Abstract
Candida auris is a multidrug-resistant pathogen against which echinocandins are the drug of choice. However, information on how the chitin synthase inhibitor nikkomycin Z influences the killing activities of echinocandins against C. auris is currently lacking. We determined the killing activities of anidulafungin and micafungin (0.25, 1, 8, 16 and 32 mg/L each) with and without nikkomycin Z (8 mg/L) against 15 isolates representing four C. auris clades (South Asian n = 5; East Asian n = 3; South African n = 3; South American n = 4, two of which were of environmental origin). Two and one isolates from the South Asian clade harbored mutations in the hot-spot 1 (S639Y and S639P) and 2 (R1354H) regions of the FKS1 gene, respectively. The anidulafungin, micafungin and nikkomycin Z MIC ranges were 0.015-4, 0.03-4 and 2->16 mg/L, respectively. Anidulafungin and micafungin alone exerted weak fungistatic activity against wild-type isolates and the isolate with a mutation in the hot-spot 2 region of FKS1 but was ineffective against the isolates with a mutation in the hot-spot 1 region. The nikkomycin Z killing curves were always similar to their respective controls. Twenty-two of sixty (36.7%) anidulafungin plus nikkomycin Z and twenty-four of sixty (40%) micafungin plus nikkomycin Z combinations produced at least 100-fold decreases in the CFUs (synergy), with a 41.7% and 20% fungicidal effect, respectively, against wild-type isolates. Antagonism was never observed. Similar results were found with the isolate with a mutation in hot-spot 2 of FKS1, but the combinations were ineffective against the two isolates with prominent mutations in hot-spot 1 of FKS1. The simultaneous inhibition of β-1,3 glucan and chitin synthases in wild-type C. auris isolates produced significantly greater killing rates than either drug alone. Further studies are warranted to verify the clinical efficacy of echinocandin plus nikkomycin Z combinations against echinocandin susceptible C. auris isolates.
1. Introduction
According to the World Health Organization, Candida auris is a critical priority fungal pathogen [1]. This classification underlines the need for novel and highly effective therapies against this emerging microorganism, which is associated with hospital outbreaks and the rapid emergence of resistance against conventional antifungal agents [2,3,4,5,6]. Recent guidelines recommend echinocandins as the drug of choice for severe C. auris infections [2,3,4,7,8]. However, clade-specific mortality is still high, especially among patients with COVID-19 infection, where 66%, 60% and 50% mortality rates were reported with the South American, South Asian, and South African clades, respectively [9,10,11]. Moreover, reports of echinocandin-resistant isolates emerging during treatment are also increasing [1,2,4]. Although antimicrobial peptides, metals, nanoparticles and natural compounds (i.e., quorum-sensing molecules) are often promising new approaches, their translation to clinical practice is often delayed due to the lack of safety and efficacy data in mammals [12,13,14,15]. To address the low activity of antifungal agents, numerous combinational therapies have been proposed, based mostly on in vitro data. Suggested combinations include multiple antifungal agents together, antifungal agents with antibiotics and antifungal agents combined with nonantimicrobial drugs [12,13,14,15].
Although nikkomycin Z is an obvious choice for this purpose, as phase I clinical results that support its tolerability in humans up to 2000 mg single per os dose are available [16,17,18], data on its activity against C. auris are scarce. Nikkomycin Z is a peptidyl nucleoside derived from Streptomyces tendae, with a novel mechanism of action targeting chitin synthesis of the fungal cell wall. It shows considerable activity against hard-to-treat fungi, such as Blastomyces and Coccidioides [18]. While the activity of nikkomycin Z against common Candida species alone is mediocre at best, synergism was commonly observed in combination with echinocandins, resulting in faster killing [18,19]. In animal models, nikkomycin Z was well tolerated. However, after intravenous and oral administration only a short drug half-life (15 and 60 min, respectively) was detected. Development is limited only by financial issues related to large-scale production and commercialization [18].
The aim of our study was to investigate the interaction of nikkomycin Z with micafungin and anidulafungin using a time–kill methodology and to assess whether this combination can overcome the echinocandin tolerance of C. auris isolates belonging to various lineages.
2. Materials and Methods
2.1. Isolates
Fifteen clinical isolates representing four of the five described C. auris clades (South Asian n = 5; East Asian n = 3; South African n = 3; South American n = 4) were used in this study. Two isolates from the South American clade were derived from the hospital environment. The type strain (NCPF 13029 = CBS 10913; East Asian clade) was also included (Table 1). C. auris isolates were identified by a combination of ribosomal DNA gene sequencing targeting the 28S rRNA and/or ITS1 regions, which was also used for clade delineation [20,21,22]. Two days before the experiments, the isolates were subcultured using Sabouraud agar and screened on CHROMagar Candida (Becton Dickinson) to ensure the purity of the Candida isolates.

Table 1.
MIC values of anidulafungin (ANI), micafungin (MICA) and nikkomycin Z (NIK) in RPMI 1640 against Candida auris isolates and type strain. MICs were determined using the CLSI broth microdilution method.
