Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring
Abstract
1. Introduction
2. Methods
3. Sample Pretreatment—Solid Phase-Based Microextraction Techniques
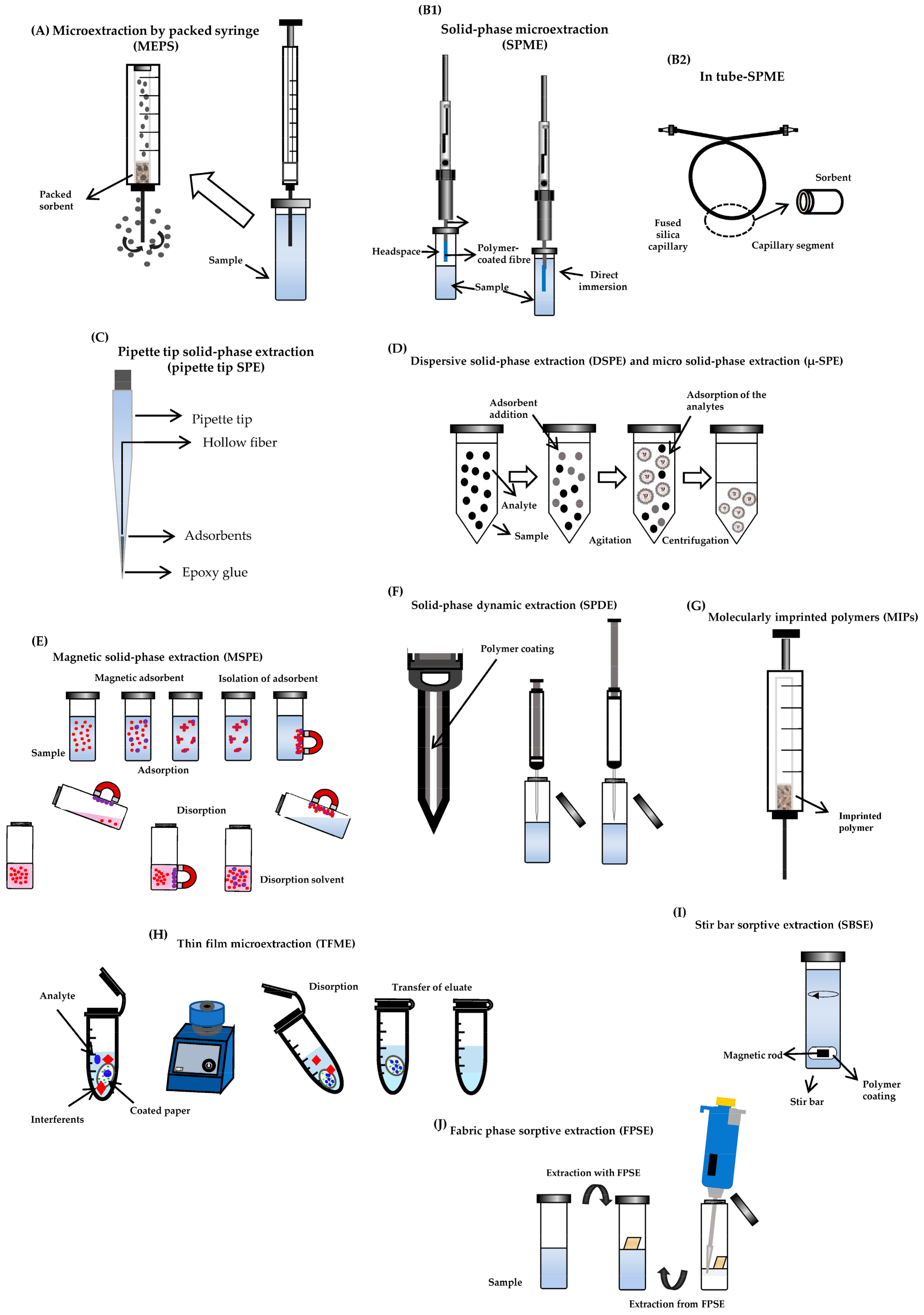
3.1. Microextraction by Packed Sorbent
3.2. Solid-Phase Microextraction and in-Tube Solid-Phase Microextraction
3.3. Pipette Tip Solid-Phase Extraction, Micro Solid-Phase Extraction and Dispersive Solid-Phase Extraction or Matrix Solid-Phase Dispersion
3.4. Magnetic Solid-Phase Extraction and Solid-Phase Dynamic Extraction
3.5. Molecularly Imprinted Polymers and Thin Film Microextraction
3.6. Stir Bar Sorptive Extraction and Fabric Phase Sorptive Extraction
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adaway, J.E.; Keevil, B.G. Therapeutic Drug Monitoring and LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 883–884, 33–49. [Google Scholar] [CrossRef]
- Eliasson, E.; Lindh, J.D.; Malmström, R.E.; Beck, O.; Dahl, M.L. Therapeutic Drug Monitoring for Tomorrow. Eur. J. Clin. Pharmacol. 2013, 69, 25–32. [Google Scholar] [CrossRef] [PubMed]
- International Association of Therapeutic Drug Monitoring and Clinical Toxicology IATDMCT. Available online: https://www.iatdmct.org/ (accessed on 10 March 2023).
- Kang, J.S.; Lee, M.H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Avataneo, V.; D’Avolio, A.; Cusato, J.; Cantù, M.; De Nicolò, A. LC-MS Application for Therapeutic Drug Monitoring in Alternative Matrices. J. Pharm. Biomed. Anal. 2019, 166, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Psillakis, E.; Pedersen-Bjergaard, S.; Ozkan, S. Separation Science: The State of the Art: Analytical Chemistry: There Is No Green Like More Green. LCGC Eur. 2022, 35, 438–439. [Google Scholar] [CrossRef]
- Kokosa, J.M.; Przyjazny, A. Green Microextraction Methodologies for Sample Preparations. Green Anal. Chem. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Microextraction by Packed Sorbent (MEPS). Trends Anal. Chem. 2015, 67, 34–44. [Google Scholar] [CrossRef]
- Moein, M.M.; Said, R.; Abdel-Rehim, M. Microextraction by Packed Sorbent. Bioanalysis 2015, 7, 2155–2161. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Gonçalves, J.; Porto-Figueira, P.; Figueira, J.A.; Alves, V.; Perestrelo, R.; Medina, S.; Câmara, J.S. Current Trends on Microextraction by Packed Sorbent—Fundamentals, Application Fields, Innovative Improvements and Future Applications. Analyst 2019, 144, 5048–5074. [Google Scholar] [CrossRef]
- Rosado, T.; Gallardo, E.; Vieira, D.N.; Barroso, M. Microextraction by Packed Sorbent. In Microextraction Techniques in Analytical Toxicology; Jain, R., Singh, R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 71–115. ISBN 9781003128298. [Google Scholar]
- Berthod, L.; Roberts, G.; Mills, G.A. A Solid-Phase Extraction Approach for the Identification of Pharmaceutical–Sludge Adsorption Mechanisms. J. Pharm. Anal. 2014, 4, 117–124. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.M.; Borrull, F. Materials for Solid-Phase Extraction of Organic Compounds. Separations 2019, 6, 27. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by Packed Sorbent (MEPS): A Tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef]
- Lourenço, D.; Sarraguça, M.; Alves, G.; Coutinho, P.; Araujo, A.R.T.S.; Rodrigues, M. A Novel HPLC Method for the Determination of Zonisamide in Human Plasma Using Microextraction by Packed Sorbent Optimised by Experimental Design. Anal. Methods 2017, 9, 5910–5919. [Google Scholar] [CrossRef]
- Ventura, S.; Rodrigues, M.; Pousinho, S.; Falcão, A.; Alves, G. Determination of Lamotrigine in Human Plasma and Saliva Using Microextraction by Packed Sorbent and High Performance Liquid Chromatography–Diode Array Detection: An Innovative Bioanalytical Tool for Therapeutic Drug Monitoring. Microchem. J. 2017, 130, 221–228. [Google Scholar] [CrossRef]
- Magalhães, P.; Alves, G.; Llerena, A.; Falcão, A. Therapeutic Drug Monitoring of Fluoxetine, Norfluoxetine and Paroxetine: A New Tool Based on Microextraction by Packed Sorbent Coupled to Liquid Chromatography. J. Anal. Toxicol. 2017, 41, 631–638. [Google Scholar] [CrossRef]
- Szultka-Mlynska, M.; Buszewski, B. Electrochemical Oxidation of Selected Immunosuppressants and Identification of Their Oxidation Products by Means of Liquid Chromatography and Tandem Mass Spectrometry (EC-HPLC-MS/MS). J. Pharm. Biomed. Anal. 2019, 176, 112799. [Google Scholar] [CrossRef]
- Cruz, J.C.; de Faria, H.D.; Figueiredo, E.C.; Queiroz, M.E.C. Restricted Access Carbon Nanotube for Microextraction by Packed Sorbent to Determine Antipsychotics in Plasma Samples by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2020, 412, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Marasca, C.; Protti, M.; Mandrioli, R.; Atti, A.R.; Armirotti, A.; Cavalli, A.; De Ronchi, D.; Mercolini, L. Whole Blood and Oral Fluid Microsampling for the Monitoring of Patients under Treatment with Antidepressant Drugs. J. Pharm. Biomed. Anal. 2020, 188, 1–9. [Google Scholar] [CrossRef]
- Ahmed, H.; Wahbi, A.A.; Elmongy, H.; Amini, A.; Koyi, H.; Branden, E.; Abdel-Rehim, M. Determination and Pharmacokinetics of Omeprazole Enantiomers in Human Plasma and Oral Fluid Utilizing Microextraction by Packed Sorbent and Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Anal. Chem. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, Y. Simple, Rapid, and Cost-Effective Microextraction by the Packed Sorbent Method for Quantifying of Urinary Free Catecholamines and Metanephrines Using Liquid Chromatography-Tandem Mass Spectrometry and Its Application in Clinical Analysis. Anal. Bioanal. Chem. 2020, 412, 2763–2775. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, Y.; Zhao, R. Quantitative Measurement of Plasma Free Metanephrines by a Simple and Cost-Effective Microextraction Packed Sorbent with Porous Graphitic Carbon and Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Methods Chem. 2021, 2021, 8821276. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. (Ed.) Handbook of Solid Phase Microextraction; Elsevier: Oxford, UK, 2012; ISBN 9780123914491. [Google Scholar]
