Controlled Drug Release from Nanoengineered Polysaccharides
Abstract
1. Introduction
2. Principles and Kinetics of Drug Release: A Brief Review
- Zero-order: By releasing medication at a constant rate and keeping drug concentrations within the therapeutic window for a longer period, zero-order drug delivery systems can solve problems with immediate-release and first-order systems. This release profile can be used to lower dosage requirements, lessen dosing intervals, and improve receptor binding, post-receptor effects, and chemical interactions in terms of pharmacodynamics [87];
- First-order: Various therapeutic agents’ absorption and/or elimination have been described using this model. However, using a basic theory to define first-order kinetics is challenging. In this sense, first-order release states that the kinetic release rate depends on how the drug concentration changes over time [88];
- Higuchi model: The model defines drug release from insoluble matrices as a function of the square root of time, related to the Fickian diffusion equation. The slope of the plot gives the Higuchi dissolution constant [89];
- Hixson-Crowell model: This is a cube root law that deals with the dissolution rate that is normalized with respect to the decrease in solid surface area as a function of time. Adaptable to matrices where there is a change in the surface area and diameter of particles or tablets. It assumes no shape change as the suspended solid dissolves; its surface decreases by two-thirds of its weight [90];
- Baker-Lonsdale: It is a modified Higuchi model and describes the drug release from spherical matrices [91];
- Korsmeyer-Peppas model: This model was established specifically for the release of drugs from polymeric matrices like hydrogels [92]. As a power law, a comprehensive semi-empirical equation that establishes an exponential relationship between the release and the time. Modified forms have also been employed that contain the latency time, which marks the launch of drug release from the matrix;
- Hopfenberg model: It models and correlates drug release from surface-eroding polymers and assumes that the surface area remains constant during the degradation process. Good for drug release from slabs, spheres, and infinite cylinders displaying heterogeneous erosion [93];
- Poiseuille’s law of laminar flow. It can model drug release from membrane matrices, such as monolithic osmotic tablet systems. It is used for drug release from swelling gels or tables through orifices via pressure difference [93];
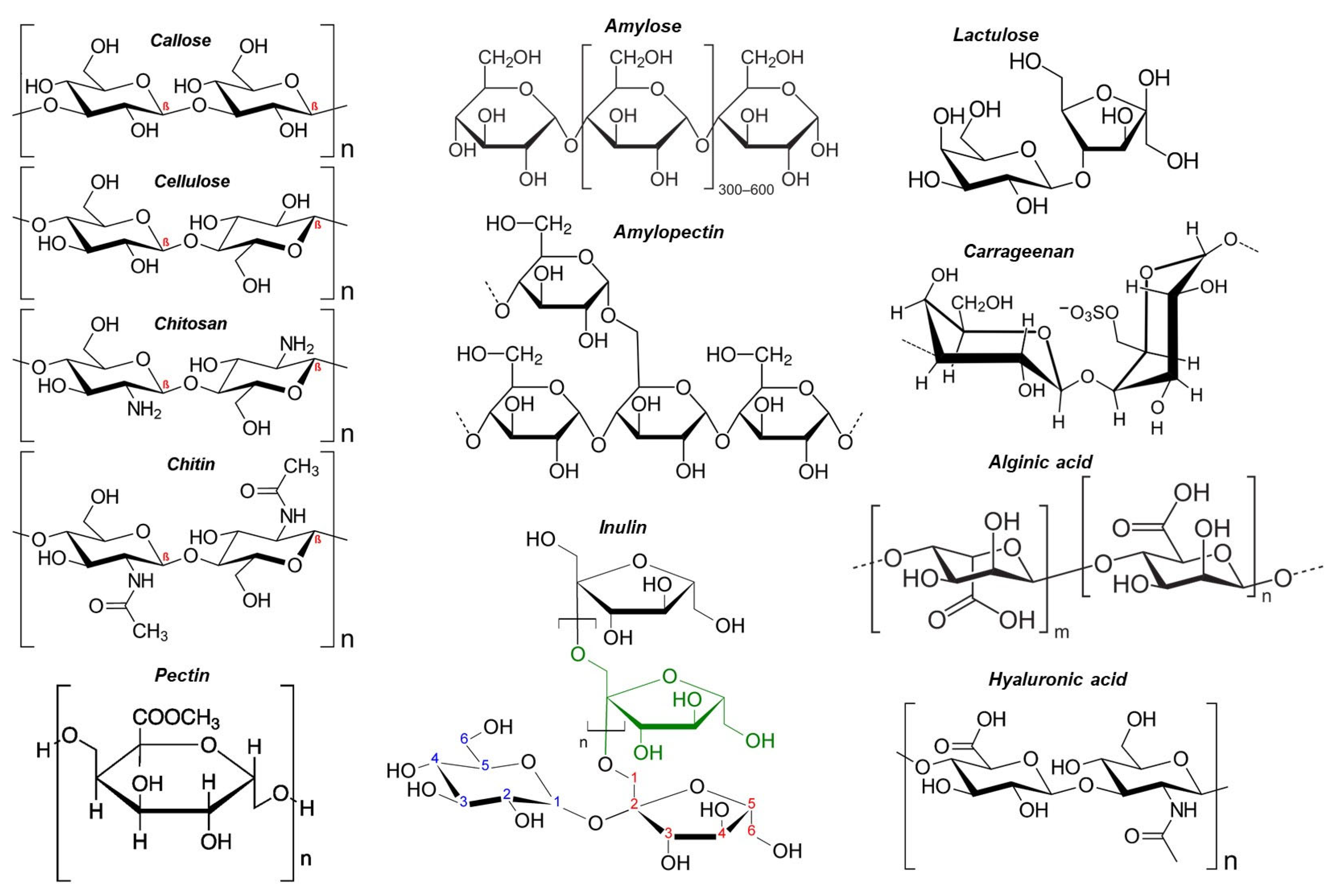
3. Drugs and Their Properties Encapsulated by Polysaccharides
4. Polysaccharide Encapsulated Natural Extracts and Release
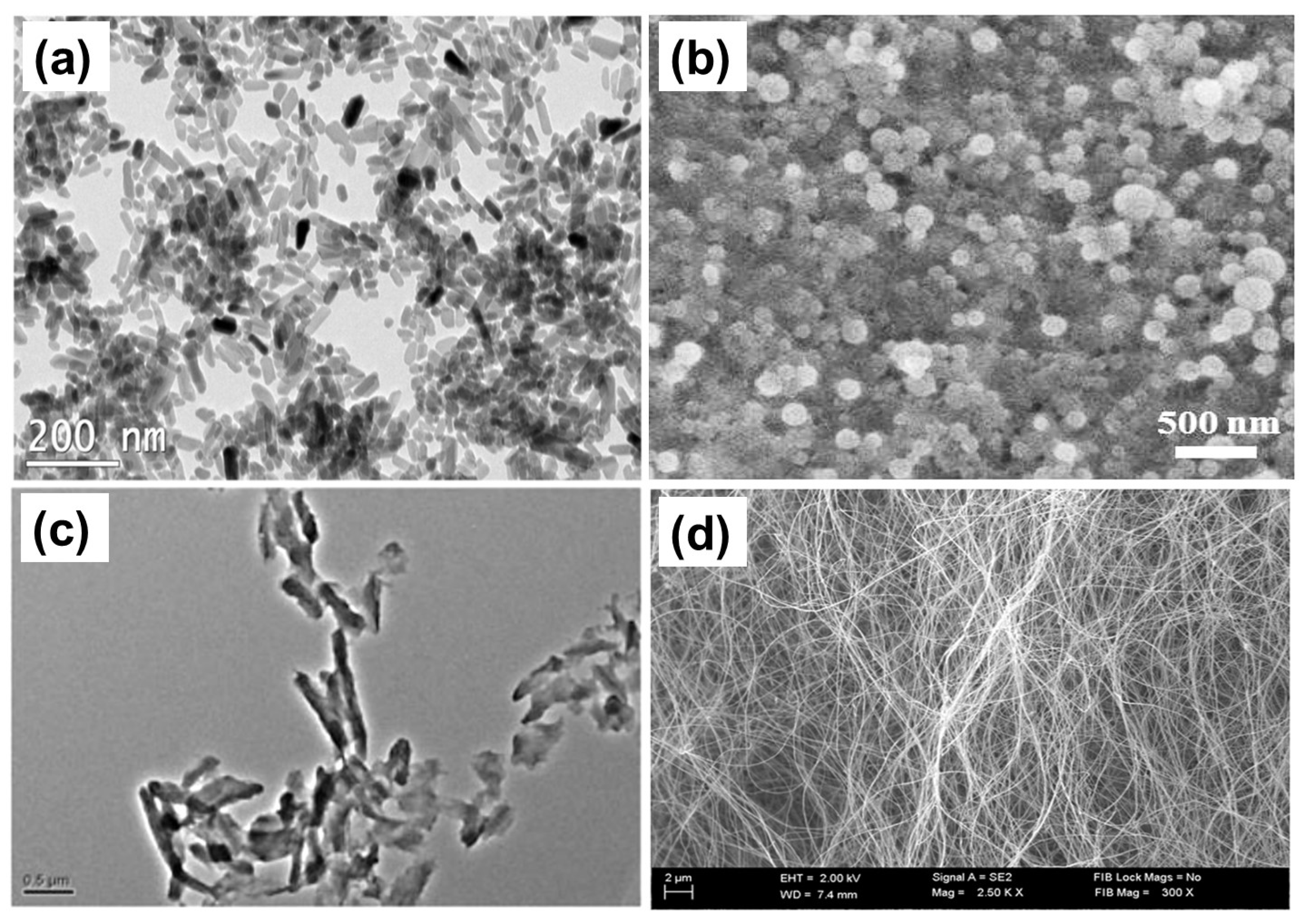
5. Drug Release from Polysaccharide-Based Nanofibers
6. Drug Release from Polysaccharide-Based Nanoparticles
7. Pharmacological Activity of Polysaccharides and Their Stimulus Release Properties
8. Biopharmaceutics and Pharmacokinetics Considerations
9. Statistical Analysis of Release Profiles
10. Summary and Future Trends
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.A.; Ferreira, R.B. Antimicrobial polysaccharides obtained from natural sources. Future Microbiol. 2022, 17, 701–716. [Google Scholar] [CrossRef]
- Chen, L.C.; Fan, Z.Y.; Wang, H.Y.; Wen, D.C.; Zhang, S.Y. Effect of polysaccharides from adlay seed on anti-diabetic and gut microbiota. Food Funct. 2019, 10, 4372–4380. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pang, W.; Bai, S.; Zheng, Z.; Wu, X. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement. Altern. Med. 2013, 13, 267. [Google Scholar] [CrossRef]
- BeMiller, J.N. (Ed.) 4-Polysaccharides: Occurrence, Structures, and Chemistry. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–101. [Google Scholar] [CrossRef]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Bayer, G.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Antiseptic povidone-iodine encapsulating edible phospholipid gels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126537. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, bio-medical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Badwan, A.A.; Rashid, I.; Al Omari, M.M.; Darras, F.H. Chitin and chitosan as direct compression excipients in pharmaceutical applications. Mar. Drugs 2015, 13, 1519–1547. [Google Scholar] [CrossRef] [PubMed]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Esher, S.K.; Ost, K.S.; Kohlbrenner, M.A.; Pianalto, K.M.; Telzrow, C.L.; Campuzano, A.; Nichols, C.B.; Munro, C.; Wormley, F.L., Jr.; Alspaugh, J.A. Defects in intracellular trafficking of fungal cell wall synthases lead to aberrant host immune recognition. PLoS Pathog. 2018, 14, e1007126. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Yadav, H.; Karthikeyan, C. Natural polysaccharides: Structural features and properties. In Polysaccharide Carriers for Drug Delivery; Woodhead Publishing: Sawston, UK, 2019; pp. 1–17. [Google Scholar]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Jensen, J.K.; Sørensen, S.O.; Harholt, J.; Geshi, N. Biosynthesis of pectin. Physiol. Plant. 2007, 129, 283–295. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Weerapol, Y.; Nunthanid, J.; Sungthongjeen, S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007, 67, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Micro-Biol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Albuquerque, P.B.S.; de Oliveira, W.F.; dos Santos Silva, P.M.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based controlled release systems for therapeutics de-livery and tissue engineering: From bench to bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Valente, S.A.; Silva, L.M.; Lopes, G.R.; Sarmento, B.; Coimbra, M.A.; Passos, C.P. Polysaccharide-based formula-tions as potential carriers for pulmonary delivery–a review of their properties and fates. Carbohydr. Polym. 2022, 277, 118784. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A review of current applications and upcoming potentiali-ties). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. Natural polysaccharides as electroactive polymers. Appl. Microbiol. Biotechnol. 2005, 67, 735–745. [Google Scholar] [CrossRef]
- Boddohi, S.; Kipper, M.J. Engineering nanoassemblies of polysaccharides. Adv. Mater. 2010, 22, 2998–3016. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine pol-ysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef]
- Petit, E.; Delattre, C.; Papy-Garcia, D.; Michaud, P. Chondroitin sulfate lyases: Applications in analysis and glycobiology. Adv. Pharmacol. 2006, 53, 167–186. [Google Scholar]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Han, G.; Wang, F. Recent advances in polysaccharides for osteoarthritis therapy. Eur. J. Med. Chem. 2017, 139, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal polysaccharides, novel application, and outlook. In Algae Based Polymers, Blends, and Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Yang, M.; Lin, H.B.; Gong, S.; Chen, P.Y.; Geng, L.L.; Zeng, Y.M.; Li, D.Y. Effect of astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine 2014, 70, 81–86. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L.; Ren, J.; Li, H.; Li, X.; Yu, S.; Zhang, W.; Fan, W. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Xue, H.; Gan, F.; Zhang, Z.; Hu, J.; Chen, X.; Huang, K. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int. J. Biol. Macromol. 2015, 81, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef]
