Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review
Abstract
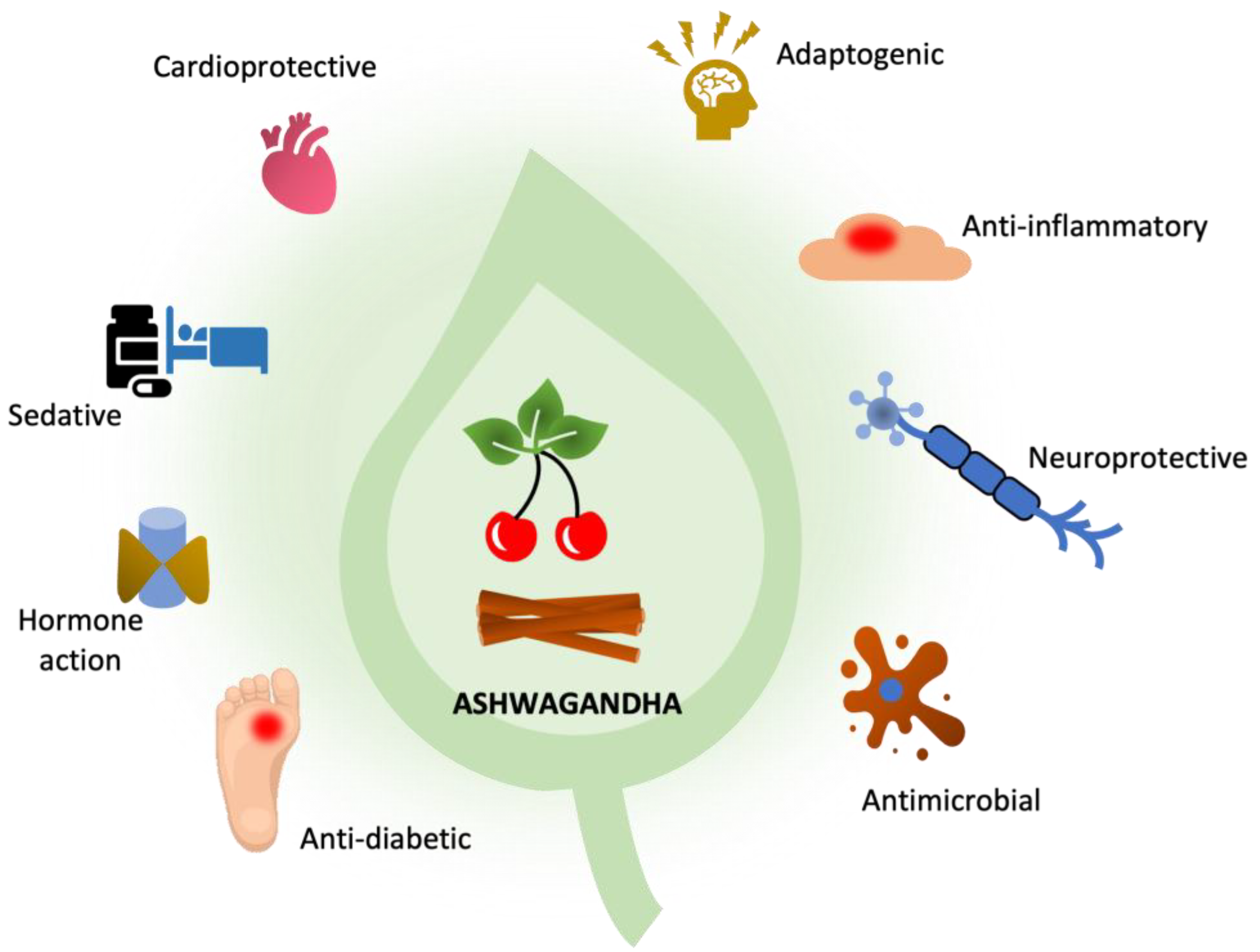
1. Introduction
2. Active Compounds
3. Biological Activity
3.1. Neuroprotective and Anti-Neurodegenerative Effects
3.1.1. Ashwagandha Use in Alzheimer’s Disease
3.1.2. Ashwagandha Use in Parkinson’s Disease
- Genetic conditions;
- Endo- and exogenous toxic factors;
- Neuroinfections;
- Oxidative stress;
- Reduced growth factors;
- The sum of the action of several of the above factors.
3.1.3. Use of Ashwagandha in the Treatment of Huntington’s Disease
3.2. Treatment of Obsessive-Compulsive Disorder, Alcohol Withdrawal Syndrome
3.3. Anti-Inflammatory/Immunomodulatory Effects
3.4. Antibacterial Properties
3.5. Support for Infertility Treatment
3.6. Anticancer Effects
3.7. Antidiabetic Activity
3.8. Cardioprotective Properties
3.9. Treatment of Sleep Disorders
3.10. Anxiolytic and Anti-Stress Effects
3.11. Adaptogenic Effect
3.12. Treatment of Hypothyroidism
3.13. Increase Muscle Strength
3.14. Other Effects of Ashwagandha
4. Safety of Use
5. Contraindications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on Ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed]
- Połumackanycz, M.; Forencewicz, A.; Wesołowski, M.; Viapiana, A. Ashwagandha (Withania somnifera L.)—The plant with proven health-promoting properties. Farm. Pol. 2020, 76, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- John, J. Therapeutic potential of Withania somnifera: A report on phyto-pharmacological properties. Int. J. Pharm. Sci. Res. 2014, 5, 2131–2148. [Google Scholar]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania Somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Kurapati, K.R.V.; Atluri, V.S.R.; Samikkannu, T.; Nair, M.P.N. Ashwagandha (Withania somnifera) reverses β-amyloid1-42 induced toxicity in human neuronal cells: Implications in HIV-associated neurocognitive disorders (HAND). PLoS ONE 2013, 8, e77624. [Google Scholar] [CrossRef]
- Pandey, A.; Bani, S.; Dutt, P.; Satti, N.K.; Suri, K.A.; Qazi, G.N. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef]
- Das, R.; Rauf, A.; Akhter, S.; Islam, M.N.; Emran, T.B.; Mitra, S.; Khan, I.N.; Mubarak, M.S. Role of Withaferin A and Its Derivatives in the Management of Alzheimer’s Disease: Recent Trends and Future Perspectives. Molecules 2021, 26, 3696. [Google Scholar] [CrossRef]
- Atluri, V.S.R.; Tiwari, S.; Rodriguez, M.; Kaushik, A.; Yndart, A.; Kolishetti, N.; Yatham, M.; Nair, M. Inhibition of Amyloid-Beta production, associated neuroinflammation, and Histone Deacetylase 2-mediated epigenetic modifications prevent neuropathology in Alzheimer’s disease in vitro Model. Front. Aging Neurosci. 2020, 11, 342. [Google Scholar] [CrossRef]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Deane, R.; Sagare, A.P.; Bell, R.D.; Winkler, E.A. Low-density lipoprotein receptor-related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J. Neurochem. 2010, 115, 1077–1089. [Google Scholar] [CrossRef]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef]
- Rauch, J.N.; Luna, G.; Guzman, E.; Audouard, M.; Challis, C.; Sibih, Y.E.; Leshuk, C.; Hernandez, I.; Wegmann, S.; Hyman, B.T. LRP1 is a master regulator of tau uptake and spread. Nature 2020, 580, 381–385. [Google Scholar] [CrossRef]
- Dubey, S.; Kallubai, M.; Subramanyam, R. Improving the inhibition of β-amyloid aggregation by withanolide and withanoside derivatives. Int. J. Biol. Macromol. 2021, 173, 56–65. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Withanoside IV and its active metabolite, sominone, attenuate Aβ(25–35)-induced neurodegeneration. Eur. J. Neurosci. 2006, 23, 1417–1426. [Google Scholar] [CrossRef]
- Rabhi, C.; Arcile, G.; Le Goff, G.; Da Costa Noble, C.; Ouazzani, J. Neuroprotective effect of CR-777, a glutathione derivative of Withaferin A, obtained through the bioconversion of Withania somnifera (L.) Dunal extract by the fungus Beauveria bassiana. Molecules 2019, 24, 4599. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, G.; Patnaik, R. Withanolide a penetrates brain via intra-nasal administration and exerts neuroprotection in cerebral ischemia reperfusion injury in mice. Xenobiotica 2020, 50, 957–966. [Google Scholar] [CrossRef]
- Singh, M.; Ramassamy, C. In vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J. Nutr. Sci. 2017, 6, e54. [Google Scholar] [CrossRef]
- Bhargava, P.; Malik, V.; Liu, Y.; Ryu, J.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Molecular Insights Into Withaferin-A-Induced Senescence: Bioinformatics and Experimental Evidence to the Role of NFκB and CARF. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Jelińska, A.; Muszalska, I.C. Chemia Leków z Elementami Chemii Medycznej dla Studentów Farmacji i Farmaceutów; Uniwersytet Medyczny im. Karola Marcinkowskiego: Poznan, Poland, 2018. [Google Scholar]
- Ahmad, M.; Saleem, S.; Ahmad, A.S.; Ansari, M.A.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum. Exp. Toxicol. 2005, 24, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, J.; Thongtan, T.; Kumrapich, B.; Saisawang, C.; Ketterman, A.J. Neuroprotective effects of Withania somnifera in the SH-SY5Y Parkinson cell model. Heliyon 2021, 7, e08172. [Google Scholar] [CrossRef] [PubMed]
- RajaSankar, S.; Manivasagam, T.; Sankar, V.; Prakash, S.; Muthusamy, R.; Krishnamurti, A.; Surendran, S. Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J. Ethnopharmacol. 2009, 125, 369–373. [Google Scholar] [CrossRef]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S. Functional and morphological correlates in the Drosophila LRRK2 loss-of-function model of Parkinson’s disease: Drug effects of Withania somnifera (Dunal) administration. PLoS ONE 2016, 11, e0146140. [Google Scholar] [CrossRef]
- RajaSankar, S.; Manivasagam, T.; Surendran, S. Ashwagandha leaf extract: A potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci. Lett. 2009, 454, 11–15. [Google Scholar] [CrossRef]
- Rojas, N.G.; Cesarini, M.E.; Peker, G.; Da Prat, G.A.; Etcheverry, J.L.; Gatto, E.M. Review of Huntington’s Disease: From Basics to Advances in Diagnosis and Treatment. J. Neurol. Res. 2022, 12, 93–113. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J. Med. Food 2009, 12, 591–600. [Google Scholar] [CrossRef]
