Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense
Abstract
1. Introduction
2. Lactoferrin (LF)
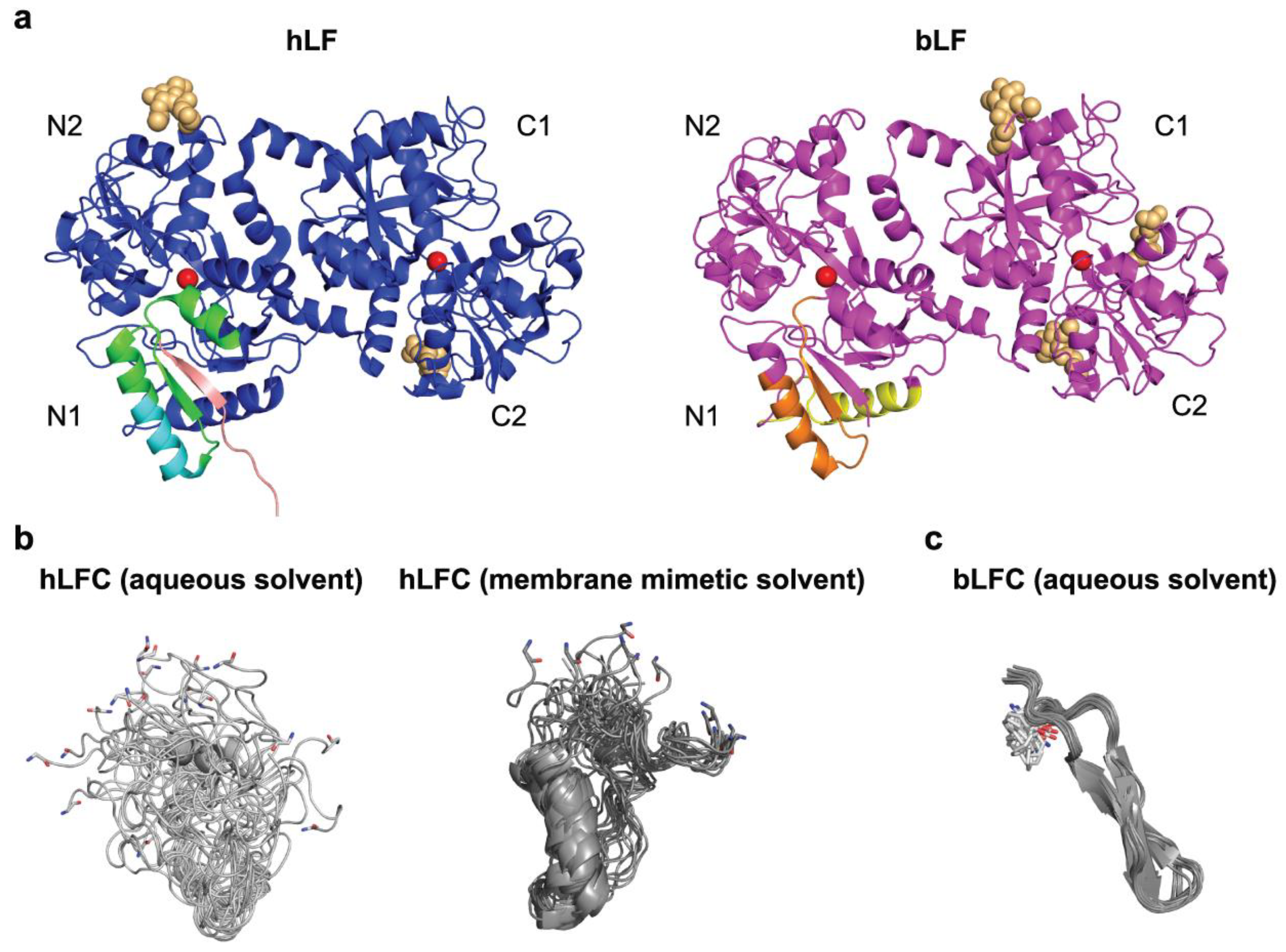
2.1. Structure of LF
2.2. Tissue Distribution of LF
2.3. Receptors of LF
| LF Receptor | Expressed by | Selected References |
|---|---|---|
| LRP-1 (CD91) | Multiple cell types (fibroblasts, osteoblasts, myeloid cells) | [55,56,57,72] |
| CXCR4 (CD184) | Leukocytes, epithelial cells, platelets | [58,73] |
| Intelectin-1 (omentin-1) | Intestinal epithelial cells | [59,60] |
| CD14 | Monocytes, macrophages, neutrophils | [61,74] |
| TLR2 (CD282), TLR4 (CD284) | Myeloid cells, endothelial cells | [62,63,75] |
| DC-SIGN (CD209) | Myeloid cells (DCs, certain macrophage types) | [64,76,77] |
| Nucleolin | Proliferating cells | [66] |
| HSPGs | Broadly expressed on various cells and as extracellular matrix macromolecules | [11,67,68] |
3. LF-Derived Bioactive Natural and Synthetic Peptides
3.1. Lactoferricin (LFC) and Derived Peptides
3.2. Lactoferrampin (LFA)
4. LF and LFC in Host Defense
4.1. LF in Iron Homeostasis
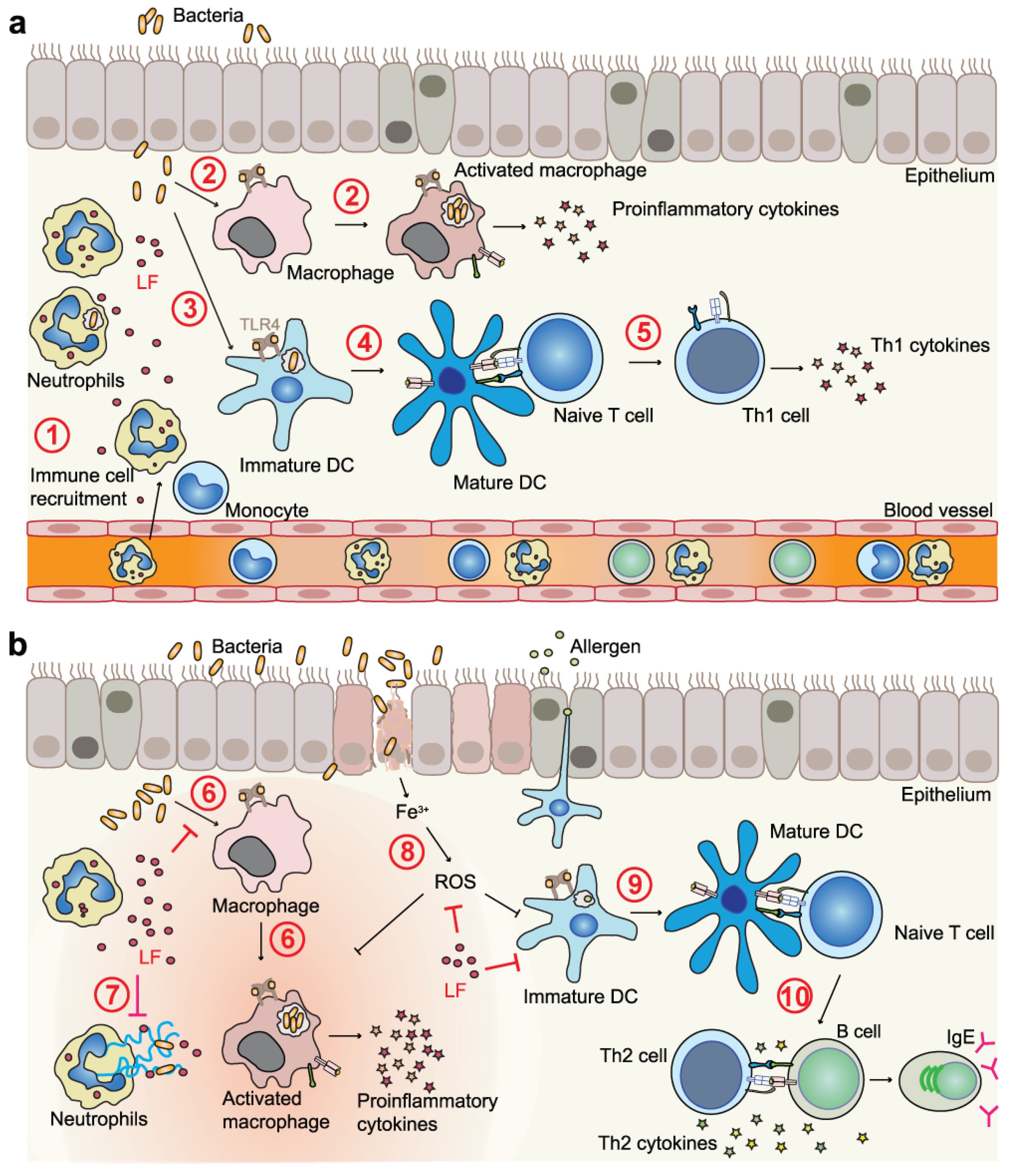
4.2. LF and LFC in Immunomodulation
4.3. LF and LFC as Inhibitors of Serine Proteases
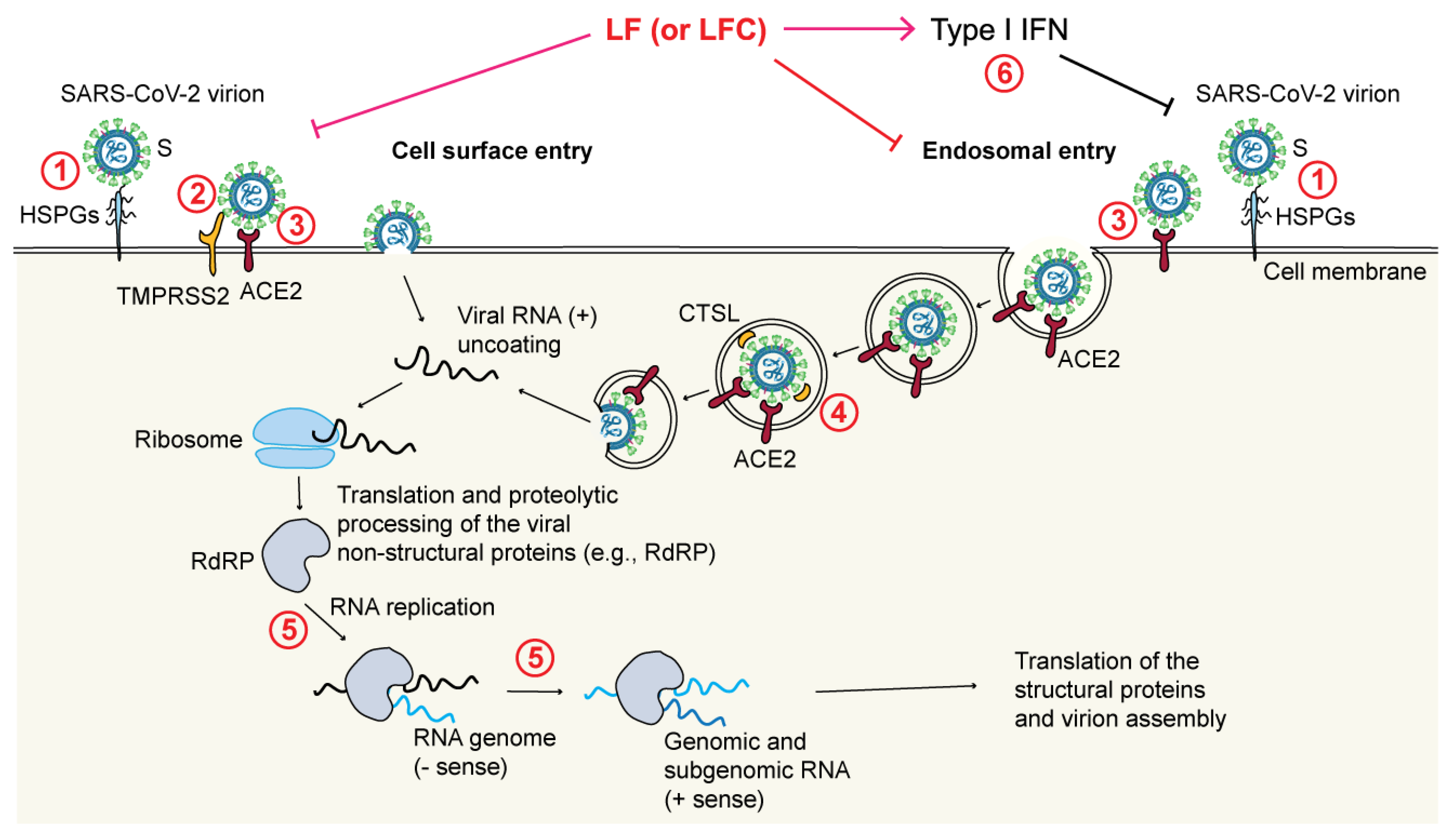
4.4. Direct Antiviral Activities of LF and LFC
- Through blockade of the TMPRSS2-mediated virus priming [178]. This mechanism has been observed in particular for LFC, both synthetic and natural, but not for full-length LF, again pointing to differences between the released LFC and the corresponding region encompassed within the N-terminus of intact LF;
- Through blockade of the cathepsin L (CTSL)-mediated virus priming [194]. In this case, a bLF hydrolysate showed an inhibitory effect toward CTSL (a cysteine protease that primes SARS-CoV-2 in endosomes) that resulted in a decreased infection rate by a SARS-CoV-2 pseudovirus;
- Through enhancement of IFN responses [195]. It has been shown that bLF enhances the expression of IFN-β and downstream IFN-stimulated genes (e.g., MX1 and IFITM3), all of which are known to exert antiviral effects;
- Through maintenance of iron homeostasis [198];
- Possibly also through inhibition of the main viral protease Mpro, also called 3CLpro [199].
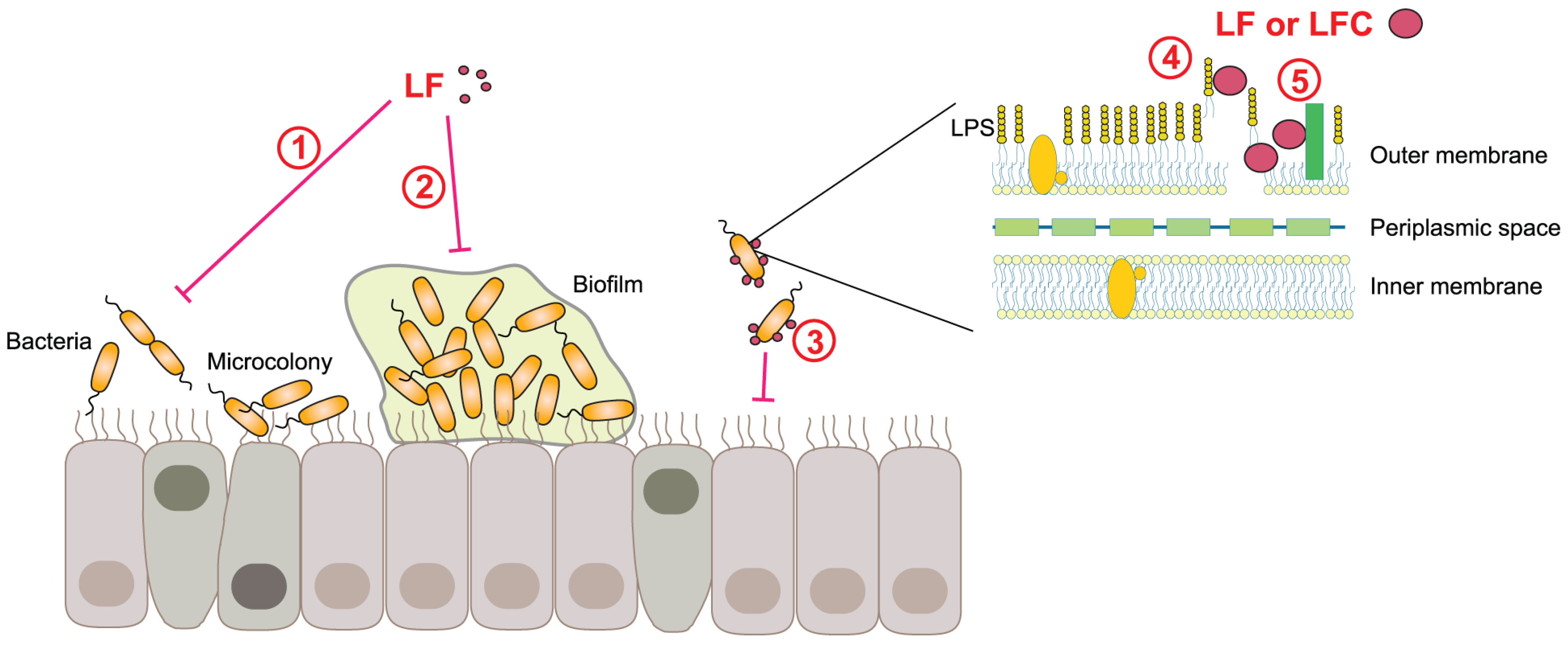
4.5. Antibacterial, Antifungal, and Antiparasitic Activities of LF and LFC
4.6. Antitumor Activities of LF and LFC
5. LF in Clinical Trials
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.; Alvarado, R.; Phinney, B.; Lonnerdal, B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011, 10, 1746–1754. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Tan, L.T.; Law, J.W.; Hong, K.W.; Ratnasingam, V.; Ab Mutalib, N.S.; Lee, L.H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.M. Nanoformulation of lactoferrin potentiates its activity and enhances novel biotechnological applications. Int. J. Biol. Macromol. 2020, 165, 970–984. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A. Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta 2012, 1820, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Sorensen, S.P.L. The proteins in whey. C. R. Des. Trav. Lab. Carlsberg Ser. Chim. 1940, 23, 55–99. [Google Scholar]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Boukria, O.; El Hadrami, E.M.; Sameen, A.; Sahar, A.; Khan, S.; Safarov, J.; Sultanova, S.; Leriche, F.; Ait-Kaddour, A. Biochemical, Physicochemical and Sensory Properties of Yoghurts Made from Mixing Milks of Different Mammalian Species. Foods 2020, 9, 1722. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gomez, C.; Ramirez-Texcalco, L.S.; Torres-Lopez, E.; Ramirez-Mondragon, R.; Hernandez-Ramirez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Mazurier, J. A critical review of the roles of host lactoferrin in immunity. Biometals 2010, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Kaczynska, K.; Kleczkowska, P.; Bukowska-Osko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon-A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Embleton, N.D.; Berrington, J.E. Clinical Trials of Lactoferrin in the Newborn: Effects on Infection and the Gut Microbiome. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M. Milk-derived proteins and peptides in clinical trials. Postep. Hig. Med. Dosw. 2013, 67, 800–816. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Conte, M.P.; Campione, E.; Bianchi, L.; Valenti, P. An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection. Biometals 2022, 1–20. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, e301–e308. [Google Scholar] [CrossRef]
- Anderson, B.F.; Baker, H.M.; Dodson, E.J.; Norris, G.E.; Rumball, S.V.; Waters, J.M.; Baker, E.N. Structure of human lactoferrin at 3.2-A resolution. Proc. Natl. Acad. Sci. USA 1987, 84, 1769–1773. [Google Scholar] [CrossRef]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rumball, S.V.; Baker, E.N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature 1990, 344, 784–787. [Google Scholar] [CrossRef]
- Ianiro, G.; Rosa, L.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G.; Cutone, A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. Biometals 2022, 1–26. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A. Metal and anion binding sites in lactoferrin and related proteins. Pure Appl. Chem. 1990, 62, 1067–1070. [Google Scholar] [CrossRef]
- Anghel, L.; Radulescu, A.; Erhan, R.V. Structural aspects of human lactoferrin in the iron-binding process studied by molecular dynamics and small-angle neutron scattering. Eur. Phys. J. E Soft Matter. 2018, 41, 109. [Google Scholar] [CrossRef] [PubMed]
- Majka, G.; Spiewak, K.; Kurpiewska, K.; Heczko, P.; Stochel, G.; Strus, M.; Brindell, M. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013, 405, 5191–5200. [Google Scholar] [CrossRef]
- Ying, L.; Furmanski, P. Iron binding to human lactoferrin alters reactivity of the protein with plant lectins. Biochem. Biophys. Res. Commun. 1993, 196, 686–691. [Google Scholar] [CrossRef]
- Aisen, P.; Leibman, A. Lactoferrin and transferrin: A comparative study. Biochim. Biophys. Acta Protein Struct. 1972, 257, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, P.H.; Geerts, M.E.; van Veen, H.A.; Mericskay, M.; de Boer, H.A.; Nuijens, J.H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997, 328, 145–151. [Google Scholar] [CrossRef] [PubMed]
- El Yazidi-Belkoura, I.; Legrand, D.; Nuijens, J.; Slomianny, M.C.; van Berkel, P.; Spik, G. The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT29-18-C1 cells is mediated through basic residues located in the N-terminus. Biochim. Biophys. Acta Gen. Subj. 2001, 1568, 197–204. [Google Scholar] [CrossRef]
- Zlatina, K.; Galuska, S.P. The N-glycans of lactoferrin: More than just a sweet decoration. Biochem. Cell Biol. 2021, 99, 117–127. [Google Scholar] [CrossRef]
