Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Cancer Cell Membranes
2.4. Characterization
2.5. Biocompatibility Assay
2.6. Drug Loading and Release
2.7. Cellular Uptake and Homologous Targeting
2.8. Antitumor Activity
2.9. Statistical Analysis
3. Results and Discussion
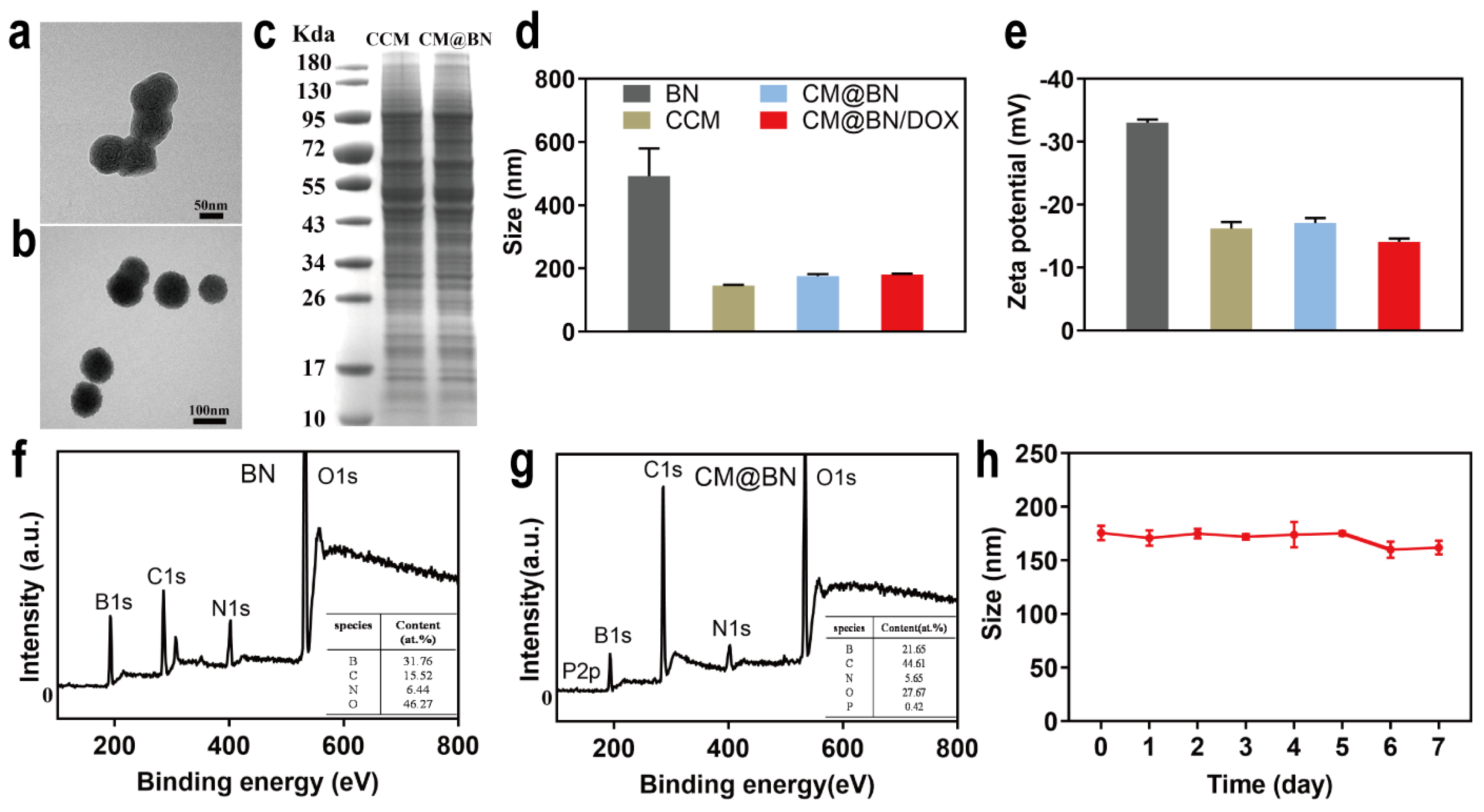
3.1. Preparation and Characterization of CM@BN
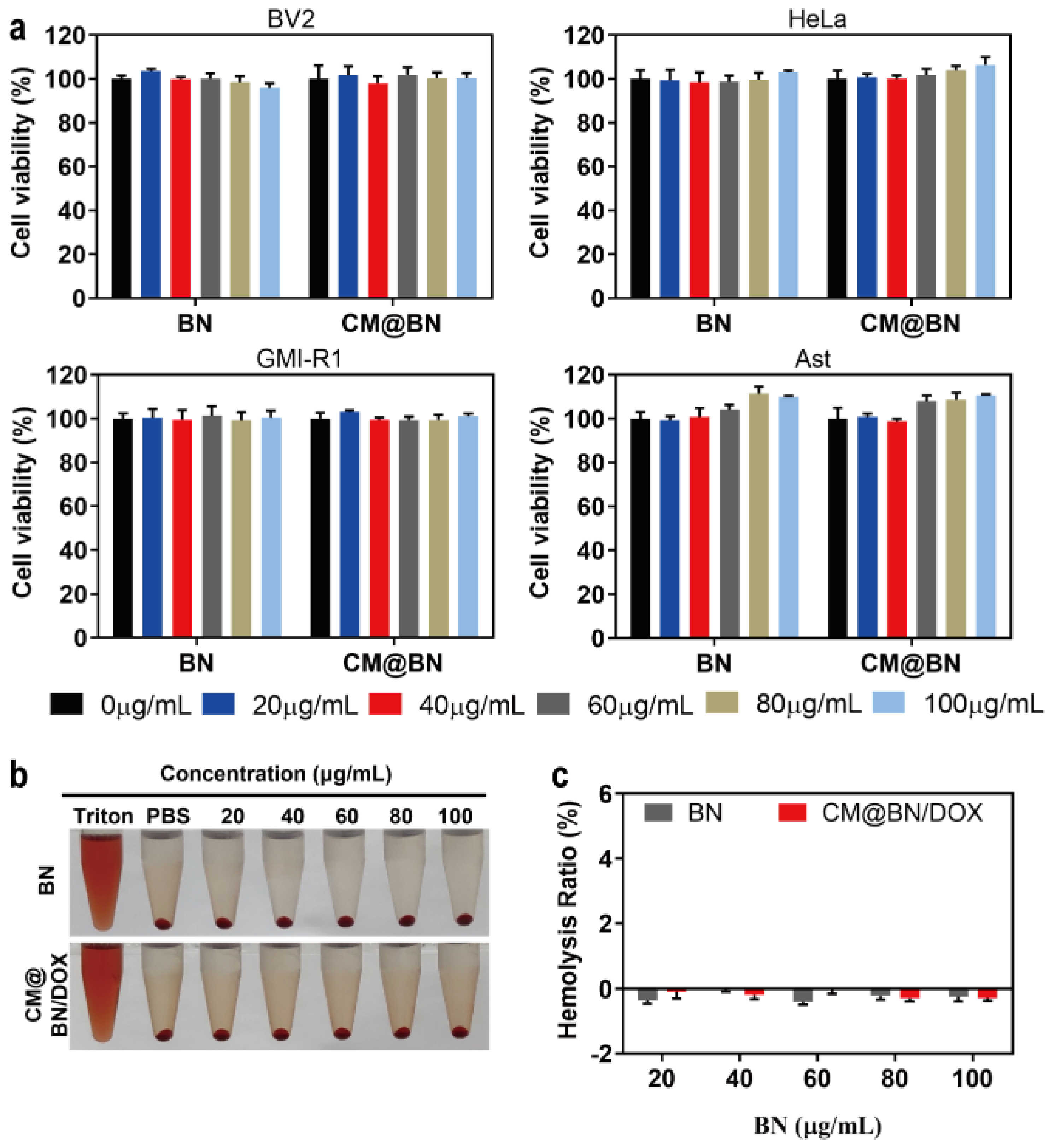
3.2. Biocompatibility Assay
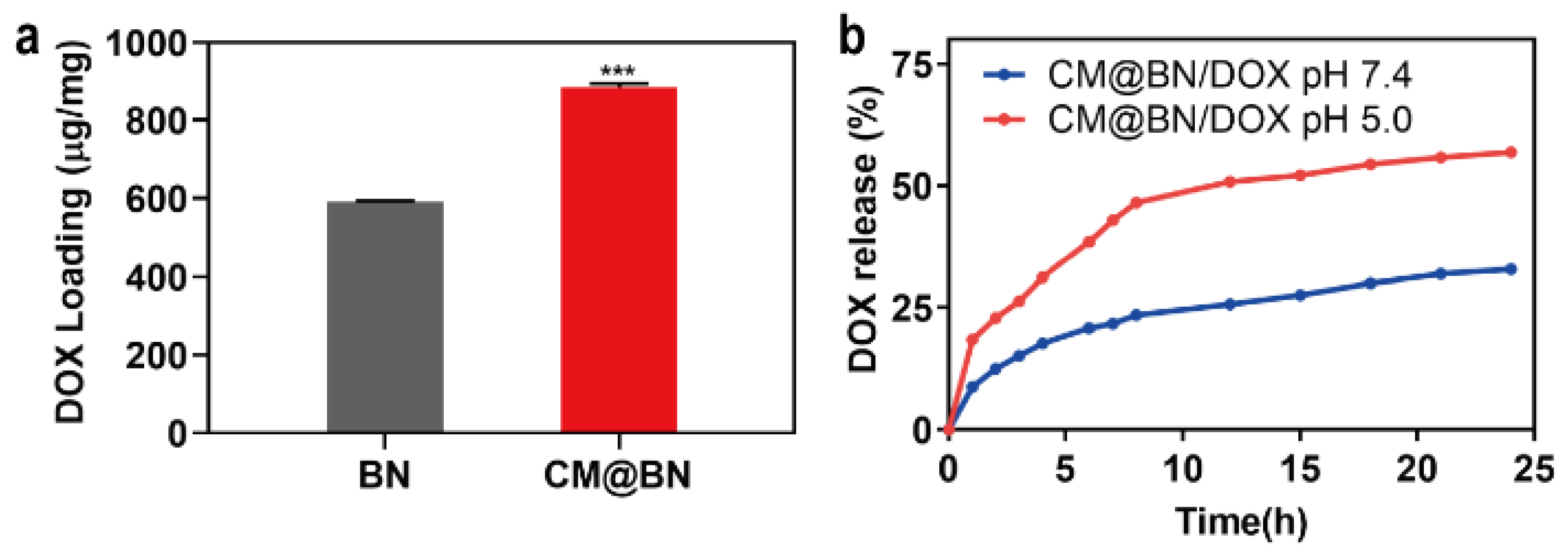
3.3. Drug Loading and Release
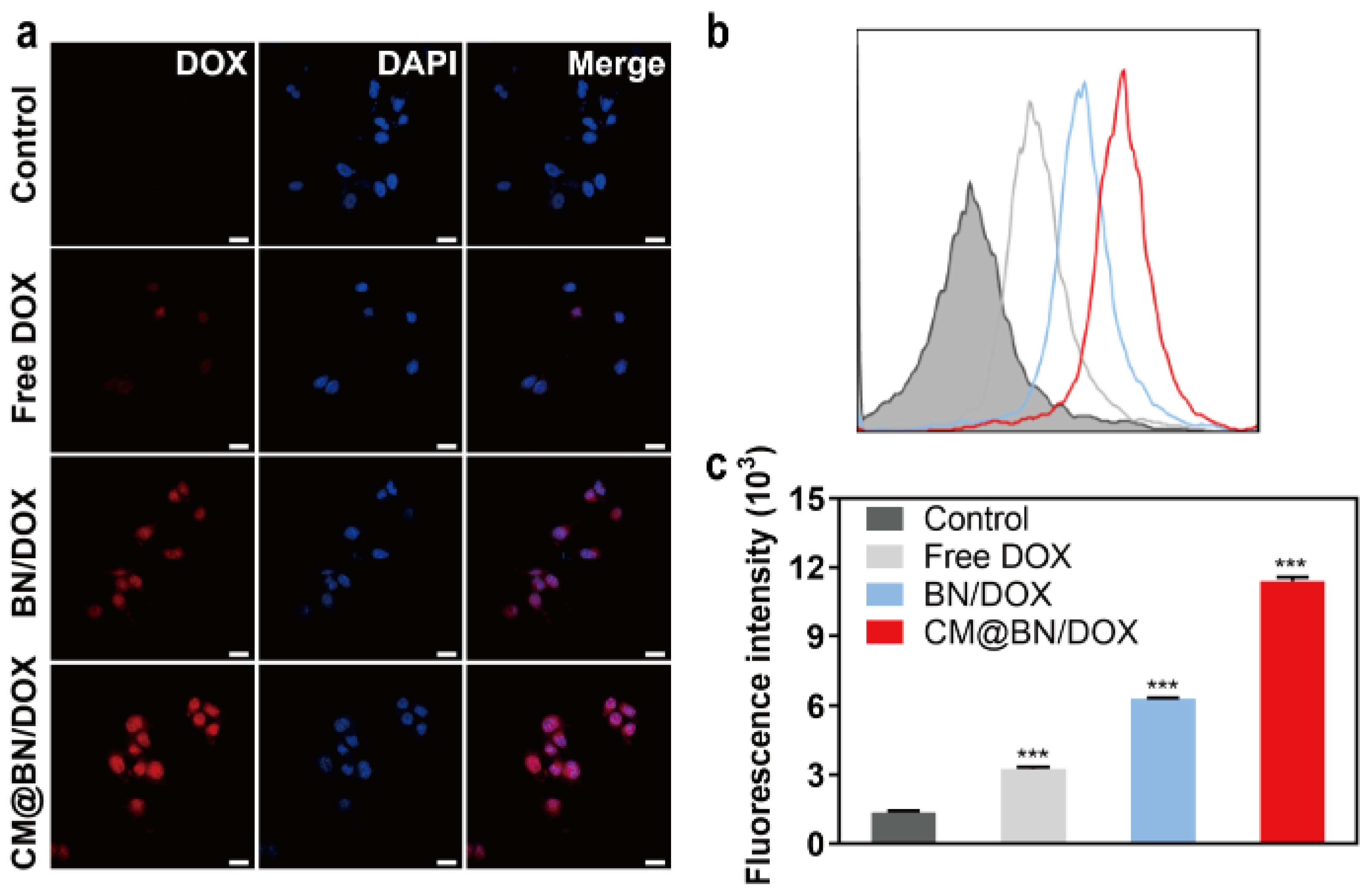
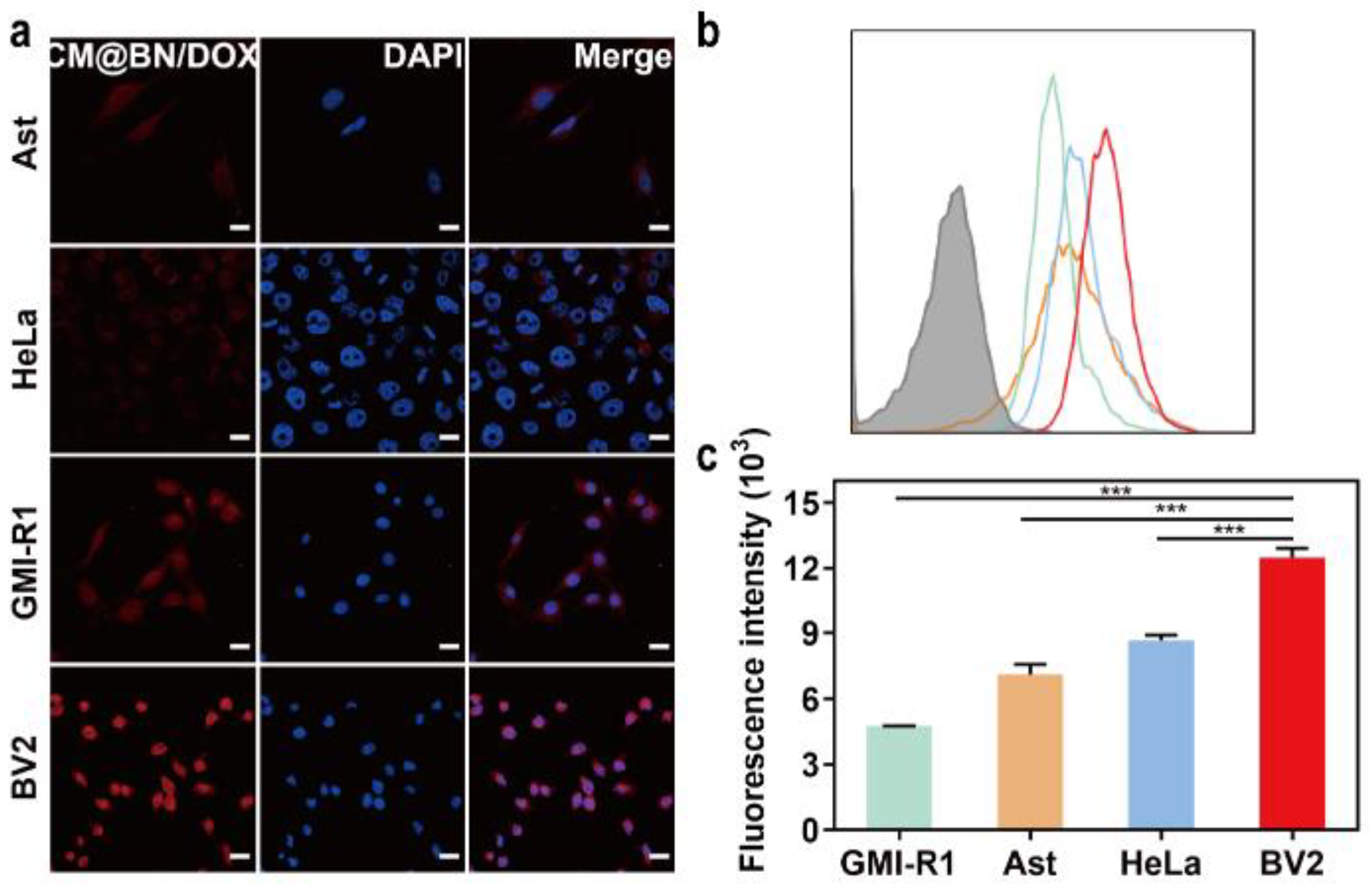
3.4. In Vitro Cellular Uptake and Targeting Ability
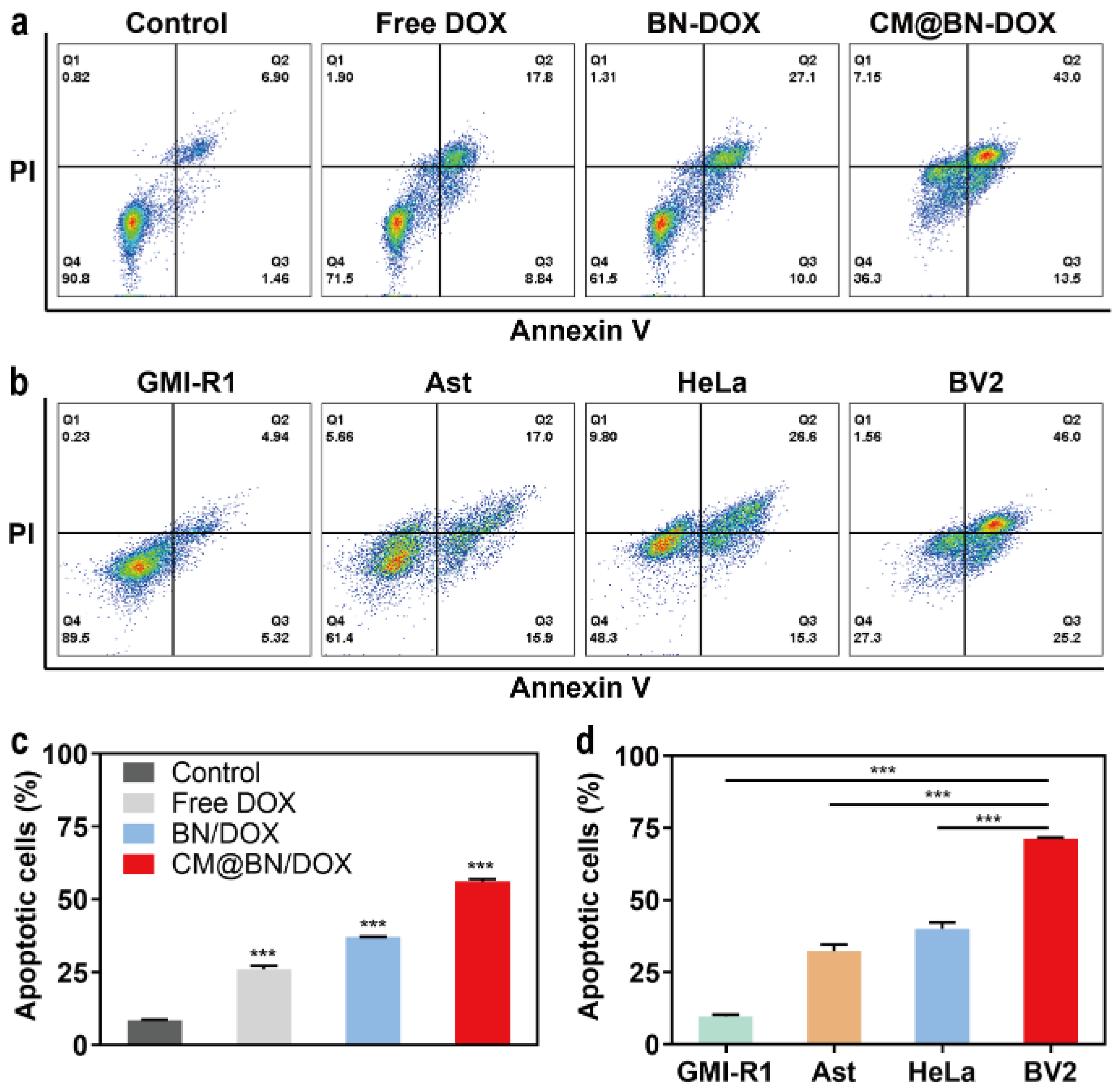
3.5. In Vitro Antitumor Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chugh, V.; Vijaya Krishna, K.; Pandit, A. Cell Membrane-Coated Mimics: A Methodological Approach for Fabrication, Characterization for Therapeutic Applications, and Challenges for Clinical Translation. ACS Nano 2021, 15, 17080–17123. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.; Goyal, N.; Gupta, S. Synthesizing and Optimizing Rutile TiO2 Nanoparticles for Magnetically Guided Drug Delivery. Int. J. Nanomed. 2022, 17, 3147–3161. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Liu, X.; Chen, Y.; Zhao, P.; Chen, C.; Yan, R.; Li, M.; Fan, T.; Altine, B.; et al. One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1-targeted combination cancer therapy. Theranostics 2020, 10, 2918–2929. [Google Scholar] [CrossRef]
