiation of lysozyme under conditions in which the
radicals were the oxidizing agent [
21,
34]. Franzini et al. reported that lysozyme aggregates (28 and 42 kDa) appeared above the dose of 20 Gy absorbed during radiolysis, and their amount was correlated with the increase in DT emission, which suggests the formation of a covalent bond between tyrosine residues in the lysozyme structure [
35]. In our experiments, the excitation of the lysozyme solution with light that does not excite endogenous luminophores (λ
exc > 380 nm) leads to emission in the spectral range 460–560 nm. Lysozyme is an example of a protein for which, in addition to the formation of covalent bonds as a result of the attack of
radicals, aggregates emitting light in the visible range have been observed.
Figure 7B shows the excitation spectra recorded for the emission wavelength at 410 or 450 nm for a lysozyme after reaction with
radicals. The band centered at about 330 nm for the detection wavelength of 410 nm is typical for dityrosine. DT bridging is accompanied with the formation of light-emitting aggregates in the low-energy part of the spectrum (an emission band extending over 375 nm, maximum band at ≈3.1 eV). The similar excitation spectra were obtained for the N
2O-saturated solution containing 70 μM HSA and 0.1 M NaN
3 after irradiation [
4] (Figure 18 of Ref. [
4]).
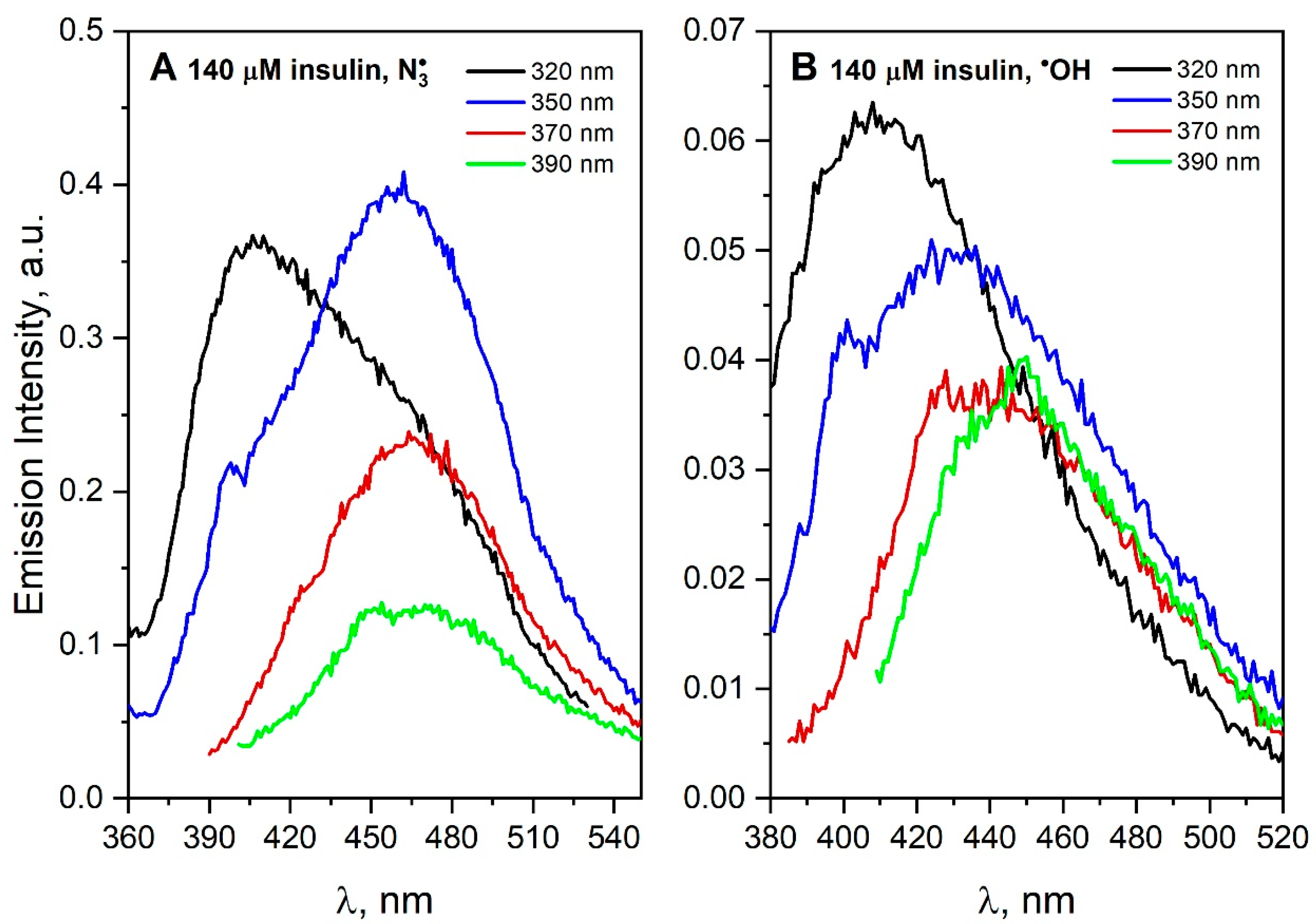
The molecular basis of insulin aggregation is relevant for modeling the amyloidogenesis process, which is involved in many pathologies [
36]. Insulin is known to form polymorphic amyloid fibrils depending on aggregating conditions. It was shown that the appearance of the intrinsic fluorescence of insulin fibrils is strongly dependent on the protonation state of N- and C-termini in the fibrils [
37]. Photochemical analysis of the measurement data obtained for saturated N
2O and irradiated (1485 Gy) insulin solution (140 μM) containing NaN
3 (0.1 M) shows that DT and new intermolecular hydrogen and/or ionic bonds were formed in the solution (
Figure 8A). The excitation of the solution with ~320 nm light leads to a wide emission band of insulin aggregates (suggest two populations of aggregates). What is important to note is that insulin does not contain any tryptophan residues in polypeptide chains. The scavenging of
•OH radical by insulin also leads to species other than DT, as evidenced by a broad, less intense red-shifted band (λ
max = 440–450 nm, λ
exc = 370–390 nm,
Figure 8B). In addition, the results of near and far UV-CD spectroscopy described in the literature [
38] show that exposure of insulin to UV also leads to structural damage, including insulin dimerization by cross-linking dityrosine or breaking the disulfide bond. When insulin reacts with
•OH or