- Spik, G.; Coddeville, B.; Montreuil, J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie 1988, 70, 1459–1469. [Google Scholar] [CrossRef]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lonnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. 2012, 11, M111 015248. [Google Scholar] [CrossRef]
- Valk-Weeber, R.L.; Eshuis-de Ruiter, T.; Dijkhuizen, L.; van Leeuwen, S.S. Dynamic Temporal Variations in Bovine Lactoferrin Glycan Structures. J. Agric. Food Chem. 2020, 68, 549–560. [Google Scholar] [CrossRef]
- Van Berkel, P.H.C.; Geerts, M.E.J.; Vanveen, H.A.; Kooiman, P.M.; Pieper, F.R.; Deboer, H.A.; Nuijens, J.H. Glycosylated and Unglycosylated Human Lactoferrins Both Bind Iron and Show Identical Affinities towards Human Lysozyme and Bacterial Lipopolysaccharide, but Differ in Their Susceptibilities towards Tryptic Proteolysis. Biochem. J. 1995, 312, 107–114. [Google Scholar] [CrossRef]
- Zheng, F.; Du, Y.M.; Lin, X.S.; Zhou, L.Q.; Bai, Y.; Yu, X.B.; Voglmeir, J.; Liu, L. N-Glycosylation Plays an Essential and Species-Specific Role in Anti-Infection Function of Milk Proteins Using Listeria monocytogenes as Model Pathogen. J. Agric. Food Chem. 2019, 67, 10774–10781. [Google Scholar] [CrossRef]
- Ward, P.P.; Mendoza-Meneses, M.; Mulac-Jericevic, B.; Cunningham, G.A.; Saucedo-Cardenas, O.; Teng, C.T.; Conneely, O.M. Restricted spatiotemporal expression of lactoferrin during murine embryonic development. Endocrinology 1999, 140, 1852–1860. [Google Scholar] [CrossRef]
- Yanaihara, A.; Mitsukawa, K.; Iwasaki, S.; Otsuki, K.; Kawamura, T.; Okai, T. High concentrations of lactoferrin in the follicular fluid correlate with embryo quality during in vitro fertilization cycles. Fertil. Steril. 2007, 87, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.T. Lactoferrin gene expression and regulation: An overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.T.; Gladwell, W.; Beard, C.; Walmer, D.; Teng, C.S.; Brenner, R. Lactoferrin gene expression is estrogen responsive in human and rhesus monkey endometrium. Mol. Hum. Reprod. 2002, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Dikovskaya, M.A.; Trunov, A.N.; Chernykh, V.V.; Korolenko, T.A. Cystatin C and lactoferrin concentrations in biological fluids as possible prognostic factors in eye tumor development. Int. J. Circumpolar Health 2013, 72, 21087. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, G.T.; Cho, I.H.; Sim, S.M.; Yang, J.M.; Lee, D.Y. An antimicrobial protein, lactoferrin exists in the sweat: Proteomic analysis of sweat. Exp. Dermatol. 2011, 20, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Aranda, P.; Perez, M.D.; Calvo, M. Concentration of lactoferrin and transferrin throughout lactation in cow’s colostrum and milk. Biol. Chem. Hoppe Seyler. 1988, 369, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Welty, F.K.; Smith, K.L.; Schanbacher, F.L. Lactoferrin concentration during involution of the bovine mammary gland. J. Dairy Sci. 1976, 59, 224–231. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef]
- Berliner, N.; Hsing, A.; Graubert, T.; Sigurdsson, F.; Zain, M.; Bruno, E.; Hoffman, R. Granulocyte colony-stimulating factor induction of normal human bone marrow progenitors results in neutrophil-specific gene expression. Blood 1995, 85, 799–803. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–658. [Google Scholar] [CrossRef]
- Bennett, R.M.; Kokocinski, T. Lactoferrin Turnover in Man. Clin. Sci. 1979, 57, 453–460. [Google Scholar] [CrossRef]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef]
- Zhou, X.L.; Xu, W.; Tang, X.X.; Luo, L.S.; Tu, J.F.; Zhang, C.J.; Xu, X.; Wu, Q.D.; Pan, W.S. Fecal lactoferrin in discriminating inflammatory bowel disease from irritable bowel syndrome: A diagnostic meta-analysis. BMC Gastroenterol. 2014, 14, 121. [Google Scholar] [CrossRef]
- Fillebeen, C.; Ruchoux, M.M.; Mitchell, V.; Vincent, S.; Benaissa, M.; Pierce, A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor alpha or 1-methyl-4-phenylpyridinium treatment. Mol. Brain Res. 2001, 96, 103–113. [Google Scholar] [CrossRef]
- Bournazou, I.; Pound, J.D.; Duffin, R.; Bournazos, S.; Melville, L.A.; Brown, S.B.; Rossi, A.G.; Gregory, C.D. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Investig. 2009, 119, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Takahashi, H.; Mizumachi, K.; Takezawa, T. Low density lipoprotein receptor-related protein (LRP) is required for lactoferrin-enhanced collagen gel contractile activity of human fibroblasts. J. Biol. Chem. 2003, 278, 22112–22118. [Google Scholar] [CrossRef]
- Willnow, T.E.; Goldstein, J.L.; Orth, K.; Brown, M.S.; Herz, J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J. Biol. Chem. 1992, 267, 26172–26180. [Google Scholar] [CrossRef]
- Grey, A.; Banovic, T.; Zhu, Q.; Watson, M.; Callon, K.; Palmano, K.; Ross, J.; Naot, D.; Reid, I.R.; Cornish, J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol. Endocrinol. 2004, 18, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Aoki, R.; Uchida, R.; Tajima, A.; Aoki-Yoshida, A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochem. Cell Biol. 2017, 95, 57–63. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol. Pharm. Bull. 2008, 31, 1605–1608. [Google Scholar] [CrossRef]
- Ward, P.P.; Mendoza-Meneses, M.; Cunningham, G.A.; Conneely, O.M. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell. Biol. 2003, 23, 178–185. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell. Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qin, Z.; Ye, Q.; Chen, P.; Wang, Z.; Yan, Q.; Luo, Z.; Liu, X.; Zhou, Y.; Xiong, W.; et al. Lactoferrin suppresses the Epstein-Barr virus-induced inflammatory response by interfering with pattern recognition of TLR2 and TLR9. Lab. Investig. 2014, 94, 1188–1199. [Google Scholar] [CrossRef]
- Carthagena, L.; Becquart, P.; Hocini, H.; Kazatchkine, M.D.; Bouhlal, H.; Belec, L. Modulation of HIV Binding to Epithelial Cells and HIV Transfer from Immature Dendritic Cells to CD4 T Lymphocytes by Human Lactoferrin and its Major Exposed LF-33 Peptide. Open Virol. J. 2011, 5, 27–34. [Google Scholar] [CrossRef]
- Mazurier, J.; Legrand, D.; Hu, W.L.; Montreuil, J.; Spik, G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur. J. Biochem. 1989, 179, 481–487. [Google Scholar] [CrossRef]
- Legrand, D.; Vigie, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.C.; Carpentier, M.; Briand, J.P.; Mazurier, J.; Hovanessian, A.G. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Elass-Rochard, E.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Role of heparan sulphate proteoglycans in the regulation of human lactoferrin binding and activity in the MDA-MB-231 breast cancer cell line. Eur. J. Cell Biol. 1998, 77, 344–351. [Google Scholar] [CrossRef]