2.2. Whole Genome Sequencing
Library preparation was performed using the tagmentation-based Illumina DNAFlex Library Prep kit (Illumina, San Diego, CA, USA), according to the manufacturer’s protocol. Paired-end 300 bp sequencing was executed on an Illumina MiSeq instrument. The raw sequencing reads were aligned to the C. auris B8441 reference genome using the Burrows–Wheeler Aligner algorithm. The genetic variants (single-nucleotide polymorphisms, mutations and indel variants) were determined using the GATK algorithm. The library preparations, sequencing and data analysis were performed at the Genomic Medicine and Bioinformatics Core Facility of the University of Debrecen, Hungary [23]. Two isolates (28 and 208) from the South Asian clade harbored mutations (S639Y and S639P, respectively) in hot-spot 1 of the FKS1 gene region, as determined by whole genome sequencing. In the case of isolate 20 (South Asian clade), a mutation (R1354H) was found in hot-spot 2 of the FKS1 gene. The remaining 12 isolates showed wild-type genotypes (Table 1).
2.3. Antifungal Susceptibility Testing
We conducted the MIC assays in U-bottom, tissue culture-treated microtiter test plates (TPP Techno Plastic Products AG, Switzerland; cat. no. 92097). Anidulafungin and micafungin were obtained from Molcan Corporation (Richmond Hill, ON, Canada). Nikkomycin Z was purchased from Sigma (Budapest, Hungary). Antifungals were dissolved in 100% DMSO and diluted further in RPMI 1640 to final concentrations between 0.015 and 8 mg/L for anidulafungin or micafungin and 0.03 and 16 mg/L for nikkomycin Z. The starting inoculum was ~103 CFU/mL. Plates were incubated at 35 °C, and MICs were read visually after 24 h using the partial inhibition criterion [24,25]. The MICs were determined at least in duplicate. For categorization, tentative MIC breakpoints, as suggested by the Centers for Disease Control and Prevention, were used: susceptible ≤ 2 mg/L for both anidulafungin and micafungin [7]. Clinical breakpoints or epidemiological cutoff values have not been published for nikkomycin Z against Candida species.
2.4. Time–Kill Studies
The anidulafungin and micafungin killing curves were determined with all isolates in RPMI 1640 at 0.25, 1, 8, 16 and 32 mg/L [21]. As previous pharmacokinetic studies have shown that nikkomycin Z concentrations in serum did not exceed 8 mg/L, the highest nikkomycin Z concentration tested was 8 mg/L [17,18]. Independently of measured nikkomycin Z MICs, the highest concentration of echinocandins tested were 32 mg/L for all isolates. The starting inocula were 2–6 × 105 CFU/mL. The samples were removed at 0, 4, 8, 12 and 24 h, serially diluted ten-fold, plated (4 × 30 mL) onto a single Sabouraud dextrose agar and incubated at 35 °C for 48 h. The limit of detection was 50 CFU/mL. Fungicidal or fungistatic activity was defined as ≥3 log CFU/mL or <3 log CFU/mL changes in the viable cell count compared to the starting inoculum. All experiments were performed twice; the means of the resulting data are presented. The killing kinetics at the tested concentrations were analyzed, as described previously [21]. Positive killing rate (k) values indicate killing, and negative k values indicate growth. The mean times to achieve a 99.9% reduction of the starting inoculum (T99.9 = 3/k) were calculated from the k values for each isolate and concentration [21].
2.5. Combination of Anidulafungin and Micafungin plus Nikkomycin Z in Time–Kill Studies
Nikkomycin Z was added at a single, fixed (8 mg/L) concentration to 0.25, 1, 8, 16 and 32 mg/L of anidulafungin and micafungin [19]. The efficacy was determined as described above, and the T99.9 values were calculated as well. Synergy and antagonism were defined, respectively, as a 100-fold increase or decrease in killing compared with the killing of the most active single antifungal. If the change was less than 100-fold, the interaction was considered additive. Interactions were determined after 24 h [22].
2.6. Statistical Analysis
One-way ANOVA with Tukey’s post-testing was used to analyze differences in the killing kinetics among isolates and concentrations of anidulafungin and micafungin with or without nikkomycin Z. The t-test (with Welch’s correction, where appropriate) was used for the same anidulafungin and micafungin concentrations to test differences in the killing kinetics of anidulafungin plus nikkomycin Z and micafungin plus nikkomycin Z [19,21].
3. Results
3.1. Anidulafungin, Micafungin and Nikkomycin Z MIC Values against four Candida auris Clades
The anidulafungin and micafungin MICs (with the exception of isolate 28, South Asian clade, with a mutation in hot-spot 1 of the FKS1 gene) were not higher than the tentative MIC breakpoints suggested by the CDC [7]. The micafungin MICs were the same or 1–3 dilutions higher than the anidulafungin MICs (Table 1).
The nikkomycin Z MICs ranged from 2 to >16 mg/L; 5 of 15 of the isolates were not inhibited, even at 16 mg/L (Table 1).
3.1.1. Nikkomycin Z Killing Activity against four Candida auris Clades
Regardless of the isolate and clade, nikkomycin Z did not produce even transient CFU decreases at the tested concentrations; the largest decrease measured was 0.17 log CFU in the case of isolate 20 (MIC = 2 mg/L, South Asian clade). The time–kill curves for isolates 20 and I-156 (MIC = 4 mg/L) (South Asian and South American clades, respectively) are shown in Supplementary Materials Figure S1.
