Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Temperature-Responsive Self-Folding Placebo Feedstock
2.3. SSE 3D Printing
2.4. Temperature-Responsive Shape Memory Programming
2.5. ML Modeling
2.5.1. Data Splitting Strategy
2.5.2. Construction of ML Models
2.5.3. Model Performance Criterion
2.5.4. Assessment of Optimized Batch
2.6. Rheology Measurement
2.7. Raman Spectroscopy
2.8. DSC
2.9. TGA
2.10. XRD
2.11. SEM
2.12. Percent Entrapment Efficiency
2.13. In Vitro PCM Release Study
2.14. PCM Release Kinetic Study
3. Results and Discussion
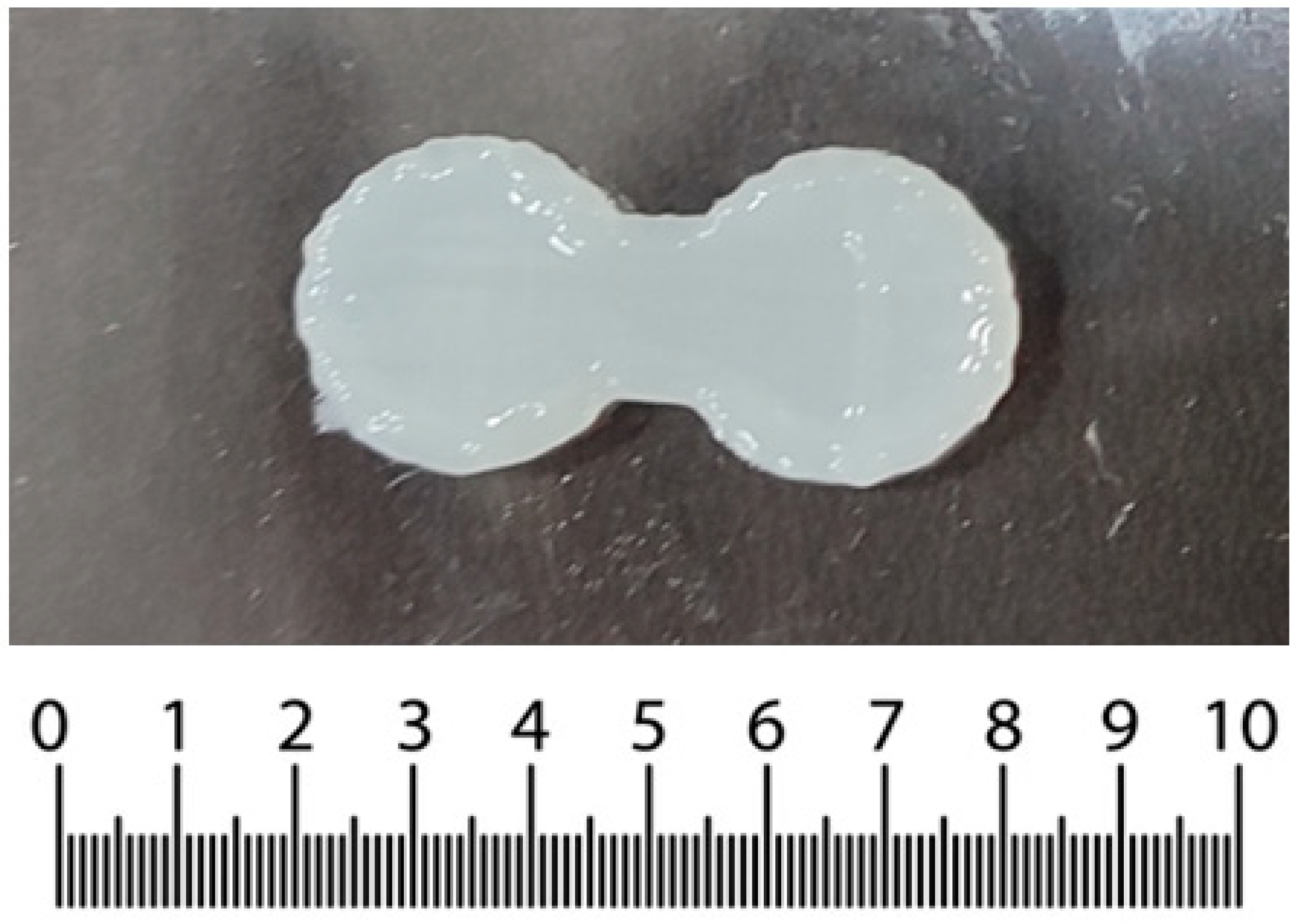
3.1. SSE of 3D Construct
3.2. Temperature-Responsive Shape Recovery
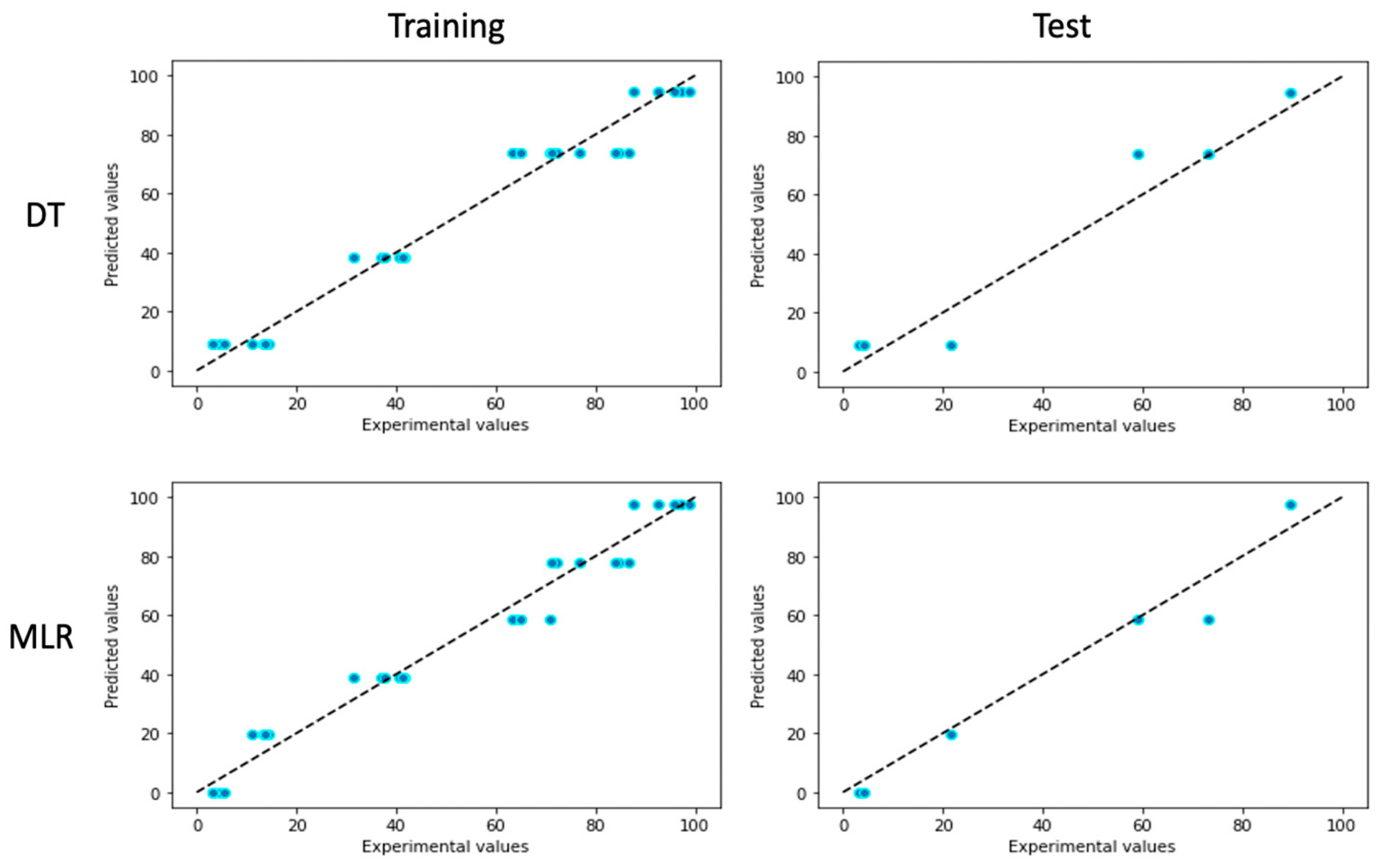
3.3. Comparison of Different ML Models
3.4. Assessment of Optimized Batch
3.5. Rheology Measurement
3.6. SSE of Optimized Formulation
3.7. Raman Analysis
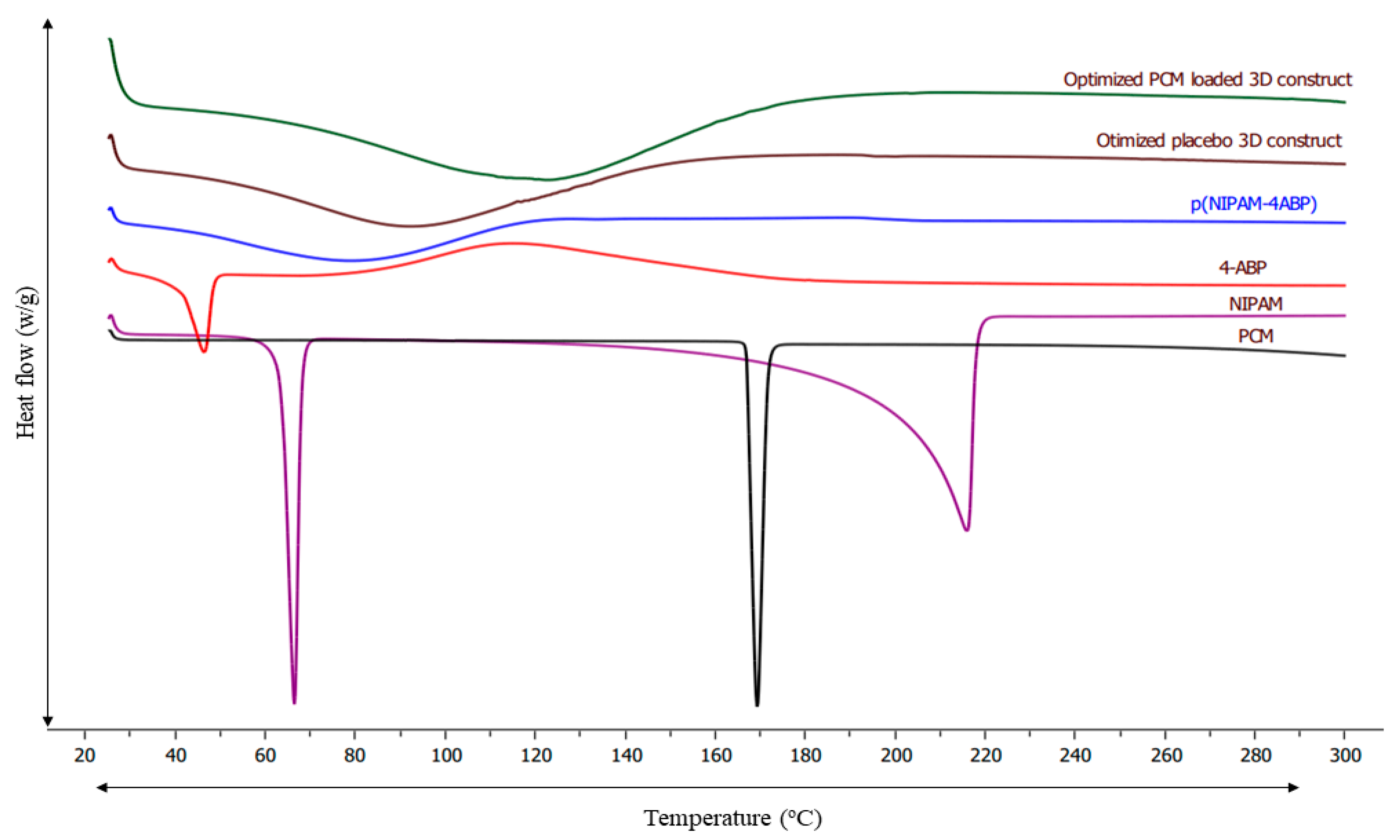