radicals, in both cases, two populations of aggregates were observed. The insulin (51-residue peptide) monomer consists of two polypeptide chains linked by two disulfide bonds. For this reason, we can expect that certain aromatic groups of insulin will be more readily available for attack by the radical in contrast to more complex systems as proteins. It is reported that at least one tyrosine residue must be accessible to
•OH radicals in the monomer of insulin [
39]. In the case of the insulin molecule,
can also react with accessible cysteine, although the reaction is about 100 times slower than its reactions with Trp and Tyr. Recently, amyloid aggregation has been studied at neutral pH, which more closely resembles the physiological conditions [
37]. A very interesting observation was the detection of the fluorescence spectrum of the insulin solution after excitation with 350 nm light, with maximum centered at 440 nm. The authors proposed that intrinsic blue-green fluorescence can be used to study different fibrillization pathways. Moreover, this intrinsic fluorescence may offer an alternative method for the detection of the amyloid formation without using external probes.
Recently, our studies on albumin aggregation suggest that the appearance of visible fluorescence does not require the presence of aromatic residues or a conjugated π-electron system. The same conclusion concerning the origins of intrinsic fluorescence of insulin amyloids was drawn by Iannuzzi et al. [
37]. Radiolysis of insulin under oxidative conditions leads to the formation of aggregates characterized by a new emission of light. However, there is a significant difference in the position of the emission bands of insulin after reaction with hydroxyl or azide radicals. This is probably due to the different mechanism of
and
•OH radicals with insulin. The excitation spectrum recorded after the reaction of the
radical with insulin, with wavelength 350 nm, is similar to that of the obtained for insulin amyloids [
37]. In our opinion, redox processes associated with insulin oxidation leads to the formation of aggregates whose emission spectrum corresponds to that of insulin amyloids. Further, our work is well underway to clarify whether the spectral convergence is to some extent due to the involvement of covalent bonds in the formation of insulin amyloids.
Ovalbumin is the major protein component of egg white. The OVA polypeptide chain consists of 385 amino acid residues (44.3 kDa). The emission spectra of neat OVA solutions in the concentration range of 60–180 µM has been registered to study the self-aggregation process of ovalbumin. The formation of OVA self-aggregates has been observed after excitation of albumin solutions with 320 nm light (λ
max = 400 nm). The results of these experiments show that the light emission of protein aggregates depend linearly on the concentration of OVA (see
Figure S7 in
Supplementary Materials). We obtained the same result for HSA [
4] (Figure 1 of Ref. [
4]).