3.1.2. Anidulafungin and Anidulafungin plus Nikkomycin Z Killing Activities against Four Candida auris Clades
South Asian Clade
Anidulafungin showed fungistatic effect against the two wild-type isolates, and the highest CFU decreases (1.4 log) and k values (0.22 1/h) were found with isolate 27 at 8 and 32 mg/L (Table 2 and Figure 1). In the case of isolate 20, with a mutation in hot-spot 2 of FKS1, the k values were negative at 0.25 and 1 mg/L (−0.09 and −0.12 1/h, respectively). For the two isolates (28 and 208) with prominent mutations in hot-spot 1 of FKS1, anidulafungin produced a weak fungistatic effect in the first 4–8 h, with prominent regrowth and k values that were always negative after 24 h (Table 2, Figure 1 and Supplementary Materials Figure S2). The addition of 8 mg/L of nikkomycin Z to anidulafungin increased the k values for the two wild-type isolates (Table 2 and Figure 1). With isolate 196, synergy (more than 2 log CFU decreases) was noted with a fungicidal effect from 1 to 32 mg/L anidulafungin plus 8 mg/L nikkomycin Z (T99.9 ranged from 4.7 to 8.7 h) at all tested concentrations (Table 2). Although the nikkomycin Z MICs for isolates 20 and 27 were higher than 16 mg/L, 8–32 mg/L anidulafungin plus 8 mg/L nikkomycin Z was fungicidal in the case of isolate 27 (T99.9 ranged from 2.6 to 5.8 h, Supplementary Materials Table S1), and the k values were positive at all tested combinations (k value ranges were 0.05–0.07 1/h) in the case of isolate 20. However, synergy was not observed for these isolates.

Table 2.
Maximum changes in log CFU/mL compared to the starting inoculum in time–kill studies at different anidulafungin (mg/L), and anidulafungin (mg/L) plus 8 mg/L nikkomycin Z concentrations (mg/L) for the four Candida auris clades. Data in bold and underlined indicate a synergistic (more than 2 log CFU decreases in CFU) and fungicidal effect (at least 3 log decreases in CFU), respectively.
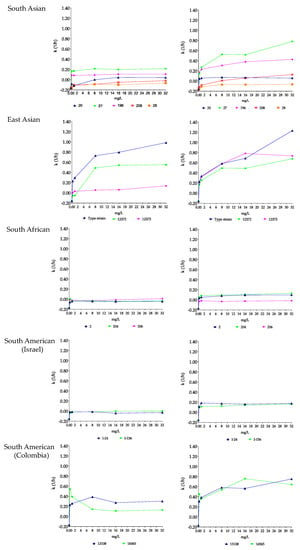
Figure 1.
Killing rate (k) values of anidulafungin (left) and anidulafungin plus nikkomycin Z (right) in RPMI 1640 against South Asian (isolates 20, 27, 28, 196 and 208), East Asian (type strain = NCPF 13029 = CBS 10913, and isolates 12372 and 12373), South African (isolates 2, 204 and 206) and South American (isolates I-24 and I-156 from Israel and hospital environmental isolates 13108 and 16565 from Colombia) clades. Anidulafungin was tested at 0.25, 1, 8, 16 and 32 mg/L with (8 mg/L) and without nikkomycin Z. Positive and negative k values indicate a decrease and increase, respectively, in viable cell numbers. Error bars were omitted for better visualization of the graphics.
In the case of isolate 28, the addition of nikkomycin Z to anidulafungin produced only transient CFU decreases after 4 h, followed by marked regrowth after 24 h (0.54–1.42 log CFU/mL increases; k values ranged from −0.07 to −0.15 1/h) (Table 2 and Figure 1). In contrast, in the case of isolate 208, 8–32 mg/L anidulafungin plus 8 mg/L nikkomycin Z produced positive k values; the highest k value (0.13 1/h) was found with 32 mg/L anidulafungin plus 8 mg/L nikkomycin Z (Table 2, Figure 1 and Supplementary Materials Figure S2).
East Asian Clade
Anidulafungin showed concentration-dependent killing activity against the type strain with a 3 log CFU decrease at 32 mg/L (T99.9 was 3.04 h, Supplementary Materials Table S1). Against the remaining two isolates, anidulafungin was fungistatic and regrowth was always observed (Table 2 and Figure 1). In combination experiments, 16–32 mg/L anidulafungin with nikkomycin Z produced a rapid fungicidal effect (T99.9 = 1.7−2.9 h) against the type strain (Figure 1 and Supplementary Materials Table S1). Nikkomycin Z increased the killing activity of anidulafungin at all concentrations against isolates 12372 and 12373; 1–32 mg/L anidulafungin plus 8 mg/L nikkomycin Z proved to be synergistic against both isolates (Table 2). Moreover, 8–32 mg/L anidulafungin plus 8 mg/L nikkomycin Z produced a fungicidal effect (Figure 1 and Figure 2, Supplementary Materials Table S1).
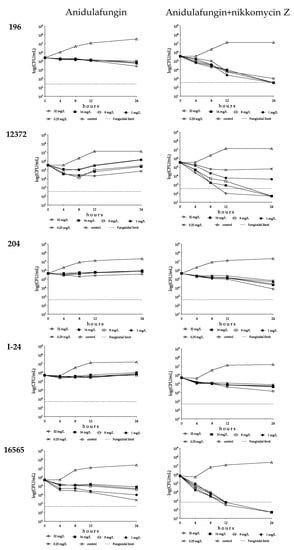
Figure 2.