- Thorne, R.G.; Lakkaraju, A.; Rodriguez-Boulan, E.; Nicholson, C. In vivo diffusion of lactoferrin in brain extracellular space is regulated by interactions with heparan sulfate. Proc. Natl. Acad. Sci. USA 2008, 105, 8416–8421. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Ageeva, K.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B. Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals 2009, 22, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Yamniuk, A.P.; Burling, H.; Vogel, H.J. Thermodynamic characterization of the interactions between the immunoregulatory proteins osteopontin and lactoferrin. Mol. Immunol. 2009, 46, 2395–2402. [Google Scholar] [CrossRef]
- Zwirzitz, A.; Reiter, M.; Skrabana, R.; Ohradanova-Repic, A.; Majdic, O.; Gutekova, M.; Cehlar, O.; Petrovcikova, E.; Kutejova, E.; Stanek, G.; et al. Lactoferrin is a natural inhibitor of plasminogen activation. J. Biol. Chem. 2018, 293, 8600–8613. [Google Scholar] [CrossRef]
- Cappelletti, M.; Presicce, P.; Calcaterra, F.; Mavilio, D.; Della Bella, S. Bright expression of CD91 identifies highly activated human dendritic cells that can be expanded by defensins. Immunology 2015, 144, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, Z.Y.; Groopman, J.E. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood 1998, 92, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Machacek, C.; Fischer, M.B.; Stockinger, H. Differentiation of human monocytes and derived subsets of macrophages and dendritic cells by the HLDA10 monoclonal antibody panel. Clin. Transl. Immunol. 2016, 5, e55. [Google Scholar] [CrossRef]
- Ando, K.; Hasegawa, K.; Shindo, K.; Furusawa, T.; Fujino, T.; Kikugawa, K.; Nakano, H.; Takeuchi, O.; Akira, S.; Akiyama, T.; et al. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010, 277, 2051–2066. [Google Scholar] [CrossRef]
- Stax, M.J.; Mouser, E.E.; van Montfort, T.; Sanders, R.W.; de Vries, H.J.; Dekker, H.L.; Herrera, C.; Speijer, D.; Pollakis, G.; Paxton, W.A. Colorectal mucus binds DC-SIGN and inhibits HIV-1 trans-infection of CD4+ T-lymphocytes. PLoS ONE 2015, 10, e0122020. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Machacek, C.; Charvet, C.; Lager, F.; Le Roux, D.; Platzer, R.; Leksa, V.; Mitulovic, G.; Burkard, T.R.; Zlabinger, G.J.; et al. Extracellular Purine Metabolism Is the Switchboard of Immunosuppressive Macrophages and a Novel Target to Treat Diseases with Macrophage Imbalances. Front. Immunol. 2018, 9, 852. [Google Scholar] [CrossRef]
- Troost, F.J.; Saris, W.H.; Brummer, R.J. Orally ingested human lactoferrin is digested and secreted in the upper gastrointestinal tract in vivo in women with ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef]
- Kuwata, H.; Yip, T.T.; Tomita, M.; Hutchens, T.W. Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin. Biochim. Biophys. Acta 1998, 1429, 129–141. [Google Scholar] [CrossRef]
- Brines, R.D.; Brock, J.H. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta 1983, 759, 229–235. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.; Brummer, R.J. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Spik, G.; Brunet, B.; Mazurier-Dehaine, C.; Fontaine, G.; Montreuil, J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Paediatr. Scand. 1982, 71, 979–985. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Bellamy, W.; Wakabayashi, H.; Takase, M.; Kawase, K.; Shimamura, S.; Tomita, M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993, 182, 97–105. [Google Scholar] [CrossRef]
- Kuhnle, A.; Galuska, C.E.; Zlatina, K.; Galuska, S.P. The Bovine Antimicrobial Peptide Lactoferricin Interacts with Polysialic Acid without Loss of Its Antimicrobial Activity against Escherichia coli. Animals 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.N.; Demcoe, A.R.; Jenssen, H.; Gutteberg, T.J.; Vogel, H.J. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob. Agents Chemother. 2005, 49, 3387–3395. [Google Scholar] [CrossRef]
- Hwang, P.M.; Zhou, N.; Shan, X.; Arrowsmith, C.H.; Vogel, H.J. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry 1998, 37, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.; Rekdal, O.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar] [CrossRef]
- Jenssen, H.; Andersen, J.H.; Uhlin-Hansen, L.; Gutteberg, T.J.; Rekdal, O. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 2004, 61, 101–109. [Google Scholar] [CrossRef]
- Pei, J.; Xiong, L.; Chu, M.; Guo, X.; Yan, P. Effect of intramolecular disulfide bond of bovine lactoferricin on its molecular structure and antibacterial activity against Trueperella pyogenes separated from cow milk with mastitis. BMC Vet. Res. 2020, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- Arredondo-Beltran, I.G.; Ramirez-Sanchez, D.A.; Zazueta-Garcia, J.R.; Canizalez-Roman, A.; Angulo-Zamudio, U.A.; Velazquez-Roman, J.A.; Bolscher, J.G.M.; Nazmi, K.; Leon-Sicairos, N. Antitumor activity of bovine lactoferrin and its derived peptides against HepG2 liver cancer cells and Jurkat leukemia cells. Biometals 2023, 1–17. [Google Scholar] [CrossRef]
- Han, F.F.; Gao, Y.H.; Luan, C.; Xie, Y.G.; Liu, Y.F.; Wang, Y.Z. Comparing bacterial membrane interactions and antimicrobial activity of porcine lactoferricin-derived peptides. J. Dairy Sci. 2013, 96, 3471–3487. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, L.T.; Berge, G.; Sveinbjornsson, B.; Svendsen, J.S.; Vorland, L.H.; Rekdal, O. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res. 2002, 22, 2703–2710. [Google Scholar] [PubMed]
- El-Baky, N.A.; Elkhawaga, M.A.; Abdelkhalek, E.S.; Sharaf, M.M.; Redwan, E.M.; Kholef, H.R. De novo expression and antibacterial potential of four lactoferricin peptides in cell-free protein synthesis system. Biotechnol. Rep. 2021, 29, e00583. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int. Immunopharmacol. 2014, 20, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, M.I.; Groenink, J.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef]
- Van der Kraan, M.I.; Nazmi, K.; Teeken, A.; Groenink, J.; van’t Hof, W.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2005, 386, 137–142. [Google Scholar] [CrossRef]
- Haney, E.F.; Lau, F.; Vogel, H.J. Solution structures and model membrane interactions of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim. Biophys. Acta 2007, 1768, 2355–2364. [Google Scholar] [CrossRef]
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.; Vogel, H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 2009, 91, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Nazmi, K.; Bolscher, J.G.; Vogel, H.J. Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim. Biophys. Acta 2012, 1818, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Bolscher, J.G.; Adao, R.; Nazmi, K.; van den Keybus, P.A.; van’t Hof, W.; Nieuw Amerongen, A.V.; Bastos, M.; Veerman, E.C. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 2009, 91, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xiong, W.; Hu, Q.; Zuo, P.; Shao, B.; Lan, F.; Lu, X.; Xu, Y.; Xiong, S. Lactoferrin-derived peptides and Lactoferricin chimera inhibit virulence factor production and biofilm formation in Pseudomonas aeruginosa. J. Appl. Microbiol. 2010, 109, 1311–1318. [Google Scholar] [CrossRef]