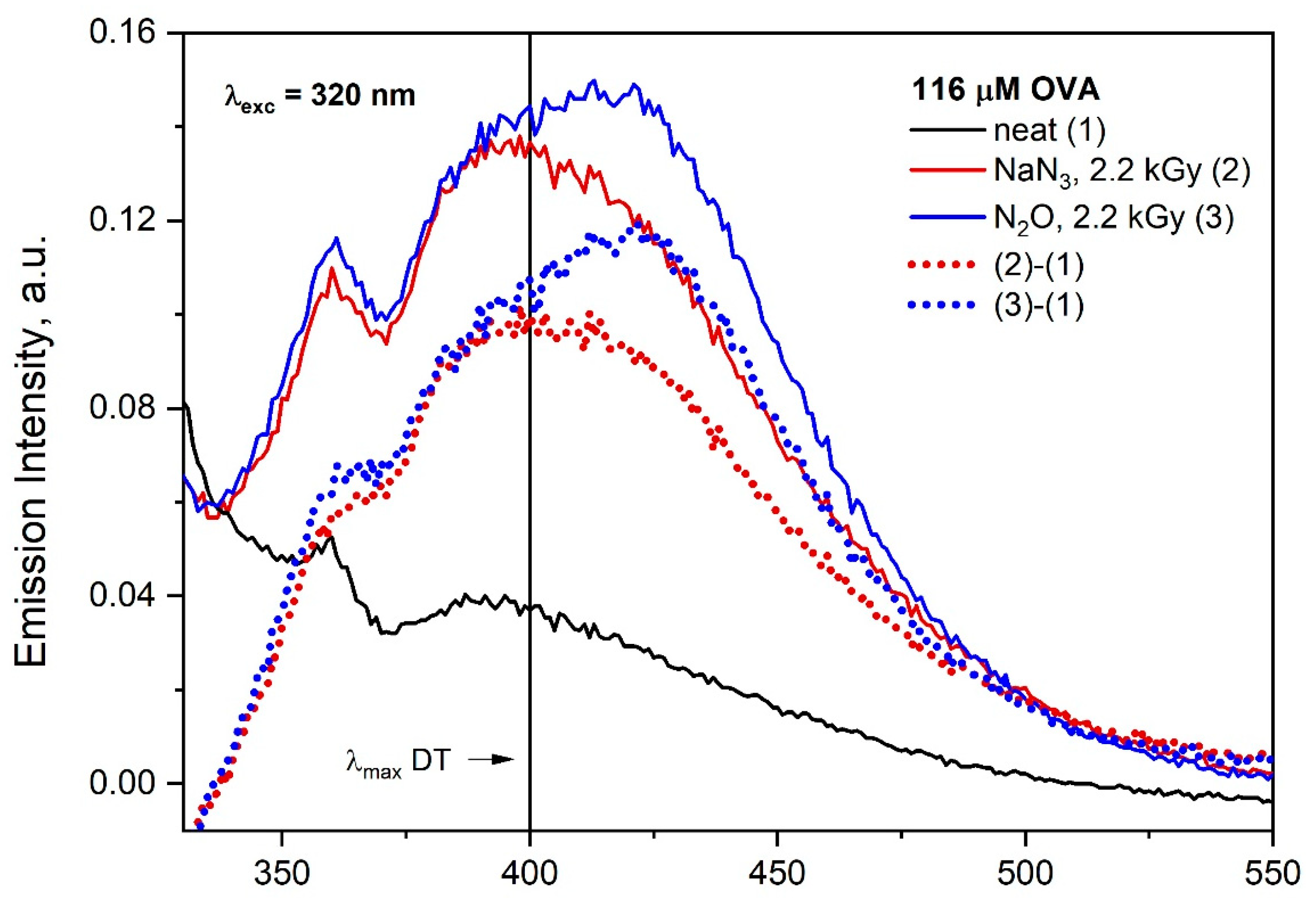
Steady-state radiolysis was applied to study the influence of the type of oxidizing radicals on the aggregation process of OVA. The emission spectra of the aqueous solution containing 116 μM OVA in the quartz cell were recorded after irradiation with dose 2200 Gy and are presented in
Figure S8. The excitation of irradiated aqueous OVA solution (in the absence and presence of NaN
3) with the 320 nm light leads to the emission with maximum at 400 nm (
) and 420 nm (
•OH), as shown in
Figure S8. The maximum of emission of OVA aggregates generated in the irradiated solution by
radicals was at the same spectral range as in the case of self-aggregatesemission (before irradiation). The overlapping of the emission bands of both DT and self-aggregates makes it very difficult to identify the formation of dityrosine in OVA solution after irradiation in optical measurements. The excitation of both solutions (irradiated in the presence and absence of NaN
3) with a wavelength above 320 nm results in emissions above 400 nm. Based on
Figure S8, at first glance, the OVA spectra after irradiation under different conditions do not differ. More detailed analysis showed that the mechanism of the azide and hydroxyl radicals’ reactions with OVA differs significantly.
In the case of
, the wide band is due to the overlapping of the emission of the DT and OVA aggregates (λ
exc = 320 nm). We are aware that the reaction of two TyrO
• radicals generated in different OVA molecules does not always lead to the formation of fluorescent tyrosine dimer. Regardless of this, in order to achieve a correct identification of DT and aggregates generated by ionizing radiation, it is recommended to analyze the differential spectra obtained by subtracting the OVA emission spectrum recorded before and after irradiation (especially for concentrated albumin solutions) (
Figure 9).
When OVA reacts with hydroxyl radical, a similar broad emission band was observed as in the case of the reaction of the azide radical with OVA, but the maximum of this band is red shifted (near 420 nm). As mentioned above, the correction of emission spectra is required. Subtracting the OVA emission spectrum recorded before and after irradiation allowed to distinguish the emission of albumin aggregates. In the case of •OH radicals, species other than DT are formed (maximum of emission at near 420 nm).