- Joshi, T.; Kumar, V.; Kaznacheyeva, E.V.; Jana, N.R. Withaferin A Induces Heat Shock Response and Ameliorates Disease Progression in a Mouse Model of Huntington’s Disease. Mol. Neurobiol. 2021, 58, 3992–4006. [Google Scholar] [CrossRef]
- Fakhri, S.; Piri, S.; Moradi, S.Z.; Khan, H. Phytochemicals Targeting Oxidative Stress, Interconnected Neuroinflammatory, and Neuroapoptotic Pathways Following Radiation. Curr. Neuropharmacol. 2022, 20, 836–856. [Google Scholar] [CrossRef]
- Leder, S.; Siwiak-Kobayashi, M.; Nerwice, W.B.A. Psychiatria, Podręcznik dla Studentów Medycyny; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2016. [Google Scholar]
- Borkowska, A. Znaczenie zaburzeń funkcji poznawczych i możliwości ich oceny w chorobach psychicznych. Psychiatr. Prakt. Klin. 2009, 2, 30–40. [Google Scholar]
- Borkowska, A. Dysfunkcje poznawcze w zaburzeniu obsesyjno-kompulsyjnym. Psychiatr. Psychol. Klin. 2005, 5, 66–78. [Google Scholar]
- Jahanbakhsh, S.P.; Manteghi, A.A.; Emami, S.A.; Mahyari, S.; Gholampour, B.; Mohammadpour, A.H.; Sahebkar, A. Evaluation of the efficacy of Withania somnifera (Ashwagandha) root extract in patients with obsessive-compulsive disorder: A randomized double-blind placebo-controlled trial. Complement. Ther. Med. 2016, 27, 25–29. [Google Scholar] [CrossRef]
- Kaurav, B.P.S.; Wanjari, M.M.; Chandekar, A.; Chauhan, N.S.; Upmanyu, N. Influence of Withania somnifera on obsessive compulsive disorder in mice. Asian Pac. J. Trop. Med. 2012, 5, 380–384. [Google Scholar] [CrossRef]
- Gupta, G.L.; Rana, A.C. Effect of Withania somnifera Dunal in ethanol-induced anxiolysis and withdrawal anxiety in rats. Indian J. Exp. Biol. 2008, 46, 470–475. [Google Scholar]
- Haque, I.M.; Mishra, A.; Kalra, B.S.; Chawla, S. Role of Standardized Plant Extracts in Controlling Alcohol Withdrawal Syndrome—An Experimental Study. Brain Sci. 2021, 11, 919. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Rasool, M.; Varalakshmi, P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul. Pharmacol. 2006, 44, 406–410. [Google Scholar] [CrossRef]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Withania Somnifera as a Potential Anxiolytic and Anti-inflammatory Candidate Against Systemic Lipopolysaccharide-Induced Neuroinflammation. Neuromol. Med. 2018, 20, 343–362. [Google Scholar] [CrossRef]
- Kanjilal, S.; Gupta, A.K.; Patnaik, R.S.; Dey, A. Analysis of Clinical Trial Registry of India for Evidence of Anti-Arthritic Properties of Withania somnifera (Ashwagandha). Altern. Ther. Health Med. 2021, 27, 58–66. [Google Scholar] [PubMed]
- Davis, L.; Kuttan, G. Immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2000, 71, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, A.; Shukla, H.; Benny, I.R.; Tharakan, M.; George, L.; Koshy, S. Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract—A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. J. Clin. Med. 2021, 10, 3644. [Google Scholar] [CrossRef] [PubMed]
- Rawat, V.; Bisht, P. Antibacterial activity of Withania somnifera against Gram-positive isolates from pus samples. Ayu 2014, 35, 330. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Gupta, R.P. In vitro antibacterial effect of Withania somnifera root extract on Escherichia coli. Vet. World 2015, 8, 57. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P. Evaluation of antimicrobial efficacy of flavonoids of Withania somnifera L. Indian J. Pharm. Sci. 2011, 73, 473. [Google Scholar]
- Alam, N.; Hossain, M.; Mottalib, M.A.; Sulaiman, S.A.; Gan, S.H.; Khalil, M.I. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Mwitari, P.G.; Ayeka, P.A.; Ondicho, J.; Matu, E.N.; Bii, C.C. Antimicrobial Activity and Probable Mechanisms of Action of Medicinal Plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS ONE 2013, 8, e65619. [Google Scholar] [CrossRef]
- Owais, M.; Sharad, K.S.; Shehbaz, A.; Saleemuddin, M. Antibacterial efficacy of Withania somnifera (Ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 2005, 12, 229–235. [Google Scholar] [CrossRef]
- Pandit, S.; Chang, K.W.; Jeon, J.G. Effects of Withania somnifera on the growth and virulence properties of Streptococcus mutans and Streptococcus sobrinus at sub-MIC levels. Anaerobe 2013, 19, 1–8. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Dayakar, A.; Veronica, J.; Sundar, S.; Maurya, R. An in vitro study of apoptotic like death in Leishmania donovani promastigotes by withanolides. Parasitol. Int. 2013, 62, 253–261. [Google Scholar] [CrossRef]
- Girish, K.S.; Machiah, K.D.; Ushanandini, S.; Kumar, K.H.; Nagaraju, S.; Govindappa, M.; Vedavathi, M.; Kemparaju, K. Antimicrobial properties of a non-toxic glycoprotein (WSG) from Withania somnifera (Ashwagandha). J. Basic Microbiol. 2006, 46, 365–374. [Google Scholar] [CrossRef]
- Murugan, R.; Rajesh, R.; Seenivasan, B.; Haridevamuthu, B.; Sudhakaran, G.; Guru, A.; Rajagopal, R.; Kuppusamy, P.; Juliet, A.; Gopinath, P. Withaferin A targets the membrane of Pseudomonas aeruginosa and mitigates the inflammation in zebrafish larvae; an in vitro and in vivo approach. Microb. Pathog. 2022, 172, 105778. [Google Scholar] [CrossRef]
- Dikasso, D.; Makonnen, E.; Debela, A.; Abebe, D.; Urga, K.; Makonnen, W.; Assefa, A.; Melaku, D.; Makonnen, W. In vivo anti-malarial activity of hydroalcoholic extracts from Asparagus africanus Lam In mice infected with Plasmodium berghei. Ethiop. J. Health Dev. 2007, 20, 112–118. [Google Scholar] [CrossRef]
- Łukaszuk, K.; Kozioł, K.; Jakiel, G.; Jakimiuk, A.; Jędrzejczak, P.; Kuczyński, W.; Kurzawa, R.; Pawelczyk, L.; Radwan, M.; Spaczyński, R. Diagnostyka i leczenie niepłodności—Rekomendacje Polskiego Towarzystwa Medycyny Rozrodu i Embriologii (PTMRiE) oraz Polskiego Towarzystwa Ginekologów i Położników (PTGP). Ginekol. Perinatol. Prakt. 2018, 3, 112–140. [Google Scholar]
- Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Rajender, S.; Shankhwar, S.N.; Singh, V.; Dalela, D. Withania somnifera Improves Semen Quality in Stress-Related Male Fertility. Evid. Based. Complement. Alternat. Med. 2011, 2011, 576962. [Google Scholar] [CrossRef]