- Flores-Villasenor, H.; Canizalez-Roman, A.; Reyes-Lopez, M.; Nazmi, K.; de la Garza, M.; Zazueta-Beltran, J.; Leon-Sicairos, N.; Bolscher, J.G. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 2010, 23, 569–578. [Google Scholar] [CrossRef]
- Ruangcharoen, S.; Suwannarong, W.; Lachica, M.; Bolscher, J.G.M.; Nazmi, K.; Khunkitti, W.; Taweechaisupapong, S. Killing activity of LFchimera on periodontopathic bacteria and multispecies oral biofilm formation in vitro. World J. Microbiol. Biotechnol. 2017, 33, 167. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Diaz, H.; Canizalez-Roman, A.; Nepomuceno-Mejia, T.; Gallardo-Vera, F.; Hornelas-Orozco, Y.; Nazmi, K.; Bolscher, J.G.; Carrero, J.C.; Leon-Sicairos, C.; Leon-Sicairos, N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017, 95, 82–90. [Google Scholar] [CrossRef]
- Lopez-Soto, F.; Leon-Sicairos, N.; Nazmi, K.; Bolscher, J.G.; de la Garza, M. Microbicidal effect of the lactoferrin peptides lactoferricin17-30, lactoferrampin265-284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals 2010, 23, 563–568. [Google Scholar] [CrossRef]
- Roth-Walter, F. Iron-Deficiency in Atopic Diseases: Innate Immune Priming by Allergens and Siderophores. Front. Allergy 2022, 3, 859922. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal Lactoferrin Administration Decreases Oxidative Stress in the Amniotic Fluid of Pregnant Women: An Open-Label Randomized Pilot Study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, A.J.; Kim, G.Y.; Jo, A.; Lee, J.E.; Leem, S.H.; Yoon, J.H.; Ye, S.K.; Chung, J.W. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J. Microbiol. Biotechnol. 2017, 27, 1877–1884. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Zhu, N.; Xu, Z.; Wang, Y.; Qu, Y.; Wang, J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J. Neurochem. 2020, 152, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron and infection. Microbiol. Rev. 1978, 42, 45–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.L.; Chen, C.L.; Tong, X.; Li, Y.H.; Yang, K.; Lv, H.T.; Xu, J.Y.; Qin, L.Q. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Sorensen, O.E.; Theilgaard-Monch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; de la Rosa, G.; Tewary, P.; Oppenheim, J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009, 30, 531–537. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [CrossRef]
- Leitch, E.C.; Willcox, M.D.P. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J. Med. Microbiol. 1999, 48, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Kanyshkova, T.G.; Semenov, D.V.; Buneva, V.N.; Nevinsky, G.A. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 1999, 451, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Britigan, B.E.; Lewis, T.S.; Waldschmidt, M.; McCormick, M.L.; Krieg, A.M. Lactoferrin binds CpG-containing oligonucleotides and inhibits their immunostimulatory effects on human B cells. J. Immunol. 2001, 167, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Magura, C.E.; Hurley, W.L. Heparin-binding properties of lactoferrin and lysozyme. Comp. Biochem. Physiol. B 1992, 103, 889–895. [Google Scholar] [CrossRef]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.S.; Mazurier, J.; Spik, G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [CrossRef]
- Mattsby-Baltzer, I.; Roseanu, A.; Motas, C.; Elverfors, J.; Engberg, I.; Hanson, L.A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 1996, 40, 257–262. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef]
- Wisgrill, L.; Wessely, I.; Spittler, A.; Forster-Waldl, E.; Berger, A.; Sadeghi, K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018, 192, 315–324. [Google Scholar] [CrossRef]
- Na, Y.J.; Han, S.B.; Kang, J.S.; Yoon, Y.D.; Park, S.K.; Kim, H.M.; Yang, K.H.; Joe, C.O. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 2004, 4, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Du, X.; Jiang, R. Biological activities of commercial bovine lactoferrin sources. Biochem. Cell Biol. 2021, 99, 35–46. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Chen, C.Y.; Castro, G.A. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation 2000, 24, 33–44. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef]
- Zagulski, T.; Lipinski, P.; Zagulska, A.; Broniek, S.; Jarzabek, Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 1989, 70, 697–704. [Google Scholar] [PubMed]
- Nebermann, L.; Dohler, J.R.; Perlick, L. Treatment of enterogenic endotoxinemia with lactoferrin in rats. Langenbecks Arch. Surg. 2001, 386, 146–149. [Google Scholar] [CrossRef]
- Talukder, M.J.; Harada, E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can. J. Physiol. Pharmacol. 2007, 85, 200–208. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [CrossRef]
- Wu, H.; Fan, L.; Gao, Y.; Wang, J.; Zheng, N. The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-kappaB/PPAR Signaling Pathway. Foods 2022, 11, 3378. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.X.; Liu, Y.; Xi, E.Z.; An, J.J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Miyakawa, M.; Tada, A.; Oda, H.; Motobayashi, H.; Iwabuchi, S.; Tamura, S.; Tanaka, M.; Hashimoto, S. Lactoferrin and its digestive peptides induce interferon-alpha production and activate plasmacytoid dendritic cells ex vivo. Biometals 2022, 1–11. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef]
- Perdijk, O.; van Neerven, R.J.J.; van den Brink, E.; Savelkoul, H.F.J.; Brugman, S. Bovine Lactoferrin Modulates Dendritic Cell Differentiation and Function. Nutrients 2018, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, Q.; Cheng, G.; Liu, X.; Liu, S.; Luo, J.; Zhang, A.; Bian, L.; Chen, J.; Lv, J.; et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int. J. Mol. Med. 2015, 36, 363–368. [Google Scholar] [CrossRef]
- Welsh, K.J.; Hwang, S.A.; Boyd, S.; Kruzel, M.L.; Hunter, R.L.; Actor, J.K. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis 2011, 91, S105–S113. [Google Scholar] [CrossRef]
- Actor, J.K.; Nguyen, T.K.T.; Wasik-Smietana, A.; Kruzel, M.L. Modulation of TDM-induced granuloma pathology by human lactoferrin: A persistent effect in mice. Biometals 2022, 1–13. [Google Scholar] [CrossRef]
- Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Oral recombinant human or mouse lactoferrin reduces Mycobacterium tuberculosis TDM induced granulomatous lung pathology. Biochem. Cell Biol. 2017, 95, 148–154. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin administration into the nostril alleviates murine allergic rhinitis and its mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chuang, K.C.; Chen, S.W.; Chao, Y.H.; Yen, C.C.; Yang, S.H.; Chen, W.; Chang, K.H.; Chang, Y.K.; Chen, C.M. Lactoferrin Ameliorates Ovalbumin-Induced Asthma in Mice through Reducing Dendritic-Cell-Derived Th2 Cell Responses. Int. J. Mol. Sci. 2022, 23, 14185. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Shin, S.Y.; Kim, J.H.; Lee, H.Y.; Palikhe, N.S.; Ye, Y.M.; Kim, S.H.; Park, H.S. Serum lactoferrin level as a serologic biomarker for allergic rhinitis. Clin. Exp. Allergy 2010, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Nagasaki, K.; Chea, C.; Ando, T.; Ayuningtyas, N.F.; Inubushi, T.; Ishikado, A.; Imanaka, H.; Sugiyama, E.; Takahashi, I.; et al. Oral administration of bovine lactoferrin suppresses the progression of rheumatoid arthritis in an SKG mouse model. PLoS ONE 2022, 17, e0263254. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Yen, C.C.; Chang, W.H.; Tung, M.C.; Chen, H.L.; Liu, H.C.; Liao, C.H.; Lan, Y.W.; Chong, K.Y.; Yang, S.H.; Chen, C.M. Lactoferrin Protects Hyperoxia-Induced Lung and Kidney Systemic Inflammation in an In Vivo Imaging Model of NF-kappaB/Luciferase Transgenic Mice. Mol. Imaging Biol. 2020, 22, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.K.; Ganeshnarayan, K.; Markowitz, K.; Schreiner, H.; Furgang, D.; Fine, D.H.; Velliyagounder, K. Lactoferrin knockout mice demonstrates greater susceptibility to Aggregatibacter actinomycetemcomitans-induced periodontal disease. J. Periodontol. 2013, 84, 1690–1701. [Google Scholar] [CrossRef]
- Velusamy, S.K.; Fine, D.H.; Velliyagounder, K. Prophylactic effect of human lactoferrin against Streptococcus mutans bacteremia in lactoferrin knockout mice. Microbes Infect. 2014, 16, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zheng, Y.; Fan, S.; Qin, Z.; Li, N.; Tang, A.; Ai, F.; Zhang, X.; Bian, Y.; Dang, W.; et al. Lactoferrin deficiency promotes colitis-associated colorectal dysplasia in mice. PLoS ONE 2014, 9, e103298. [Google Scholar] [CrossRef]
- Velliyagounder, K.; Alsaedi, W.; Alabdulmohsen, W.; Markowitz, K.; Fine, D.H. Oral lactoferrin protects against experimental candidiasis in mice. J. Appl. Microbiol. 2015, 118, 212–221. [Google Scholar] [CrossRef]
- Montezuma, S.R.; Dolezal, L.D.; Rageh, A.A.; Mar, K.; Jordan, M.; Ferrington, D.A. Lactoferrin Reduces Chorioretinal Damage in the Murine Laser Model of Choroidal Neovascularization. Curr. Eye Res. 2015, 40, 946–953. [Google Scholar] [CrossRef]
- Zhang, G.H.; Mann, D.M.; Tsai, C.M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect. Immun. 1999, 67, 1353–1358. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Shen, J.; Xiao, G.; van Wijnen, A.J.; Im, H.J. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J. Cell. Physiol. 2013, 228, 447–456. [Google Scholar] [CrossRef]
- Yan, D.; Kc, R.; Chen, D.; Xiao, G.; Im, H.J. Bovine lactoferricin-induced anti-inflammation is, in part, via up-regulation of interleukin-11 by secondary activation of STAT3 in human articular cartilage. J. Biol. Chem. 2013, 288, 31655–31669. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ellman, M.B.; Yan, D.; An, H.S.; Kc, R.; Li, X.; Chen, D.; Xiao, G.; Cs-Szabo, G.; Hoskin, D.W.; et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J. Cell. Physiol. 2013, 228, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C.; et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.; Clark, R.F.; Hoskin, D.W.; Power Coombs, M.R. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front. Biosci. Landmark Ed. 2022, 27, 43. [Google Scholar] [CrossRef]
- Rybarczyk, J.; Kieckens, E.; De Zutter, L.; Remon, J.P.; Vanrompay, D.; Cox, E. Effects of lactoferrin treatment on Escherichia coli O157:H7 rectal colonization in cattle. Vet. Microbiol. 2017, 202, 38–46. [Google Scholar] [CrossRef]
- Rybarczyk, J.; Khalenkow, D.; Kieckens, E.; Skirtach, A.G.; Cox, E.; Vanrompay, D. Lactoferrin translocates to the nucleus of bovine rectal epithelial cells in the presence of Escherichia coli O157:H7. Vet. Res. 2019, 50, 75. [Google Scholar] [CrossRef]
- Keragala, C.B.; Medcalf, R.L. Plasminogen: An enigmatic zymogen. Blood 2021, 137, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Noring, R.; Epstein, M.P.; McCloud, B.; Hu, R.; Limentani, S.A.; Rogers, R.A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1995, 171, 1258–1265. [Google Scholar] [CrossRef]
- Koenigs, A.; Hammerschmidt, C.; Jutras, B.L.; Pogoryelov, D.; Barthel, D.; Skerka, C.; Kugelstadt, D.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J. Biol. Chem. 2013, 288, 25229–25243. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Loy, J.A.; Tang, J.; Zhang, X.C. Crystal structure of the catalytic domain of human plasmin complexed with streptokinase. Science 1998, 281, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Prior, P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J. Biol. Chem. 2021, 296, 100135. [Google Scholar] [CrossRef] [PubMed]
- Huggins, D.J. Structural analysis of experimental drugs binding to the SARS-CoV-2 target TMPRSS2. J. Mol. Graph. Model. 2020, 100, 107710. [Google Scholar] [CrossRef]
- Tse, L.V.; Marcano, V.C.; Huang, W.; Pocwierz, M.S.; Whittaker, G.R. Plasmin-mediated activation of pandemic H1N1 influenza virus hemagglutinin is independent of the viral neuraminidase. J. Virol. 2013, 87, 5161–5169. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Skrabana, R.; Gebetsberger, L.; Tajti, G.; Barath, P.; Ondrovicova, G.; Prazenicova, R.; Jantova, N.; Hrasnova, P.; Stockinger, H.; et al. Blockade of TMPRSS2-mediated priming of SARS-CoV-2 by lactoferricin. Front. Immunol. 2022, 13, 958581. [Google Scholar] [CrossRef]
- Krzyzowska, M.; Janicka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Grobelny, J.; Szymanski, P. Lactoferrin-Conjugated Nanoparticles as New Antivirals. Pharmaceutics 2022, 14, 1862. [Google Scholar] [CrossRef]
- Groot, F.; Geijtenbeek, T.B.; Sanders, R.W.; Baldwin, C.E.; Sanchez-Hernandez, M.; Floris, R.; van Kooyk, Y.; de Jong, E.C.; Berkhout, B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN--gp120 interaction. J. Virol. 2005, 79, 3009–3015. [Google Scholar] [CrossRef]
- Chen, J.M.; Fan, Y.C.; Lin, J.W.; Chen, Y.Y.; Hsu, W.L.; Chiou, S.S. Bovine Lactoferrin Inhibits Dengue Virus Infectivity by Interacting with Heparan Sulfate, Low-Density Lipoprotein Receptor, and DC-SIGN. Int. J. Mol. Sci. 2017, 18, 1957. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Jenssen, H.; Sandvik, K.; Gutteberg, T.J. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004, 74, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, A.; Jenssen, H.; Nordstrom, I.; Eriksson, K. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012, 93, 340–345. [Google Scholar] [CrossRef]