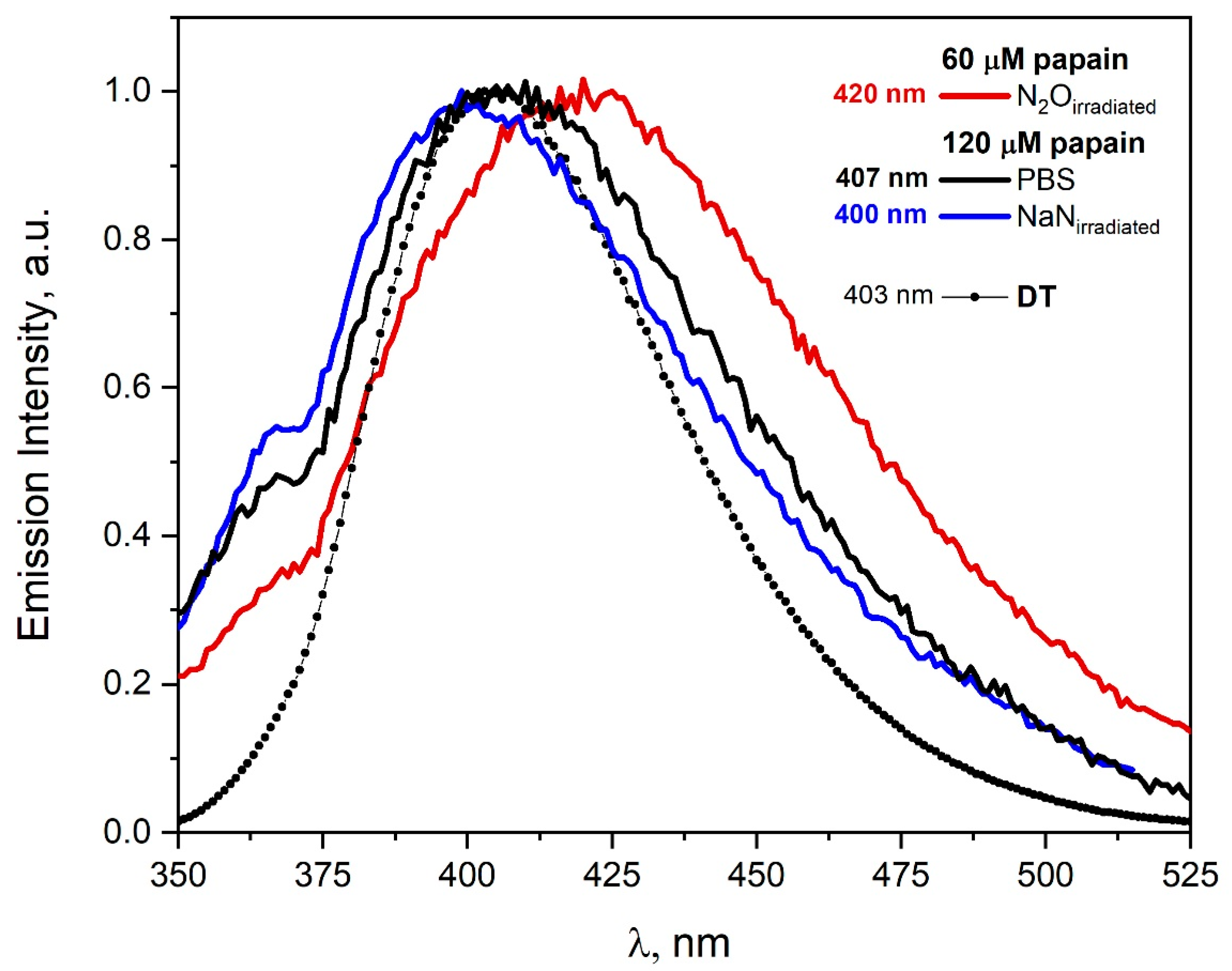
The emission spectra recorded for the excitation wavelength 320 nm before and after irradiation of the papain solutions are presented in
Figure 10. The emission spectra of irradiated solutions differ slightly depending on the type of reactive species used. As a result of the reaction of azide radicals with papain, tyrosine residues are mainly modified and DT is formed in contrast to
•OH radicals, where other species are formed (λ
max = 420 nm). The emission spectrum of spontaneous papain dimers does not differ significantly (in contrast to HSA solution) from the emission spectra of aggregates obtained after the reaction of azide radicals with papain. The spectroscopic method allows to distinguish spontaneous dimers to a very limited extent from those generated in the reaction of
•OH radicals with papain. In particular, the analysis of emission spectra related to DT formation (attack of azide radicals on papain) is virtually impossible due to the similarity of the emission band of papain self-aggregates to the DT band. It is worth emphasizing that the application of the emission detection method is useful for the observation of the participation of
•OH radicals in the reaction with papain. The redshift of the band maximum recorded after irradiation of the papain solution saturated with N
2O (λ
max = 420 nm) indicates that formed aggregates are not stabilized only by DT.