- Su, Y.; Wang, K.; Li, Y.; Song, W.; Xin, Y.; Zhao, W.; Tian, J.; Ren, L.; Lu, L. Sorafenib-loaded polymeric micelles as passive targeting therapeutic agents for hepatocellular carcinoma therapy. Nanomedicine 2018, 13, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist 2008, 13, 248–260. [Google Scholar] [CrossRef]
- Tong, Q.; Qiu, N.; Ji, J.; Ye, L.; Zhai, G. Research Progress in Bioinspired Drug Delivery Systems. Expert Opin. Drug Deliv. 2020, 17, 1269–1288. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Y.; Wu, S.; Yan, M.; Cao, Y.; Mou, N.; Wang, G.; Sun, D.; Wu, W. Cell membrane camouflaged biomimetic nanoparticles: Focusing on tumor theranostics. Mater. Today Bio 2022, 14, 100228. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, R.; Yodsanit, N.; Ye, M.; Wang, Y.; Wang, B.; Guo, L.W.; Kent, K.C.; Gong, S. Hydrogen peroxide-responsive platelet membrane-coated nanoparticles for thrombus therapy. Biomater. Sci. 2021, 9, 2696–2708. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Wang, Q.; Gao, J.; Chen, J.; Tan, H.; Li, S.; Wang, Z.; Weng, X.; Yang, H.; et al. Targeted immunomodulation therapy for cardiac repair by platelet membrane engineering extracellular vesicles via hitching peripheral monocytes. Biomaterials 2022, 284, 121529. [Google Scholar] [CrossRef] [PubMed]