- Guo, Z.; Lou, Y.; Kong, M.; Luo, Q.; Liu, Z.; Wu, J. A systematic review of phytochemistry, pharmacology and pharmacokinetics on Astragali radix: Implications for Astragali radix as a personalized medicine. Int. J. Mol. Sci. 2019, 20, 1463. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Seo, D.B.; Park, J.Y.; Park, J.; Shin, K.S. Immunostimulating and antimetastatic effects of polysaccha-rides purified from ginseng berry. Am. J. Chin. Med. 2019, 47, 823–839. [Google Scholar] [CrossRef]
- Xie, J.T.; Wu, J.A.; Mehendale, S.; Aung, H.H.; Yuan, C.S. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine 2004, 11, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, extraction and biomedical properties of polysaccha-rides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.W.; Lim, D.S.; Lee, S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobi, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2012, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, S.; Luo, S.; Liao, H.; Wang, Y.; Deng, X.; Ma, F.; Ma, C.W.; Zhou, L. Identification of genes underlying the enhancement of immunity by a formula of lentinan, pachymaran and tremelia polysaccharides in immunosuppressive mice. Sci. Rep. 2018, 8, 10082. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B.K.S. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, L.; Zhang, L. Molecular Basis for Poria cocos Mushroom Polysaccharide Used as an Antitumor Drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar] [PubMed]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Frases, S.; Guimaräes, A.J.; Rivera, J.; Casadevall, A. Evidence for branching in cryptococcal capsular poly-saccharides and consequences on its biological activity. Mol. Microbiol. 2011, 79, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial dextran-hydrolyzing enzymes: Fundamentals and applica-tions. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Microvascular pressure and functional capillary density in extreme he-modilution with low-and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. American journal of physiology. Heart Circ. Physiol. 2004, 287, H363–H373. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Farè, S. Biopolymer-based strategies in the design of smart medical devices and artificial organs. Int. J. Artif. Organs 2018, 41, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ferreiro, A.; Barcia, M.G.; Gil-Martínez, M.; Vieites-Prado, A.; Lema, I.; Argibay, B.; Méndez, J.B.; Lamas, M.J.; Otero-Espinar, F.J. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2015, 94, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.A.; Bunt, C.R.; Green, C.R.; Alany, R.G. In-vitro and in-vivo evaluation of car-rageenan/methylcellulose polymeric systems for transscleral delivery of macromolecules. Eur. J. Pharm. Sci. 2011, 44, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Fernández-Valle, M.E.; Aranaz, I.; Heras, A. pH-and Temperature-Sensitive Chitosan Hydrogels: Swelling and MRI Studies. Macromol. Chem. Phys. 2011, 212, 887–895. [Google Scholar] [CrossRef]
- Mandal, S.; Thimmasetty, M.K.; Prabhushankar, G.L.; Geetha, M.S. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Lobel, E.; Trevgoda, A.; Peled, Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J. Control. Release 1997, 44, 201–208. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Wang, A. pH-responsive carboxymethylcellulose-g-poly (sodium acry-late)/polyvinylpyrrolidone semi-IPN hydrogels with enhanced responsive and swelling properties. Macromol. Res. 2011, 19, 57–65. [Google Scholar] [CrossRef]
- Jin, R.; Hiemstra, C.; Zhong, Z.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hy-aluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005, 34, 4312–4314. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Hemmati, K.; Ghaemy, M. Synthesis of nanohydrogels based on tragacanth gum biopolymer and investigation of swelling and drug delivery. Int. J. Biol. Macromol. 2016, 82, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, E.M.; Pino, E.N.; Ainslie, K.M. Acetalated dextran: A tunable and acid-labile biopolymer with facile synthesis and a range of applications. Chem. Rev. 2017, 117, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, C.; Zhang, Z.; Ren, K.; Chen, X. Injectable, biomolecule-responsive polypeptide hydrogels for cell en-capsulation and facile cell recovery through triggered degradation. ACS Appl. Mater. Interfaces 2016, 8, 30692–30702. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef]
- Epstein-Barash, H.; Orbey, G.; Polat, B.E.; Ewoldt, R.H.; Feshitan, J.; Langer, R.; Borden, M.A.; Kohane, D.S. A microcomposite hydrogel for repeated on-demand ultrasound-triggered drug delivery. Biomaterials 2010, 31, 5208–5217. [Google Scholar] [CrossRef]
- Selegård, R.; Aronsson, C.; Brommesson, C.; Dånmark, S.; Aili, D. Folding driven self-assembly of a stimuli-responsive peptide-hyaluronan hybrid hydrogel. Sci. Rep. 2017, 7, 7013. [Google Scholar] [CrossRef]
- Akhgari, A.; Abbaspour, M.R.; Rezaee, S.; Kuchak, A. Evaluation of the swelling, erosion and drug release from polysaccharide matrix tablets based on pectin and inulin. Jundishapur J. Nat. Pharm. Prod. 2011, 6, 51–58. [Google Scholar]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Xu, X.; Weng, Y.; Xu, L.; Chen, H. Sustained release of Avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. Int. J. Biol. Macromol. 2013, 60, 272–276. [Google Scholar] [CrossRef]
- Langer, R.S.; Wise, D.L. Medical Applications of Controlled Release; CRC Press LLC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bussemer, T.; Otto, I.; Bodmeier, R. Pulsatile drug-delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2001, 18, 1–26. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Alginate-based composite sponges as gastroretentive carriers for curcumin-loaded self-microemulsifying drug delivery systems. Sci. Pharm. 2017, 85, 11. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Laracuente, M.L.; Marina, H.Y.; McHugh, K.J. Zero-order drug delivery: State of the art and future pro-spects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Christoper, G.P. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Ofori-Kwakye, K.; Mfoafo, K.A.; Kipo, S.L.; Kuntworbe, N.; El Boakye-Gyasi, M. Development and evaluation of natural gum-based extended release matrix tablets of two model drugs of different water solubilities by direct compres-sion. Saudi Pharm. J. 2016, 24, 82–91. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M. P Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Katzhendler, I.; Hoffman, A.; Goldberger, A.; Friedman, M. Modeling of drug release from erodible tablets. J. Pharm. Sci. 1997, 86, 110–115. [Google Scholar] [CrossRef]
- Lu, E.X.; Jiang, Z.Q.; Zhang, Q.Z.; Jiang, X.G. A water-insoluble drug monolithic osmotic tablet system utilizing gum arabic as an osmotic, suspending and expanding agent. J. Control. Release 2003, 92, 375–382. [Google Scholar] [CrossRef]
- Kosmidis, K.; Macheras, P. On the dilemma of fractal or fractional kinetics in drug release studies: A comparison between Weibull and Mittag-Leffler functions. Int. J. Pharm. 2018, 543, 269–273. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Kranz, H.; Jürgens, K.; Pinier, M.; Siepmann, J. Drug release from MCC-and carrageenan-based pellets: Experiment and theory. Eur. J. Pharm. Biopharm. 2009, 73, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in biomedical research and development—A review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysac-charides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and char-acterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS PharmSciTech 2017, 18, 936–946. [Google Scholar] [CrossRef]