We assume that papain dimers are mainly responsible for the new emission, in the cases of both
•OH and
radicals. The literature data indicate that papain exists in aqueous solution as a mixture of monomer and dimer, the relative amounts being a function of protein concentration. In addition, electrophoretic and sedimentation studies indicate that papain also has a tendency to aggregate in dimers after oxidation [
40]. Our DLS measurements confirm this thesis. Analysis of the dynamic light scattering measurement (DLS) after irradiation of N
2O-saturated solution containing papain (70 μM) or solution containing papain (70 μM) and NaN
3 (0.1M) did not confirm the formation of high-molecular aggregates. In the irradiated solutions of papain under oxidizing conditions, papain monomers and dimers are mainly present (d~5 nm). Our measurements are in agreement with the literature [
41].
Resonance light scattering (RLS) is a sensitive method for studying protein aggregation. The DLS results showed that the largest aggregates were generated as a result of the attack of the
•OH radical on β-lactoglobulin (diameters of several tens of nm), while in the remaining solutions, the size of the nanostructures was below 10 nm. The results of the RLS measurements are in excellent agreement with those obtained by the DLS method. The data in
Figure S9 show a spectacular 50-fold increase in the RLS signal, resulting from the presence of large aggregates generated as a result of the reaction of
•OH radicals with β-lactoglobulin molecules.
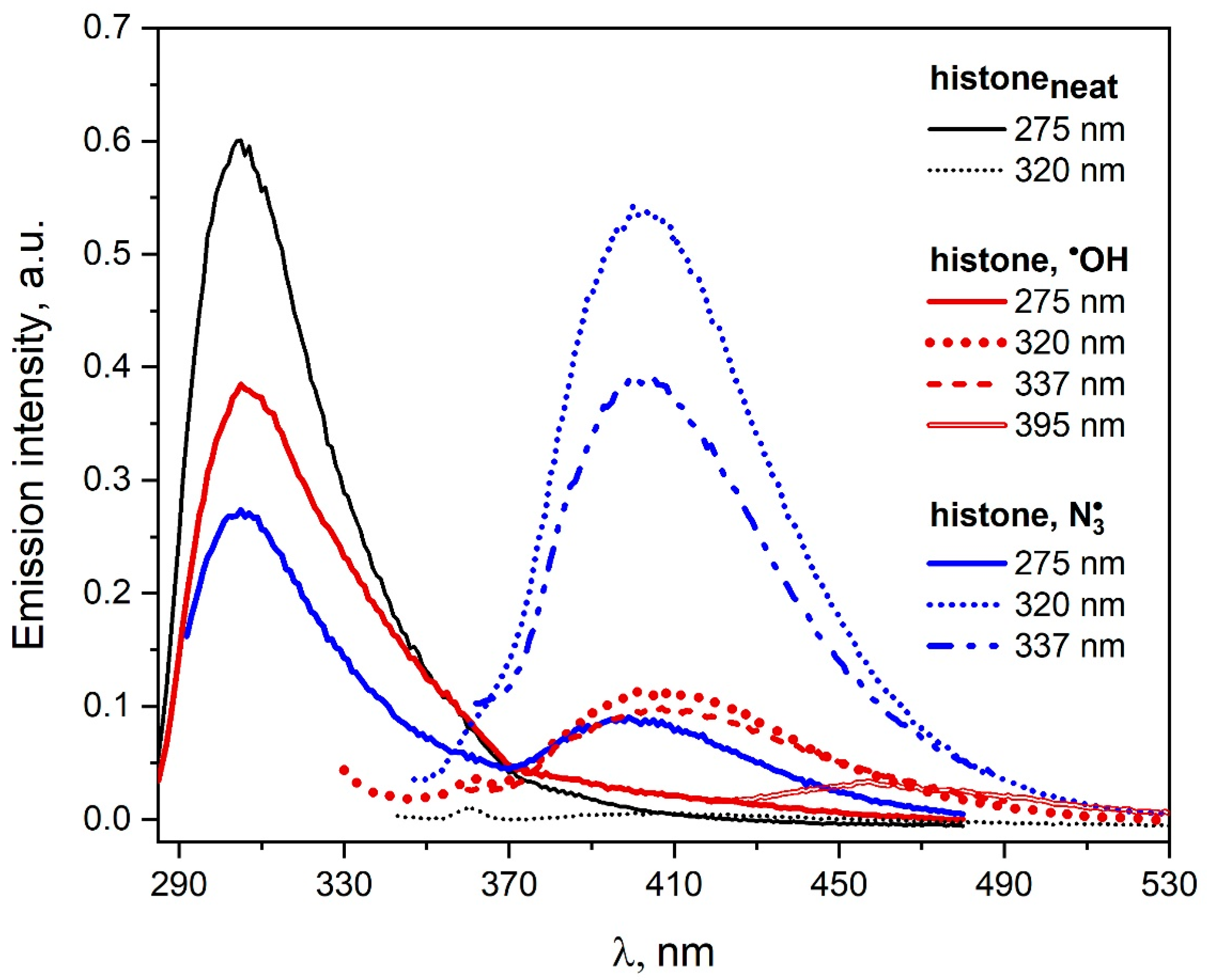
The histone solutions (containing or not containing 0.1 M NaN
3) saturated with N
2O and irradiated with a dose of 1650 Gy were analyzed spectroscopically by measuring the emission and excitation emission spectra. The emission spectra recorded before and after irradiation of the histone solutions are presented in
Figure 11. As a result of the reaction of
radicals with histone, tyrosine residues are mainly modified, and DT is formed.