3.8. DSC Analysis
3.9. TGA Analysis
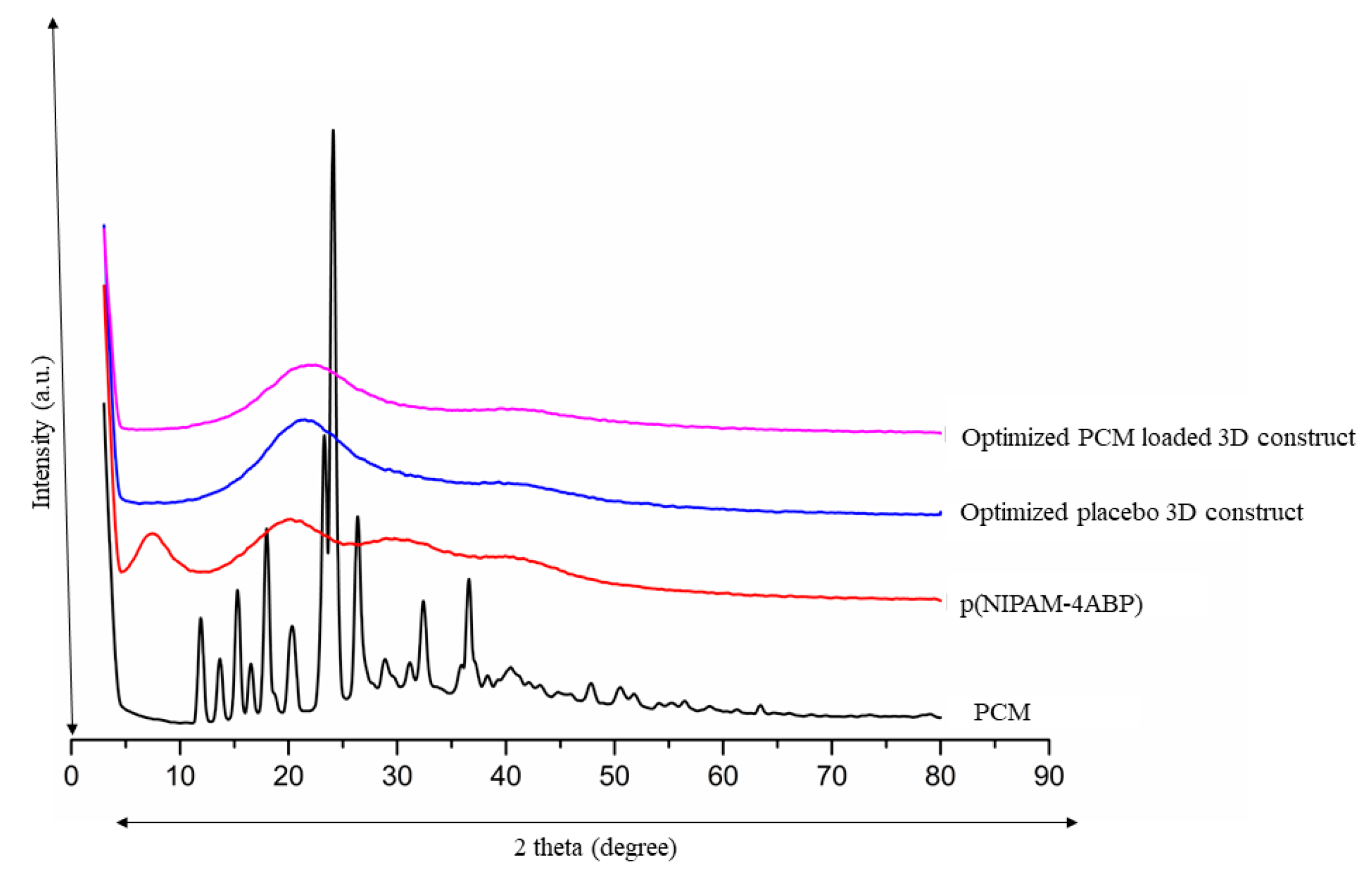
3.10. XRD Analysis
3.11. SEM
3.12. Percent Entrapment Efficiency
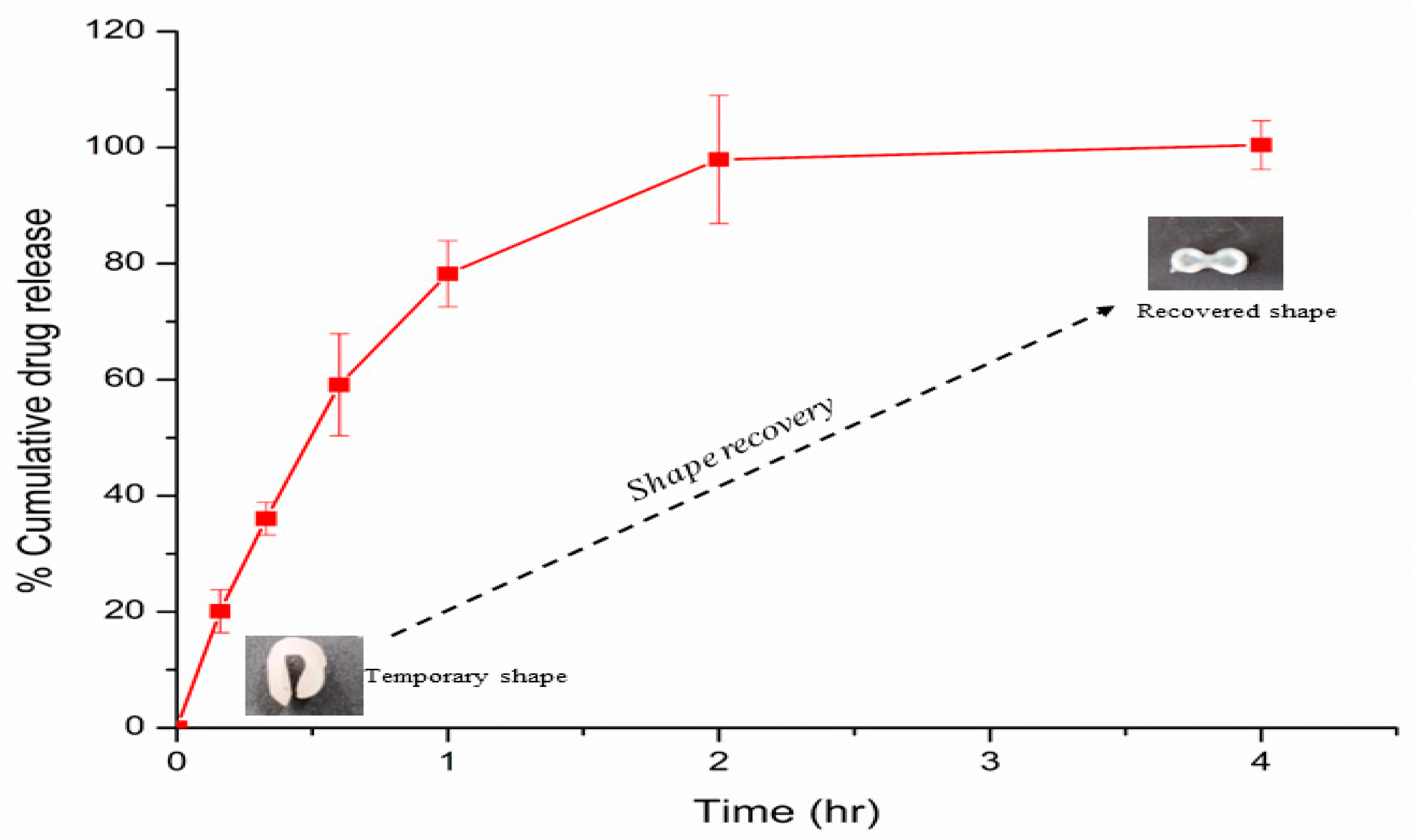
3.13. In Vitro PCM Release Study
3.14. PCM Release Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4D | Four-Dimensional |
| ML | Machine Learning |
| SSE | Semi-solid extrusion |
| PCM | Paracetamol |
| PCL | Polycaprolactone |
| PNIPAM | poly(N-isopropyl acrylamide) |
| 4-ABP | 4-Acryloyloxybenzophenone |
| p(NIPAM-4-ABP) | poly(N-isopropyl acrylamide)-4-Acryloyloxybenzophenone |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| MAE | Mean absolute error |
| RMSE | Root means square error |
| MLR | Multiple linear regression |
| DT | Decision tree |
References
- Zhou, W.; Qiao, Z.; Nazarzadeh Zare, E.; Huang, J.; Zheng, X.; Sun, X.; Shao, M.; Wang, H.; Wang, X.; Chen, D. 4D-printed dynamic materials