- Malviya, R.; Tyagi, A.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.; Sundram, S.; Karupiah, S.; Chakravarthi, S.; Meenakshi, D.U.; Gupta, N.; et al. Fabrication and Characterization of Chitosan—Tamarind Seed Polysaccharide Composite Film for Transdermal Delivery of Pro-tein/Peptide. Polymers 2021, 13, 1531. [Google Scholar] [CrossRef]
- Macleod, G.S.; Fell, J.T.; Collett, J.H.; Sharma, H.L.; Smith, A.M. Selective drug delivery to the colon using pectin: Chitosan: Hydroxypropyl methylcellulose film coated tablets. Int. J. Pharm. 1999, 187, 251–257. [Google Scholar] [CrossRef]
- Zarandona, I.; Barba, C.; Guerrero, P.; de la Caba, K.; Maté, J. Development of chitosan films containing β-cyclodextrin inclusion complex for controlled release of bioactives. Food Hydrocoll. 2020, 104, 105720. [Google Scholar] [CrossRef]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, G.; Orasugh, J.T.; Saha, N.R.; Roy, I.; Bhattacharyya, A.; Chattopadhyay, A.K.; Rana, D.; Chattopadhyay, D. Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J. Chem. 2017, 41, 15312–15319. [Google Scholar] [CrossRef]
- Kim, S.; Liu, Y.; Gaber, M.W.; Bumgardner, J.D.; Haggard, W.O.; Yang, Y. Development of chitosan–ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 145–155. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (Chitosan/Guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef] [PubMed]
- Riccio, B.V.F.; Klosowski, A.B.; Prestes, E.; de Sousa, T.B.; de Assunção Morais, L.C.; Lemes, B.M.; Beltrame, F.L.; Campos, P.M.; Ferrari, P.C. Chitosan/nanocellulose-based bionanocomposite films for controlled betamethasone and silver sulfadiazine delivery. J. Appl. Polym. Sci. 2021, 138, 50468. [Google Scholar] [CrossRef]
- Moon, M.S. Essential basic bacteriology in managing musculoarticuloskeletal infection: Bacterial anatomy, their be-havior, host phagocytic activity, immune system, nutrition, and antibiotics. Asian Spine J. 2019, 13, 343. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Kroschinsky, F.; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef]
- Wang, R.; Lu, D.; Wang, H.; Zou, H.; Bai, T.; Feng, C.; Lin, Q. “Kill-release” antibacterial polysaccharides multilayer coating based therapeutic contact lens for effective bacterial keratitis treatment. RSC Adv. 2021, 11, 26160–26167. [Google Scholar] [CrossRef]
- Deng, H.; Sun, J.; Yu, Z.; Guo, Z.; Xu, C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C 2021, 118, 111530. [Google Scholar] [CrossRef]
- Xie, L.; Wei, H.; Kou, L.; Ren, L.; Zhou, J. Antibiotic drug release behavior of poly (vinyl alcohol)/sodium alginate hydrogels. Mater. Werkst. 2020, 51, 850–855. [Google Scholar] [CrossRef]
- Kunkit, N.; Deekaikam, T.; Chaimuang, S.; Pekkoh, J.; Manokruang, K. Physical hydrogels prepared from cationically modified pectin with tunable sol-gel phase transition behaviors. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 131–141. [Google Scholar] [CrossRef]
- Madhumathi, K.; Rekha, L.J.; Kumar, T.S. Tailoring antibiotic release for the treatment of periodontal infrabony defects using bioactive gelatin-alginate/apatite nanocomposite films. J. Drug Deliv. Sci. Technol. 2018, 43, 57–64. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Gottesmann, M.; Goycoolea, F.M.; Steinbacher, T.; Menogni, T.; Hensel, A. Smart drug delivery against Helicobacter pylori: Pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 2020, 104, 5943–5957. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and charac-terization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Wang, F.; Yang, S.; Yuan, J.; Gao, Q.; Huang, C. Effective method of chitosan-coated alginate nanoparticles for target drug delivery applications. J. Biomater. Appl. 2016, 31, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Zarkesh, M.; Nozhat, Z. Application of bacterial nanocellulose in cancer drug delivery: A review. Curr. Pharm. Des. 2021, 27, 3656–3665. [Google Scholar] [CrossRef]
- Junka, A.; Żywicka, A.; Chodaczek, G.; Dziadas, M.; Czajkowska, J.; Duda-Madej, A.; Bartoszewicz, M.; Mikołajewicz, K.; Krasowski, G.; Szymczyk, P.; et al. Potential of biocellulose carrier impregnated with essential oils to fight against biofilms formed on Hydroxyapatite. Sci. Rep. 2019, 9, 1256. [Google Scholar] [CrossRef]
- Giri, T.K.; Kumar, K.; Alexander, A.; Badwaik, H.; Tripathi, D.K. A novel and alternative approach to controlled release drug delivery system based on solid dispersion technique. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 147–159. [Google Scholar] [CrossRef]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The multitarget activity of natural extracts on cancer: Synergy and xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: From encapsulation to application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Kumar, M.; Sinhal, A.; Saifi, A. Recent development in novel drug delivery systems of herbal drugs. Int. J. Green Pharm. 2011, 5. [Google Scholar] [CrossRef]
- Enrico, C. Nanotechnology-based drug delivery of natural compounds and phytochemicals for the treatment of cancer and other diseases. Stud. Nat. Prod. Chem. 2019, 62, 91–123. [Google Scholar]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Ceseracciu, L.; Guzman-Puyol, S.; Paul, U.C.; Alfaro-Pulido, A.; Grande, C.; Vezzulli, L.; Bandiera, T.; Bertorelli, R.; Russo, D.; et al. Antimicrobial, antioxidant, and waterproof RTV silicone-ethyl cellulose composites containing clove essential oil. Carbohydr. Polym. 2018, 192, 150–158. [Google Scholar] [CrossRef]
- Setti, C.; Suarato, G.; Perotto, G.; Athanassiou, A.; Bayer, I.S. Investigation of in vitro hydrophilic and hydrophobic dual drug release from polymeric films produced by sodium alginate-MaterBi® drying emulsions. Eur. J. Pharm. Biopharm. 2018, 130, 71–82. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit. Rev.™ Ther. Drug Carr. Syst. 2010, 27. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int. J. Bio-Log. Macromol. 2011, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural ex-tracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Ahmed, M.A.E.; Faten, A.E.E.; Emad, A.S.; Hany, A.E.S. Traditional medicinal plants research in Egypt: Studies of antioxidant and anticancer activities. J. Med. Plants Res. 2012, 6, 689–703. [Google Scholar]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- De Souza, E.J.D.; Kringel, D.H.; Dias, A.R.G.; da Rosa Zavareze, E. Polysaccharides as wall material for the en-capsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, S.; Fan, S.; Ma, Y.; Li, D.; Tao, Y.; Han, Y. Encapsulation of bioactive polyphenols by starch and their impacts on gut microbiota. Curr. Opin. Food Sci. 2021, 38, 102–111. [Google Scholar] [CrossRef]