- Prieto-Fernandez, E.; Egia-Mendikute, L.; Vila-Vecilla, L.; Bosch, A.; Barreira-Manrique, A.; Lee, S.Y.; Garcia-Del Rio, A.; Antonana-Vildosola, A.; Jimenez-Lasheras, B.; Moreno-Cugnon, L.; et al. Hypoxia reduces cell attachment of SARS-CoV-2 spike protein by modulating the expression of ACE2, neuropilin-1, syndecan-1 and cellular heparan sulfate. Emerg. Microbes Infect. 2021, 10, 1065–1076. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Beljaars, L.; van der Strate, B.W.; Bakker, H.I.; Reker-Smit, C.; van Loenen-Weemaes, A.M.; Wiegmans, F.C.; Harmsen, M.C.; Molema, G.; Meijer, D.K. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antivir. Res. 2004, 63, 197–208. [Google Scholar] [CrossRef]
- Carvalho, C.A.M.; Casseb, S.M.M.; Goncalves, R.B.; Silva, E.V.P.; Gomes, A.M.O.; Vasconcelos, P.F.C. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2017, 98, 1749–1754. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Bonaccorsi di Patti, M.C.; Iacovelli, F.; Conte, M.P.; Ianiro, G.; Romeo, A.; Campione, E.; Bianchi, L.; Valenti, P.; et al. Lactoferrin Binding to SARS-CoV-2 Spike Glycoprotein Blocks Pseudoviral Entry and Relieves Iron Protein Dysregulation in Several In Vitro Models. Pharmaceutics 2022, 14, 2111. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Sugiyama, K.; Tanaka, T.; Tanaka, K.; Sekihara, H.; Shimotohno, K.; Kato, N. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem. Biophys. Res. Commun. 1998, 245, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, A.; Ikeda, M.; Naganuma, A.; Nakamura, T.; Inudoh, M.; Tanaka, K.; Kato, N. Identification of a lactoferrin-derived peptide possessing binding activity to hepatitis C virus E2 envelope protein. J. Biol. Chem. 2003, 278, 10162–10173. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.A.G.; Clemens, R.A.; Pressman, P.; Zaigham, M.; Davies, K.J.A.; Naidu, A.S. COVID-19 during Pregnancy and Postpartum: Antiviral Spectrum of Maternal Lactoferrin in Fetal and Neonatal Defense. J. Diet. Suppl. 2020, 19, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- Patil, D.; Chen, S.; Fogliano, V.; Madadlou, A. Hydrolysis improves the inhibition efficacy of bovine lactoferrin against infection by SARS-CoV-2 pseudovirus. Int. Dairy J. 2023, 137, 105488. [Google Scholar] [CrossRef]
- Mirabelli, C.; Wotring, J.W.; Zhang, C.J.; McCarty, S.M.; Fursmidt, R.; Pretto, C.D.; Qiao, Y.; Zhang, Y.; Frum, T.; Kadambi, N.S.; et al. Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105815118. [Google Scholar] [CrossRef]
- Lai, X.; Yu, Y.; Xian, W.; Ye, F.; Ju, X.; Luo, Y.; Dong, H.; Zhou, Y.H.; Tan, W.; Zhuang, H.; et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience 2022, 25, 104136. [Google Scholar] [CrossRef] [PubMed]
- He, S.T.; Qin, H.; Guan, L.; Liu, K.; Hong, B.; Zhang, X.; Lou, F.; Li, M.; Lin, W.; Chen, Y.; et al. Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model. J. Med. Virol. 2023, 95, e28281. [Google Scholar] [CrossRef]
- Naidu, S.A.G.; Clemens, R.A.; Naidu, A.S. SARS-CoV-2 Infection Dysregulates Host Iron (Fe)-Redox Homeostasis (Fe-R-H): Role of Fe-Redox Regulators, Ferroptosis Inhibitors, Anticoagulants, and Iron-Chelators in COVID-19 Control. J. Diet. Suppl. 2022, 20, 1–60. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Yu, Z.; Wu, S.; Ding, L.; Liu, J. Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-CoV-2. Lebensm. Wiss. Technol. 2022, 154, 112684. [Google Scholar] [CrossRef]
- Wotring, J.W.; Fursmidt, R.; Ward, L.; Sexton, J.Z. Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern. J. Dairy Sci. 2022, 105, 2791–2802. [Google Scholar] [CrossRef]
- Avalos-Gomez, C.; Ramirez-Rico, G.; Ruiz-Mazon, L.; Sicairos, N.L.; Serrano-Luna, J.; de la Garza, M. Lactoferrin: An Effective Weapon in the Battle Against Bacterial Infections. Curr. Pharm. Des. 2022, 28, 3243–3260. [Google Scholar] [CrossRef] [PubMed]
- Sallmann, F.R.; Baveye-Descamps, S.; Pattus, F.; Salmon, V.; Branza, N.; Spik, G.; Legrand, D. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. Binding characteristics and biological effects. J. Biol. Chem. 1999, 274, 16107–16114. [Google Scholar] [CrossRef]
- Ellison, R.T., 3rd; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Dashper, S.G.; Pan, Y.; Veith, P.D.; Chen, Y.Y.; Toh, E.C.; Liu, S.W.; Cross, K.J.; Reynolds, E.C. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob. Agents Chemother. 2012, 56, 1548–1556. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Clearly, T.G. Lactoferrin disruption of bacterial type III secretion systems. Biometals 2004, 17, 257–260. [Google Scholar] [CrossRef]
- Dierick, M.; Ongena, R.; Vanrompay, D.; Devriendt, B.; Cox, E. Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine. Pharmaceutics 2022, 14, 1778. [Google Scholar] [CrossRef]
- Silva, T.; Magalhaes, B.; Maia, S.; Gomes, P.; Nazmi, K.; Bolscher, J.G.; Rodrigues, P.N.; Bastos, M.; Gomes, M.S. Killing of Mycobacterium avium by lactoferricin peptides: Improved activity of arginine- and D-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014, 58, 3461–3467. [Google Scholar] [CrossRef]
- Hossain, F.; Moghal, M.M.R.; Islam, M.Z.; Moniruzzaman, M.; Yamazaki, M. Membrane potential is vital for rapid permeabilization of plasma membranes and lipid bilayers by the antimicrobial peptide lactoferricin B. J. Biol. Chem. 2019, 294, 10449–10462. [Google Scholar] [CrossRef]
- Aguilera, O.; Ostolaza, H.; Quiros, L.M.; Fierro, J.F. Permeabilizing action of an antimicrobial lactoferricin-derived peptide on bacterial and artificial membranes. FEBS Lett. 1999, 462, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.; Spiller, C.; Moriarty, L.C.; Patel, A.; Gant, V.; Odell, E.W.; Evans, R.W. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol. Lett. 2004, 233, 193–199. [Google Scholar] [CrossRef]
- Hossain, F.; Dohra, H.; Yamazaki, M. Effect of membrane potential on entry of lactoferricin B-derived 6-residue antimicrobial peptide into single Escherichia coli cells and lipid vesicles. J. Bacteriol. 2021, 203, e00021. [Google Scholar] [CrossRef] [PubMed]
- Wuersching, S.N.; Huth, K.C.; Hickel, R.; Kollmuss, M. Targeting antibiotic tolerance in anaerobic biofilms associated with oral diseases: Human antimicrobial peptides LL-37 and lactoferricin enhance the antibiotic efficacy of amoxicillin, clindamycin and metronidazole. Anaerobe 2021, 71, 102439. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, J.S.; Lee, D.G. Lactoferricin B like peptide triggers mitochondrial disruption-mediated apoptosis by inhibiting respiration under nitric oxide accumulation in Candida albicans. IUBMB Life 2020, 72, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Frontera, L.S.; Moyano, S.; Quassollo, G.; Lanfredi-Rangel, A.; Ropolo, A.S.; Touz, M.C. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018, 8, 18020. [Google Scholar] [CrossRef]
- Haversen, L.A.; Engberg, I.; Baltzer, L.; Dolphin, G.; Hanson, L.A.; Mattsby-Baltzer, I. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 2000, 68, 5816–5823. [Google Scholar] [CrossRef]