- Nasimi Doost Azgomi, R.; Nazemiyeh, H.; Bazargani, H.S.; Fazljou, S.M.B.; Nejatbakhsh, F.; Jazani, A.M.; AsrBadr, Y.A.; Zomorrodi, A. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: A triple-blind randomised clinical trial. Andrologia 2018, 50, e13041. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod. Biomed. Online 2018, 36, 311–326. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Durg, S.; Shivaram, S.B.; Bavage, S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine 2018, 50, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Dongre, S.; Langade, D.; Bhattacharyya, S. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Improving Sexual Function in Women: A Pilot Study. Biomed Res. Int. 2015, 2015, 284154. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Srivastava, M.K.; Pathak, A.K. Effect of standardized root extract of Ashwagandha (Withania somnifera) on well-being and sexual performance in adult males: A randomized controlled trial. Health Sci. Rep. 2022, 5, e741. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Chander, H.; Munshi, A. Mechanisms of anti-tumor activity of Withania somnifera (Ashwagandha). Nutr. Cancer 2021, 73, 914–926. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Rao, A.S.; Nandal, A.; Kumar, S.; Ganaie, S.A.; Narasihman, B. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J. Ethnopharmacol. 2021, 270, 113704. [Google Scholar] [CrossRef]
- Vashi, R.; Patel, B.M.; Goyal, R.K. Keeping abreast about Ashwagandha in breast cancer. J. Ethnopharmacol. 2021, 269, 113759. [Google Scholar] [CrossRef]
- Nagy, Z.; Cheung, B.B.; Tsang, W.; Tan, O.; Herath, M.; Ciampa, O.C.; Shadma, F.; Carter, D.R.; Marshall, G.M. Withaferin A activates TRIM16 for its anti-cancer activity in melanoma. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef]
- Jawarneh, S.; Talib, W.H. Combination of Ashwagandha Water Extract and Intermittent Fasting as a Therapy to Overcome Cisplatin Resistance in Breast Cancer: An in vitro and in vivo Study. Front. Nutr. 2022, 9, 863619. [Google Scholar] [CrossRef]
- Azab, K.S.; Maarouf, R.E.; Abdel-Rafei, M.K.; El Bakary, N.M.; Thabet, N.M. Withania somnifera (Ashwagandha) root extract counteract acute and chronic impact of γ-radiation on liver and spleen of rats. Hum. Exp. Toxicol. 2022, 41, 9603271221106344. [Google Scholar] [CrossRef]
- Jain, S.; Pandhi, P.; Singh, A.P.; Malhotra, S. Efficacy of standardised herbal extracts in type 1 diabetes—An experimental study. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 23–33. [Google Scholar] [CrossRef]
- Kyathanahalli, C.N.; Manjunath, M.J. Oral supplementation of standardized extract of Withania somnifera protects against diabetes-induced testicular oxidative impairments in prepubertal rats. Protoplasma 2014, 251, 1021–1029. [Google Scholar] [CrossRef]
- Thakur, D.A.; Dey, A.; Chatterjee, S.; Kumar, V. Reverse Ayurvedic Pharmacology of Ashwagandha as an Adaptogenic Anti-Diabetic Plant: A Pilot Study. Curr. Tradit. Med. 2015, 1, 51–61. [Google Scholar] [CrossRef]
- Udayakumar, R.; Kasthurirengan, S.; Mariashibu, T.S.; Rajesh, M.; Anbazhagan, V.R.; Kim, S.C.; Ganapathi, A.; Choi, C.W. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009, 10, 2367–2382. [Google Scholar] [CrossRef]
- Udayakumar, R.; Kasthurirengan, S.; Vasudevan, A.; Mariashibu, T.S.; Rayan, J.J.S.; Choi, C.W.; Ganapathi, A.; Kim, S.C. Antioxidant effect of dietary supplement Withania somnifera L. reduce blood glucose levels in alloxan-induced diabetic rats. Plant Foods Hum. Nutr. 2010, 65, 91–98. [Google Scholar] [CrossRef]
- Tekula, S.; Khurana, A.; Anchi, P.; Godugu, C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018, 106, 1428–1440. [Google Scholar] [CrossRef]
- Surya Ulhas, R.; Malaviya, A. In-silico validation of novel therapeutic activities of withaferin a using molecular docking and dynamics studies. J. Biomol. Struct. Dyn. 2022, 39, 1–12. [Google Scholar] [CrossRef]
- Andallu, B.; Radhika, B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J. Exp. Biol. 2000, 38, 607–609. [Google Scholar]
- Visavadiya, N.P.; Narasimhacharya, A. Hypocholesteremic and antioxidant effects of Withania somnifera (Dunal) in hypercholesteremic rats. Phytomedicine 2007, 14, 136–142. [Google Scholar] [CrossRef]
- Agnihotri, A.P.; Sontakke, S.D.; Thawani, V.R.; Saoji, A.; Goswami, V.S.S. Effects of Withania somnifera in patients of schizophrenia: A randomized, double blind, placebo controlled pilot trial study. Indian J. Pharmacol. 2013, 45, 417–418. [Google Scholar]
- Nayak, S.; Nayak, S.; Panda, B.K.; Das, S. A Clinical Study on management of stress in type-2 diabetes mellitus (Madhumeha) with Ashwagandha (Withania Somnifera). Ayushdhara 2015, 2, 413–417. [Google Scholar]
- Usharani, P.; Fatima, N.; Kumar, C.U.; Kishan, P. Evaluation of a highly standardized Withania somnifera extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double blind, placebo controlled study. Int. J. Ayurveda Pharma Res. 2014, 2, 22–32. [Google Scholar]
- Mohanty, I.R.; Arya, D.S.; Gupta, S.K. Withania somnifera provides cardioprotection and attenuates ischemia-reperfusion induced apoptosis. Clin. Nutr. 2008, 27, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. Biomed Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef]
- Guo, R.; Gan, L.; Lau, W.B.; Yan, Z.; Xie, D.; Gao, E.; Christopher, T.A.; Lopez, B.L.; Ma, X.; Wang, Y. Withaferin A Prevents Myocardial Ischemia/Reperfusion Injury by Upregulating AMP-Activated Protein Kinase-Dependent B-Cell Lymphoma2 Signaling. Circ. J. 2019, 83, 1726–1736. [Google Scholar] [CrossRef]
- Michalik, A.; Jarzyna, R. Kluczowa rola kinazy białkowej aktywowanej przez AMP (AMPK) w procesach starzenia. Postepy Biochem. 2016, 62, 459–471. [Google Scholar]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Woods, A.; Vertommen, D.; Neumann, D.; Turk, R.; Bayliss, J.; Schlattner, U.; Wallimann, T.; Carling, D.; Rider, M.H. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J. Biol. Chem. 2003, 278, 28434–28442. [Google Scholar] [CrossRef]
- Siemiński, M.; Skorupa, Ł.; Wiśniewska-Skorupa, K. Diagnostyka i terapia bezsenności w praktyce ogólnolekarskiej Część I: Epidemiologia, patomechanizm i diagnostyka bezsenności. Forum Med. Rodz. 2018, 12, 242–251. [Google Scholar]