- Kataoka, H.; Saito, K. Recent Advances in SPME Techniques in Biomedical Analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Current Developments and Future Trends in Solid-Phase Microextraction Techniques for Pharmaceutical and Biomedical Analyses. Anal. Sci. 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Kataoka, H. In-Tube Solid-Phase Microextraction: Current Trends and Future Perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and Applications of Capillary Microextraction Techniques: A Review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ueta, I.; Ogawa, M.; Abe, A.; Yogo, K.; Shirai, S.; Jinno, K. Fiber-Packed Needle-Type Sample Preparation Device Designed for Gas Chromatographic Analysis. Anal. Bioanal. Chem. 2009, 393, 861–869. [Google Scholar] [CrossRef]
- Barroso, M.; Moreno, I.; Da Fonseca, B.; Queiroz, J.A.; Gallardo, E. Role of Microextraction Sampling Procedures in Forensic Toxicology. Bioanalysis 2012, 4, 1805–1826. [Google Scholar] [CrossRef]
- Melo, L.P.; Queiroz, R.H.C.; Queiroz, M.E.C. Automated Determination of Rifampicin in Plasma Samples by In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2454–2458. [Google Scholar] [CrossRef]
- Chaves, A.R.; Queiroz, M.E.C. Immunoaffinity In-Tube Solid Phase Microextraction Coupled with Liquid Chromatography with Fluorescence Detection for Determination of Interferon α in Plasma Samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 928, 37–43. [Google Scholar] [CrossRef]
- Grecco, C.F.; Miranda, L.F.C.; Costa Queiroz, M.E. Aminopropyl Hybrid Silica Monolithic Capillary Containing Mesoporous SBA-15 Particles for in-Tube SPME-HILIC-MS/MS to Determine Levodopa, Carbidopa, Benserazide, Dopamine, and 3-O-Methyldopa in Plasma Samples. Microchem. J. 2020, 157, 105106. [Google Scholar] [CrossRef]
- Li, J.; An, J.; Jiang, Y. Development of a Method of Hollow Fiber-Based Solid-Phase Microextraction Followed by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of Five Antipsychotics in Human Whole Blood and Urine. J. Chromatogr. A 2020, 1620, 461000. [Google Scholar] [CrossRef] [PubMed]
- Looby, N.; Vasiljevic, T.; Reyes-Garcés, N.; Roszkowska, A.; Bojko, B.; Wąsowicz, M.; Jerath, A.; Pawliszyn, J. Therapeutic Drug Monitoring of Tranexamic Acid in Plasma and Urine of Renally Impaired Patients Using Solid Phase Microextraction. Talanta 2021, 225, 121945. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, P.; Jalilian, N.; Ebrahimzadeh, H.; Amini, S. Trace-Level Monitoring of Anti-Cancer Drug Residues in Wastewater and Biological Samples by Thin-Film Solid-Phase Micro-Extraction Using Electrospun Polyfam/Co-MOF-74 Composite Nanofibers Prior to Liquid Chromatography Analysis. J. Chromatogr. A 2021, 1655, 462484. [Google Scholar] [CrossRef]
- Nazdrajić, E.; Tascon, M.; Rickert, D.A.; Gómez-Ríos, G.A.; Kulasingam, V.; Pawliszyn, J.B. Rapid Determination of Tacrolimus and Sirolimus in Whole Human Blood by Direct Coupling of Solid-Phase Microextraction to Mass Spectrometry via Microfluidic Open Interface. Anal. Chim. Acta 2021, 1144, 53–60. [Google Scholar] [CrossRef]
- Schaefer, V.D.; de Lima Feltraco Lizot, L.; Hahn, R.Z.; Schneider, A.; Antunes, M.V.; Linden, R. Simple Determination of Valproic Acid Serum Concentrations Using BioSPME Followed by Gas Chromatography-Mass Spectrometric Analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1167, 122574. [Google Scholar] [CrossRef]
- Jing, Y.; Singh, V.; Chen, L.; Pawliszyn, J. High-Throughput Biomonitoring of Organophosphate Flame-Retardant Metabolites in Urine via 96-Blade Solid-Phase Microextraction Coupled with Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Talanta 2021, 232, 122466. [Google Scholar] [CrossRef]
- Roy, K.S.; Nazdrajić, E.; Shimelis, O.I.; Ross, M.J.; Chen, Y.; Cramer, H.; Pawliszyn, J. Optimizing a High-Throughput Solid-Phase Microextraction System to Determine the Plasma Protein Binding of Drugs in Human Plasma. Anal. Chem. 2021, 93, 11061–11065. [Google Scholar] [CrossRef]
- Bojko, B.; Looby, N.; Olkowicz, M.; Roszkowska, A.; Kupcewicz, B.; Reck dos Santos, P.; Ramadan, K.; Keshavjee, S.; Waddell, T.K.; Gómez-Ríos, G.; et al. Solid Phase Microextraction Chemical Biopsy Tool for Monitoring of Doxorubicin Residue during in Vivo Lung Chemo-Perfusion. J. Pharm. Anal. 2021, 11, 37–47. [Google Scholar] [CrossRef]
- Luckwell, J.; Beal, A. Automated Micropipette Tip-Based SPE in Quantitative Bioanalysis. Bioanalysis 2011, 3, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, Z.G.; Feng, Y.Q. Porous Monoliths: Sorbents for Miniaturized Extraction in Biological Analysis. Anal. Bioanal. Chem. 2011, 399, 3345–3357. [Google Scholar] [CrossRef] [PubMed]
- Naing, N.N.; Tan, S.C.; Lee, H.K. Micro-Solid-Phase Extraction. In Solid-Phase Extraction; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–471. ISBN 9780128169070. [Google Scholar]
- Hamidi, S.; Taghvimi, A.; Mazouchi, N. Micro Solid Phase Extraction Using Novel Adsorbents. Crit. Rev. Anal. Chem. 2021, 51, 103–114. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Recent Developments in Matrix Solid-Phase Dispersion Extraction. J. Chromatogr. A 2010, 1217, 2521–2532. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Laganà, A. Recent Advances and Developments in Matrix Solid-Phase Dispersion. Trends Anal. Chem. 2015, 71, 186–193. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. A Review on the Recent Progress in Matrix Solid Phase Dispersion. Molecules 2018, 23, 2767. [Google Scholar] [CrossRef]
- Hasegawa, C.; Kumazawa, T.; Uchigasaki, S.; Lee, X.P.; Sato, K.; Terada, M.; Kurosaki, K. Determination of Dextromethorphan in Human Plasma Using Pipette Tip Solid-Phase Extraction and Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2011, 401, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Hamidi, S. Dispersive Micro-Solid-Phase Extraction Using Carbon-Based Adsorbents for the Sensitive Determination of Verapamil in Plasma Samples Coupled with Capillary Electrophoresis. J. Sep. Sci. 2017, 40, 3318–3326. [Google Scholar] [CrossRef]
- Simões, N.S.; de Oliveira, H.L.; da Silva, R.C.S.; Teixeira, L.S.; Sales, T.L.S.; de Castro, W.V.; de Paiva, M.J.N.; Sanches, C.; Borges, K.B. Hollow Mesoporous Structured Molecularly Imprinted Polymer as Adsorbent in Pipette-Tip Solid-Phase Extraction for the Determination of Antiretrovirals from Plasma of HIV-Infected Patients. Electrophoresis 2018, 39, 2581–2589. [Google Scholar] [CrossRef]