Our measurements are consistent with those described in the literature [
42,
43]. It was reported that
•OH or
radicals generated by short electron beam pulses with the reaction of histone were causing intramolecular crossing. In the case of
•OH radicals, essentially non-tyrosine moieties of protein were involved. When
radicals react with histone during the pulse radiolysis coupling of tyrosine, radicals occur mainly within a single protein molecule. In the case of
•OH radical attack on histone, a new emission was observed with a maximum near 400 nm after excitation with 320 nm light of the irradiated solution. The intensity of this band is much lower (5.5 times) compared to the analogous intensity of the spectrum recorded after the reaction of
radicals with protein. It should be noted that the excitation of saturated N
2O and irradiated histone solution with light at above 320 nm leads to emission in the spectral range of 430–530 nm. In the case of
radicals, the new emission in this spectral range is negligible. These results suggest that the reaction of
•OH radicals with histone leads to the formation of both intra- and intermolecular bonds between histone molecules.
The use of a more accurate measurement technique based on light scattering should help distinguish intra- and intermolecular bonds between tyrosine residues. Analysis of the light scattering intensity (LSI) after irradiation of N
2O-saturated solution containing histone indicate intramolecular cross-linking of tyrosine residue [
44]. It is notable that intermolecular cross-linking mainly involves non-tyrosine histone moieties. The azide radicals react rather selectively with several histone residues, especially with tyrosine and cysteine moieties. When azide radicals react with the histone, the LSI increased weakly and the formation of dimers was strongly impeded.
3.3. The Formation of Dityrosine in Peptide Solution by Ionizing Radiation
Recent studies have provided the first indication that HSA can be used as a drug delivery system for peptide analogs of insulin hot spots, inhibiting insulin aggregation [
36]. It was shown that the binding of VEALYL (short peptide with amino acids sequence: Ala-Glu-Ala-Leu-Tyr-Leu) by HSA prevents the formation of amyloid fibrils. In this work, we conducted studies on the influence of oxidative stress on properties of VEALYL, which may help to understand the aggregation process of more complex systems as proteins and enzymes. It is easy to predict that the key amino acid involved in scavenging oxidative radicals in the case of VEALYL is tyrosine. Reactions of