Time–kill plots of anidulafungin (left) and anidulafungin plus nikkomycin Z (right) in RPMI 1640 against Candida auris isolates 196 (South Asian clade), 12372 (East Asian clade), 204 (South African clade), I-24 (bloodstream isolate from Israel, South American clade) and 16565 (hospital environmental isolate from Colombia, South American clade). Anidulafungin was tested at 0.25, 1, 8, 16 and 32 mg/L with (8 mg/L) and without nikkomycin Z.
South African Clade
Anidulafungin against the South African clade produced a weak fungistatic effect with k values that were almost always negative (Figure 1 and Figure 2, Table 2). A total of 8 mg/L nikkomycin Z increased the killing activity of anidulafungin against isolates 2 and 204 (k values were positive at all tested combinations; Figure 1 and Figure 2, Table 2), with synergy in the case of isolate 204 at 32 mg/L anidulafungin plus 8 mg/L nikkomycin Z (Table 2). Transient CFU decreases were found in the case of isolate 206 in the first 4–8 h, but after 24 h regrowth (0.08–0.20 CFU increases) was observed at the tested combinations (Figure 1 and Table 2).
South American Clade
Anidulafungin showed a negligible fungistatic effect against the isolates from Israel, with regrowth always observed (CFU increase ranges were 0.10–0.30 and 0.16–0.56 for isolates I-24 and I-156, respectively) (Figure 1 and Figure 2, Table 2). Nikkomycin Z significantly increased the killing activity of anidulafungin, with k values that were always positive at all tested combinations for both isolates (Figure 1 and Figure 2). In the case of isolate I-156, synergy was observed when 32 mg/L anidulafungin was combined with 8 mg/L nikkomycin Z (Table 2).
Anidulafungin was fungistatic against the environmental isolates of the South American clade, with higher k values at lower concentrations in the case of isolate 16565 (mini-paradoxical effect) (Figure 1 and Table 2). The combination of anidulafungin with nikkomycin Z produced a rapid fungicidal effect (T99.9 value ranges for isolates 13108 and 16565 were 1.1–6.6 and 2.6–5.6 h, respectively) (Figure 1 and Figure 2, Supplementary Materials Table S1). Moreover, 9 of the 10 drug combinations were synergistic (Table 2).
3.1.3. Micafungin and Micafungin plus Nikkomycin Z Killing Activities against Four Candida auris Clades
South Asian Clade
With isolates 27 and 196, the CFU decreases were higher at 0.25 or 1 mg/L than at 8–32 mg/L (mini-paradoxical effect); the highest k value was measured with isolate 27 at 1 mg/L (k = 0.66 1/h) (Figure 3 and Figure 4, and Table 3). Micafungin produced small CFU decreases with prominent regrowth (0.32–0.85 log increases) and negative k values at 1–32 mg/L against isolate 20 (R1354H mutation in hot-spot 2 of FKS1). The killing activity of micafungin against this isolate increased significantly in the presence of 8 mg/L nikkomycin Z at all concentrations (k value ranges were 0.08–0.11 1/h), with synergy at 0.25–1 mg/L micafungin plus 8 mg/L nikkomycin Z (Figure 3 and Table 3). For isolates 27 and 196, four of ten drug combinations showed synergy (Table 3). Moreover, in the case of isolate 27, a slow fungicidal effect (T99.9 = 18.1 h) was observed at 32 mg/L micafungin plus 8 mg/L nikkomycin Z (Figure 4, Table 3 and Supplementary Materials Table S2). In contrast, against isolates 28 and 208 (mutations in hot-spot 1 of FKS1), micafungin with or without nikkomycin Z produced only transient CFU decreases in the first 4 h of exposure with prominent regrowth and negative k values after 24 h (Figure 3 and Table 3).
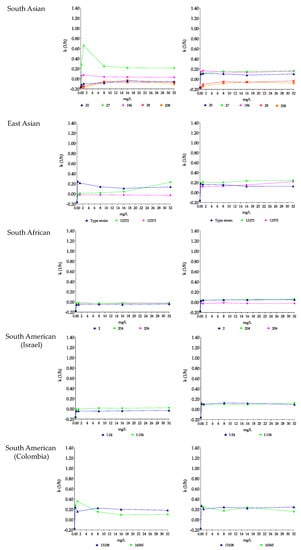
Figure 3.
Killing rate (k) values of micafungin (left) and micafungin plus nikkomycin Z (right) in RPMI 1640 against South Asian (isolates 20, 27, 28, 196 and 208), East Asian (type strain = NCPF 13029 = CBS 10913, and isolates 12372 and 12373), South African (isolates 2, 204 and 206) and South American (isolates I-24 and I-156 from Israel and hospital environmental isolates 13108 and 16565 from Colombia) clades. Micafungin was tested at 0.25, 1, 8, 16 and 32 mg/L with (8 mg/L) and without nikkomycin Z. Positive and negative k values indicate a decrease and increase, respectively, in viable cell numbers. Error bars were omitted for better visualization of the graphics.
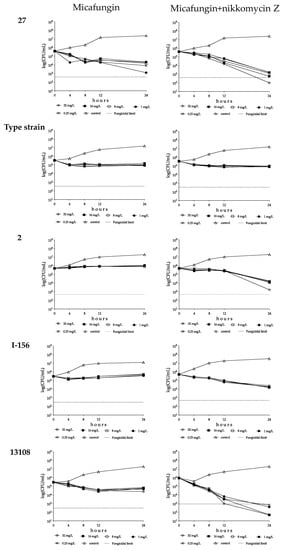
Figure 4.