in biomedical applications: Chemistry, challenges, and their future perspectives in the clinical sector. J. Med. Chem. 2020, 63, 8003–8024. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, P.; Chaudhari, V.S.; Banerjee, S. Customized 3D-printed hollow capsular device filled with norfloxacin-loaded micropellets for controlled-release delivery. Drug Deliv. Transl. Res. 2022, 13, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 173, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, N.; Chen, W.; Wang, C.-H.; Kruzic, J.J.; Li, X. A review on additive manufacturing of shape-memory materials for biomedical applications. Jom 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.M.; Kang, S.F.; Wu, X.L.; Lu, H.B.; Fu, J.; Cui, H. From 3D to 4D printing: Approaches and typical applications. J. Mech. Sci. Technol. 2015, 29, 4281–4288. [Google Scholar] [CrossRef]
- Zakharchenko, S.; Puretskiy, N.; Stoychev, G.; Stamm, M.; Ionov, L. Temperature controlled encapsulation and release using partially biodegradable thermo-magneto-sensitive self-rolling tubes. Soft Matter 2010, 6, 2633–2636. [Google Scholar] [CrossRef]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; von Klitzing, R.; Volodkin, D. Cooling-triggered release from mesoporous poly (n-isopropylacrylamide) microgels at physiological conditions. ACS Appl. Mater. Interfaces 2020, 12, 57401–57409. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing-Encapsulated Polycaprolactone—Thermoplastic Polyurethane with High Shape Memory Performances. Adv. Eng. Mater. 2022, 25, 2201309. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Soltanmohammadi, K.; Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 4D printing of PET-G via FDM including tailormade excess third shape. Manuf. Lett. 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375–6384. [Google Scholar] [CrossRef]