- Koller, D.; Zubiaur, P.; Saiz-Rodríguez, M.; Abad-Santos, F.; Wojnicz, A. Simultaneous Determination of Six Antipsychotics, Two of Their Metabolites and Caffeine in Human Plasma by LC-MS/MS Using a Phospholipid-Removal Microelution-Solid Phase Extraction Method for Sample Preparation. Talanta 2019, 198, 159–168. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, Q.; Chen, M.; Shi, J.; Huang, X.; Kong, Q.; Long, D.; Chen, Z.; Yan, S. Determination of 18 Antibiotics in Urine Using LC-QqQ-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1105, 176–183. [Google Scholar] [CrossRef]
- Koller, D.; Vaitsekhovich, V.; Mba, C.; Steegmann, J.L.; Zubiaur, P.; Abad-Santos, F.; Wojnicz, A. Effective Quantification of 11 Tyrosine Kinase Inhibitors and Caffeine in Human Plasma by Validated LC-MS/MS Method with Potent Phospholipids Clean-up Procedure. Application to Therapeutic Drug Monitoring. Talanta 2020, 208, 120450. [Google Scholar] [CrossRef] [PubMed]
- Khosrowshahi, E.M.; Khosrowshahi, B.L.; Farajzadeh, M.A.; Jouyban, A.; Tuzen, M.; Mogaddam, M.R.A.; Nemati, M. Application of Microcrystalline Cellulose as an Efficient and Cheap Sorbent for the Extraction of Metoprolol from Plasma and Wastewater before HPLC-MS/MS Determination. Biomed. Chromatogr. 2022, 36, e5371. [Google Scholar] [CrossRef]
- Pinto, M.A.L.; de Souza, I.D.; Queiroz, M.E.C. Determination of Drugs in Plasma Samples by Disposable Pipette Extraction with C18-BSA Phase and Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 139, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, J.; Lee, J.; Lee, J.; Lee, H.S.; Shin, Y.; Kim, J.H. Method for the Simultaneous Analysis of 300 Pesticide Residues in Hair by LC-MS/MS and GC-MS/MS, and Its Application to Biomonitoring of Agricultural Workers. Chemosphere 2021, 277, 130215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, H.; Feng, G.; Yang, L.; Zhao, L.; Li, R.; Zhao, R. Sensitive HPLC-DMS/MS/MS Method Coupled with Dispersive Magnetic Solid Phase Extraction Followed by in Situ Derivatization for the Simultaneous Determination of Multiplexing Androgens and 17-Hydroxyprogesterone in Human Serum and Its Application to Patients with Polycystic Ovarian Syndrome. Clin. Chim. Acta 2023, 538, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic Solid-Phase Extraction Using Metal-Organic Frameworks (MOFs) and Their Derived Carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Laganà, A. Recent Applications of Magnetic Solid-Phase Extraction for Sample Preparation. Chromatographia 2019, 82, 1251–1274. [Google Scholar] [CrossRef]
- Ansari, S. Application of Magnetic Molecularly Imprinted Polymer as a Versatile and Highly Selective Tool in Food and Environmental Analysis: Recent Developments and Trends. Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Che, D.; Cheng, J.; Ji, Z.; Zhang, S.; Li, G.; Sun, Z.; You, J. Recent Advances and Applications of Polydopamine-Derived Adsorbents for Sample Pretreatment. Trends Anal. Chem. 2017, 97, 1–14. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.L.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.S.; Lin, J.M. Recent Advances in Graphene-Based Magnetic Composites for Magnetic Solid-Phase Extraction. Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent Advances in Facile Synthesis and Applications of Covalent Organic Framework Materials as Superior Adsorbents in Sample Pretreatment. Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep Eutectic Solvents: An Overview on Their Interactions with Water and Biochemical Compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Ahmed, N.; Amjad, M.W.; Hussain, M.A.; Elsherif, M.A.; Ejaz, H.; Alotaibi, N.H. Covalent Organic Frameworks (COFs) as Multi-Target Multifunctional Frameworks. Polymers 2023, 15, 267. [Google Scholar] [CrossRef]
- Ji, X.; Cui, L.; Xu, Y.; Liu, J. Non-Covalent Interactions for Synthesis of New Graphene Based Composites. Compos. Sci. Technol. 2015, 106, 25–31. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Liu, Z. Boronate Affinity Materials for Separation and Molecular Recognition: Structure, Properties and Applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Liu, T.; Shi, S.; Russell, T.P. Host–Guest Molecular Recognition at Liquid–Liquid Interfaces. Engineering 2021, 7, 603–614. [Google Scholar] [CrossRef]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the Interface of Nucleic Acid Aptamers and Binding Targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern Trends in Solid Phase Extraction: New Sorbent Media. Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Cai, P.; Xiong, X.; Li, D.; Zhou, Y.; Xiong, C. Magnetic Solid-Phase Extraction Coupled with UHPLC-MS/MS for Four Antidepressants and One Metabolite in Clinical Plasma and Urine Samples. Bioanalysis 2020, 12, 35–52. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, Y.; Guo, D.; Bi, X.; Hou, X. Surface Molecularly Imprinted Polymer Based on Core-Shell Fe3O4@MIL-101(Cr) for Selective Extraction of Phenytoin Sodium in Plasma. Anal. Chim. Acta 2020, 1128, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, M.; Zhao, L. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Simultaneous Determination of 12 Anti-Tumor Drugs in Human Plasma and Its Application in Therapeutic Drug Monitoring. J. Pharm. Biomed. Anal. 2021, 206, 114380. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, G. A UPLC-MS/MS Method for Simultaneous Determination of Eight Special-Grade Antimicrobials in Human Plasma and Application in TDM. J. Pharm. Biomed. Anal. 2022, 220, 114964. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Zhao, L. Development and Validation of an UPLC-MS/MS Method for Simultaneous Determination of Fifteen Targeted Anti-Cancer Drugs in Human Plasma and Its Application in Therapeutic Drug Monitoring. J. Pharm. Biomed. Anal. 2022, 212, 114517. [Google Scholar] [CrossRef]
- Zhou, T.; Deng, Z.; Wang, Q.; Li, H.; Li, S.; Xu, X.; Zhou, Y.; Sun, S.; Xuan, C.; Tian, Q.; et al. Magnetic Molecularly Imprinted Polymers for the Rapid and Selective Extraction and Detection of Methotrexatein Serum by HPLC-UV Analysis. Molecules 2022, 27, 6084. [Google Scholar] [CrossRef]