Time–kill plots of micafungin (left) and micafungin plus nikkomycin Z (right) in RPMI 1640 against isolates 27 (South Asian clade), NCPF 13029 = CBS 10913 (type strain, East Asian clade), 2 (South African clade), I-156 (bloodstream isolate from Israel, South American clade) and 13108 (hospital environmental isolate from Colombia, South American clade). Micafungin was tested at 0.25, 1, 8, 16 and 32 mg/L with (8 mg/L) and without nikkomycin Z.

Table 3.
Maximum changes in log CFU/mL compared to the starting inoculum in time–kill studies at different micafungin (mg/L), and micafungin (mg/L) plus 8 mg/L nikkomycin Z concentrations (mg/L) for the four Candida auris clades. Data in bold and underlined indicate a synergistic (more than 2 log CFU decreases in CFU) and fungicidal effect (at least 3 log decreases in CFU), respectively.
East Asian Clade
Micafungin was fungistatic against isolates from the East Asian clade, with regrowth almost always observed (Table 3). In the case of the type strain, the k values at 0.25–1 mg/L were significantly higher than at 8–32 mg/L (0.21–24 1/h and 0.11–0.14 1/h, respectively, Figure 3 and Figure 4). For isolate 12373, the k values were negative at all tested concentrations (Figure 3). In the combination experiments, for the type strain nikkomycin Z did not significantly alter killing (mini-paradoxical effect) compared to micafungin alone (Figure 3 and Figure 4). In contrast, for isolates 12372 and 12373, 8 of 10 drug combinations led to synergy, and a slow fungicidal effect (T99.9 = 23.7 h) was noticed with isolate 12373 at 32 mg/L micafungin plus 8 mg/L nikkomycin Z (Table 3 and Supplementary Materials Table S2).
South African Clade
Micafungin showed a weak fungistatic effect against the three isolates from this clade, with growth or transient CFU decreases (k values were always negative for the three isolates) (Figure 3 and Figure 4, and Table 3). Nikkomycin Z significantly increased the killing activity of micafungin at all tested concentrations with isolates 2 and 204. Moreover, in the case of isolate 2, synergy was noticed at the two highest micafungin concentrations (Figure 3 and Figure 4, and Table 3). In the case of isolate 206, similar to anidulafungin plus nikkomycin Z combinations, the k values remained negative at all tested combinations (k value ranges were from −0.01 to −0.03 1/h) (Figure 3).
South American Clade
Micafungin generated a weak fungistatic effect against clinical isolates; the k values were positive only in the case of isolate I-156 at 8–32 mg/L (0.01–0.02 1/h) (Figure 3 and Figure 4). The micafungin k values significantly increased with nikkomycin Z; at all tested concentrations micafungin plus nikkomycin Z produced concentration-independent killing activities (k value ranges were 0.09–0.12 1/h for both isolates) without a synergistic effect (Figure 3 and Figure 4, and Table 3).
Micafungin against the environmental isolates was fungistatic, and the k values were lower at 8–32 mg/L than at 0.25 or 1 mg/L (mini-paradoxical effect) (Figure 3 and Figure 4, and Table 3). Adding nikkomycin Z to micafungin produced a fungicidal effect with synergy in all combinations (10 of 10) for both isolates (Figure 3 and Figure 4, Table 3 and Supplementary Materials Table S2).
4. Discussion
The anidulafungin and micafungin MICs were lower than the tentative breakpoint (≤2 mg/L) suggested by the CDC for all tested wild-type isolates and two of three isolates with FKS mutations, a finding which may suggest the need to re-examine the breakpoints. With the exception of anidulafungin against the type strain (NCPF 13029 = CBS 10913) at 32 mg/L (Table 2), both tested echinocandins showed fungistatic activities against wild-type C. auris clinical isolates and the isolate with a mutation in hot-spot 2 of FKS1 with frequent regrowth or, to a lesser extent, decreased killing activities at higher drug concentrations (mini-paradoxical effect). The two echinocandins were ineffective against the isolates with the known resistance mutation in hot-spot 1 of FKS1. Nikkomycin Z alone was not fungistatic against C. auris isolates, even at high drug concentrations (8–16× MIC values; Figure 1); the killing curves were always similar to their respective controls, even against isolates with lower MICs (2–4 mg/L). The killing activities of anidulafungin and micafungin against the wild-type isolates were significantly increased in the presence of nikkomycin Z with the exception of isolate 206 (South African clade). The extent varied considerably in an isolate-dependent manner, ranging from a slight increase in the maximum CFU decrease to a weak fungistatic effect turning to rapid fungicidality. The mini-paradoxical effect was also eliminated with nikkomycin Z, except in the case of micafungin against the type strain. It is notable that 22 of 60 (36.7%) anidulafungin plus nikkomycin Z and 24 of 60 (40%) micafungin plus nikkomycin Z combinations resulted in at least 100-fold decreases in CFUs (synergistic interaction) against wild-type isolates. Moreover, these combinations frequently resulted in fungicidal activity when nikkomycin Z was combined with anidulafungin (25/60 = 41.7%) or micafungin (12/60 = 20%) (Table 2 and Table 3). Notably, an antagonistic interaction was never observed. Nikkomycin Z significantly increased the killing activities of the two echinocandins against the isolate with an R1354H mutation in hot-spot 2 of FKS1, sometimes with synergism. Even against one of the two isolates with the established mutations in hot-spot 1 of FKS1 (isolate 208), the 32 mg/L anidulafungin plus 8 mg/L nikkomycin Z produced CFU decreases without regrowth (Supplementary Materials Figure S2).