- Kelgane, S.B.; Salve, J.; Sampara, P.; Debnath, K. Efficacy and Tolerability of Ashwagandha Root Extract in the Elderly for Improvement of General Well-being and Sleep: A Prospective, Randomized, Double-blind, Placebo-controlled Study. Cureus 2020, 12, e7083. [Google Scholar] [CrossRef]
- Kaushik, M.K.; Kaul, S.C.; Wadhwa, R.; Yanagisawa, M.; Urade, Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS ONE 2017, 12, e0172508. [Google Scholar] [CrossRef]
- Vernon, M.K.; Dugar, A.; Revicki, D.; Treglia, M.; Buysse, D. Measurement of non-restorative sleep in insomnia: A review of the literature. Sleep Med. Rev. 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Sarsour, K.; Van Brunt, D.L.; Johnston, J.A.; Foley, K.A.; Morin, C.M.; Walsh, J.K. Associations of nonrestorative sleep with insomnia, depression, and daytime function. Sleep Med. 2010, 11, 965–972. [Google Scholar] [CrossRef]
- Roth, T. What is the nature of nonrestorative sleep? Sleep Med. 2010, 10, 963–964. [Google Scholar] [CrossRef]
- Roth, T.; Zammit, G.; Lankford, A.; Mayleben, D.; Stern, T.; Pitman, V.; Clark, D.; Werth, J.L. Nonrestorative sleep as a distinct component of insomnia. Sleep 2010, 33, 449–458. [Google Scholar] [CrossRef]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of Ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef]
- Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005, 25, 117–129. [Google Scholar] [CrossRef]
- Gaine, M.E.; Chatterjee, S.; Abel, T. Sleep Deprivation and the Epigenome. Front. Neural Circuits 2018, 12, 14. [Google Scholar] [CrossRef]
- Abrams, R.M. Sleep Deprivation. Obstet. Gynecol. Clin. North Am. 2015, 42, 493–506. [Google Scholar] [CrossRef]
- Suganya, K.; Kayalvizhi, E.; Yuvaraj, R.; Chandrasekar, M.; Kavitha, U.; Suresh, K.K. Effect of Withania Somnifera on the antioxidant and neurotransmitter status in sleep deprivation induced Wistar rats. Bioinformation 2020, 16, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D. An investigation into the stress-relieving and pharmacological actions of an Ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Kirby, J.B.; O’Connor, J.; Lindsay, K.G.; Hutchins, A.; Harris, M. The Perceived Impact of Ashwagandha on Stress, Sleep Quality, Energy, and Mental Clarity for College Students: Qualitative Analysis of a Double-Blind Randomized Control Trial. J. Med. Food 2022, 25, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Lindsay, K.; Baker, C.; Kirby, J.; Hutchins, A.; Harris, M. The Impact of Ashwagandha on Stress, Sleep Quality, and Food Cravings in College Students: Quantitative Analysis of a Double-Blind Randomized Control Trial. J. Med. Food 2022, 25, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Fuladi, S.; Emami, S.A.; Mohammadpour, A.H.; Karimani, A.; Manteghi, A.A.; Sahebkar, A. Assessment of the Efficacy of Withania somnifera Root Extract in Patients with Generalized Anxiety Disorder: A Randomized Double-blind Placebo-Controlled Trial. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 191–196. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef]
- Pratte, M.A.; Nanavati, K.B.; Young, V.; Morley, C.P. An alternative treatment for anxiety: A systematic review of human trial results reported for the Ayurvedic herb Ashwagandha (Withania somnifera). J. Altern. Complement. Med. 2014, 20, 901–908. [Google Scholar] [CrossRef]
- Karasek, M. Dehydroepiandrosterone (DHEA) in postmenopausal women. Prz. Menopauzalny 2005, 9, 8. [Google Scholar]
- Jarząbek-Bielecka, G.; Plagens-Rotman, K.; Kędzia, W.; Religioni, U.; Merks, P. Znaczenie DHEA w zdrowiu seksualnym w okresie przekwitania. Farm. Pol. 2020, 76, 537–540. [Google Scholar] [CrossRef]
- Gopukumar, K.; Thanawala, S.; Somepalli, V.; Rao, T.S.S.; Thamatam, V.B.; Chauhan, S. Efficacy and Safety of Ashwagandha Root Extract on Cognitive Functions in Healthy, Stressed Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, H.; Mishra, R.; Manchanda, S.; Gupta, M.; Saini, V.; Sharma, A.; Kaur, G. Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol. Cell. Biochem. 2017, 427, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J.M.; Brar, J.; Rai, A.; Chengappa, K.N.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2019, 31, 123–129. [Google Scholar]
- Candelario, M.; Cuellar, E.; Reyes-Ruiz, J.M.; Darabedian, N.; Feimeng, Z.; Miledi, R.; Russo-Neustadt, A.; Limon, A. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015, 171, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.; Cottiglia, F.; Maccioni, E.; Talani, G.; Sanna, E.; Bassareo, V.; Kasture, S.B.; Acquas, E. The biologically active compound of Withania somnifera (L.) Dunal, docosanyl ferulate, is endowed with potent anxiolytic properties but devoid of typical benzodiazepine-like side effects. J. Psychopharmacol. 2021, 35, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Marathe, P.A.; Satam, S.D.; Raut, S.B.; Shetty, Y.C.; Pooja, S.G.; Raut, A.A.; Kale, P.P.; Rege, N.N. Effect of Withania somnifera (L.) Dunal aqueous root extract on reinstatement using conditioned place preference and brain GABA and dopamine levels in alcohol dependent animals. J. Ethnopharmacol. 2021, 274, 113304. [Google Scholar] [CrossRef]
- Chengappa, K.N.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive use of a standardized extract of Withania somnifera (Ashwagandha) to treat symptom exacerbation in schizophrenia: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2018, 79, 22496. [Google Scholar] [CrossRef]