- Ailuno, G.; Baldassari, S.; Lai, F.; Florio, T.; Caviglioli, G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells 2020, 9, 2569. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, C. Research Progress of Cell Membrane Biomimetic Nanoparticles for Tumor Therapy. Nanoscale Res. Lett. 2022, 17, 36. [Google Scholar] [CrossRef]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Singh, N. Red blood cells membrane-derived nanoparticles: Applications and key challenges in their clinical translation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1776. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Joshi, A.; Mohanty, S.; Singh, N. Polymeric micelles coated with hybrid nanovesicles enhance the therapeutic potential of the reversible topoisomerase inhibitor camptothecin in a mouse model. Acta Biomater. 2021, 121, 579–591. [Google Scholar] [CrossRef]
- Fan, L.; Wei, A.; Gao, Z.; Mu, X. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomed. Pharmacother. 2023, 161, 114451. [Google Scholar] [CrossRef]
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: A promising biomimetic nanoplatforms for cancer theranostics. J. Control. Release 2022, 348, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.; Zhou, C.; Lin, Z.; He, Q. Leukocyte Membrane-Coated Liquid Metal Nanoswimmers for Actively Targeted Delivery and Synergistic Chemophotothermal Therapy. Research 2020, 2020, 3676954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Liu, C.; Hao, M.; Kong, C.; Zhao, X.; Gao, Y.; Zhang, Y.; Cui, W.; Zhang, C.; et al. B16 Membrane-Coated Vesicles for Combined Photodynamic Therapy and Immunotherapy Shift Immune Microenvironment of Melanoma. Int. J. Nanomed. 2022, 17, 855–868. [Google Scholar] [CrossRef]
- Fan, R.; He, S.; Wang, Y.; Qiao, J.; Liu, H.; Galstyan, L.; Ghazaryan, A.; Cai, H.; Feng, S.; Ni, P.; et al. Targeted delivery of a PROTAC induced PDEδ degrader by a biomimetic drug delivery system for enhanced cytotoxicity against pancreatic cancer cells. Am. J. Cancer Res. 2022, 12, 1027–1041. [Google Scholar]
- Liu, Y.; Sukumar, U.K.; Kanada, M.; Krishnan, A.; Massoud, T.F.; Paulmurugan, R. Camouflaged Hybrid Cancer Cell-Platelet Fusion Membrane Nanovesicles Deliver Therapeutic MicroRNAs to Presensitize Triple-Negative Breast Cancer to Doxorubicin. Adv. Funct. Mater. 2021, 31, 2103600. [Google Scholar] [CrossRef]
- Ihsanullah, I. Boron nitride-based materials for water purification: Progress and outlook. Chemosphere 2021, 263, 127970. [Google Scholar] [CrossRef]
- Pan, D.; Su, F.; Liu, H.; Ma, Y.; Das, R.; Hu, Q.; Liu, C.; Guo, Z. The Properties and Preparation Methods of Different Boron Nitride Nanostructures and Applications of Related Nanocomposites. Chem. Rec. 2020, 20, 1314–1337. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Açikyildiz, M.; Tatar, A.; Geyikoğlu, F. Synthesis, characterization and cytotoxicity of boron nitride nanoparticles: Emphasis on toxicogenomics. Cytotechnology 2019, 71, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Sharker, S.M. Hexagonal Boron Nitrides (White Graphene): A Promising Method for Cancer Drug Delivery. Int. J. Nanomed. 2019, 14, 9983–9993. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhi, C.; Hanagata, N.; Yamaguchi, M.; Bando, Y.; Golberg, D. Boron nitride nanotubes functionalized with mesoporous silica for intracellular delivery of chemotherapy drugs. Chem. Commun. 2013, 49, 7337–7339. [Google Scholar] [CrossRef] [PubMed]
- Ailuno, G.; Balboni, A.; Caviglioli, G.; Lai, F.; Barbieri, F.; Dellacasagrande, I.; Florio, T.; Baldassari, S. Boron Vehiculating Nanosystems for Neutron Capture Therapy in Cancer Treatment. Cells 2022, 11, 4029. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Wang, W.; Lin, J.; Xing, C.; Chai, R.; Abbas, S.; Song, T.; Tang, C.; Huang, Y. Fe3O4 nanoparticle-coated boron nitride nanospheres: Synthesis, magnetic property and biocompatibility study. Ceram. Int. 2017, 43, 6371–6376. [Google Scholar] [CrossRef]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorg. Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef]