- Weisany, W.; Yousefi, S.; Tahir, N.A.R.; Zadeh, N.G.; McClements, D.J.; Adhikari, B.; Ghasemlou, M. Targeted delivery and controlled released of essential oils using nanoencapsulation: A review. Adv. Colloid Interface Sci. 2022, 303, 102655. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Huang, B.Q.; Tsai, G.J.; Hsieh, H.Y.; Sung, H.W.; Mi, F.L. Characterization of tea cate-chins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from pro-tein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Thangavel, K. Synthesis and characterization of nano-encapsulated catechin by mo-lecular inclusion with beta-cyclodextrin. J. Food Eng. 2012, 111, 255–264. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Ozdemir, N.; Hill, L.E.; Gomes, C.L. Synthesis and characterization of nano-encapsulated black pepper oleoresin using hydroxypropyl beta-cyclodextrin for antioxidant and antimicrobial applications. J. Food Sci. 2013, 78, N1913–N1920. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, A.; Liu, M.; Li, X.; Guo, H.; Zuo, C.; Li, Y. The intestine-responsive lysozyme nanoparticles-in-oxidized starch microgels with mucoadhesive and penetrating properties for improved epithelium absorption of quercetin. Food Hydrocoll. 2020, 99, 105309. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Castro-Rosas, J.; Rangel-Vargas, E.; Navarro-Cortez, R.O.; Cabrera-Canales, Z.E.; Díaz-Batalla, L.; Martínez-Bustos, F.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. A modified Achira (Canna indica L.) starch as a wall material for the encapsulation of Hibiscus sabdariffa extract using spray drying. Food Res. Int. 2019, 119, 547–553. [Google Scholar] [CrossRef]
- Ji, S.; Jia, C.; Cao, D.; Muhoza, B.; Zhang, X. Formation, characterization and properties of resveratrol-dietary fiber composites: Release behavior, bioaccessibility and long-term storage stability. LWT 2020, 129, 109556. [Google Scholar] [CrossRef]
- Athira, G.K.; Jyothi, A.N. Cassava starch-poly (vinyl alcohol) nanocomposites for the controlled delivery of cur-cumin in cancer prevention and treatment. Starch-Stärke 2015, 67, 549–558. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž., Tešić; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Assadpour, E.; Esfanjani, A.F.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Li, Z.; Huang, D.; Nong, L.; Ning, Z.; Hu, Z.; Xu, C.; Yan, J.K. Pectin-decorated selenium nanoparticles as a nanocarrier of curcumin to achieve enhanced physicochemical and biological properties. IET Nanobiotechnol. 2019, 13, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Manjusha, V.; Sekhar, V.C. A new biodegradable nano cellulose-based drug delivery system for pH-controlled delivery of curcumin. Int. J. Biol. Macromol. 2021, 183, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Raza, W.; Meena, A. Lemongrass derived cellulose nanofibers for controlled release of curcumin and its mechanism of action. Ind. Crops Prod. 2021, 173, 114099. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The biopolymer bacterial nanocel-lulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Shikhi-Abadi, P.G.; Irani, M. A review on the applications of electrospun chitosan nanofibers for the cancer treat-ment. Int. J. Biol. Macromol. 2021, 183, 790–810. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chi-tosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Turan, C.U.; Guvenilir, Y. Electrospun poly (ω-pentadecalactone-co-ε-caprolactone)/gelatin/chitosan ternary nan-ofibers with antibacterial activity for treatment of skin infections. Eur. J. Pharm. Sci. 2022, 170, 106113. [Google Scholar] [CrossRef]
- Hadjianfar, M.; Semnani, D.; Varshosaz, J. Polycaprolactone/chitosan blend nanofibers loaded by 5-fluorouracil: An approach to anticancer drug delivery system. Polym. Adv. Technol. 2018, 29, 2972–2981. [Google Scholar] [CrossRef]
- Feng, K.; Li, C.; Wei, Y.S.; Zong, M.H.; Wu, H.; Han, S.Y. Development of a polysaccharide based multi-unit nanofiber mat for colon-targeted sustained release of salmon calcitonin. J. Colloid Interface Sci. 2019, 552, 186–195. [Google Scholar] [CrossRef]
- Celebioglu, A.; Saporito, A.F.; Uyar, T. Green Electrospinning of Chitosan/Pectin Nanofibrous Films by the Incor-poration of Cyclodextrin/Curcumin Inclusion Complexes: pH-Responsive Release and Hydrogel Features. ACS Sustain. Chem. Eng. 2022, 10, 4758–4769. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Al-Enizi, A.; Aldalbahi, A.; El-Hamshary, H.; El-Newehy, M.H. Core-shell nanofibers from poly (vinyl alcohol) based biopolymers using emulsion electrospinning as drug delivery system for cephalexin drug. J. Macromol. Sci. A 2020, 58, 130–144. [Google Scholar] [CrossRef]
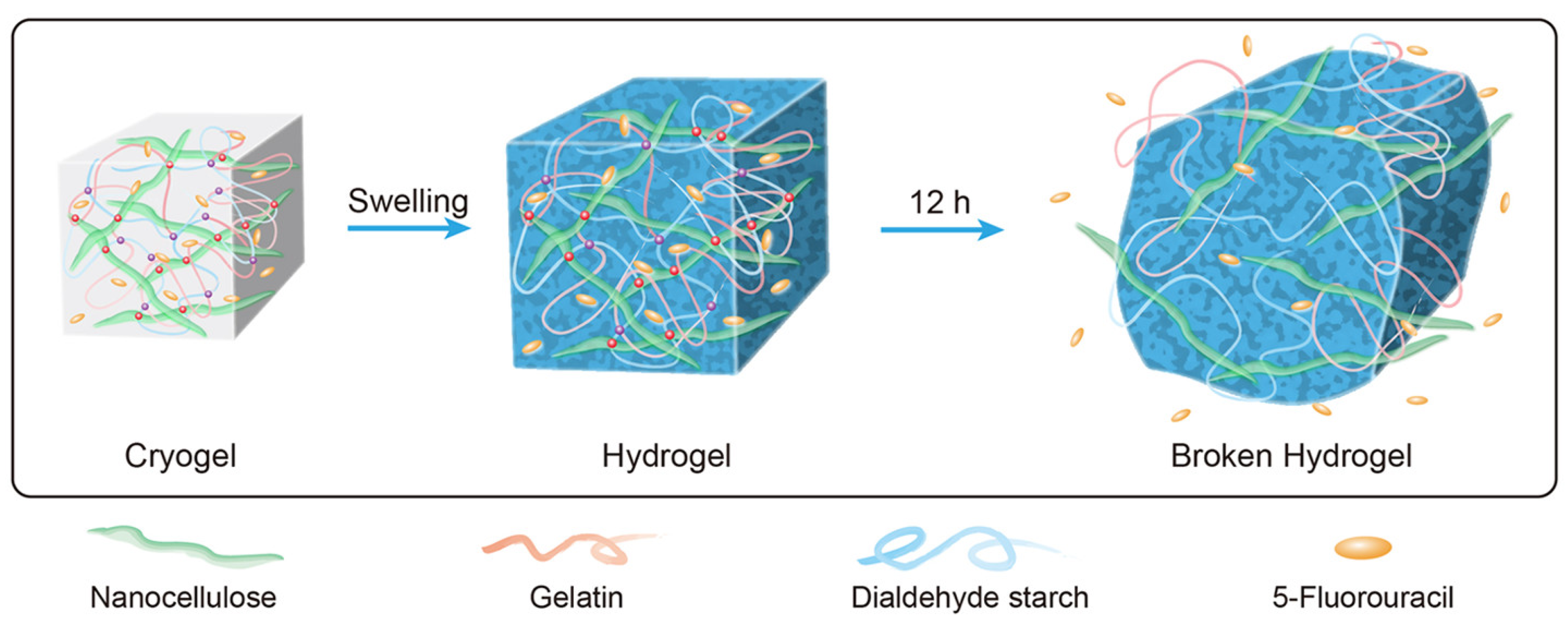
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/gelatin composite cryogels for controlled drug release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Moydeen, A.M.; Padusha, M.; Thamer, B.M.; Ahamed, N.A.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M.H. Single-nozzle core-shell electrospun nanofibers of pvp/dextran as drug delivery system. Fibers Polym. 2019, 20, 2078–2089. [Google Scholar] [CrossRef]
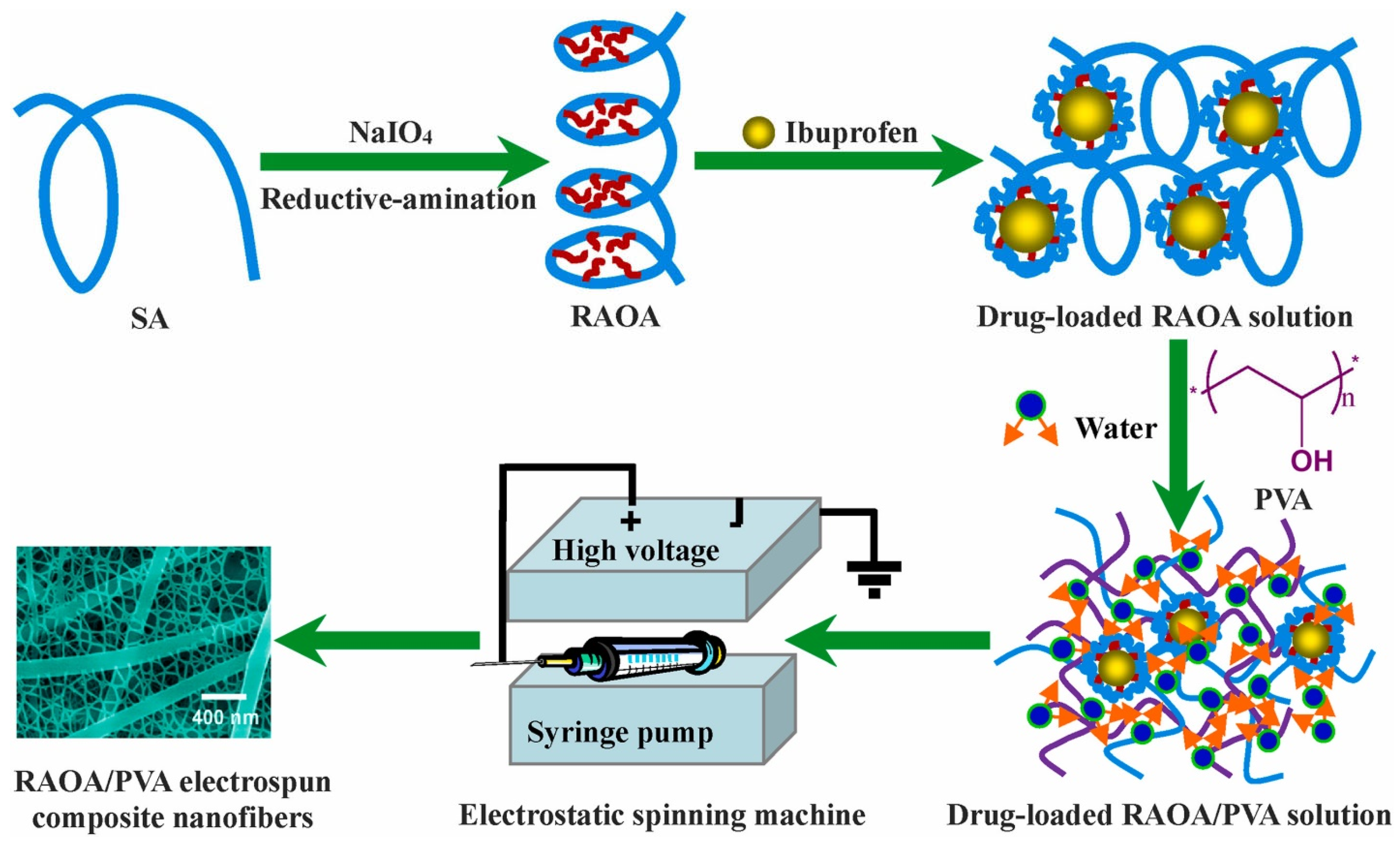
- Chen, X.; Zhu, Q.; Wen, Y.; Li, Z.; Cao, S.; Yan, H.; Lin, Q. Chemical modification of alginate via the oxida-tion-reductive amination reaction for the development of alginate derivative electrospun composite nanofibers. J. Drug Deliv. Sci. Technol. 2022, 68, 103113. [Google Scholar] [CrossRef]
- Zupančič, Š. Core-shell nanofibers as drug-delivery systems. Acta Pharm. 2019, 69, 131–153. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–shell fibers: Design, roles, and controllable release strategies in tissue engineering and drug delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Su, Y.; Su, Q.; Liu, W.; Jin, G.; Mo, X.; Ramakrishn, S. Dual-drug encapsulation and release from core–shell nano-fibers. J. Biomater. Sci. Polym. Ed. 2012, 23, 861–871. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch na-noparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
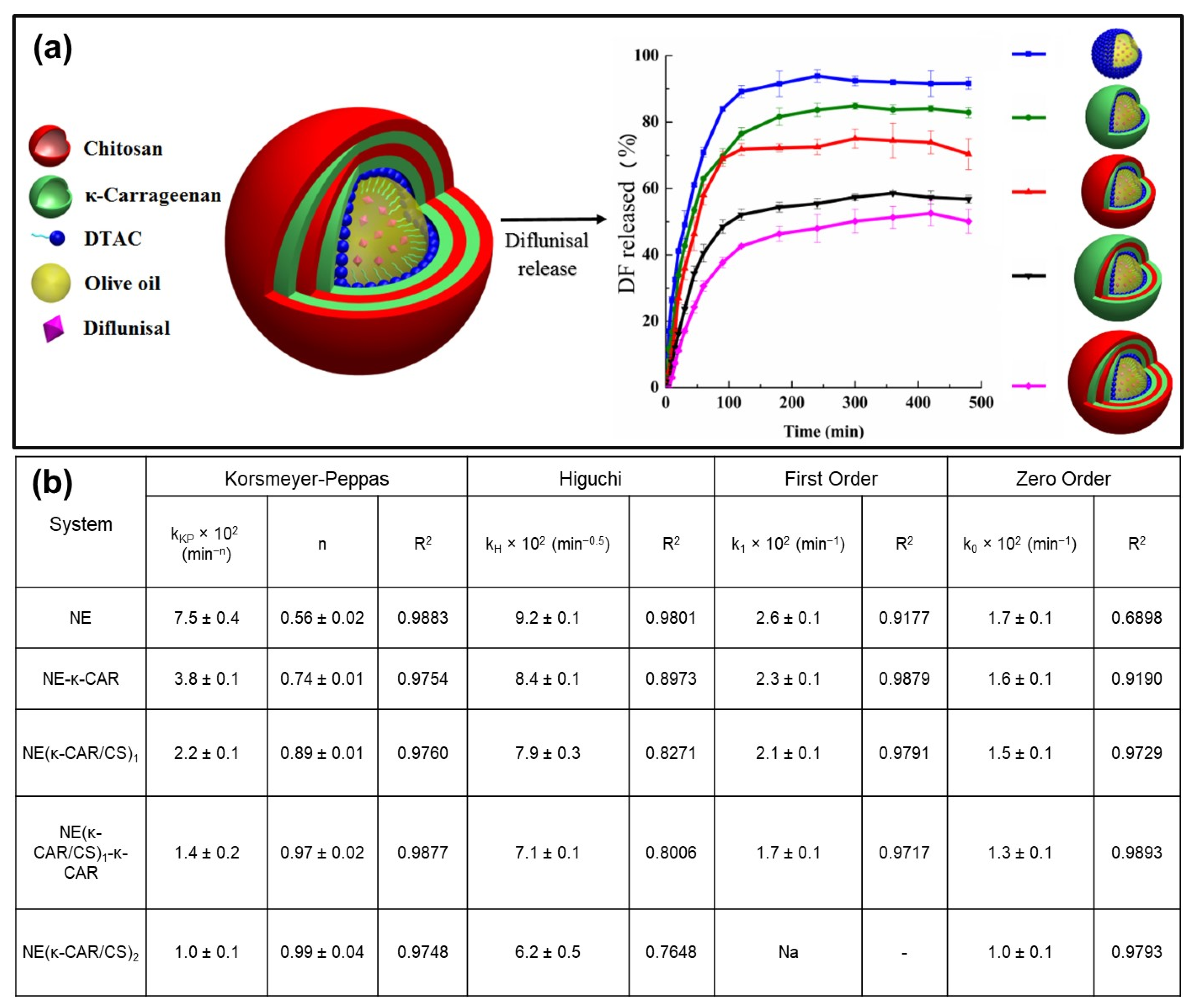
- Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martínez-Barbosa, M.E.; Vélaz, I.; Tánori, J. Drug release properties of diflunisal from layer-by-layer self-assembled κ-carrageenan/chitosan nanocapsules: Effect of de-posited layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef]
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. Effect of natural and synthetic surfactants on poly-saccharide nanoparticles: Hydrophobic drug loading, release, and cytotoxic studies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123618. [Google Scholar] [CrossRef]
- Santoyo-Aleman, D.; Sanchez, L.T.; Villa, C.C. Citric-acid modified banana starch nanoparticles as a novel vehicle for β-carotene delivery. J. Sci. Food Agric. 2019, 99, 6392–6399. [Google Scholar] [CrossRef]
- Tao, X.; Zhan, L.; Huang, Y.; Li, P.; Liu, B.; Chen, P. Preparation, characterization and evaluation of capsaicin-loaded indica rice starch nanoparticles. Food Chem. 2022, 386, 132692. [Google Scholar] [CrossRef]
- Ergin, A.D.; Bayindir, Z.S.; Ozcelikay, A.T.; Yuksel, N. A novel delivery system for enhancing bioavailability of S-adenosyl-l-methionine: Pectin nanoparticles-in-microparticles and their in vitro-in vivo evaluation. J. Drug Deliv. Sci. Technol. 2021, 61, 102096. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Marras-Marquez, T.; Peña, J.; Veiga-Ochoa, M.D. Robust and versatile pectin-based drug delivery sys-tems. Int. J. Pharm. 2015, 479, 265–276. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 42. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Sábio, R.M.; de Souza, M.P.C.; Fernandes, R.P.; de Oliveira, A.G.; Chorilli, M. Cellulose nanofibers improve the performance of retrograded starch/pectin microparticles for colon-specific delivery of 5-ASA. Pharmaceutics 2021, 13, 1515. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A bioinspired alginate-gum arabic hydrogel with mi-cro-/nanoscale structures for controlled drug release in chronic wound healing. ACS Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Pramod, P.S.; Shah, R.; Chaphekar, S.; Balasubramanian, N.; Jayakannan, M. Polysaccharide nano-vesicular mul-tidrug carriers for synergistic killing of cancer cells. Nanoscale 2014, 6, 11841–11855. [Google Scholar] [CrossRef] [PubMed]