Our results are consistent with previous observations on the South African clade that echinocandins are weakly fungistatic [21,26]. Unfortunately, data on the in vitro pharmacodynamics of echinocandins against other clades are still limited. Nikkomycin Z MIC values for isolates of the South Asian and South American clades were similar to each other and lower than those observed with isolates from the East Asian clade. For isolates of the South African clade, the MIC values were lower than those reported for 100 isolates tested by Bentz et al. [16]. The lack of time–kill studies with nikkomycin Z alone or with echinocandins against different C. auris clades in that study precluded a comparison with our results.
Our results confirmed the positive interaction between echinocandins and nikkomycin Z using a time–kill methodology [19,27,28]. Poester et al. combined micafungin or caspofungin with nikkomycin Z (nikkomycin Z MICs were higher than 64 mg/L for all isolates) against 11 C. auris isolates using the checkerboard method. Micafungin with nikkomycin Z showed additive and synergistic interactions with six of seven and one of seven isolates (all belonging to the South Asian clade), respectively. The combination of caspofungin with nikkomycin Z proved to be synergistic against all five tested isolates of the South Asian clade and against the one tested isolate of the South African clade. However, killing studies were again not performed, and echinocandin resistant isolates were not tested [27].
An important strength of our study is that whole genome sequencing was performed with all isolates used in this study and included not only wild-type isolates but isolates with known mutations in hot-spots 1 and 2 of FKS1. One possible limitation is that the number of the isolates tested per some clades was relatively low and nikkomycin Z was tested only at 8 mg/L. However, in the killing studies, nikkomycin Z was tested with both echinocandins at five different concentrations (75 combinations of nikkomycin Z with each of anidulafungin and micafungin). Moreover, the time–kill methodology, in contrast to the checkerboard method, is suitable for measuring the ≥99.9 CFU decreases (i.e., fungicidal endpoint) and for distinguishing this from a fungistatic effect. The next-generation echinocandin rezafungin was not available for this study, and we did not test the third approved echinocandin, caspofungin, which may be regarded as another limitation. However, caspofungin susceptibility testing is not recommended using broth microdilution methodologies because of the significant interlaboratory variability observed using broth-based methods [29].
Briano et al. reported a significant association between multisite C. auris (South Asian clade) colonization (skin, respiratory and/or urinary) and development of C. auris candidemia in intensive care units during the COVID-19 outbreak (Genoa, Northern Italy), with a 27% crude 30-day mortality rate [30]. Alarmingly, 7 of 27 patients developed late recurrent candidemia during echinocandin treatment, and the MICs of caspofungin (4 mg/L) and amphotericin B (2 mg/L) increased against these isolates (in one case each). Patients with isolates showing low MICs to echinocandins were treated again with echinocandins, while the patient with an isolate that exhibited a high MIC to caspofungin was treated with anidulafungin plus flucytosine. The mortality was 57% (four of seven), suggesting that the killing activity of echinocandins against C. auris was weak among those critically ill patients [30]. Other authors have also reported septic metastatic complications (i.e., spondylodiscitis, meningitis and endo- and pericarditis) during echinocandin treatment [6,31]. The poor eradication of the fungus from the bloodstream seems, thus, fully in accordance with the weak in vitro fungistatic activity of echinocandins against wild-type C. auris [21,26].
Higher daily echinocandin doses may increase the cure rate among patients with invasive candidiasis. Although larger daily echinocandin doses were well tolerated in clinical situations, a significantly better cure rate was not detected [32,33]. Combination therapy is another strategy to combat invasive fungal diseases, including C. auris infections. Amphotericin B and its lipid formulations, voriconazole, isavuconazole and even flucytosine, have been added to the echinocandins to increase the cure rate with invasive C. auris infections. However, the full clinical significance of combination therapy is still unknown [12,15,18,34].
The mechanism responsible for the weak in vitro killing activity of echinocandins against C. auris is not well understood but may be related to aggregate formation by C. auris cells, as demonstrated both in vitro and in vivo [20,22]. Isolates of the South Asian clade have been reported to possess a higher basal chitin content in the cell wall compared to C. albicans, C. tropicalis and C. guilliermondii, as well as a higher basal expression of CHS2 genes [35,36]. Pezotti et al. reported larger amounts of α-1, 3-glucans but smaller amounts of chitin in isolates from the East Asian and South African clades compared to their C. albicans counterparts using Raman spectroscopic methods [37]. As echinocandins inhibit β-1, 3-glucan but not α-1, 3-glucan synthesis, the relatively lower amount of β-1, 3-glucan in C. auris might explain the weaker inhibition of C. auris growth compared to C. albicans and other Candida species. However, echinocandin-induced cell wall damage increases chitin synthase activity with the resulting increased amounts of chitin in the cell wall compensating for the decreased β-1, 3-glucan levels [19]. In the current study, the simultaneous inhibition of β-1, 3-glucan and chitin synthases significantly increased the killing rate against the four clades, with the exception of one isolate from the South African clade, suggesting that depleting one of the cell wall components is insufficient for killing, and depleting both seems to impair the viability significantly, which would explain the synergism observed between the two echinocandins and nikkomycin Z. Synergism was found in the case of an isolate with mutations in hot-spot 2 of FKS1, which also exhibited low echinocandin MICs. The lack of synergism between the two echinocandins and nikkomycin Z against the two isolates with prominent mutations in hot-spot 1 of FKS1 can be explained by a high basal or inducible chitin content compared to the wild-type isolates [38]. However, 8 mg/L of nikkomycin Z significantly increased the killing activity of 32 mg/L anidulafungin in the case of isolate 208 (with an HS1 S639P mutation), which had a low anidulafungin MIC (0.5 mg/L). This suggests that different echinocandin resistance mutations may affect synergy differently.