- Niskanen, J.; Zhang, I.; Xue, Y.; Golberg, D.; Maysinger, D.; Winnik, F.M. Boron nitride nanotubes as vehicles for intracellular delivery of fluorescent drugs and probes. Nanomedicine 2016, 11, 447–463. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, S.; Yan, T.; Zhi, C.; Gao, X.D.; Hanagata, N. Polyethyleneimine-functionalized boron nitride nanospheres as efficient carriers for enhancing the immunostimulatory effect of CpG oligodeoxynucleotides. Int. J. Nanomed. 2015, 10, 5343–5353. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano. 2014, 8, 6123–6130. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Yan, T.; Huang, D.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated boron nitride nanospheres for targeted delivery of anticancer drugs. Int. J. Nanomed. 2016, 11, 4573–4582. [Google Scholar] [CrossRef]
- Feng, S.; Li, H.; Ren, Y.; Zhi, C.; Huang, Y.; Chen, F.; Zhang, H. RBC membrane camouflaged boron nitride nanospheres for enhanced biocompatible performance. Colloids Surf. B Biointerfaces 2020, 190, 110964. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Bando, Y.; Huang, Y.; Zhi, C.; Golberg, D. Synthetic Routes and Formation Mechanisms of Spherical Boron Nitride Nanoparticles. Adv. Funct. Mater. 2008, 18, 3653–3661. [Google Scholar] [CrossRef]