- Remenapp, A.; Coyle, K.; Orange, T.; Lynch, T.; Hooper, D.; Hooper, S.; Conway, K.; Hausenblas, H.A. Efficacy of Withania somnifera supplementation on adult’s cognition and mood. J. Ayurveda Integr. Med. 2022, 13, 100510. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence based efficacy of adaptogens in fatigue Evidence-Based Efficacy of Adaptogens in Fatigue, and Molecular Mechanisms Related to their Stress-Protective Activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Muruganandam, A.V. Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol. Biochem. Behav. 2003, 75, 547–555. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A.K.; Chandan, B.K.; Gupta, D.K.; Bhutani, K.K.; Anand, K.K. Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withania somnifera Dun. Phytother. Res. 2001, 15, 311–318. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Mourad, H.H.; Mannaa, F.A.; Morsy, F.A.; Hassan, L.K.; Taher, R.F. Role of Ashwagandha methanolic extract in the regulation of thyroid profile in hypothyroidism modeled rats. Mol. Biol. Rep. 2019, 46, 3637–3649. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Changes in thyroid hormone concentrations after administration of Ashwagandha root extract to adult male mice. J. Pharm. Pharmacol. 1998, 50, 1065–1068. [Google Scholar] [CrossRef]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Shenoy, S.; Chaskar, U.; Sandhu, J.S.; Paadhi, M.M. Effects of eight-week supplementation of Ashwagandha on cardiorespiratory endurance in elite Indian cyclists. J. Ayurveda Integr. Med. 2012, 3, 209–214. [Google Scholar] [CrossRef]
- Choudhary, B.; Shetty, A.; Langade, D.G. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in improving cardiorespiratory endurance in healthy athletic adults. Ayu 2015, 36, 63–68. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Kedia, A.W.; Sandrock, J.E.; Raub, B.J.; Kerksick, C.M.; Lopez, H.L. Effects of an aqueous extract of Withania somnifera on strength training adaptations and recovery: The STAR trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef]
- Shree, P.; Mishra, P.; Selvaraj, C.; Singh, S.K.; Chaube, R.; Garg, N.; Tripathi, Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)—A molecular docking study. J. Biomol. Struct. Dyn. 2022, 40, 190–203. [Google Scholar] [CrossRef]
- Khanal, P.; Chikhale, R.; Dey, Y.N.; Pasha, I.; Chand, S.; Gurav, N.; Ayyanar, M.; Patil, B.M.; Gurav, S. Withanolides from Withania somnifera as an immunity booster and their therapeutic options against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 5295–5308. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. Drug Des. Devel. Ther. 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- Minhas, U.; Minz, R.; Bhatnagar, A. Prophylactic effect of Withania somnifera on inflammation in a non-autoimmune prone murine model of lupus. Drug Discov. Ther. 2011, 5, 195–201. [Google Scholar] [CrossRef]
- Xu, K.; Shi, H.; Du, Y.; Ou, J. Withaferin A inhibits proliferation of human endometrial cancer cells via transforming growth factor-β (TGF-β) signalling. 3 Biotech 2021, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.-M.; Chang, P.M.-H.; Cheng, T.-S.; Kuo, Y.-L.; Wu, A.T.-H.; Tran, T.-H.; Yang, Y.-H.; Chen, J.-M.; Tsai, Y.-C.; Chu, Y.-S.; et al. Identification of Withaferin A as a Potential Candidate for Anti-Cancer Therapy in Non-Small Cell Lung Cancer. Cancers 2019, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, G.; Kumar, B.A.; Lakshman, M.; Manvitha, V.; Kumar, B.K. Adaptogenic and immunomodulatory activity of Ashwagandha root extract: An experimental study in an equine model. Front. Vet. Sci. 2020, 7, 541112. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Ahamed, R.; Rajesh, S.; George, T.; Mohanan, M.; Augustine, P. Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs. World J. Hepatol. 2020, 12, 574–595. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S.; Avula, B.; Khan, I.A.; Jonasson, J.G.; Ghabril, M.; Hayashi, P.H.; Navarro, V. Ashwagandha-induced liver injury: A case series from Iceland and the US drug-induced liver injury network. Liver Int. 2020, 40, 825–829. [Google Scholar] [CrossRef]
- Ireland, P.J.; Hardy, T.; Burt, A.D.; Donnelly, M.C. Drug-induced hepatocellular injury due to herbal supplement Ashwagandha. J. R. Coll. Physicians Edinb. 2021, 51, 363–366. [Google Scholar] [CrossRef]
- Suryawanshi, G.; Abdallah, M.; Thomson, M.; Desai, N.; Chauhan, A.; Lim, N. Ashwagandha-Associated Acute Liver Failure Requiring Liver Transplantation. Am. J. Ther. 2023, 30, e80–e83. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef]
- Prabu, P.C.; Panchapakesan, S. Prenatal developmental toxicity evaluation of Withania somnifera root extract in Wistar rats. Drug Chem. Toxicol. 2015, 38, 50–56. [Google Scholar] [CrossRef]
- Kamal, H.I.; Patel, K.; Brdak, A.; Heffernan, J.; Ahmad, N. Ashwagandha as a Unique Cause of Thyrotoxicosis Presenting With Supraventricular Tachycardia. Cureus 2022, 14, e23494. [Google Scholar] [CrossRef]
- Brar, G.K.; Malhotra, M. Ashwagandha (Withania somnifera)—A herb with versatile medicinal properties empowering human physical and mental health. J. Pre-Clin. Clin. Res. 2021, 15, 129–133. [Google Scholar]
- Bassareo, V.; Porru, S.; Rosas, M.; Frau, R.; Talani, G.; Sanna, E.; Acquas, E.; Gioacchino, M. New Insights on the Effects of Withania somnifera on the Motivational Properties of Addictive Drugs. In Proceedings of the XVII-National Congress of the Italian Neuroscience Society, Turin, Italy, 4 May 2017. [Google Scholar]
- Akhgarjand, C.; Asoudeh, F.; Bagheri, A.; Kalantar, Z.; Vahabi, Z.; Shab-bidar, S.; Rezvani, H.; Djafarian, K. Does Ashwagandha supplementation have a beneficial effect on the management of anxiety and stress? A systematic review and meta-analysis of randomized controlled trials. Phyther. Res. 2022, 36, 4115–4124. [Google Scholar] [CrossRef]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. Investigation of CYP3A4 and CYP2D6 Interactions of Withania somnifera and Centella asiatica in Human Liver Microsomes. Phytother. Res. 2015, 29, 785–790. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]

| Disease | Target | Likely Mechanism of Action | Type of Study | Observed Activity | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | The use of MTM to investigate how it affected the expression of the genes involved in neural plasticity and HDAC2 in an Alzheimer’s disease cell culture model. | An sp1 inhibitor called MTM prevented HDAC2 overexpression and resulted in much lower HDAC2 gene and protein expression, which restored the expression of synaptic plasticity genes in SH-APP cells. | In vitro model system | Inhibition of amyloid-beta production and activated B cells (NF-κB)-associated neuroinflammatory molecules’ gene expression—prevention of neuroinflammation and neurodegeneration | [10] |