- Kang, L.; Lin, C.; Ning, F.; Sun, X.; Zhang, M.; Zhang, H.; Wang, Y.; Hu, P. Rapid Determination of Folic Acid and Riboflavin in Urine by Polypyrrole Magnetic Solid-Phase Extractant Combined Ultra-Performance Liquid Chromatography. J. Chromatogr. A 2021, 1648, 462192. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Zhou, C.; Yang, L.; Zhai, S.; Yang, P.; Zhao, R.; Li, R. Magnetic Solid Phase Extraction Followed by In-Situ Derivatization with Core–Shell Structured Magnetic Graphene Oxide Nanocomposite for the Accurate Quantification of Free Testosterone and Free Androstenedione in Human Serum. J. Chromatogr. B 2022, 1196, 123188. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Recent Developments and Applications of Microextraction Techniques in Drug Analysis. Anal. Bioanal. Chem. 2010, 396, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Rossbach, B.; Kegel, P.; Letzel, S. Application of Headspace Solid Phase Dynamic Extraction Gas Chromatography/Mass Spectrometry (HS-SPDE-GC/MS) for Biomonitoring of n-Heptane and Its Metabolites in Blood. Toxicol. Lett. 2012, 210, 232–239. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M.; Otárola-Jiménez, J. Magnetic Solid-Phase Extraction Using Carbon Nanotubes as Sorbents: A Review. Anal. Chim. Acta 2015, 892, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Kepekci-Tekkeli, S.E.; Durmus, Z.; Kepekci-Tekkeli, S.E.; Durmus, Z. Magnetic Solid Phase Extraction Applications Combined with Analytical Methods for Determination of Drugs in Different Matrices Review. J. Chil. Chem. Soc. 2019, 64, 4448–4458. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymers-Based Microextraction Techniques. Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin Film Microextraction: Towards Faster and More Sensitive Microextraction. Trends Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Tascon, M.; Reyes-Garcés, N.; Boyacl, E.; Poole, J.; Pawliszyn, J. Quantitative Analysis of Biofluid Spots by Coated Blade Spray Mass Spectrometry, a New Approach to Rapid Screening. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zou, J.; Cai, P.S.; Xiong, C.M.; Ruan, J.L. Preparation of Magnetic ODS-PAN Thin-Films for Microextraction of Quetiapine and Clozapine in Plasma and Urine Samples Followed by HPLC-UV Detection. J. Pharm. Biomed. Anal. 2016, 125, 319–328. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Ali, T.A.; Abdallah, A.B. Molecularly Imprinted Polymer Based GCE for Ultra-Sensitive Voltammetric and Potentiometric Bio Sensing of Topiramate. Anal. Sci. 2021, 37, 955–962. [Google Scholar] [CrossRef]
- Shahhoseini, F.; Langille, E.A.; Azizi, A.; Bottaro, C.S. Thin Film Molecularly Imprinted Polymer (TF-MIP), a Selective and Single-Use Extraction Device for High-Throughput Analysis of Biological Samples. Analyst 2021, 146, 3157–3168. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Xin, Y.; Li, Q.; Liu, W. Core-Shell Nanocomposite of Flower-like Molybdenum Disulfide Nanospheres and Molecularly Imprinted Polymers for Electrochemical Detection of Anti COVID-19 Drug Favipiravir in Biological Samples. Microchim. Acta 2022, 189, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, J.; Qu, J.; Huang, J.; Tan, R.; Yu, Y.; Wu, J.; Yang, J.; Li, Y.; et al. A Bifunctional Electrochemical Sensor for Simultaneous Determination of Electroactive and Non-Electroactive Analytes: A Universal yet Very Effective Platform Serving Therapeutic Drug Monitoring. Biosens. Bioelectron. 2022, 208, 114233. [Google Scholar] [CrossRef] [PubMed]
- Włodarski, R.; Żuchowska, K.; Filipiak, W. Quantitative Determination of Unbound Piperacillin and Imipenem in Biological Material from Critically Ill Using Thin-Film Microextraction-Liquid Chromatography-Mass Spectrometry. Molecules 2022, 27, 926. [Google Scholar] [CrossRef]
- Sardaremelli, S.; Hasanzadeh, M.; Razmi, H. Chemical Binding of Horseradish Peroxidase Enzyme with Poly Beta-Cyclodextrin and Its Application as Molecularly Imprinted Polymer for the Monitoring of H2O2 in Human Plasma Samples. J. Mol. Recognit. 2021, 34, e2884. [Google Scholar] [CrossRef] [PubMed]
- Abdollahiyan, P.; Mohammadzadeh, A.; Hasanzadeh, M. Chemical Binding of Molecular-Imprinted Polymer to Biotinilated Antibody: Utilization of Molecular Imprinting Polymer as Intelligent Synthetic Biomaterials toward Recognition of Carcinoma Embryonic Antigen in Human Plasma Sample. J. Mol. Recognit. 2021, 34, e2897. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.A.; Etxebarria, N.; Zuloaga, O. Stir-Bar Sorptive Extraction: A View on Method Optimisation, Novel Applications, Limitations and Potential Solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef] [PubMed]
- Hasan, C.K.; Ghiasvand, A.; Lewis, T.W.; Nesterenko, P.N.; Paull, B. Recent Advances in Stir-Bar Sorptive Extraction: Coatings, Technical Improvements, and Applications. Anal. Chim. Acta 2020, 1139, 222–240. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Y.; Zhang, Q.; Zang, L.; Chen, B.; Hu, B. Stir Bar Sorptive Extraction and Its Application. J. Chromatogr. A 2021, 1637, 461810. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in Sol-Gel Microextraction Phases for Solvent-Free Sample Preparation in Analytical Chemistry. Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Kabir, A.; Samanidou, V. Fabric Phase Sorptive Extraction: A Paradigm Shift Approach in Analytical and Bioanalytical Sample Preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef]
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric Phase Sorptive Extraction: Current State of the Art and Future Perspectives. Separations 2018, 5, 40. [Google Scholar] [CrossRef]
- Ruan, X.; Xing, L.; Peng, J.; Li, S.; Song, Y.; Sun, Q. A Simplified Fabric Phase Sorptive Extraction Method for the Determination of Amphetamine Drugs in Water Samples Using Liquid Chromatography-Mass Spectrometry. RSC Adv. 2020, 10, 10854–10866. [Google Scholar] [CrossRef] [PubMed]
- Balbão, M.S.; Bertucci, C.; Bergamaschi, M.M.; Queiroz, R.H.C.; Malfará, W.R.; Dreossi, S.A.C.; de Paula Mello, L.; Queiroz, M.E.C. Rifampicin Determination in Plasma by Stir Bar-Sorptive Extraction and Liquid Chromatography. J. Pharm. Biomed. Anal. 2010, 51, 1078–1083. [Google Scholar] [CrossRef]
- Catai, A.P.F.; Picheli, F.P.; Carrilho, E.; Queiroz, M.E.C. Assessing Stir Bar Sorptive Extraction and Microextraction by Packed Sorbent for Determination of Selective Serotonin Reuptake Inhibitor Antidepressants in Plasma Sample by Non-Aqueous Capillary Electrophoresis. J. Braz. Chem. Soc. 2013, 24, 1635–1641. [Google Scholar] [CrossRef]