- Fais, R.; Rizzato, C.; Franconi, I.; Tavanti, A.; Lupetti, A. Synergistic Activity of the Human Lactoferricin-Derived Peptide hLF1-11 in Combination with Caspofungin against Candida Species. Microbiol. Spectr. 2022, 10, e0124022. [Google Scholar] [CrossRef]
- Sanchez-Gomez, S.; Ferrer-Espada, R.; Stewart, P.S.; Pitts, B.; Lohner, K.; Martinez de Tejada, G. Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol. 2015, 15, 137. [Google Scholar] [CrossRef]
- Morici, P.; Fais, R.; Rizzato, C.; Tavanti, A.; Lupetti, A. Inhibition of Candida albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide hLF1-11. PLoS ONE 2016, 11, e0167470. [Google Scholar] [CrossRef]
- Harris, M.R.; Coote, P.J. Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro. Int. J. Antimicrob. Agents 2010, 35, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Stallmann, H.P.; Lyaruu, D.M.; Joosten, U.; von Eiff, C.; van Nieuw Amerongen, A.; Wuisman, P.I. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 2005, 49, 2438–2444. [Google Scholar] [CrossRef]
- Shaper, M.; Hollingshead, S.K.; Benjamin, W.H., Jr.; Briles, D.E. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected]. Infect. Immun. 2004, 72, 5031–5040. [Google Scholar] [CrossRef]
- Yadav, R.; Noinaj, N.; Ostan, N.; Moraes, T.; Stoudenmire, J.; Maurakis, S.; Cornelissen, C.N. Structural Basis for Evasion of Nutritional Immunity by the Pathogenic Neisseriae. Front. Microbiol. 2019, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Ostan, N.K.; Yu, R.H.; Ng, D.; Lai, C.C.; Pogoutse, A.K.; Sarpe, V.; Hepburn, M.; Sheff, J.; Raval, S.; Schriemer, D.C.; et al. Lactoferrin binding protein B-a bi-functional bacterial receptor protein. PLoS Pathog. 2017, 13, e1006244. [Google Scholar] [CrossRef]
- Yadav, R.; Govindan, S.; Daczkowski, C.; Mesecar, A.; Chakravarthy, S.; Noinaj, N. Structural insight into the dual function of LbpB in mediating Neisserial pathogenesis. eLife 2021, 10, e71683. [Google Scholar] [CrossRef] [PubMed]
- Morgenthau, A.; Livingstone, M.; Adamiak, P.; Schryvers, A.B. The role of lactoferrin binding protein B in mediating protection against human lactoferricin. Biochem. Cell Biol. 2012, 90, 417–423. [Google Scholar] [CrossRef]
- Schryvers, A.B. Targeting bacterial transferrin and lactoferrin receptors for vaccines. Trends Microbiol. 2022, 30, 820–830. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, W.; Tang, H.; Ye, Q.; Liao, Q.; Zhou, Y.; Wu, M.; Xiong, W.; Zheng, Y.; Guo, X.; et al. Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene 2013, 32, 4273–4283. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, X.; Wang, J.; Ye, Q.; Zheng, X.; Peng, Q.; Zheng, Y.; Liu, P.; Zhang, X.; Li, Z.; et al. Lactoferrin deficiency induces a pro-metastatic tumor microenvironment through recruiting myeloid-derived suppressor cells in mice. Oncogene 2020, 39, 122–135. [Google Scholar] [CrossRef]
- Xiao, Y.; Monitto, C.L.; Minhas, K.M.; Sidransky, D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004, 10, 8683–8686. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell. Biochem. 1999, 74, 486–498. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendon, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sanchez, E.A.; Arevalo-Gallegos, S.; Varela-Ramirez, A.; Rascon-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Z.; Zhang, W.; Xiong, W.; Wu, M.; Tan, Y.; Yi, W.; Xiao, L.; Li, X.; Huang, C.; et al. Lactotransferrin: A candidate tumor suppressor-Deficient expression in human nasopharyngeal carcinoma and inhibition of NPC cell proliferation by modulating the mitogen-activated protein kinase pathway. Int. J. Cancer 2008, 123, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis 2004, 25, 1961–1966. [Google Scholar] [CrossRef]
- Rocha, V.P.; Campos, S.P.C.; Barros, C.A.; Trindade, P.; Souza, L.R.Q.; Silva, T.G.; Gimba, E.R.P.; Teodoro, A.J.; Goncalves, R.B. Bovine Lactoferrin Induces Cell Death in Human Prostate Cancer Cells. Oxidative Med. Cell. Longev. 2022, 2022, 2187696. [Google Scholar] [CrossRef] [PubMed]
- Luzi, C.; Brisdelli, F.; Iorio, R.; Bozzi, A.; Carnicelli, V.; Di Giulio, A.; Lizzi, A.R. Apoptotic effects of bovine apo-lactoferrin on HeLa tumor cells. Cell Biochem. Funct. 2017, 35, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Yamamoto, Y.; Ashino, H.; Oikawa, T.; Hazato, T.; Tsuda, H.; Iigo, M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer 2004, 111, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ayuningtyas, N.F.; Chea, C.; Ando, T.; Saninggar, K.E.; Tanimoto, K.; Inubushi, T.; Maishi, N.; Hida, K.; Shindoh, M.; Miyauchi, M.; et al. Bovine Lactoferrin Suppresses Tumor Angiogenesis through NF-kappaB Pathway Inhibition by Binding to TRAF6. Pharmaceutics 2023, 15, 165. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, M.; Luo, C.C.; Wang, J.Q.; Zheng, N. Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef]
- Cutone, A.; Colella, B.; Pagliaro, A.; Rosa, L.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Di Bartolomeo, S.; Musci, G. Native and iron-saturated bovine lactoferrin differently hinder migration in a model of human glioblastoma by reverting epithelial-to-mesenchymal transition-like process and inhibiting interleukin-6/STAT3 axis. Cell. Signal. 2020, 65, 109461. [Google Scholar] [CrossRef]
- Costantini, E.; Di Nicola, M.; Marchioni, M.; Aielli, L.; Reale, M.; Ships, L. Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells. Int. J. Environ. Res. Public Health 2022, 19, 16193. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Nguyen, P.T.; Imanaka, H.; Takata, T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018, 507, 142–147. [Google Scholar] [CrossRef]
- Wolf, J.S.; Li, G.; Varadhachary, A.; Petrak, K.; Schneyer, M.; Li, D.; Ongkasuwan, J.; Zhang, X.; Taylor, R.J.; Strome, S.E.; et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin. Cancer Res. 2007, 13, 1601–1610. [Google Scholar] [CrossRef]
- Spadaro, M.; Curcio, C.; Varadhachary, A.; Cavallo, F.; Engelmayer, J.; Blezinger, P.; Pericle, F.; Forni, G. Requirement for IFN-gamma, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Cancer Res. 2007, 67, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, W. Inhibitory effects of human lactoferrin on U14 cervical carcinoma through upregulation of the immune response. Oncol. Lett. 2014, 7, 820–826. [Google Scholar] [CrossRef]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W., Jr.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer 2004, 111, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, R.; Przepiorski, A.; Reddy, S.; Palmano, K.P.; Krissansen, G.W. “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice. BMC Cancer 2012, 12, 591. [Google Scholar] [CrossRef]