| Removal of toxic Aβ peptide in the brain | Decreased expression of RAGE and clusterin. Selective down-regulation of liver LRP and degradation of Aβ. | In vivo mouse model | Increased levels of sLRP in plasma, enhanced expression of LRP and NEP in the liver, concomitant increase in plasma Aβ42/40 and decrease in brain Aβ monomer levels—reversal of behavioural dysfunctions | [11,12] | |
| The ability of Withania somnifera derivatives to improve the fibril formation of amyloid-β 42 in Alzheimer’s disease | Interaction with the hydrophobic core of amyloid-β 1–42 during the oligomeric stage, thus inhibiting further interaction with the monomers and reduces aggregation | In vitro studies | Decrease in apoptotic cells and reactive oxygen species. | [15] | |
| Neuroprotective effect of Withaferin A | Cell toxicity signaling pathway inhibition involving PI3K/mtor pathway | In vitro studies | Protection of dopaminergic and cortical neurons. Increase in cell survivor. | [17] | |
| Withanolide A’s ability to penetrate the brain and protect against cerebral ischemia-reperfusion damage | Inhibition of matrix metalloproteinases-2 (MMP-2). Lowered elevated levels of glutamate and GABA. However, more studies required to properly elucidate the mechanism of action. | In vivo mouse model | Decrease in apoptotic and necrotic cell death. Reduction in morphological damage of brain tissues. Reduced cerebral infarction and oedema. Restored blood-brain barrier disruption. | [18] | |
| Parkinson’s disease | Protection of neuronal injury in Parkinson’s disease and physiological abnormalities | Upregulation Of DA receptors after lesioning. Use of gpx which uses H202 to oxidize GSH in order to defend against hydrogen peroxide toxicity and detoxify free radicals and lipid peroxides. Further studies required for description of mechanism especially for dosage form. Interference with oxidative damage. | In vivo mouse model | Reversal of toxic effects of 6-OHDA, muscle, and locomotive activity. Increase in striatal content and dopaminergic D2 receptor populations in striatum. Improvement of enzyme activity hence physiological functions | [22,26] |
| W. somnifera root extract, protective effects against 6-OHDA-induced toxicity in the human neuroblastoma SH-SY5Y cell line | Increase in glutathione peroxidase activity and thioltransferase activity. Modulation of oxidative response proteins and the control of redox regulation via S-glutathionylation | In vitro cell lines | Increase in ATP levels and decrease in protein-glutathionylation levels in the cells. | [23] | |
| Effect of W. somnifera on catecholamines and physiological abnormalities | Induction of catecholamines, antioxidants, and translation of proteins hence Cell growth | In vivo mouse model | Increased DA, DOPAC and HVA levels and normalized TBARS levels in the corpus striatum. Improved motor function | [24] | |
| Evaluation of the neuroprotective effects of W. somnifera extract on the LRRK2 loss-of-function | Decrease in PSP amplitude and suppression of mutation by reversal of mutation-related loss of mitochondrial structural integrity | In vivo Drosophila melanogaster model | Improved motor and muscle activity. Protection against mitochondria degeneration | [25] | |
| Huntington’s disease | Restoration of biochemical alterations caused by 3-NP | Not yet fully understood and require further research | In vivo mouse model | Restoration of mitochondrial enzyme activity and antioxidant enzymes in striatum and cortex of the brain, reversal of muscle impairment, reduction of lipid peroxidation, nitrate, and dehydrogenase enzymes | [28] |
| Elongation of lifespan with administration of Withaferin A | Activation of HSF1 and induction of HSR chaperones | In vivo mouse model | Decrease in inflammatory process and mutant huntingtin aggregates. Improvement of striatal function | [29] | |
| Treatment of obsessive-compulsive disorder, alcohol withdrawal syndrome | Alleviating symptoms of obsessive-compulsive disorder | Not yet fully understood and require further research—likely impact on the serotonin system | Randomized double-blind placebo-controlled trial | Significantly greater effect of W. somnifera in alleviation severity of OCD assessed using Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) in a randomized double-blind placebo-controlled trial | [35] |
| In vivo rat model | Decrease in marble hiding behaviour activity behavioural activity without affecting motor activity in rats | [36] | |||
| In vivo rat model | Beneficial effects on controlling behavioural changes, anxiety and seizures in alcohol withdrawal symptoms in rats, and improve locomotor activity. | [38] | |||
| In vivo rat model | Alleviation of withdrawal anxiety due to chronic alcohol consumption, | [37] | |||
| Anti-inflammatory/immunomodulatory effects mi | Alleviating inflammation and improving immunity | Not yet fully understood and require further research—likely Inhibition of lipopolysaccharide (LPS)-activated NFκB, P38 and JNK/SAPK MAPK pathways, regulating cytokines | In vivo mouse model | Inhibition of the NF-κB and MAPK (mitogen-activated protein kinase) pathways by de-creasing the expression of pro-inflammatory cytokines, including interleukin (IL)-8, IL-6, tumour necrosis factor (TNF-α), IL-1β, and IL-12, and increasing the expression of anti-inflammatory cytokines | [41] |
| In vivo mouse model | Potent inhibitory effect on proteinuria, nephritis, and other inflammatory markers such as cytokines including interleukin (IL)-6 and tumour necrosis factor (TNF)-α, nitric oxide (NO), and ROS in a mouse model of lupus | [133] | |||
| In vivo rat model | Changes in the concentrations of a number of serum proteins such as α2 glycoprotein, acute phase protein α1 and prealbumin were demonstrated, along with a significant reduction in inflammation | [40] | |||
| In vivo mouse model | Increase in the total number of white blood cells and bone marrow cells, as well as to increase the titre of circulating antibodies and antibody-producing cells, and to stimulate the production of immune cells and phagocytosis of macrophages | [44] | |||
| In vivo rat model | Changes in the concentrations of serum proteins such as α2 glycoprotein, acute phase protein α1 and prealbumin with a significant reduction in inflammation | [40] | |||