- Fan, W.; He, M.; You, L.; Zhu, X.; Chen, B.; Hu, B. Water-Compatible Graphene Oxide/Molecularly Imprinted Polymer Coated Stir Bar Sorptive Extraction of Propranolol from Urine Samples Followed by High Performance Liquid Chromatography-Ultraviolet Detection. J. Chromatogr. A 2016, 1443, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric Phase Sorptive Extraction-High Performance Liquid Chromatography-Photo Diode Array Detection Method for Simultaneous Monitoring of Three Inflammatory Bowel Disease Treatment Drugs in Whole Blood, Plasma and Urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.A.; Nakahara, T.T.; Bervelieri Madeira, T.; Bortholazzi Almeida, M.; Maffei Monteiro, A.; de Almeida Silva, M.; Carrilho, E.; Piccoli de Melo, L.G.; Nixdorf, S.L. Optimization and Validation of an SBSE-HPLC-FD Method Using Laboratory-Made Stir Bars for Fluoxetine Determination in Human Plasma. Biomed. Chromatogr. 2019, 33, e4398. [Google Scholar] [CrossRef]
- Gazioglu, I.; Evrim Kepekci Tekkeli, S.; Tartaglia, A.; Aslan, C.; Locatelli, M.; Kabir, A. Simultaneous Determination of Febuxostat and Montelukast in Human Plasma Using Fabric Phase Sorptive Extraction and High Performance Liquid Chromatography-Fluorimetric Detection. J. Chromatogr. B 2022, 1188, 123070. [Google Scholar] [CrossRef]
- Tiris, G.; Gazioglu, I.; Furton, K.G.; Kabir, A.; Locatelli, M. Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography for the Determination of Favipiravir in Human Plasma and Breast Milk. J. Pharm. Biomed. Anal. 2023, 223, 115131. [Google Scholar] [CrossRef]
- Locatelli, M.; Furton, K.G.; Tartaglia, A.; Sperandio, E.; Ulusoy, H.I.; Kabir, A. An FPSE-HPLC-PDA Method for Rapid Determination of Solar UV Filters in Human Whole Blood, Plasma and Urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1118–1119, 40–50. [Google Scholar] [CrossRef]
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| MEPS |
|
|
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Zonisamide | 100 μL of plasma | Protein precipitation with 400 μL of ice-cold acetonitrile, agitation, centrifugation, evaporation and reconstitution with 100 μL of 0.1 M phosphate buffer solution (pH 8). MEPS (C18): conditioning with 3 × 200 μL of methanol and 3 × 200 μL of water; sample load (2 × 100 μL) at 10 μL/s; elution with 2 × 30 μL of acetonitrile and dilution with 90 μL of water. | HPLC-DAD | n.a. and 0.2 μg/mL | 0.2–80 μg/mL | [16] |
| Lamotrigine | 100 μL of plasma and saliva | Protein precipitation with 400 μL of ice-cold acetonitrile, agitation, centrifugation, evaporation and reconstitution with 200 μL of 0.3% triethylaminewater solution (pH 6). MEPS (C18): conditioning with 3 × 200 μL of methanol and 3 × 200 μL of water; sample load (3×) at 10 μL/s; washing with 200 μL of water; elution with 2 × 30 μL methanol and dilution with 90 μL of water. | HPLC-DAD | n.a. and 0.1 μg/mL for both samples | 0.1–20 μg/mL for both samples | [17] |
| Fluoxetine, Norfluoxetine, Paroxetine | 500 μL of plasma | Protein precipitation with 1.5 mL of acetonitrile, agitation, centrifugation, evaporation and reconstitution with 500 μL of 50 mM sodium phosphate monobasic anhydrous aqueous solution (pH 4). MEPS (C18): conditioning with 3 × 200 μL of methanol and 2 × 200 μL of water; sample load (3×) of the entire volume; washing with 2 × 200 μL of 5% aqueous ammonium hydroxide solution; elution with 5 × 200 µL of methanol with 1% formic acid. | HPLC-FLD | 5 and 20 ng/mL (Fluoxetine, Norfluoxetine), 1 and 5 ng/mL (Paroxetine) | 20–750 ng/mL (Fluoxetine, Norfluoxetine), 5–750 ng/mL (Paroxetine) | [18] |
| Cyclosporine A, Everolimus, Mycophenolic acid, Sirolimus, Tacrolimus | 150 μL of serum | MEPS (C18): conditioning with 200 μL of methanol and 200 μL of water; sample load (3 × 150 μL) at 5 μL/s and air-dried; elution with 300 μL of methanol with formic acid. | LC-MS/MS (ESI) | 0.021 and 0.063 ng/mL (Cyclosporine A), 0.023 and 0.068 ng/mL (Everolimus), 0.027 and 0.092 ng/mL (Mycophenolic acid), 0.029 and 0.098 ng/mL (Sirolimus), 0.031 and 0.113 ng/mL (Tacrolimus) | 1–50 ng/mL for all the compounds | [19] |
| Chlorpromazine, Clozapine, Olanzapine, Quetiapine | 100 μL of plasma | The sample is diluted with 400 μL of borate buffer solution (10 mmol/mL, pH 9). MEPS (RACNT): conditioning with 2 × 100 μL of acetonitrile and 2 × 100 μL of water; sample load (3×); washing with 150 μL of water; elution with 2 × 100 μL of acetonitrile. | UHPLC–MS/MS (ESI) | n.a. and 10 ng/mL for all the compounds | 10–700 ng/mL (Chlorpromazine, Clozapine, Quetiapine), 10–200 ng/mL (Olanzapine) | [20] |
| Sertraline, Norsertraline, Fluoxetine, Norfluoxetine, Citalopram, N-desmethylcitalopram, N,N-desmethylcitalopram, Vortioxetine | 100 μL of blood and oral fluid | VAMS (20 µL): absorption for 5 s, drying for 1 h at room temperature and ultrasound-assisted extraction for 20 min in 1 mL of methanol. Subsequently, for the solution resulting from the blood sample: evaporation, centrifugation and reconstitution with 100 μL of HPLC mobile phase (65:35, v:v of 33 mM aqueous phosphate buffer, pH 3 containing 0.3% triethylamine:acetonitrile) and for the solution resulting from the oral fluid sample: centrifugation. MEPS (C2): activation with 3 × 100 μL of methanol; conditioning with 3 × 100 μL of water; sample load (10×) at 5 μL/s; washing with 2 × 100 μL of water and 100 μL (10 mM, pH 9) of carbonate buffer:methanol (90:10, v:v) at 20 μL/s; elution with 3 × 200 μL of methanol at 5 μL/s. | HPLC-UV-FL | 2.5 and 7 ng/mL (Sertraline, Norsertraline), 3 and 10 ng/mL (Fluoxetine, Norfluoxetine), 0.3 and 1 ng/mL (Citalopram, N-desmethylcitalopram, N,N-desmethylcitalopram), 1.5 and 5 ng/mL (Vortioxetine) for blood samples; 1.5 and 5 ng/mL (Sertraline, Norsertraline), 2.5 and 7 ng/mL (Fluoxetine, Norfluoxetine), 0.3 and 1 ng/mL (Citalopram, N-desmethylcitalopram, N,N-desmethylcitalopram), 1 and 3 ng/mL (Vortioxetine) for oral fluid samples | 7–500 ng/mL (Sertraline, Norsertraline), 10–750 ng/mL (Fluoxetine, Norfluoxetine), 1–200 ng/mL (Citalopram, N-desmethylcitalopram, N,N-desmethylcitalopram), 5–500 ng/mL (Vortioxetine) for blood samples; 5–500 ng/mL (Sertraline, Norsertraline), 7–750 ng/mL (Fluoxetine, Norfluoxetine), 1–200 ng/mL (Citalopram, N-desmethylcitalopram, N,N-desmethylcitalopram), 3–500 ng/mL (Vortioxetine) for oral fluid samples | [21] |