Since chitin is not found in mammals, the effects of nikkomycin Z are highly selective for fungi. The side effects are minimal, as demonstrated in preclinical and multidose human safety phase I trials [16,17,18]. Though nikkomycin Z alone is ineffective to treat Candida infections, earlier works and this study have shown that nikkomycin Z in combination with echinocandins produced synergistic or additive interactions without antagonism against Candida species [19]. The coadministration of nikkomycin Z with echinocandins might improve the outcome of invasive C. auris infections, particularly in immunosuppressed patients, warranting more efforts towards the cost-effective production and commercialization of nikkomycin Z [17,18].
5. Conclusions
Nikkomycin Z alone has no measurable killing activity, while anidulafungin and micafungin showed weak or moderately fungistatic activity against wild-type C. auris. The killing activities of anidulafungin and micafungin in the presence of nikkomycin Z significantly increased against C. auris at clinically attainable concentrations without antagonism, with the exception of one isolate from the South African clade. Our results suggest that by adding nikkomycin Z to anidulafungin or micafungin, the combination may be sufficiently potent to successfully treat invasive C. auris infections with the wild-type FKS genotype at clinically attainable concentrations. Further studies are needed to verify the clinical efficacy of echinocandin plus nikkomycin Z combinations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15051365/s1, Figure S1: Time-kill curves of nikkomycin Z in RPMI-1640 against isolates 20 (South Asian clade) and I-156 (bloodstream isolate from Israel, South American clade; Figure S2: Time-kill plots of anidulafungin, and anidulafungin plus nikkomycin Z in RPMI-1640 against isolates 28 and 208; Table S1: Time (hours) to reach 99.9% growth reduction (T99.9 = 3/k) from the starting inocula at different anidulafungin and anidulafungin plus 8 mg/L nikkomycin Z concentrations in RPMI-1640 against 4 Candida auris clades. Table S2: Time (hours) to reach 99.9% growth reduction (T99.9 = 3/k) from the starting inocula at different micafungin and micafungin plus 8 mg/L nikkomycin Z concentrations in RPMI-1640 against 4 Candida auris clades.
Author Contributions
Conceptualization, A.A., A.M.B. and L.M.; Methodology, A.A., D.B., Z.T., B.B. and G.U.; Investigation, R.K., L.F., G.K. and L.M.; Writing—Original Draft Preparation, A.A., Z.T. and G.K.; Supervision, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
R. Kovacs was supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences. This research was supported by the Hungarian National Research, Development and Innovation Office (NKFIH FK138462). R. Kovacs was supported by the UNKP- 21-5 New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data shown and discussed in this paper have been deposited in the NCBI GenBank with the following BioProject no.: PRJNA865124.
Conflicts of Interest
L. Majoros has received conference travel grants from MSD, Cidara, Astellas and Pfizer. The other authors have none to declare.
References
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Khojasteh, S.; Jafarzdeh, J.; Hosseini, S.A.; Haghani, I.; Turki, H.; Gharehbolagh, S.A.; Abastabar, M.; Mahmoudi, S. Candida auris and COVID-19: A health threatening combination. Curr. Med Mycol. 2022, 8, 44–50. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Osei, S.J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen 2018, 7, e578. [Google Scholar] [CrossRef]
- Armstrong, P.A.; Rivera, S.M.; Escandon, P.; Caceres, D.H.; Chow, N.; Stuckey, M.J.; Diaz, J.; Gomez, A.; Velez, N.; Espinosa-Bode, A.; et al. Hospital-associated multicenter outbreak of emerging fungus Candida auris, Colombia, 2016. Emerg. Infect. Dis. 2019, 25, 1339–1346. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre, A.I.; Martinez-Morel, H.; Calabuig, E.; Salavert-Lleti, M.; Ramirez, P.; Lopez-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 31 January 2023).