| In vivo rat model | Inhibition of reactive gliosis, production of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and expression of nitrooxidative stress enzymes | [60] | |||
| Randomized double-blind placebo-controlled trial | Significantly increased natural killer cell activity and cytokine levels compared to placebo | [45] | |||
| Randomized double-blind placebo-controlled trial | Significant increase in natural killer cell activity and cytokine levels in a randomized, double-blind, placebo-controlled trial | [45] | |||
| Antibacterial properties | Inhibiting the growth pathogenic bacteria | Not yet fully understood and require further research–likely disrupting bacterial cell membranes—more research needed | In vitro | Inhibition of the growth of methicillin-resistant Staphylococcus aureus and Enterococcus spp. | [46] |
| In vitro | Effective inhibition the growth of Staphylococcus aureus, Proteus mirabilis, Escherichia coli and Pseudomonas aeruginosa. | [122] | |||
| In vitro | Effective inhibition the growth of Escherichia coli, Salmonella typhi, Citrobacter freundii, Pseudomonas aeruginosa and Klebsiella pneumoniae. | [49] | |||
| In vitro | Effective treatment for salmonellosis, as it significantly alleviates the course of infection following infection with this pathogen | [51] | |||
| In vitro | Significantly slowness the growth of bacteria present in the oral cavity, such as Streptococcus mutant and Streptococcus sobrinus | [52] | |||
| In vitro | Induction of cell death (acts on promastigotes) of Leishamania donovani by activating the process of apoptosis | [53] | |||
| In vitro | Antifungal properties against some fungal species; it inhibits Candida albicans | [48] | |||
| In vitro | Antifungal properties of Withania somnifera glycoprotein from its root tubers, against Aspergillus flavus, Fusarium oxysporum, Fusarium verticilloides, and antibacterial properties against Clavibacter michiganensis subsp. michiganensis | [54] | |||
| In vivo mouse model | Effective in the treatment of malaria, significantly reducing parasitaemia | [56] | |||
| Support for infertility treatment | Improve the quality of semen | Not yet fully understood and require further research—likely effect on the GABA receptors, thus facilitating the expression of GnRH expression; structural similarity to testosterone and thus imparted the benefits of male steroidal hormones; regulation of oxidative stress | In vivo study | Decrease in stress, improved the level of antioxidants and improved overall semen quality | [58] |
| Randomized, double-blind, placebo-controlled study | Statistically significant increase in the total DISF-M (the derogatis interview for sexual functioning-male) scores | [65] | |||
| Triple-blind randomised clinical trial | Increased mean sperm count and progressive motility and improved sperm morphology compared to the baseline | [59] | |||
| Clinical trial | Repair of disturbed plasma concentrations of lactate, alanine, citrate, GPC, histidine and phenylalanine and restores semen quality | [60] | |||
| A randomized controlled trial | Significant subjective perception of sexual well-being and assisted in increasing serum testosterone levels in the participants. | [65] | |||
| Anticancer effects | Inhibition of cancer cell proliferation | Not yet fully understood and require further research | In vitro | Activation by withaferin A the TRIM16 protein, leads to the degradation of cancer-related proteins and ultimately induces cell death in melanoma cells | [68] |
| In vitro | Effectiveness of withaferin a in the treatment of melanoma by compound induces apoptosis reduction cell proliferation and inhibits melano-ma cell migration | [69] | |||
| In vitro/In vivo | Inhibited GBM growth in vitro and In vivo and triggered intrinsic apoptosis of GBM cells | [70] | |||
| In vitro | Inhibition of proliferation of human endometrial cancer cells by Withaferin A through the modulation of TGF-β signaling and the inhibition of TGF-β dependent Smad2 phosphorylation | [134] | |||
| In vitro | Withaferin A alone or in combination with standard chemotherapy is a potential treatment option for EGFR (epidermal growth factor receptor) wild-type lung cancer and may decrease the occurrence of cisplatin resistance by inhibiting lung CSCs (cancer stem-like cell). | [135] | |||
| In vitro/In vivo mouse model | combination of extract and intermittent fasting decrease cancer cell proliferation through apoptosis induction, while also reducing cisplatin-induced toxicity in the liver and kidney. | [71] | |||
| In vivo rat model | protective effect against acute and chronic gamma radiation-induced damage to the liver and spleen tissues of rats | [72] | |||
| Antidiabetic activity | Lowering blood sugar levels | Not yet fully understood and require further research—likely Improving insulin sensitivity, stimulating beta cells, reducing inflammation, and protecting against oxidative stress, inhibition of α-glucosidase | In vivo rat model | Decrease in fasting blood glucose levels in rats with STZ-induced hyperglycaemia | [73] |
| In vivo rat model | Improvement diabetes-induced testicular dysfunction in pre-adolescent rats. | [74] | |||
| In vivo rat model | Efficacy against elevated plasma glucose, insulin and cortisol levels and changes in adrenal and spleen weights in diabetic animals | [80] | |||
| Double-blind randomized control trial | Improve antioxidant parameters and lipid profile, and demonstrate the tolerability and safety | [84] | |||
| Treatment of sleep disorders | Improve the quality and length of sleep | Not yet fully understood and require further research—likely effect on GABAergic activity | In vivo mouse model | Significant induction of NREM (Non-Rapid Eye Movement) sleep in research on mice | [93] |
| In vivo rat model | significant reduction in the levels of free radicals, lipid peroxidation, and an increase in the levels of antioxidant enzymes in the sleep-deprived rat group | [102] | |||
| Randomized, double-blind, placebo-controlled study | Improvement in the general wellbeing, sleep quality, and mental alertness in a prospective, randomized, double-blind, placebo-controlled study | [92] | |||