| (R)- and (S)- Omeprazole | 100 μL of plasma and oral fluid | The sample is diluted with water (1:4, v:v). MEPS (C8): conditioning with 100 μL of ethanol and 100 μL of water; sample load (6×); washing with 5% 2 × 100 µL of methanol in water; elution with 2 × 250 µL of ethanol. | LC-MS/MS (ESI) | 0.1 and 0.4 ng/mL (calculated limits) for both samples and compounds | 25–600 ng/mL (plasma) and 25–300 ng/mL (oral fluid) for both compounds | [22] |
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| SPME and in-tube SPME |
|
|
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Rifampicin | 500 µL of plasma | Protein precipitation with acetonitrile at a 2:1 ratio (v:v), agitation and centrifugation; the supernatant is collected, dried, resuspended with 0.5 mL of buffer solution and vortexed. In-tube SPME: extraction is performed with 10 draw/eject cycles; sample solution draw/eject volume of 200 µL; pH of the buffer solution of 7; draw/eject flow rate at 315 µL/min. | HPLC-UV | n.a. and 0.1 µg/mL | 0.1–100 µg/mL | [33] |
| Interferon α2a | 250 µL of plasma | The sample is diluted with 250 µL of phosphate buffer solution (0.025 mol/L, pH 6) and vortexed. In-tube SPME (immunoaffinity capillary): extraction is performed with 20 draw/eject cycles (150 µL), at a linear flow rate of 315 µL/min, using online desorption (dynamic desorption) by redirecting the mobile phase through the capillary. | HPLC-FD | n.a. and 0.006 MIU/mL | 0.006–3 MIU/mL | [34] |
| Levodopa, Carbidopa, Benserazide, Dopamine, 3-O-methyldopa | 200 µL of plasma | Protein precipitation with acetonitrile at a 1:2 ratio (v:v), agitation, centrifugation and filtration of the supernatant (400 μL). In-tube SPME (aminopropyl hybrid silica monolithic capillary containing mesoporous Santa Barbara Amorphous (SBA-15) particles) | HILIC-MS/MS (ESI) | n.a. and 22 ng/mL (Levodopa), n.a. and 33 ng/mL (Carbidopa), n.a. and 170 ng/mL (Benserazide), n.a. and 1.2 ng/mL (Dopamine), n.a. and 10 ng/mL (3-O-methyldopa) | 22–2000 ng/mL (Levodopa), 33–2000 ng/mL (Carbidopa), 170–2000 ng/mL (Benserazide), 1.2–2000 ng/mL (Dopamine), 10–2000 ng/mL (3-O-methyldopa) | [35] |
| Perphenazine, Chlorpromazine, Chlorprothixene, Promethazine, Trifluoperazine | 500 µL of blood and 10 mL of urine | Hollow fiber-based SPME:
| UPLC-MS/MS (ESI) | 12.5 and 25 pg/mL (Perphenazine, Chlorprothixene, Promethazine), 6.25 and 12.5 pg/mL (Chlorpromazine, Trifluoperazine) for blood samples, 12.5 and 25 pg/mL (Perphenazine), 6.25 and 12.5 pg/mL (Chlorpromazine, Chlorprothixene, Promethazine, Trifluoperazine) for urine samples | 25–1 × 104 pg/mL (Perphenazine, Chlorprothixene, Promethazine), 12.5–1 × 104 pg/mL (Chlorpromazine, Trifluoperazine) for blood samples, 25–1 × 104 pg/mL (Perphenazine), 12.5–1 × 104 pg/mL (Chlorpromazine, Chlorprothixene, Promethazine, Trifluoperazine) for urine samples | [36] |
| Tranexamic acid | n.a. of plasma and urine | SPME (hydrophilic-lipophilic balance (HLB) coated)
| LC-MS/MS (n.a.) | 10 μg/mL for plasma samples, 25 μg/mL for urine samples | 10–1000 μg/mL for plasma samples, 25–1000 μg/mL for urine samples | [37] |
| Sorafenib, Dasatinib, Erlotinib hydrochloride | 2 mL of plasma, serum and n.a. of urine | 100 μL of hydrochloric acid (12 mol/L) and 100 μL of trifluoracetic acid is mixed with 2 mL of plasma which is agitated, centrifuged, and its supernatant separated and diluted with water (2:8, v:v), and the acid solution (pH 1) is neutralized with sodium hydroxide (0.01 mol/L) and filtered through a PVDF membrane. An amount equal to 2 mL of acetonitrile is added to 2 mL of serum which is centrifuged and its supernatant separated, filtered and diluted with water (2:8, v:v) before extraction. The urine sample is centrifuged, filtered and diluted with water (5:5, v:v) before extraction. TF-SPME (polyfam/Co-MOF-74 composite nanofibers): the piece of sorbent (1 cm2) is cut from the nanofiber sheet and submerged in 10 mL of acetonitrile for 10 min for conditioning; it is immersed in 20 mL of the sample solution (optimum pH 10) for adsorption under agitation for 10 min; the sorbent is transferred to a vial, to which 500 µL of alkaline methanol is added, plus stirring for 7 min for the desorption process. | HPLC-UV | 0.03 and 0.1 μg/L (Sorafenib), 0.15 and 0.5 μg/L (Dasatinib), 0.2 and 0.5 μg/L (Erlotinib hydrochloride) for all the samples | 0.1–1500 μg/L (Sorafenib), 0.5–1500 μg/L (Dasatinib, Erlotinib hydro-chloride) for all the samples | [38] |
| Tacrolimus, Sirolimus, Everolimus, Cyclosporine A | 200 µL of blood | The sample is subjected to the mechanical lysis process of three freeze-thaw cycles (1 min in liquid nitrogen and 1 min in an ice bath); the sample is further subjected to an additional chemical lysis process with 1.3 mL of a lysing solution of zinc sulfate:acetonitrile:water (6:3:1, v:v:v). BioSPME (Oasis® hydrophilic-lipophilic balance (HLB) particles): extraction for 60 min at 55 °C; the fiber is rinsed in water for 5 s; the fiber is placed into the pre-filled MOI chamber; the desorbed analytes are introduced into the equipment. | MOI-MS/MS (ESI) | 0.3 and 0.8 ng/mL (Tacrolimus, Cyclosporine A), 0.2 and 0.7 ng/mL (Sirolimus), 0.3 and 1 ng/mL (Everolimus) | 1–50 ng/mL (Tacrolimus, Sirolimus, Everolimus), 2.5–500 ng/mL (Cyclosporine A) | [39] |
| Valproic acid | 50 µL of plasma | BioSPME (LC Tips C18): conditioning with 200 μL of a mixture of methanol:water (50:50, v:v) for 20 min under homogenization in an orbital shaker; 50 μL of the sample and 150 μL of hydrochloric acid 0.1 M is added in a polypropylene tube, followed by homogenization in an orbital shaker for 30 min; elution of the LC Tips C18 by adding the tips to a GC autosampler vial containing 150 μL of methanol, performing another homogenization step for 30 min. | GC-MS (n.a.) | n.a. and 10 mg/L | 10–150 mg/L | [40] |
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| pipette tip SPE |
|
|
| µ-SPE |
|
|
| DSPE |
|
|
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Dextromethorphan | 100 µL of plasma | 300 μL of water and 50 μL of 1 mol/L glycine-sodium hydroxide buffer (pH 10) are added to the sample; the mixture is centrifuged, and the supernatant is reserved. Pipette tip SPE (MonoTip C18 tips (C18-bonded monolithic silica gel)): conditioning with 200 µL of methanol and 200 µL of water; extraction with 200 μL of the prepared supernatant performed for 20 sequential aspirating/dispensing cycles; washing with 200 µL of water; discarding the eluate; tip drying for 30 s; elution with 100 µL of methanol for 5 aspirating/dispensing cycles. | GC-MS (EI) | 1.25 and 2.5 ng/mL | 2.5–320 ng/mL | [52] |