- Shastri, P.S.; Shankarnarayan, S.A.; Oberoi, J.; Rudramurthy, S.M.; Wattal, C.; Chakrabarti, A. Candida auris candidaemia in an intensive care unit—Prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 2020, 57, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Elizondo, M.T.; Medrano, R.; Bocanegra, M.C.; Lara, C.E.; Gaona, N.; Castilleja, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill Coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e0114621. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Samaranayake, L. Emerging and future strategies in the management of recalcitrant Candida auris. Med. Mycol. 2022, 60, myac008. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheng, L.W.; Chan, K.L.; Tam, C.C.; Mahoney, N.; Friedman, M.; Shilman, M.M.; Land, K.M. Antifungal Drug Repurposing. Antibiotics 2020, 9, 812. [Google Scholar] [CrossRef]
- Fioriti, S.; Brescini, L.; Pallotta, F.; Canovari, B.; Morroni, G.; Barchiesi, F. Antifungal Combinations against Candida Species: From Bench to Bedside. J. Fungi 2022, 8, 1077. [Google Scholar] [CrossRef]
- Bentz, M.L.; Nunnally, N.; Lockhart, S.R.; Sexton, D.J.; Berkow, E.L. Antifungal activity of nikkomycin Z against Candida auris. J. Antimicrob. Chemother. 2021, 76, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Nix, D.E.; Swezey, R.R.; Hector, R.; Galgiani, J.N. Pharmacokinetics of Nikkomycin Z after Single Rising Oral Doses. Antimicrob. Agents Chemother. 2009, 53, 2517–2521. [Google Scholar] [CrossRef]
- Larwood, D.J. Nikkomycin Z-Ready to meet the promise? J. Fungi 2020, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, J.; Földi, R.; Bayegan, S.; Kardos, G.; Majoros, L. Effect of nikkomycin Z and 50% human serum on the killing activity of high-concentration caspofungin against Candida species using time-kill methodology. J. Chemother. 2012, 24, 18–25. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. Msphere 2016, 18, e00189-16. [Google Scholar] [CrossRef]
- Kovács, R.; Tóth, Z.; Locke, J.B.; Forgacs, L.; Kardos, G.; Nagy, F.; Borman, A.M.; Majoros, L. Comparison of in vitro killing activity of rezafungin, anidulafungin, caspofungin, and micafungin against four Candida auris clades in RPMI-1640 in the absence and presence of human serum. Microorganisms 2021, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Forgács, L.; Borman, A.M.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]
- Balla, N.; Kovács, F.; Balázs, B.; Borman, A.M.; Bozó, A.; Tóth, Z.; Kobaissi, O.; Majoros, L.; Kovács, R. Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms. Antibiotics 2022, 11, 1601. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Dudiuk, C.; Berrio, I.; Leonardelli, F.; Morales-Lopez, S.; Theill, L.; Macedo, D.; Rodriguez, J.Y.; Salcedo, S.; Marin, A.; Gamarra, S.; et al. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. J. Antimicrob. Chemother. 2019, 74, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Poester, V.R.; Munhoz, L.S.; Benelli, J.L.; Melo, A.M.; Al-Hatmi, A.M.S.; Larwood, D.J.; Martinez, M.; Stevens, D.A.; Xavier, M.O. Initial Results of the International Efforts in Screening New Agents against Candida auris. J. Fungi 2022, 8, 771. [Google Scholar] [CrossRef]
- Sandovsky-Losica, H.; Shwartzman, R.; Lahat, Y.; Segal, E. Antifungal activity against Candida albicans of nikkomycin Z in combination with caspofungin, voriconazole or amphotericin B. J. Antimicrob. Chemother. 2008, 62, 635–637. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Arendrup, M.C.; Pfaller, M.A.; Bonfietti, B.; Bustamente, E.; Canton, E.; Cuenca, M.; Dannaoui, E.; Fothergill, A.; Fuller, J. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: Should the clinical laboratory be testing this agent? Antimicrob. Agents Chemother. 2013, 57, 5836–5842. [Google Scholar] [CrossRef]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Alatoom, A.; Sartawi, M.; Lawlor, K.; AbdelWareth, L.; Thomsen, J.; Nusair, A.; Mirza, I. Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates. Int. J. Infect. Dis. 2018, 70, 36–37. [Google Scholar] [CrossRef]
- Betts, R.F.; Nucci, M.; Talwar, D.; Gareca, M.; Telles, F.; Bedimo, R.J.; Herbrecht, R. Caspofungin high-dose study group. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin. Infect. Dis. 2009, 48, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rotstein, C.M.F.; Betts, R.F.; Nucci, M.; Talwar, D.; Waele, J.J.; Vazquez, J.S.; Dupont, B.F.; Horn, D.L.; Reboli, A.C. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 2007, 45, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Caballero, U.; Eraso, E.; Quindós, G.; Jauregizar, N. In Vitro Interaction and Killing-Kinetics of Amphotericin B Combined with Anidulafungin or Caspofungin against Candida auris. Pharmaceutics 2021, 13, 1333. [Google Scholar] [CrossRef]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; Garcia-Carnero, L.C.; Amezcua-Hernández, D.G.; E Lozoya-Pérez, N.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Shahi, G.; Kumar, M.; Skwarecki, A.S.; Edmondson, M.; Banerjee, A.; Usher, J.; Gow, N.A.; Milewski, S.; Prasad, R. Fluconazole resistant Candida auris clinical isolates have increased levels of cell wall chitin and increased susceptibility to a glucosamine-6-phosphate synthase inhibitor. Cell Surf. 2022, 8, 100076. [Google Scholar] [CrossRef]
- Pezzotti, G.; Kobara, M.; Asai, T.; Nakaya, T.; Miyamoto, N.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; Marin, E.; et al. Raman Imaging of Pathogenic Candida auris: Visualization of Structural Characteristics and Machine-Learning Identification. Front. Microbiol. 2021, 12, 769597. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Paul, R.A.; Rudramurthy, S.M.; Kashyap, N.; Bhattacharya, S.; Soman, R.; Shankarnarayan, S.A.; Chavan, D.; Singh, S.; Das, P.; et al. Impact of FKS1 Genotype on Echinocandin In Vitro Susceptibility in Candida auris and In Vivo Response in a Murine Model of Infection. Antimicrob. Agents Chemother. 2022, 66, e0165221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).