| Randomized, double-blind, placebo-controlled study | Significantly improved the quality of sleep and easier and faster to falling asleep | [84] | |||
| Randomized, double-blind, placebo-controlled study | Sleep efficiency, sleep duration and total sleep time improvements in physical, psychological, and environmental areas were also noted | [98] | |||
| Cardioprotective properties | Protection of heart cells against harmful agents | Not yet fully understood and require further research—likely anti-apoptotic properties due to an increase in AMP-activated protein kinase (AMPK) phosphorylation and an increase in the Bcl-2/Bax ratio (AMPK) and by restoring oxidative balance | In vivo rat model | A decrease in glutathione levels, a decrease in the activity of superoxide dismutase, catalase, creatinine phosphokinase, and lactate dehydrogenase albino rats in which myocardial necrosis treated with Withania Somnifera. | [85] |
| In vivo rat model | Reduction of the damage to the heart caused by ischemia induced in rats | [86] | |||
| In vivo rat model | In this study in rats, low doses of withaferin A were shown to have a cardioprotective effect by upregulating the mitochondrial anti-apoptotic pathway due to an increase in AMP-activated protein kinase (AMPK) phosphorylation and an increase in the Bcl-2/Bax ratio (AMPK). | [87] | |||
| Anxiolytic and anti-stress effects | Calming and stress-relieving effect | Not yet fully understood and require further research—likely moderating effect on the hypothalamus-pituitary-adrenal axis (HPA); antioxidant and anti-inflammatory effects | A randomized, double-blind, placebo-controlled study | Reduction in the HAM-A (Hamilton Anxiety Rating Scale) IN A randomized, double-blind, placebo-controlled study | [103] |
| A double-blind, randomized, placebo-controlled clinical study | Reduction in PSS (perceived stress scale) scores I A Double-blind, Randomized, Placebo-controlled Clinical Study | [108] | |||
| A randomized, double-blind, placebo-controlled study | A reduction in stress levels, improvement in memory and attention, sleep quality, and overall psychological well-being. | [112] | |||
| Randomized double-blind placebo-controlled trial | Potentially support SSRI therapy in patients diagnosed with GAD syndrome | [107] | |||
| Randomized, placebo-controlled clinical trial | Medium effect sizes over placebo for depression single-item and anxiety-depression cluster scores. Adverse events were mild and transient | [114] | |||
| Double-blind randomized control trial | Increased of college students’ perceived well-being through supporting sustained energy, heightened mental clarity, and enhanced sleep quality | [104] | |||
| Double-blind randomized control trial | Improvements in attention and working memory, as well as reductions in symptoms of anxiety and stress, as a result of Withania somnifera supplementation in adults | [119] | |||
| Adaptogenic effect | ability to adapt and maintain homeostasis in response to various stressors, both physical and emotional | Not yet fully understood and require further research—likely regulation of the HPA axis, antioxidant effects, immunomodulatory effects, and modulation of neurotransmitter signaling. | In vivo equine model | Adaptogenic and immunomodulatory activity in an equine model, potentially improving the health and performance of horses | [136] |
| In vivo rat model | Significant anti-stress effects, including the modulation of the HPA axis, increased levels of antioxidant enzymes, and reduced levels of lipid peroxidation, | [121] | |||
| Hypothyroidism | Increase in the concentration of thyroid hormones | Not yet fully understood and require further research | A double-blind, randomized placebo-controlled trial | Effective normalisation of serum thyroid indices during the 8-week treatment period | [123] |
| In vivo rat model | Significant parameter improvements in serum TSH level, serum glucose, Il-6, body weight gain, hepatic and renal MDA and NO, the values of GSH, gpx and Na+/K+-atpase, and improvement in thyroid histology | [124] | |||
| In vivo mouse model | Significant reduction of hepatic lipid peroxidation, whereas the activity of antioxidant enzymes such as superoxide dismutase and catalase were increased | [125] | |||
| Increase muscle strength | Not yet fully understood and require further research, likely increase in testosterone levels, reductions in muscle damage and inflammation, and antioxidant and anti-inflammatory effects. | A double-blind, randomized placebo-controlled trial | Ashwagandha supplementation association with increased muscle strength and endurance in older adults who performed resistance training. | [126] | |
| A double-blind, randomized placebo-controlled trial | Increase in cardiorespiratory endurance and an improvement in quality of life | [128] | |||
| A double-blind, randomized placebo-controlled trial | Extract of Ashwagandha on muscle strength, power, and recovery in healthy men who engaged in resistance training. | [129] | |||
| A double-blind, randomized placebo-controlled trial | Significant improvements in cardiorespiratory endurance measures of the elite Indian cyclists | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. https://doi.org/10.3390/pharmaceutics15041057
Mikulska P, Malinowska M, Ignacyk M, Szustowski P, Nowak J, Pesta K, Szeląg M, Szklanny D, Judasz E, Kaczmarek G, et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics. 2023; 15(4):1057. https://doi.org/10.3390/pharmaceutics15041057
Chicago/Turabian StyleMikulska, Paulina, Marta Malinowska, Miłosz Ignacyk, Paweł Szustowski, Joanna Nowak, Karolina Pesta, Monika Szeląg, Damian Szklanny, Eliza Judasz, Gabriela Kaczmarek, and et al. 2023. "Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review" Pharmaceutics 15, no. 4: 1057. https://doi.org/10.3390/pharmaceutics15041057
APA StyleMikulska, P., Malinowska, M., Ignacyk, M., Szustowski, P., Nowak, J., Pesta, K., Szeląg, M., Szklanny, D., Judasz, E., Kaczmarek, G., Ejiohuo, O. P., Paczkowska-Walendowska, M., Gościniak, A., & Cielecka-Piontek, J. (2023). Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics, 15(4), 1057. https://doi.org/10.3390/pharmaceutics15041057