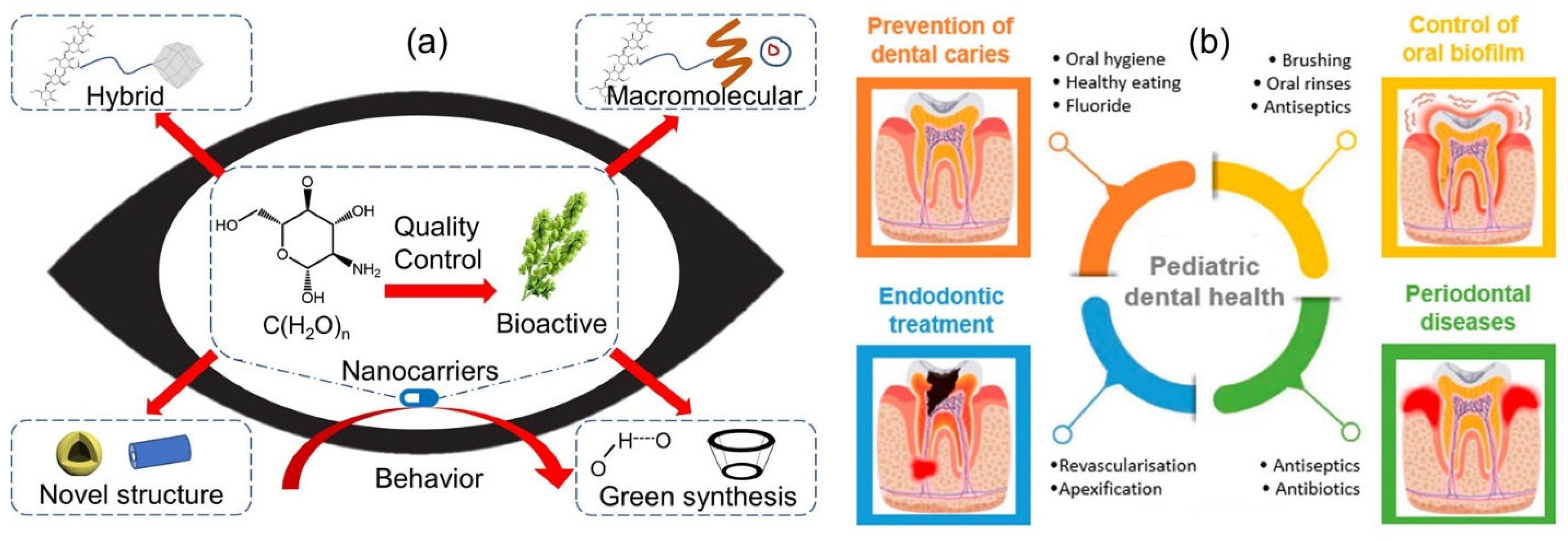
- Katsarov, P.; Shindova, M.; Lukova, P.; Belcheva, A.; Delattre, C.; Pilicheva, B. Polysaccharide-Based Micro-and Nanosized Drug Delivery Systems for Potential Application in the Pediatric Dentistry. Polymers 2021, 13, 3342. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of dextran as nanoscale drug carriers. Nanomedicine 2018, 13, 3149–3158. [Google Scholar] [CrossRef]
- Yu, H.; Wu, W.; Lin, X.; Feng, Y. Polysaccharide-based nanomaterials for ocular drug delivery: A perspective. Front. Bioeng. Biotechnol. 2020, 8, 601246. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, N.; Rasekh, B.; Mofradnia, S.R.; Yazdian, F.; Rashedi, H.; Tavakoli, Z. Molecular dynamics studies of polysaccharide carrier based on starch in dental cavities. Int. J. Biol. Macromol. 2019, 121, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J. Recent reports on polysaccharide-based materials for drug delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, Y.; Lin, S.; Wen, L.; Wu, D.; Zhao, M.; Chen, F.; Jia, Y.; Yang, B. Structural identification of (1→6)-α-D-glucan, a key responsible for the health benefits of longan, and evaluation of anticancer activity. Biomacromolecules 2013, 14, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liao, N.; Ye, X.; Hu, Y.; Wu, D.; Guo, X.; Zhong, J.; Wu, J.; Chen, S. Isolation and structural characterization of a novel antioxidant mannoglucan from a marine bubble snail, Bullacta exarata (Philippi). Mar. Drugs 2013, 11, 4464–4477. [Google Scholar] [CrossRef]
- Nie, S.; Cui, S.W.; Xie, M.; Phillips, A.O.; Phillips, G.O. Bioactive polysaccharides from Cordyceps sinensis: Isolation, structure features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2013, 1, 38–52. [Google Scholar] [CrossRef]
- Li, L.J.; Li, M.Y.; Li, Y.T.; Feng, J.J.; Hao, F.Q.; Zhang, L. Adjuvant activity of Sargassum pallidum polysaccharides against combined Newcastle disease, infectious bronchitis and avian influenza inactivated vaccines. Mar. Drugs 2012, 10, 2648–2660. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Yan, C.; Zhao, B.; Zhao, Y.; Yang, L.; Shi, M.; Yu, H.; Li, X.; Luo, K. Polysaccharide-Based Stimulus-Responsive Nano-medicines for Combination Cancer Immunotherapy. Small 2023, 2206211. [Google Scholar] [CrossRef]
- Alvarado-Hidalgo, F.; Ramírez-Sánchez, K.; Starbird-Perez, R. Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering. Molecules 2020, 25, 5286. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla de Stéfano, J.C.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. PH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Verma, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Stimuli-responsive polysaccharides for colon-targeted drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 547–566. [Google Scholar]
- Osorio, M.; Cañas, A.; Puerta, J.; Díaz, L.; Naranjo, T.; Ortiz, I.; Castro, C. Ex vivo and in vivo biocompatibility assessment (blood and tissue) of three-dimensional bacterial nanocellulose biomaterials for soft tissue implants. Sci. Rep. 2019, 9, 10553. [Google Scholar] [CrossRef] [PubMed]
- Bon, I.; Cano-Sarabia, M.; De la Ossa, N.; Bartolí, R.; Lorenzo-Zúñiga, V. Development and characterization of a new endoscopic drug-eluting platform with proven efficacy in acute and chronic experimental colitis. Front. Med. 2020, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Gomes, D.B.; Sousa, A.; Bidarra, S.J.; Petrini, P.; Moroni, L.; Barrias, C.C.; Granja, P.L. Biofunctionalized pectin hydrogels as 3D cellular microenvironments. J. Mater. Chem. B 2015, 3, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem. Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Chen, C.; Zhao, Y.; Jia, L.; Wang, P.C. Biopharmaceutics and therapeutic potential of engineered na-nomaterials. Curr. Drug Metab. 2008, 9, 697–709. [Google Scholar] [CrossRef]
- Blakey, G.E.; Nestorov, I.A.; Arundel, P.A.; Aarons, L.J.; Rowland, M. Quantitative structure-pharmacokinetics relationships: I. Development of a whole-body physiologically based model to characterize changes in pharmacokinetics across a homologous series of barbiturates in the rat. J. Pharmacokinet. Biopharm. 1997, 25, 277–312. [Google Scholar] [CrossRef]
- Moss, D.M.; Siccardi, M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br. J. Pharmacol. 2014, 171, 3963–3979. [Google Scholar] [CrossRef]
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: A pharmacokinetic issue. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 583–599. [Google Scholar] [CrossRef]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically based pharmacokinetic modeling of nanoparticles. J. Pharm. Sci. 2019, 108, 58–72. [Google Scholar] [CrossRef]
- Li, M.; Al-Jamal, K.T.; Kostarelos, K.; Reineke, J. Physiologically based pharmacokinetic modeling of nanoparti-cles. ACS Nano 2010, 4, 6303–6317. [Google Scholar] [CrossRef]
- Cai, X.; Jin, M.; Yao, L.; He, B.; Ahmed, S.; Safdar, W.; Ahmad, I.; Cheng, D.B.; Lei, Z.; Sun, T. Physicochemical properties, pharmacokinetics, toxicology and application of nanocarriers. J. Mater. Chem. B 2023, 11, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Banerjee, P.; Leung, S.S.Y.; Yan, X. Application of pharmacokinetic-pharmacodynamic modeling in drug delivery: Development and challenges. Front. Pharmacol. 2020, 11, 997. [Google Scholar] [CrossRef]
- Karch, J. Improving on Adjusted R-squared. Collabra: Psychol. 2020, 6, 45. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. Wiley Interdiscip. Rev. Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Mayer, B.X.; Mensik, C.; Krishnaswami, S.; Derendorf, H.; Eichler, H.G.; Schmetterer, L.; Wolzt, M. Pharmacoki-netic-pharmacodynamic profile of systemic nitric oxide-synthase inhibition with L-NMMA in humans. Br. J. Clin. Pharmacol. 1999, 47, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Jelvehgari, M. Natural polysaccharide based nanoparticles for drug/gene delivery. Pharm. Sci. 2017, 23, 84–94. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Rafiee, A.; Alimohammadian, M.H.; Gazori, T.; Riazi-rad, F.; Fatemi, S.M.R.; Parizadeh, A.; Haririan, I.; Havaskary, M. Comparison of chitosan, alginate and chitosan/alginate nanoparticles with respect to their size, stability, toxicity and transfection. Asian Pac. J. Trop. Dis. 2014, 4, 372–377. [Google Scholar] [CrossRef]
- De, S.; Robinson, D. Polymer relationships during preparation of chitosan–alginate and poly-L-lysine–alginate nanospheres. J. Control. Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Nitta, S.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Zomer Volpato, F.; Almodóvar, J.; Erickson, K.; Popat, K.C.; Migliaresi, C.; Kipper, M.J. Preservation of FGF-2 bioactivity using hepa-rin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012, 8, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Termsarasab, U.; Ko, S.H.; Shim, J.S.; Chong, S.; Chung, S.-J.; Shim, C.K.; Cho, H.J.; Kim, D.D. Hyaluronic acid derivativebased self-assembled nanoparticles for the treatment of melanoma. Pharm. Res. 2012, 29, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Ubonvan, T.; Kim, D.D. Hyaluronic acid in drug delivery systems. J. Pharm. Investig. 2010, 40, 33–43. [Google Scholar] [CrossRef]