- Kondapi, A.K. Targeting cancer with lactoferrin nanoparticles: Recent advances. Nanomedicine 2020, 15, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Shankaranarayanan, J.S.; Kanwar, J.R.; Al-Juhaishi, A.J.; Kanwar, R.K. Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice. Sci. Rep. 2016, 6, 32062. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Weng, H.; Hu, G.; Wang, S.; Zhao, T.; Yao, X.; Liao, L.; Zhu, X.; Ge, Y. Lactoferrin may inhibit the development of cancer via its immunostimulatory and immunomodulatory activities (Review). Int. J. Oncol. 2021, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Leber, R.; Rinner, B.; Schaider, H.; Lohner, K.; Zweytick, D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim. Biophys. Acta 2015, 1848, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Watanabe, R.; Koike, Y.; Mitobe, M.; Shimazaki, K.; Watanabe, S.; Azuma, I. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: Involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 1997, 237, 624–628. [Google Scholar] [CrossRef]
- Jiang, R.; Lonnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef]
- Pan, W.R.; Chen, P.W.; Chen, Y.L.; Hsu, H.C.; Lin, C.C.; Chen, W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, J.; Li, W.; Li, S.; Han, X. Lactoferricin B reverses cisplatin resistance in head and neck squamous cell carcinoma cells through targeting PD-L1. Cancer Med. 2018, 7, 3178–3187. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on bovine lactoferrin. EFSA J. 2012, 10, 2701. [CrossRef]
- Zea-Vera, A.; Ochoa, T.J. Challenges in the diagnosis and management of neonatal sepsis. J. Trop. Pediatr. 2015, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- Ochoa, T.; Loli, S.; Mendoza, K.; Carcamo, C.; Bellomo, S.; Cam, L.; Castaneda, A.; Campos, M.; Jacobs, J.; Cossey, V.; et al. Effect of bovine lactoferrin on prevention of late-onset sepsis in infants <1500 g: A pooled analysis of individual patient data from two randomized controlled trials. Biochem. Cell Biol. 2021, 99, 14–19. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Akin, I.M.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.D.; Ikinciogullari, A.; Turmen, T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Bjormsjo, M.; Hernell, O.; Lonnerdal, B.; Berglund, S.K. Immunological Effects of Adding Bovine Lactoferrin and Reducing Iron in Infant Formula: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2022, 74, e65–e72. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos, M.; Prada, A.; Valdiviezo, G.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2013, 162, 349–356. [Google Scholar] [CrossRef]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sanchez, J.; Alfaro, S.; Lonnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Laffan, A.M.; McKenzie, R.; Forti, J.; Conklin, D.; Marcinko, R.; Shrestha, R.; Bellantoni, M.; Greenough, W.B., 3rd. Lactoferrin for the prevention of post-antibiotic diarrhoea. J. Health Popul. Nutr. 2011, 29, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Hablass, F.H.; Lashen, S.A.; Alsayed, E.A. Efficacy of Lactoferrin with Standard Triple Therapy or Sequential Therapy for Helicobacter pylori Eradication: A Randomized Controlled Trial. Turk. J. Gastroenterol. 2021, 32, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yoshida, A.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Masuda, Y. Effect of tablets containing lactoferrin and lactoperoxidase on gingival health in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Periodontal Res. 2019, 54, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev Res. 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Decembrino, N.; Muratore, E.; Turroni, S.; Muggeo, P.; Mura, R.; Perruccio, K.; Vitale, V.; Zecca, M.; Prete, A.; et al. Oral Lactoferrin Supplementation during Induction Chemotherapy Promotes Gut Microbiome Eubiosis in Pediatric Patients with Hematologic Malignancies. Pharmaceutics 2022, 14, 1705. [Google Scholar] [CrossRef] [PubMed]
- Lesser, G.J.; Irby, M.B.; Taylor, R.C.; Snavely, A.; Case, D.; Wang, A.; Dietrich, A.; Duncan, S. Lactoferrin supplementation for taste and smell abnormalities among patients receiving cancer chemotherapy. Support. Care Cancer 2022, 30, 2017–2025. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for iron-deficiency anemia in children with inflammatory bowel disease: A clinical trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef]
- Navarro, R.; Paredes, J.L.; Tucto, L.; Medina, C.; Angles-Yanqui, E.; Nario, J.C.; Ruiz-Cabrejos, J.; Quintana, J.L.; Turpo-Espinoza, K.; Mejia-Cordero, F.; et al. Bovine lactoferrin for the prevention of COVID-19 infection in health care personnel: A double-blinded randomized clinical trial (LF-COVID). Biometals 2022, 7, 1–10. [Google Scholar] [CrossRef]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study. Medicina 2021, 57, 842. [Google Scholar] [CrossRef]
| Virus | Mechanism of Action | Selected References |
|---|---|---|
| HIV-1 | Blockade of viral coreceptor CXCR4 of CXCR4-tropic HIV-1 strain by hLF | [58,73] |
| Inhibition of HIV-1 transfer from DCs to CD4 T cells by blockade of DC-SIGN (CD209) by bLF or hLF (and partially also by hLFC) | [64,76,180] | |
| HCMV, RCMV | Blockade of viral cell entry by hLF, bLF, hLFC, or bLFC, likely via interaction with HSPGs on target cells | [92,187] |
| Dengue virus | Blockade of viral coreceptors HSPGs, LRP-1 (CD91) and DC-SIGN (CD209) by bLF | [181] |
| HSV-1, HSV-2 | Blockade of HSPGs by hLF, bLF, hLFC, or bLFC; LFCs are more potent than LFs because of other mechanisms employed | [90,182,183] |
| Zika and Chikungunya viruses | Blockade of viral cell entry by bLF, probably via interaction with HSPGs on target cells | [188] |
| SARS-CoV-2 and other HCoVs | Inhibition of SARS-CoV-2, SARS-CoV-1, and other HCoV attachment because of the HSPG blockade by hLF or bLF | [184,185,186] |
| Blockade of SARS-CoV-2 S protein by hLF or bLF | [189] | |
| HCV | Direct blockade of HCV virions by bLF and hLF | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohradanova-Repic, A.; Praženicová, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. https://doi.org/10.3390/pharmaceutics15041056
Ohradanova-Repic A, Praženicová R, Gebetsberger L, Moskalets T, Skrabana R, Cehlar O, Tajti G, Stockinger H, Leksa V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics. 2023; 15(4):1056. https://doi.org/10.3390/pharmaceutics15041056
Chicago/Turabian StyleOhradanova-Repic, Anna, Romana Praženicová, Laura Gebetsberger, Tetiana Moskalets, Rostislav Skrabana, Ondrej Cehlar, Gabor Tajti, Hannes Stockinger, and Vladimir Leksa. 2023. "Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense" Pharmaceutics 15, no. 4: 1056. https://doi.org/10.3390/pharmaceutics15041056
APA StyleOhradanova-Repic, A., Praženicová, R., Gebetsberger, L., Moskalets, T., Skrabana, R., Cehlar, O., Tajti, G., Stockinger, H., & Leksa, V. (2023). Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics, 15(4), 1056. https://doi.org/10.3390/pharmaceutics15041056