- Young, R.E.; Graf, J.; Miserocchi, I.; Van Horn, R.M.; Gordon, M.B.; Anderson, C.R.; Sefcik, L.S. Optimizing the alignment of thermoresponsive poly (N-isopropyl acrylamide) electrospun nanofibers for tissue engineering applications: A factorial design of experiments approach. PLoS ONE 2019, 14, e0219254. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; You, J.; Zhang, N.; Yu, L.; Zhao, H.; Qian, H.-J.; Lu, Z.-Y. Controlling the Chain Folding for the Synthesis of Single-Chain Polymer Nanoparticles Using Thermoresponsive Polymers. CCS Chem. 2021, 3, 2143–2154. [Google Scholar] [CrossRef]
- Ohnsorg, M.L.; Ting, J.M.; Jones, S.D.; Jung, S.; Bates, F.S.; Reineke, T.M. Tuning PNIPAm self-assembly and thermoresponse: Roles of hydrophobic end-groups and hydrophilic comonomer. Polym. Chem. 2019, 10, 3469–3479. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Kawre, S.; Maniruzzaman, M.; Seth, K.; Banerjee, S. Thermo-responsive self-folding feedstock with excellent shape memory programming. Chem. Pap. 2023, 1–10. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Y.; Cavicchi, K.A. Sequential shapeshifting 4D printing: Programming the pathway of multi-shape transformation by 3D printing stimuli-responsive polymers. Multifunct. Mater. 2020, 3, 042002. [Google Scholar] [CrossRef]
- Kim, M.; Jang, S.; Choi, S.; Yang, J.; Kim, J.; Choi, D. Analysis of shape memory behavior and mechanical properties of shape memory polymer composites using thermal conductive fillers. Micromachines 2021, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Aghda, N.H.; Jiang, J.; Habib, A.M.; Ouyang, D.; Maniruzzaman, M. 3D bioprinted microparticles: Optimizing loading efficiency using advanced DoE technique and machine learning modeling. Int. J. Pharm. 2022, 628, 122302. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-ray and thermal analysis of selected drugs containing acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Zeng, D.; Shu, T.; Wang, S. Dual-responsive mesoporous poly-N-isopropylacrylamide-hydroxyapatite composite microspheres for controlled anticancer drug delivery. J. Sol-Gel Sci. Technol. 2021, 97, 600–609. [Google Scholar] [CrossRef]
- Bhatt, U.; Jorvekar, S.B.; Murty, U.S.; Borkar, R.M.; Banerjee, S. Extrusion 3D printing of minicaplets for evaluating in vitro & in vivo praziquantel delivery capability. Int. J. Pharm. 2023, 630, 122445. [Google Scholar]
- Brown, J.D. The Coefficient of Determination. Shiken: JALT Testing & Evaluation SIG Newsletter. March 2003, Volume 7, pp. 14–16. Available online: https://hosted.jalt.org/test/bro_16.htm (accessed on 25 March 2023).