or
•OH radicals with tyrosine over a wide range of pH and amino acid concentrations are well-known and we recently reported the detection of DT formation by steady-state and time-resolved fluorescence measurements [
4].
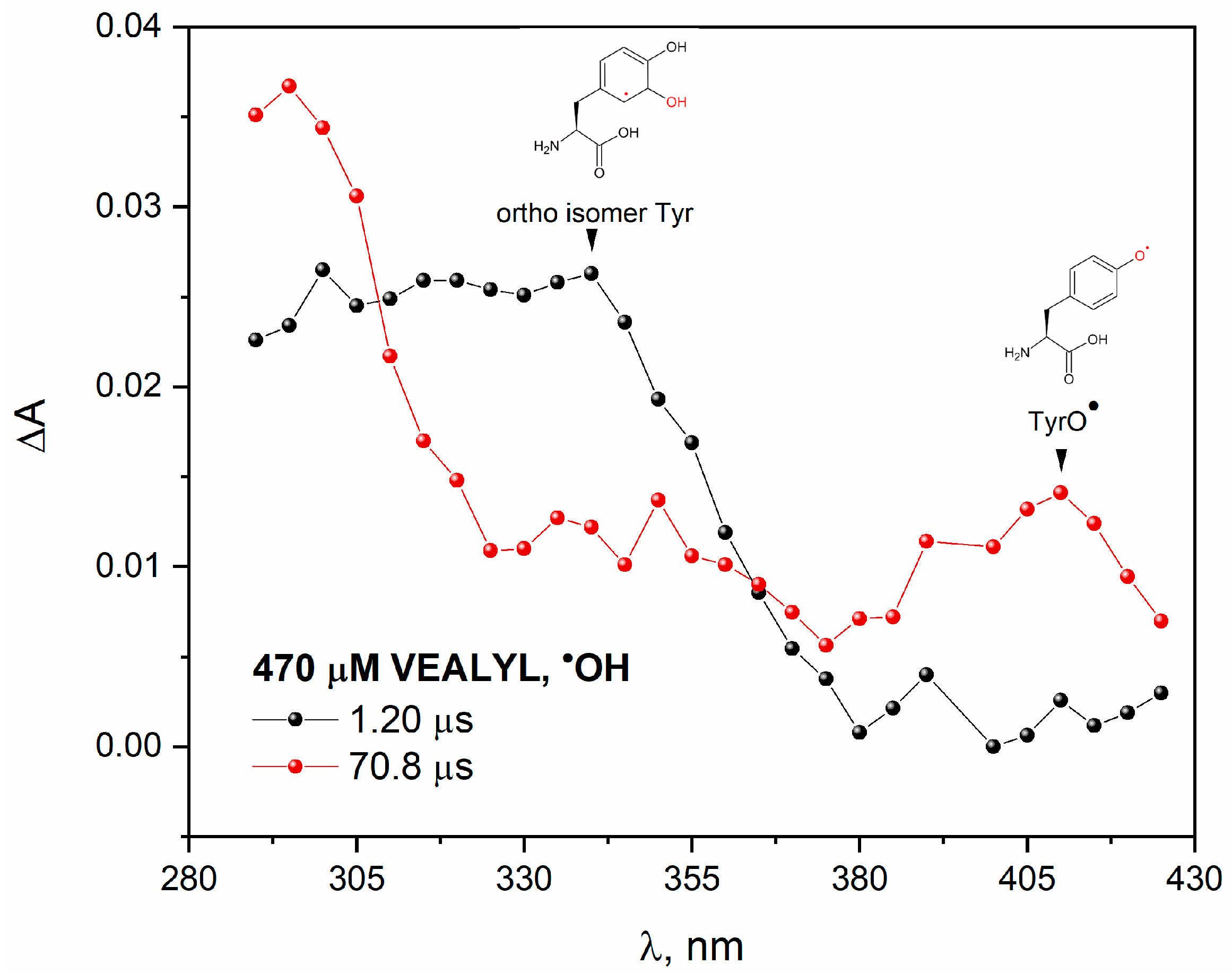
To study the reaction of an
•OH radical with VEALYL, we applied pulse radiolysis. Transient absorption spectra recorded at two selected times after an electron pulse irradiation of N
2O-saturated aqueous solution containing 70 μM VEALYL is presented in
Figure 12. The broad absorption band with maximum near 330 nm was observed immediately after irradiation (black plot). This spectrum can be attributed to the absorption of the tyrosine OH adduct on the ortho-position to the OH group. The band with a maximum at~330 nm disappears and a new transient absorption peak with maximum at 410 nm is formed (red plot). Pulse radiolysis confirmed that the reaction of a hydroxyl radical with peptide resulted in the formation of the phenoxyl radicals TyrO
•, which absorbs near 410 nm. A similar conclusion regarding the formation of DT in N
2O-saturated VEALYL solution was drawn in the case of the irradiation of H-Gly-Tyr-Gly-OH (GYG) tripeptide oxidized with
•OH radicals [
12]. Authors reported that the main route of phenoxyl radical formation consists of the addition reaction of hydroxyl radical to the phenol ring on the tyrosine side-chain and proton-catalyzed water molecule elimination.
When insulin reacts with hydroxyl radicals, similar transient absorption spectra were obtained as in the case of the reaction of
•OH with VEALYL. For insulin, the absorption band associated with the maximum at 330 and 410 nm is less intense as for the VEALYL (spectra not shown) [
39].
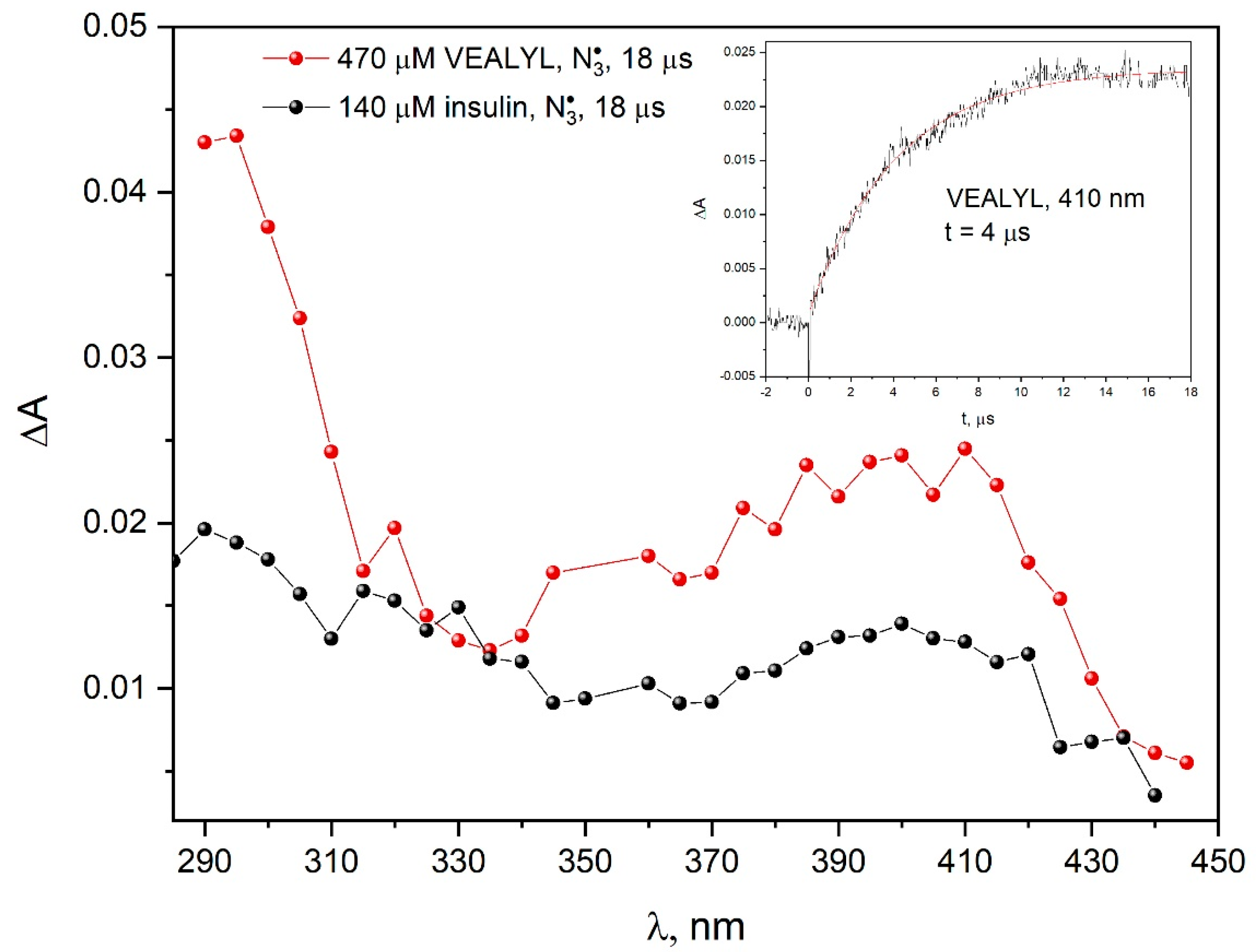
The comparison of the absorption spectra recorded during the pulse radiolysis of aqueous solutions of VEALYL or insulin containing 0.1 M NaN
3 indicate that the mechanism of oxidation of these compounds by azide radicals is similar (
Figure 13). A significant difference was observed in the 350–450 nm region. The higher absorbance in the transition spectra for VEALYL after one-electron oxidation resulted from the accessibility of tyrosine residues for
radicals. When the oxidizing radical was
•OH, we observed the formation of various types of tyrosine radicals (OH adduct on the ortho-position to the OH group and TyrO