- Shen, J.W.; Li, C.; Yang, M.Y.; Lin, J.F.; Yin, M.D.; Zou, J.J.; Wu, P.Y.; Chen, L.; Song, L.X.; Shao, J.W. Biomimetic nanoparticles: U937 cell membranes based core-shell nanosystems for targeted atherosclerosis therapy. Int. J. Pharm. 2022, 611, 121297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Zhao, R.X.; Yang, F.M.; Qi, X.T.; Ye, P.K.; Xie, M. An erythrocyte membrane-camouflaged biomimetic nanoplatform for enhanced chemo-photothermal therapy of breast cancer. J. Mater. Chem. B 2022, 10, 2047–2056. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Zhou, Q.; Chen, F.; Stenzel, M.; Gao, F.; Liu, C.; Yuan, H.; Li, H.; Jiang, Y. Self-assembled anionic and cationic Au nanoparticles with Au nanoclusters for the exploration of different biological responsiveness in cancer therapy. Nanoscale Adv. 2021, 3, 2812–2821. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Pradipta, A.R.; Ahmadi, P.; Terashima, K.; Muguruma, K.; Fujii, M.; Ichino, T.; Maeda, S.; Tanaka, K. Targeted 1,3-dipolar cycloaddition with acrolein for cancer prodrug activation. Chem. Sci. 2021, 12, 5438–5449. [Google Scholar] [CrossRef]
- Omar, M.M.; Hasan, O.A.; Zaki, R.M.; Eleraky, N.E. Externally Triggered Novel Rapid-Release Sonosensitive Folate-Modified Liposomes for Gemcitabine: Development and Characteristics. Int. J. Nanomed. 2021, 16, 683–700. [Google Scholar] [CrossRef]
- Matthews, A.T.; Soni, H.; Robinson-Freeman, K.E.; John, T.A.; Buddington, R.K.; Adebiyi, A. Doxorubicin-Induced Fetal Mesangial Cell Death Occurs Independently of TRPC6 Channel Upregulation but Involves Mitochondrial Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 7589. [Google Scholar] [CrossRef]