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE). Geosci. Model Dev. Discuss. 2014, 7, 1525–1534. [Google Scholar]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Buratti, E.; Franco, S.; Ruzicka, B. Chemical-physical behaviour of microgels made of interpenetrating polymer networks of PNIPAM and poly (acrylic acid). Polymers 2021, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Omar, J.; Boix, A.; Ulberth, F. Raman spectroscopy for quality control and detection of substandard painkillers. Vib. Spectrosc. 2020, 111, 103147. [Google Scholar] [CrossRef]
- Shekhar, S.; Mukherjee, M.; Sen, A.K. Studies on thermal and swelling properties of Poly (NIPAM-co-2-HEA) based hydrogels. Adv. Mater. Res. 2012, 1, 269. [Google Scholar] [CrossRef]
- Hurley, D.; Davis, M.; Walker, G.M.; Lyons, J.G.; Higginbotham, C.L. The effect of cooling on the degree of crystallinity, solid-state properties, and dissolution rate of multi-component hot-melt extruded solid dispersions. Pharmaceutics 2020, 12, 212. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Huang, W.M. Programming of Shape-Memory Polymers: The Temperature Memory Effect and Triple/Multiple-Shape-Memory Effect in Polymers. In Shape-Memory Polymer Device Design; Safranski, D.L., Griffis, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–137. [Google Scholar]
- Shafiei, F.; Ghavami-Lahiji, M.; Kashi, T.S.J.; Najafi, F. Drug release kinetics and biological properties of a novel local drug carrier system. Dent. Res. J. 2021, 18, 94. [Google Scholar]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Plascencia-Jatomea, M.; Higuera-Valenzuela, H.J.; Burgos-Hernández, M.; García-Flores, N.; Álvarez-Ramos, M.E.; Iriqui-Razcon, J.L.; Gonzalez, R.E.; Hernández-Abril, P.A. Synthesis and characterization of a Fe3O4@ PNIPAM-chitosan nanocomposite and its potential application in vincristine delivery. Polymers 2021, 13, 1704. [Google Scholar] [CrossRef] [PubMed]



| Code | P(NIPAM-4ABP) (%w/w) | PCL (%w/w) | Total Weight (%w/w) |
|---|---|---|---|
| P1 | 00 | 100 | 100.0 |
| P2 | 20 | 80 | |
| P3 | 40 | 60 | |
| P4 | 60 | 40 | |
| P5 | 80 | 20 | |
| P6 | 100 | 00 |
| Parameters | Optimized Value |
|---|---|
| Nozzle size | 0.4 mm |
| Distance between nozzle tip and build plate | 0.2 mm |
| Layer height | 0.2 mm |
| Printing temperature | 27.0 °C |
| Build-bed temperature | 4.0 °C |
| Printing speed | 8.0 mm/s |
| Travel speed | 8.0 mm/s |
| Retraction speed | 1.0 mm/s |
| Retraction distance | 1.0 mm |
| ML Algorithms | Training Set | Test Set | ||||
|---|---|---|---|---|---|---|
| R2 | MAE | RMSE | R2 | MAE | RMSE | |
| MLR | 0.97 | 4.89 | 5.66 | 0.957 | 5.26 | 7.05 |
| DT | 0.968 | 4.93 | 5.91 | 0.933 | 7.3 | 8.75 |
| Sample Code | P(NIPAM-4ABP) (%w/w) | PCL (%w/w) | Shape Recovery Ratio |
|---|---|---|---|
| P1 | 00 | 100 | 0.20 |
| P2 | 20 | 80 | 19.64 |
| P3 | 40 | 60 | 39.09 |
| P4 | 60 | 40 | 58.53 |
| P5 | 80 | 20 | 77.97 |
| P6 | 100 | 00 | 97.41 |
| Release Kinetic Model | R2 | n |
|---|---|---|
| Zero-order | 0.2885 | - |
| First-order | 0.9979 | - |
| Higuchi | 0.8832 | - |
| Korsmeyer–Peppas | 0.9270 | 0.376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryavanshi, P.; Wang, J.; Duggal, I.; Maniruzzaman, M.; Banerjee, S. Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling. Pharmaceutics 2023, 15, 1266. https://doi.org/10.3390/pharmaceutics15041266
Suryavanshi P, Wang J, Duggal I, Maniruzzaman M, Banerjee S. Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling. Pharmaceutics. 2023; 15(4):1266. https://doi.org/10.3390/pharmaceutics15041266
Chicago/Turabian StyleSuryavanshi, Purushottam, Jiawei Wang, Ishaan Duggal, Mohammed Maniruzzaman, and Subham Banerjee. 2023. "Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling" Pharmaceutics 15, no. 4: 1266. https://doi.org/10.3390/pharmaceutics15041266
APA StyleSuryavanshi, P., Wang, J., Duggal, I., Maniruzzaman, M., & Banerjee, S. (2023). Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling. Pharmaceutics, 15(4), 1266. https://doi.org/10.3390/pharmaceutics15041266