| Verapamil | 2 mL of plasma | Protein precipitation with 1 mL of acetonitrile, agitation and centrifugation. D-µSPE (Graphene oxide/polydopamine (PDA) and Graphene oxide/Fe3O4): 4 mg of GO/Fe3O4 and 2 mg of GO/PDA sorbents are dispersed into the sample and placed in an ultrasonic bath; drug-loaded GO/Fe3O4 is separated by applying an external magnetic field; GO/PDA sorbent is left to settle, and the supernatant is discarded; desorption with 500 µL of acetone and sonication for 5 min; separation of the sorbents and the supernatant is evaporated; the residue is redissolved in 100 μL of acetonitrile. | CE-UV | 1.2 and 5 ng/mL | 5–500 ng/mL | [53] |
| Lamivudine, Zidovudine, Efavirenz | 500 µL of plasma | The sample is centrifuged and filtered, and 1 mL of water is added to 1 mL of plasma. PT-HM-MIP-SPE (poly(MAA-co-4-VP): 20 mg of the polymer is packed into a pipette tip (1000 mL, polypropylene); washing with 250 µL of water and sample load; washing with 300 µL of hexane; elution with 500 µL of methanol; the solution is evaporated, and the residue is redissolved in 50 µL of mobile phase. | HPLC-UV | n.a. and 0.25 μg/mL (Lamivudine, Efavirenz), n.a. and 0.05 μg/mL (Zidovudine) | 0.25–10 μg/mL (Lamivudine, Efavirenz), 0.05–2 μg/mL (Zidovudine) | [54] |
| Aripiprazole, Dehydro-aripiprazole, Olanzapine, Risperidone, Paliperidone, Quetiapine, Clozapine | 200 µL of plasma | μ-SPE (PRiME HLB (hydrophilic-lipophilic balance): 290 µL of 0.2% formic acid in water (pH 1.5) is added; sample load (2 × 255 µL) into the Oasis 96-well µElution Plate; washing with 400 μL (2 × 200 μL) of 5% methanol solution with water and 2% ammonia; vacuum is applied to dryness; elution with 200 μL (2 × 100 μL) of acetonitrile:methanol:buffer (formic acid, 0.2% at pH 3) solution (8:1:1, v:v:v); the eluate is collected in a 96-well plate, and 5 μL is injected into the chromatographic system. | LC-MS/MS (ESI) | n.a. and 0.18 ng/mL (Aripiprazole), n.a. and 0.25 ng/mL (Dehydro-aripiprazole), n.a. and 1 ng/mL (Olanzapine), n.a. and 0.70 ng/mL (Risperidone), n.a. and 0.20 ng/mL (Paliperidone), n.a. and 0.50 ng/mL (Quetiapine, Clozapine) | 0.18–120 ng/mL (Aripiprazole), 0.25–80 ng/mL (Dehydro-aripiprazole), 1–100 ng/mL (Olanzapine), 0.70–60 ng/mL (Risperidone), 0.20–30 ng/mL (Paliperidone), 0.50–160 ng/mL (Quetiapine), 0.50–1000 ng/mL (Clozapine) | [55] |
| Amoxicillin, Penicillin, Tylosin tartrate, Roxithromycin, Clarithromycin, Azithromycin, Erythromycin, Chlorotetracycline hydrochloride, Terramycin, Tetracycline, Ofloxacin, Enrofloxacin, Ciprofloxacin, Norfloxacin, Olaquindox, Sulfamethazine, Sulfadiazine, Trimethoprim | 500 µL of urine | QuEChERS DSPE: the sample is transferred to a polypropylene centrifuge tube, and 3 µL of formic acid is added; the mixture is vortexed, and 1 mL of methanol is added before shaking on a thermal shaker for 30 min at 20 °C; centrifugation for 10 min at 4 °C, and 1 mL of the upper layer is transferred to a roQ QuEChERS DSPE tube (200 mg); the solution is vortexed for 5 min, centrifuged for 10 min at 4 °C, and 200 μL of the final solution is transferred to a vial and stored at −20 °C for further analysis. | LC-MS/MS (ESI) | 14.29 and 47.62 µg/L (Amoxicillin), 0.61 and 2.03 µg/L (Penicillin), 0.55 and 1.82 µg/L (Tylosin tartrate), 2 and 6.67 µg/L (Roxithromycin), 1.20 and 4 µg/L (Clarithromycin), 0.73 and 2.43 µg/L (Azithromycin), 31.43 and 104.76 µg/L (Erythromycin), 1.04 and 3.46 µg/L (Chlorotetracycline hydrochloride), 0.48 and 1.61 µg/L (Terramycin), 0.85 and 2.82 µg/L (Tetracycline), 1.79 and 5.98 µg/L (Ofloxacin), 1.42 and 4.72 µg/L (Enrofloxacin), 2.21 and 7.38 µg/L (Ciprofloxacin), 1.86 and 6.21 µg/L (Norfloxacin), 0.40 and 1.35 µg/L (Olaquindox), 0.37 and 1.23 µg/L (Sulfamethazine), 0.11 and 0.38 µg/L (Sulfadiazine), 0.14 and 0.45 µg/L (Trimethoprim) | 0.34–1100 µg/L (Amoxicillin, Chlorotetracycline hydrochloride), 0.34–550 µg/L (Penicillin, Azithromycin, Erythromycin, Terramycin, Tetracycline, Ofloxacin, Enrofloxacin, Ciprofloxacin, Norfloxacin, Olaquindox, Trimethoprim), 0.34–275 µg/L (Tylosin tartrate, Roxithromycin, Clarithromycin, Sulfamethazine, Sulfadiazine) | [56] |
| Imatinib, Dasatinib, Nilotinib, Bosutinib, Ponatinib, Ruxolitinib, Ibrutinib, Filgotinib, Tofacitinib, Baricitinib, Peficitinib | 300 µL of plasma | μ-SPE (PRiME MCX (Mixed-mode Cation exchange sorbent for bases)): 200 µL of 5% orthophosphoric acid in water is added; sample load (2 × 255 µL) into the Oasis 96-well µElution Plate; washing with 400 μL (2 × 200 μL) of 100 mM ammonium formate + 2% formic acid in aqueous solution and 400 μL (2 × 200 μL) of methanol; elution with 100 μL (2 × 50 μL) of 5% ammonium hydroxide in methanol solution (1:1, v:v) and 100 μL (1 × 100 μL) of water; after each step, vacuum is applied to dryness; the eluate is collected in a 96-well plate, and 5 μL is injected into the chromatographic system. | LC-MS/MS (ESI) | n.a. and 5 ng/mL (Imatinib), n.a. and 0.38 ng/mL (Dasatinib), n.a. and 4 ng/mL (Nilotinib), n.a. and 1 ng/mL (Bosutinib, Baricitinib), n.a. and 0.45 ng/mL (Ponatinib), n.a. and 1.50 ng/mL (Ruxolitinib, Tofacitinib), n.a. and 0.30 ng/mL (Ibrutinib), n.a. and 0.90 ng/mL (Filgotinib), n.a. and 2.50 ng/mL (Peficitinib) | 5–5000 ng/mL (Imatinib), 0.38–400 ng/mL (Dasatinib), 4–4000 ng/mL (Nilotinib), 1–600 ng/mL (Bosutinib), 0.45–500 ng/mL (Ponatinib), 1.50–500 ng/mL (Ruxolitinib), 0.30–400 ng/mL (Ibrutinib), 0.90–1200 ng/mL (Filgotinib), 1.50–250 ng/mL (Tofacitinib), 1–250 ng/mL (Baricitinib), 2.50–900 ng/mL (Peficitinib) | [57] |
| Metoprolol | 250 µL of plasma | MCC-DSPE: the sample is homogenized, and its pH is adjusted to 8; 10 µL of a zinc sulfate solution (0.05 mol/L) and 2.5 mg of MCC is added; agitation for 1 min, centrifugation for 4 min, and the supernatant is discarded; elution of the sorbent with 300 µL of methanol and agitation for 2 min; separation of the eluent by centrifugation for 5 min; evaporation and the residue is redissolved with 100 µL of the mobile phase. | HPLC-MS/MS (ESI) | 0.30 and 0.5 ng/mL | 1–1000 ng/mL | [58] |