•), and the oxidation of VEALYL and insulin with the azide radical led mainly to the formation of TyrO
•.
Emission spectra of N
2O-saturated VEALYL peptide solution (470 µM) and insulin solution (140 µM) after electron beam irradiation (1700 Gy) are presented in
Figure S10A (recorded after excitation with 320 nm light). An important observation from our steady-state radiolysis measurements was the presence of DT in the irradiated VEALYL peptide solution [
4]. The analysis of the measurement data showed that the emission intensity observed in the spectral range of 400–410 nm from DT depends on the spatial accessibility of tyrosine residues within insulin and the VEALYL peptide by
•OH radicals generated in the solution.
The structure of the VEALYL peptide contains one Tyr residue, which is exposed to an aqueous solution and reacts easily with radicals, which explains the difference in the intensity of the DT band compared to the insulin solution (14 times higher peptide emission intensity compared to insulin). In a solution, insulin has the unique trait of assuming different association states, including dimers, tetramers and hexamers [
45]. Insulin is composed of two chains, an A chain (with 21 amino acids) and a B chain (with 30 amino acids), which are linked by two disulfide bridges. Insulin contains four tyrosine residues, two of which are in the A peptide chain and other two in the B chain. In the monomer of insulin, there are four surface-exposed residues of Tyr. After self-aggregation, two Tyr residues are partially buried (Tyr16, B chain) and two residues are buried deeply within the protein structure (Tyr26, B chain). For this reason, in spectroscopic measurements, differences were observed in the intensity of DT emission between insulin and VEALYL peptide.
Figure S10B (see in the
Supplementary Materials) shows the normalized emission spectra of peptide and insulin. We confirm that peptide and insulin aggregate under oxidative stress. In both cases, covalent bonds are formed between the tyrosine residues. Insulin aggregates which form as a result of the reaction of albumin with
•OH radicals are stabilized by hydrogen bonds. The emission spectrum observed in this case is wider than the classic DT band. This is due to the overlap of dityrosine fluorescence and the emission of aggregates that are formed by a network of hydrogen and/or ionic bonds, analogous to self-aggregates.