- Hornig, S.; Bunjes, H.; Heinze, T. Preparation and characterization of nanoparticles based on dextran–drug conjugates. J. Colloid. Interface Sci. 2009, 338, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Zhang, Q.-Q.; Chen, H.L.; Li, X.M.; Jiang, Q.; Chen, M.M.; Gao, F.P.; Zhang, H.Z. Preparation and physicochemical characteristics of selfassembled nanoparticles of cholesterol succinate modified pullulan conjugates. IFMBE Proc. 2008, 19, 13–17. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in controlled drug delivery—A review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Martínez, A.; Fernández, A.; Prez, E.; Benito, M.; Teijn, J.M.; Blanco, M.D. Polysaccharide-based nanoparticles for controlled release formulations. In The Delivery of Nanoparticles; Hashim, A.A., Ed.; InTech Publisher: New York, NY, USA, 2012; pp. 185–222. [Google Scholar]
- Chiarappa, G.; Abrami, M.; Dapas, B.; Farra, R.; Trebez, F.; Musiani, F.; Grassi, G.; Grassi, M. Mathematical modeling of drug release from natural polysaccharides based matrices. Nat. Prod. Commun. 2017, 12, 1934578X1701200610. [Google Scholar] [CrossRef]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic na-nomedicine. Adv. Drug Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed]

| Classification of Polysaccharides Based on Different Natural Sources | |||
|---|---|---|---|
| Higher Plants | Algal | Animal Origin | Microbial |
| Starch | Alginates | Chitin | Dextran |
| Cellulose | Galactans | Chitosan | Gellan gum |
| Guar gum | Carrageenan | Glycosaminoglycans | Pullulan |
| Gum Arabic | Fucoidan | Hyaluronic acid | Xanthan gum |
| Locust bean gum | Ulvan (green macroalgae) | ||
| Source | Polysaccharide | Drug Form | Biological Activity and Applications |
|---|---|---|---|
| Animal | Heparin | Heparin sodium cream; heparin sodium lozenge; low molecular weight heparin sodium gel; heparin calcium for injection; heparin (sodium, calcium) injection | Anticoagulant, antiviral [29], a biosensor for thrombin [30], stabilize, deliver, and enhance growth factors like FGF-2 [31], anti-inflammatory and anti-angiogenic activity [32] |
| Chondroitin sulfate | Chondroitin sulfate tablets; chondroitin sulfate (chondroitin sulfate A sodium) capsules; chondroitin sulfate (chondroitin sulfate A sodium) injection | Coatings [31], cell growth, differentiation, morphogenesis, cell migration, and bacterial/viral infections [33], interactions with matrix proteins, activation of growth factors, regulation of angiogenesis, and melanoma cell invasion and proliferation [34], osteoarthritis [35] | |
| Hyaluronic acid | Sodium hyaluronate injection; sodium hyaluronate eye drops | Drug carriers [36], anti-arthritic [37], osteoarthritis [38] | |
| Plant | Astragalus PS | Astragalus polysaccharide injection (2-(chloromethyl)-4-(4-nitrophenyl)-1,3-thiazole) | Immunoregulatory [39], anti-oxidative [40], antiviral [41], and anti-tumor [42,43] |
| Ginseng PS | Ginseng polysaccharide injections | Immunostimulant [44], hypoglycemic [45], anti-inflammatory [46] | |
| Fucoidan PS | Active pharmaceutical ingredient | Cell proliferation and differentiation [47], immune modulation, cancer inhibition, and pathogen inhibition [48], antioxidant [49], antitumor [50], antiviral [51] | |
| Microbial | Lentinan PS | Lentinan injection; lentinan capsules; lentinus edodes mycelia polysaccharides tablets | Immunologic activities [52], antitumor [53], Hepatoprotective, and Antiviral [54] |
| Poria PS | Poria polysaccharide oral solution capsular | Antitumor [55], immunomodulation, anti-inflammation, antioxidation, anti-aging, antihepatitic, antidiabetics, and anti-hemorrhagic fever [56] | |
| Capsular PS | Vi polysaccharides typhoid vaccine; pneumococcal vaccine polyvalent; group A and C meningococcal polysaccharide vaccine | Vaccines and passive antibody therapies [57] | |
| Dextran | Dextran 40 glucose injection; dextran 70 eye drops; low molecule dextran | Biotechnological applications [58] |
| Smart Response | Biopolymer | Blend | Application | Reference |
|---|---|---|---|---|
| Sol–gel transition | Kappa carrageenan | Gellan gum | Ocular safety | [62] |
| Methylcellulose | Ophthalmic drug delivery system | [63] | ||
| Alginate | Gelrite | Ocular safety | [64] | |
| Hydroxypropyl methyl cellulose | Ophthalmic drug delivery system | [65] | ||
| – | Ophthalmic drug delivery system | [66] | ||
| Aminocaproic acid | Drug delivery | [67] | ||
| Dextran | Tyramine | Drug delivery/tissue engineering | [68] | |
| Hyaluronic acid | Tyramine | Drug delivery/tissue engineering | [69] | |
| Modified chitosan (chitosan-graft-glycolic acid) | – | Tissue engineering | [70] | |
| Swelling | Modified chitosan (N-succinyl-chitosan) | Aldehyde hyaluronic acid | Tissue engineering | [71] |
| Modified calmodulin (calcium-binding protein) | 3-[2-(trifluoromethyl)-10H-phenothiazin-10-yl]propan-1-amine | Drug delivery/microfluidic | [72] | |
| Poly(l-glutamic acid) | Phloretic acid | 3D cell culture and recovery/tissue engineering | [73] | |
| Degradation and release | Poly(l-glutamic acid) | Phloretic acid | 3D cell culture and recovery/tissue engineering | [73] |
| Alginate | – | Drug delivery | [74] | |
| Dextran | – | Drug delivery | [75] | |
| Self-assembly/folding | Peptide-hyaluronan hybrid hydrogel | – | Controlled release | [76] |
| Model Name | Equation | Measurable Variables and Definitions |
|---|---|---|
| Zero order | Qt = Cumulative amount of drug released at time t, t; Q0 = Initial drug amount in the matrix; K0 = zero-order release rate constant | |
| First-order | Qt = Cumulative amount of drug released at time t; Q0 = Initial amount of drug in the matrix; K1 = First-order release rate constant | |
| Higuchi | Qt = Cumulative amount of drug released at time t; KH = Higuchi’s release rate constant | |
| Hixson-Crowell | Qt = Cumulative amount of drug released at time t; Q0 = Initial amount of drug in the matrix; Ks = Release rate constant | |
| Baker–Lonsdale | Mt = Amount of drug released at time t; Mα = Amount of drug released at an initial time; Dm = diffusion coefficient; Cms = drug solubility in the matrix; r0 = radius of the spherical matrix; C0 = initial concentration of drug in the matrix | |
| Korsmeyer–Peppas | Mt/Mα = fraction of drug released at time t; k = kinetic constant; n = release exponent relating to transport mechanism | |
| Hopfenberg | Mt/Mα = fraction of drug dissolved; K0 = erosion rate constant; C0 = initial concentration of drug in the matrix; a0 = initial radius for matrix; n = 1, 2 and 3 for a slab, cylinder and sphere, respectively. | |
| Poiseuille’s law of laminar flow | dM/dt = drug release rate; c = concentration of drug in matrix; r = radius of orifice; η = viscosity of matrix; P1 − P2 = pressure difference between the inside and outside of the membrane. | |
| Weibull | m = fraction of the drug in solution at time t; a = time scale of the process; b = shape parameter; Ti = lag time |
| Drug | Mode of Release | Polysaccharide Matrix | Remarks | Reference |
|---|---|---|---|---|
| Dexamethasone and Levofloxacin | Opthalmic delivery | Glycol chitosan/hyalouranic acid hydrogel film | Burst release of levofloxacin followed by the sustained release for dexamethasone | [101] |
| Miconazole nitrate | Oral delivery | Chitosan-HPMC/Pectin film | Chitosan-HPMC film found to be superior as drug delivery support. | [102] |
| Peptides and proteins | Transdermal | Chitosan-tamarind seed polysaccharide composite film | The film is antimicrobial and stable. | [103] |
| Paracetamol | Colon delivery | Pectin/chitosan/hydroxyl propyl methyl cellulose films | Bimodal drug release | [104] |
| Bioactive materials | Wound dressing | Chitosan cyclodextrin inclusion complex based film | Presence of cyclodextrin prevent the loss of bioactives due to evaporation | [105] |