- Sui, J.; He, M.; Yang, Y.; Ma, M.; Guo, Z.; Zhao, M.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Reversing P-Glycoprotein-Associated Multidrug Resistance of Breast Cancer by Targeted Acid-Cleavable Polysaccharide Nanoparticles with Lapatinib Sensitization. ACS Appl. Mater. Interfaces 2020, 12, 51198–51211. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qiao, W.; Shen, Y.; Xu, H.; Fan, Y.; Liu, Y.; Lan, Y.; Gong, Y.; Chen, F.; Feng, S. Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity. Pharmaceutics 2023, 15, 1269. https://doi.org/10.3390/pharmaceutics15041269
Li H, Qiao W, Shen Y, Xu H, Fan Y, Liu Y, Lan Y, Gong Y, Chen F, Feng S. Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity. Pharmaceutics. 2023; 15(4):1269. https://doi.org/10.3390/pharmaceutics15041269
Chicago/Turabian StyleLi, Hui, Wei Qiao, Yizhe Shen, Huashan Xu, Yuan Fan, Yuxiang Liu, Yadi Lan, Yan Gong, Fuxue Chen, and Shini Feng. 2023. "Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity" Pharmaceutics 15, no. 4: 1269. https://doi.org/10.3390/pharmaceutics15041269
APA StyleLi, H., Qiao, W., Shen, Y., Xu, H., Fan, Y., Liu, Y., Lan, Y., Gong, Y., Chen, F., & Feng, S. (2023). Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity. Pharmaceutics, 15(4), 1269. https://doi.org/10.3390/pharmaceutics15041269