| Paracetyl aminophenol | In vitro | Silver loaded hydroxyl ethylacryl chitosan-sodium alginate hydrogel film | Presence of silver prolonged the drug release rate | [106] |
| Ketorolec methane | Transdermal delivery | Cellulose/nanofibril chitosan transdermal film | Sustained release of drug | [107] |
| Ellagic acid | Transdermal | Chitosan-ellagic acid-based films | Induce apoptotic death in human carcinoma cells. | [108] |
| Ciprofloxacin | In vitro | Chitosan/PVP/Guargum blended films | pH sensitive ternary blend film for the controlled release. | [109] |
| Betamethasone, Sulfadiazine | In vitro | Chitosan nanocellulose film | Ideal for wound dressings | [110] |
| Drug | Primary Effect | Spectrum | Side Effects |
|---|---|---|---|
| Ampicillin | Cidal | Broad (Gram+, some Gram−) | Allergic response, diarrhea, anemia |
| Bacitracin | Cidal | Narrow (Gram+) | Renal injury if injected |
| Carbenicillin | Cidal | Broad (Gram+, many Gram–) | Allergic responses, nausea, anemia |
| Cephalosporins | Cidal | Broad (Gram+, some Gram–) | Allergic responses, thrombophlebitis, renal injury |
| Chloramphenicol | Static | Broad (Gram+, Gram–; Rickettsia and Chlamydia) | Depressed bone marrow function, allergic reactions |
| Ciprofloxacin | Cidal | Broad (Gram+, Gram–) | Gastrointestinal upset, allergic responses |
| Clindamycin | Static | Narrow (Gram+, anaerobes) | Diarrhea |
| Dapsone | Static | Narrow (mycobacteria) | Anemia, allergic responses |
| Erythromycin | Static | Narrow (Gram+, mycoplasma) | Gastrointestinal upset, hepatic injury |
| Gentamicin | Cidal | Narrow (Gram–) | Allergic responses, nausea, loss of hearing, renal damage |
| Isoniazid | Static | Narrow (mycobacteria) | Allergic reactions, gastrointestinal upset, hepatic injury |
| Methicillin | Cidal | Narrow (Gram+) | Allergic responses, renal toxicity, anemia |
| Penicillin | Cidal | Narrow (Gram+) | Allergic responses, nausea, anemia |
| Polymyxin B | Cidal | Narrow (Gram–) | Renal damage, neurotoxic reactions |
| Rifampin | Static | Broad (Gram–, mycobacteria) | Hepatic injury, nausea, allergic responses |
| Streptomycin | Cidal | Broad (Gram+, Gram–; mycobacteria) | Allergic responses, nausea, loss of hearing, renal damage |
| Sulfonamides | Static | Broad (Gram+, Gram–) | Allergic responses, renal and hepatic injury, anemia |
| Tetracyclines | Static | Broad (Gram+, Gram–; Rickettsia and chlamydia) | Gastrointestinal upset, teeth discoloration, renal and hepatic injury |
| Trimethoprim | Cidal | Broad (Gram+, Gram–) | Allergic responses, rash, nausea, leukopenia |
| Vancomycin | Cidal | Narrow (Gram+) | Hypotension, neutropenia, kidney damage, allergic reactions |
| Active Substance | Indication | Mechanism of Action | Safety Notes |
|---|---|---|---|
| Docetaxel | Breast cancer, non-small cell lung cancer | Increased assembly of microtubule | Mutagenicity positive; Carcinogenicity is not tested |
| Paclitaxel | Soft tissue tumor | Inhibition of microtubule reorganization | Mutagenicity positive; Carcinogenicity is not tested |
| Doxorubicin | Soft tissue tumor, ovarian tumor | DNA intercalation | Mutagenicity positive; Carcinogenicity is positive |
| Cyclophosphamide | Breast cancer; ovarian cancer | DNA intercalation | Mutagenicity positive; Carcinogenicity is positive |
| Docetaxel | Breast cancer, advanced stomach cancer | Microtubule network reorganization inhibition | Mutagenicity positive Carcinogenicity not tested |
| Epirubicin | Breast cancer | DNA intercalation | Mutagenicity positive; Carcinogenicity not tested |
| 5-Fluorouracil | Head and neck cancer; breast cancer | Interferes with DNA replication | Mutagenicity positive; Carcinogenicity negative |
| Etoposide | Ewing’s sarcoma; uterine Cancer | Prevents re-ligation of the DNA strands | Mutagenicity positive; Carcinogenicity is limited |
| Rituximab | Follicular lymphomas | Bind to CD-20 | Mutagenicity is not tested Carcinogenicity is not tested |
| Oxaliplatin | Colon cancer; rectal cancer | Interfere with DNA replication | Mutagenicity positive; Carcinogenicity positive |
| Ifosfamide | Ewing’s sarcoma, germ cell tumor | Interfere with DNA replication | Mutagenicity positive; Carcinogenicity positive |
| Scientific Name | Anticancer Activity | Antioxidant Activity | ||
|---|---|---|---|---|
| Water | Ethanoic | Ethanoic | Water | |
| Atriplex sp. | 100 | 49 | 70.8 | 50.5 |
| Euphorbia paralias L. | 3.3 | 2.4 | 81.1 | 51.8 |
| Cakile maritime scop. | 89.7 | 90.8 | 56.3 | 55.6 |
| Panax quinquefolius | 64 | 2.6 | 11.7 | 56 |
| Zygophulum album L.F | 61.1 | 32.9 | 80.3 | 64.8 |
| Asparagus stipularis | 13 | 5.2 | 72.7 | 70.9 |
| Kochia indica wight | 2.88 | 1.6 | 50.4 | 72.4 |
| Retama raetam (Forssk) Webb | 2.6 | 1.4 | 80.2 | 78.1 |
| Olea europaea L. | 0 | 8.0 | 50.5 | 81.1 |
| Pituranthos tortusous | 11.2 | 14.3 | 58.4 | 81.4 |
| Limoniastrum monopetalum (L.) Boiss | 52.9 | 3.8 | 85.6 | 82 |
| Cistanche phelypaea (L.) | 37 | 100 | 50.7 | 85.6 |
| Moricandia nitens | 89.2 | 51 | 89.8 | 85.6 |
| Zygophulum simplex L. | 61.1 | 32.9 | 85.7 | 44.1 |
| Arum palaestinum | 97.3 | 19.4 | 12.7 | 43.1 |
| Anabasis artiaulata (Forssk.) Moq | 25 | 10 | 40.8 | 42.7 |
| Thymelaea hirsute (L.) Endl. | 54 | 18 | 78.6 | 35.3 |
| Astragalus pinosus. | 100 | 15.8 | 28.4 | 19.5 |
| Asphodelus microcarpus salzm | 9.1 | 1.9 | 60.3 | 49.5 |
| Solanum nigrum | 100 | 89.7 | 85.7 | 55.6 |
| Lotas polyphylles | 7.2 | 7.9 | 27.0 | 27.0 |
| Beta vulgaris | 64 | 7.0 | 41.1 | 30.3 |
| Herbs and spices | ||||
| Rosmarinus oficinalis | 80.0 | 61 | 38.4 | 65.1 |
| Camellia sinensis | 85 | 86.4 | 85.4 | 70.6 |
| Cockatiel | 9.8 | 22.9 | 56.7 | 71.4 |
| Punica granatum | 6.1 | 4 | 85.7 | 75.8 |
| Glycyrrhiza glabra | 36 | 81 | 47.4 | 84.1 |
| Capsicum annuum | 24.4 | 68.6 | 57.3 | 25.0 |
| Ocimum basilicum | 77.2 | 76.3 | 72.3 | 9.8 |
| Zingiber officinale | 47.8 | 4.9 | 55.9 | 35.5 |
| Curcuma longa | 39.4 | 72.4 | 6.4 | 43.4 |
| Cassia italca | 89.7 | 90.78 | 55.4 | 30.7 |
| Nigella sativa | 81 | 2.54 | 8.4 | 8.8 |
| Solenostemma argel | 24.66 | 95 | 41.3 | 7 |
| Parviflora | 7.83 | 1.55 | 42.7 | 40.3 |
| Matrix | Phenolic Compound | Wall Structure | Fabrication | Size Range (nm) | Target Application | Ref. |
|---|---|---|---|---|---|---|
| Cylocdextrin NPs | Curcumin & doxorubicin | Chitosan/poly(butyl cyanoacrylate) | Acidic anionic polymerization | 130–135 | Anticancer drug release | [146] |
| Cylocdextrin NPs | Catechin | Chitosan/poly(-glutamic acid) | Polyelectrolyte self-assembly | 140–150 | Controlled antioxidant release | [147] |
| Cylocdextrin NPs | Curcumin | Poly(butyl) cyanoacrylate (PBCA)/chitosan | Polymerization | 200 | Prevention of hepatic carcinoma with antiangiogenic effects | [148] |
| Cylocdextrin nanomicelles | Curcumin | β-lactoglobulin/alginate | Nano-suspension protein complexation | 280 | Sustained nutraceuticals delivery | [149] |
| Cylocdextrin NPs | Cathecin | β-cyclodextrin | Inclusion complex | 67–470 | Sustained antioxidant delivery | [150] |
| Cylocdextrin NPs | Oleoresin | Hydroxypropyl β-cyclodextrin | Inclusion complex | 100–105 | Sustained antibacterial delivery | [151] |
| Nano-starch | Quercetin | Cross-linked sodium trimetaphosphate | Self-assembly technique | 20–40 | Delivery through epithelium absorption | [152] |
| Micro-starch | Polyphenols from Hibiscus sabdariffa | Octenyl succinic anhydride | High shear homogenization | 500–800 | Sustained antibacterial effect | [153] |
| Micro-starch | Resveratrol | n/a | Solvent precipitation | 500–800 | Sustained antibacterial effect | [154] |
| Nano-starch | Curcumin | Polyvinyl alcohol | Sol-gel transformation | 300 | Controlled delivery of curcumin in cancer prevention | [155] |
| Amount of Drug (%) | 0.5 | 1 | 3 | 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | Intercept | Slope | R2 | Intercept | Slope | R2 | Intercept | Slope | R2 | Intercept | Slope | R2 |
| Zero order | 32.53 | 0.285 | 0.8642 | 34.9 | 0.332 | 0.8836 | 26.07 | 0.192 | 0.8298 | 17.77 | 0.138 | 0.8324 |
| First order | 3.47 | 0.006 | 0.7288 | 3.55 | 0.006 | 0.7679 | 3.25 | 0.005 | 0.7192 | 2.86 | 0.006 | 0.72 |
| Higuchi | 20.39 | 4.34 | 0.9724 | 20.95 | 5.023 | 0.9836 | 17.59 | 2.969 | 0.9636 | 11.68 | 2.131 | 0.9649 |
| Korsmeyer-Peppas | 1.28 | 0.262 | 0.9843 | 1.32 | 0.268 | 0.989 | 1.21 | 0.236 | 0.9906 | 1.03 | 0.245 | 0.9909 |
| Nanofiber Formulations | pH | Korsmeyer-Peppas Parameters | Mechanism of Release | |||
|---|---|---|---|---|---|---|
| PCL:Chitosan | Percent Drug (5FU) | n | a | R2 | ||
| 69:31 | 1 | 7.4 | 0.136 | 0.523 | 0.94 | Fickian diffusion |
| 4.4 | 0.1595 | 0.501 | 0.93 | |||
| 77:23 | 1 | 7.4 | 0.149 | 0.490 | 0.94 | Fickian diffusion |
| 87:13 | 1 | 7.4 | 0.143 | 0.481 | 0.93 | Fickian diffusion |
| 93:7 | 1 | 7.4 | 0.111 | 0.476 | 0.86 | Fickian diffusion |
| 100:0 | 1 | 7.4 | 0.370 | 0.151 | 0.96 | Fickian diffusion |
| Release Medium | Model | Equation | R2 | Release Kinetic | Mechanism |
|---|---|---|---|---|---|
| Simulated gastric fluid (SGF) | First order | Ln(1 − Q) = −0.01623 t − 0.08337 | 0.81752 | −0.01623 | Fickian diffusion |
| Higuchi | Q = 0.05996 t1/2 + 0.02079 | 0.96895 | 0.05996 | ||
| Weibull | LnLn [1/(1 − Q)] = 0.44996Lnt − 2.4400 | 0.93381 | 0.44996 | ||
| Ritger-Peppas | LogQ = 0.42334logt − 1.07969 | 0.92736 | 0.42334 | ||
| Simulated intestinal fluid (SIF) | First order | Ln(1 − Q) = −0.03724 − 0.12604 | 0.83910 | −0.03724 | Fickian diffusion |
| Higuchi | Q = 0.11943 t1/2 + 0.00116 | 0.97276 | 0.11943 | ||
| Weibull | LnLn[1/(1 − Q)] = 0.59929Lnt − 2.0242 | 0.91437 | 0.59929 | ||
| Ritger-Peppas | LogQ = 0.54001logt − 0.91097 | 0.89799 | 0.54001 | ||
| Simulated colonic fluid (SCF) | First order | Ln(1 − Q) = −0.12648 – 0.13138 | 0.96725 | −0.12648 | Case II transport |
| Higuchi | Q = 0.19442 t1/2 + 0.08698 | 0.95482 | 0.19442 | ||
| Weibull | LnLn[1/(1 − Q)] = 0.82806Lnt − 1.5129 | 0.98481 | 0.82806 | ||
| Ritger-Peppas | LogQ = 0.5275logt − 0.67359 | 0.92158 | 0.5275 |
| Kinetic Model | 3%Chit/30%CD- Cur-IC (pH 7.4) | 3%Chit/30%CD- Cur-IC (pH 5.4) | 2%Chit/2%Pect/ 30%CD-Cur-IC (pH 7.4) | 2%Chit/2%Pect/ 30%CD-Cur-IC (pH 5.4) |
|---|---|---|---|---|
| Zero-order | 0.4992 | 0.6901 | 0.5070 | 0.2005 |
| First-order | 0.7359 | 0.9485 | 0.7923 | 0.2181 |
| Higuchi | 0.7135 | 0.8461 | 0.7065 | 0.3727 |
| KorsmeyerPeppas | 0.6955 | 0.7148 | 0.6397 | 0.7271 |
| Diffusion exponent (n value) | 0.3813 | 0.4131 | 0.3473 | 0.3971 |
| Hixson-Crowell | 0.6602 | 0.9574 | 0.6912 | 0.2121 |
| Model | Parameter | Pea Starch | Potato Starch | Corn Starch |
|---|---|---|---|---|
| Peppas-Sahlin | R2 | 0.994 | 0.997 | 0.997 |
| AIC | 37.980 | 29.910 | 22.878 | |
| k1 | 20.868 | 10.740 | 19.305 | |
| k2 | −1.857 | −0.493 | −2.071 | |
| m | 0.325 | 0.457 | 0.226 | |
| Weibull Ti: Lag time. β: A constant related to the shape of the dissolution curve α: Scale parameter that defines the time scale. | R2 | 0.994 | 0.984 | 0.997 |
| AIC | 37.402 | 49.867 | 21.800 | |
| α | 2.845 | 5.033 | 4.379 | |
| β | 0.178 | 0.289 | 0.160 | |
| Ti | 4.342 | 3.493 | 2.651 | |
| Korsmeyer-Peppas K: A constant that depends on the dosage form characteristics. n: Release exponent that indicates the release mechanism | R2 | 0.976 | 0.966 | 0.995 |
| AIC | 51.299 | 56.493 | 25.911 | |
| k | 25.791 | 17.067 | 19.261 | |
| n | 0.161 | 0.240 | 0.146 | |
| Higuchi k: Higuchi dissolution constant. | R2 | 0.211 | 0.629 | 0.117 |
| AIC | 87.719 | 80.627 | 80.265 | |
| k | 5.258 | 4.993 | 3.660 | |
| Baker-Lonsdale kBL: A release constant. | R2 | 0.480 | 0.783 | 0.289 |
| AIC | 83.135 | 74.736 | 77.889 |
| Release Medium | Empty Cell | Mathematical Model | CAP | CAP-IRSNPs |
|---|---|---|---|---|
| 50% ethanol solution | Zero-order kinetics model | R2 | 0.425 | 0.551 |
| First-order kinetics model | R2 | 0.999 | 0.992 | |
| Higuchi model | R2 | 0.748 | 0.807 | |
| Korsmeyer-Peppas model | R2 | 0.995 | 0.873 | |
| n | 0.032 | 0.366 | ||
| Hixson-Crowell equation | R2 | 0.744 | 0.830 | |
| PBS of 1.2 | Zero-order kinetics model | R2 | 0.155 | 0.688 |
| First-order kinetics model | R2 | 0.996 | 0.996 | |
| Higuchi model | R2 | 0.293 | 0.878 | |
| Korsmeyer-Peppas model | R2 | 0.557 | 0.899 | |
| n | 0.111 | 0.434 | ||
| Hixson-Crowell equation | R2 | 0.379 | 0.961 | |
| PBS of 7.0 | Zero-order kinetics model | R2 | 0.524 | 0.461 |
| First-order kinetics model | R2 | 0.996 | 0.997 | |
| Higuchi model | R2 | 0.675 | 0.836 | |
| Korsmeyer-Peppas model | R2 | 0.828 | 0.887 | |
| n | 0.228 | 0.347 | ||
| Hixson-Crowell equation | R2 | 0.689 | 0.863 |
| Model * | Equation | R2 | R2adjusted | AIC | MSC | n |
|---|---|---|---|---|---|---|
| First-order | 0.7974 0.8280 | 0.7974 0.8280 | 66.63 39.04 | 0.8394 0.9753 | – – | |
| Hixson-Crowell | 0.6895 0.7167 | 0.6895 0.7167 | 70.48 42.03 | 0.4124 0.4763 | – – | |
| Higuchi | 0.8749 0.9730 | 0.8749 0.9730 | 62.30 27.92 | 1.321 2.8287 | – – | |
| Hopfenberg | 0.7140 0.7619 | 0.6731 0.7024 | 71.74 42.99 | 0.2722 0.3167 | – – | |
| Korsmeyer-Peppas | 0.9969 0.9937 | 0.9961 0.9921 | 16.87 21.19 | 4.378 3.9503 | 0.376 0.430 |
| Zero-Order | First-Order | Higuchi’s | Hixson-Crowell | Korsmeyer-Peppas | Kopcha | |||
|---|---|---|---|---|---|---|---|---|
| pH | R2 | R2 | R2 | R2 | R2 | n | R2 | A/B |
| 3.0 | 0.77 | 0.81 | 0.93 | 0.80 | 0.80 | 0.28 | 0.89 | 36.87 |
| 4.0 | 0.76 | 0.82 | 0.93 | 0.80 | 0.80 | 0.30 | 0.93 | 37.12 |
| 5.0 | 0.81 | 0.89 | 0.96 | 0.87 | 0.87 | 0.34 | 0.97 | 43.08 |
| 6.0 | 0.76 | 0.82 | 0.93 | 0.80 | 0.80 | 0.30 | 0.92 | 36.93 |
| 7.4 | 0.75 | 0.79 | 0.92 | 0.78 | 0.78 | 0.31 | 0.93 | 36.91 |
| Nanoparticle System | Physicochemical Properties | Comments | References |
|---|---|---|---|
| Chitosan | Shows mucoadhesive properties and ability to open tight junctions between epithelial cells | Its cationic nature mediatesdelivery of negative molecules such as DNA | [224,225] |
| Alginate | Shows mucoadhesive and gelling properties | Its anionic nature mediates deliveryof cationic agents | [226,227] |
| Heparin | An anionic and highly sulfated polysaccharide that shows anticoagulant properties | Ideal system for delivery of growthfactor | [228,229] |
| Hyaluronic acid | Affinity to water absorption and gel forming | Facilitates passive tumor targeting through CD44 receptor-mediated endocytosis | [230,231] |
| Dextran | A neutral polysaccharide with lower cytotoxicity | Degradation of nanoparticles occursby dextranase | [232] |
| Pulluan | A neutral polysaccharide produced by a specific fungus | Relative high cost of pullulan haslimited its application | [233] |
| Pectin | An anionic polysaccharide with gelling and film forming ability | Nanoparticles are degraded by pectinase secreted by bacteria present in the large intestine | [234,235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. https://doi.org/10.3390/pharmaceutics15051364
Bayer IS. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics. 2023; 15(5):1364. https://doi.org/10.3390/pharmaceutics15051364
Chicago/Turabian StyleBayer, Ilker S. 2023. "Controlled Drug Release from Nanoengineered Polysaccharides" Pharmaceutics 15, no. 5: 1364. https://doi.org/10.3390/pharmaceutics15051364
APA StyleBayer, I. S. (2023). Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics, 15(5), 1364. https://doi.org/10.3390/pharmaceutics15051364