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Venlafaxine, Paroxetine, Fluoxetine, Norfluoxetine, Sertraline | 100 µL of plasma and 1 mL of urine | The plasma sample is incubated for 30 min and diluted to 1 mL with 5 mM PBS (pH 7); the urine sample is incubated for 30 min and diluted ten times with 5 mM PBS (pH 7.0), and the pH is adjusted with sodium hydroxide. MSPE (C18-Fe3O4@SiO2 NPs (functionalized magnetic silica nanoparticles)): 20 mg of the sorbent is preconditioned with 2 mL of methanol:water (1:1, v:v) by mechanical vibration for 15 min; the magnetic particles are gathered against the wall of vial by a magnet; the magnet is removed, and the particles are dispersed again; the supernatant is removed, and 1 mL of the previously prepared sample is added; extraction by mechanical vibration for 10 min; the sorbent is gathered against the inner wall of vail by a magnet, and the supernatant is directly poured and washed with 3 × 1 mL of water; elution with 200 µL of acetonitrile:0.1% formic acid (9:1, v:v) by mechanical vibration for 10 min and filtration. | UHPLC–MS/MS (ESI) | 0.44 and 1.47 ng/mL (Venlafaxine), 0.75 and 2.46 ng/mL (Paroxetine), 0.52 and 1.7 ng/mL (Fluoxetine), 0.61 and 2.04 ng/mL (Norfluoxetine), 0.66 and 2.19 ng/mL (Sertraline) for plasma samples; 0.15 and 0.51 ng/mL (Venlafaxine), 0.40 and 1.34 ng/mL (Paroxetine), 0.21 and 0.70 ng/mL (Fluoxetine), 0.16 and 0.53 ng/mL (Norfluoxetine), 0.25 and 0.83 ng/mL (Sertraline) for urine samples | 2.5–1000 ng/mL for all the compounds and both samples | [75] |
| Phenytoin sodium | 100 µL of plasma | The sample is diluted with 300 µL of water (pH 5) and spiked with 50 µL of mobile phase. MSPE (Fe3O4@MIL-101(Cr)@MIP (molecularly imprinted polymers)): 8 mg of the sorbent is added into the solution and vortexed for 5 min; after absorption, the magnetic sorbents are separated using a magnet, and the supernatant is discarded; 500 µL of methanol is added and vortexed for 6 min; the elution solution is filtered. | HPLC-UV | n.a. and 0.05 µg/mL | 0.05–40 µg/mL | [76] |
| Cyclophosphamide, Ifosfamide, Cisplatin, Methotrexate, Pemetrexed disodium, Capecitabine, 5-fluorouracil, Gemcitabine, Doxorubicin, Fulvestrant, Tamoxifen, Irinotecan | 100 µL of plasma | MSPE: activation of the magnetic particles with 20 μL of hydrophilic–hydrophobic balance magnetic particles; 200 μL of methanol is transferred to a 96-well plate and stirred with a magnetic bar for 30 s; the activated magnetic particles are absorbed by the magnetic bar, transferred to another well plate and rinsed with 600 μL of water; 100 μL of the sample is added to another column of a 96-well plate and stirred with a magnetic bar for 30 s; elution of the drug-adsorbed magnetic particles that are absorbed by the magnetic bar are transferred to another well plate and rinsed with 600 μL of water for 30 s and then absorbed by the magnetic bar, transferred to a last column and rinsed with 800 μL of acetonitrile for 30 s to elute the analytes. | UPLC-MS/MS (ESI) | n.a. and 0.1 μg/mL (Cyclophosphamide, Ifosfamide, Cisplatin, Methotrexate, Pemetrexed disodium, Capecitabine, 5-fluorouracil, Gemcitabine), n.a. and 0.05 μg/mL (Doxorubicin, Fulvestrant, Tamoxifen, Irinotecan) | 0.10–25 μg/mL (Cyclophosphamide, Ifosfamide, Cisplatin, Methotrexate, Pemetrexed disodium, Capecitabine, 5-fluorouracil, Gemcitabine), 0.05–12.5 μg/mL (Doxorubicin, Fulvestrant, Tamoxifen, Irinotecan) | [77] |
| Linezolid, Vancomycin, Teicoplanin, Tigecycline, Imipenem, Meropenem, Voriconazole, Micafungin | 100 µL of plasma | MSPE: activation of the magnetic particles with 200 μL of methanol; 20 μL of the magnetic particles (0.1 g/mL) is introduced to a 96-well plate and stirred for 45 s; elution of methanol; the particles in the first step are transferred to another column of the 96-well plate by adsorption of the magnetic bar, and rinsed with 500 μL of water; 100 μL of the sample is added to another well plate and stirred for 45 s; elution of the drug-adsorbed magnetic particles that are transferred to another column of the 96-well plate by adsorption of the magnetic bar, rinsed with 500 μL of water and then transferred to a last column and rinsed with 600 μL of acetonitrile for 45 s to elute the analytes. | UPLC-MS/MS (ESI) | n.a. and 0.1 μg/mL (Linezolid, Teicoplanin, Tigecycline, Imipenem, Meropenem, Voriconazole Micafungin), n.a. and 0.2 μg/mL (Vancomycin) | 0.1–25 μg/mL (Linezolid, Teicoplanin, Tigecycline, Imipenem, Meropenem, Voriconazole Micafungin), 0.2–50 μg/mL (Vancomycin) | [78] |
| Aletinib, Afatinib, Apatinib, Icotinib, Dasatinib, Erlotinib, Gefitinib, Crizotinib, Lapatinib, Regorafenib, Ceritinib, Sorafenib, Vemurafenib, Imatinib, N-desmethyl imatinib | 100 µL of plasma | MSPE: activation of the magnetic particles with 150 μL of methanol; 20 μL of HLB magnetic particles is added to a 96-well plate and stirred with a magnetic bar for 30 s; the activated magnetic particles are absorbed by the magnetic bar and transferred to another column of the 96-well plate and rinsed with 600 μL of water; 100 μL of the sample is added to another well plate and stirred with a magnetic bar for 30 s; elution of the drug-adsorbed magnetic particles that are absorbed by the magnetic bar and transferred to another column of the 96-well plate, rinsed with 600 μL of water for 30 s and then absorbed by the magnetic bar and transferred to a last column and rinsed with 600 μL of acetonitrile for 30 s to elute the analytes. | UPLC-MS/MS (ESI) | n.a. and 2.5 ng/mL (Aletinib, Afatinib, Apatinib, Icotinib, Dasatinib, Crizotinib, Regorafenib, Vemurafenib, N-desmethyl imatinib), n.a. and 10 ng/mL (Erlotinib, Gefitinib, Lapatinib, Ceritinib, Sorafenib, Imatinib) | 2.5–2500 ng/mL (Aletinib, Afatinib, Apatinib, Icotinib, Dasatinib, Crizotinib, Regorafenib, Vemurafenib, N-desmethyl imatinib), 10–10,000 ng/mL (Erlotinib, Gefitinib, Lapatinib, Ceritinib, Sorafenib, Imatinib) | [79] |
| Methotrexatein | 50 µL of serum | MMIP-MSPE: 10 mL of methanol is added to the sample, the mixture is carried out by ultrasounds, centrifugation and collection of the supernatant; 10 mL of methanol is added to MMIP (100 mg) with stirring; after activation, the liquid is separated and discarded by magnetic separation, and the supernatant is added to 100 mg of MMIP; the sample is extracted and loaded by stirring at room temperature for 240 min; for magnetic separation and MMIP recovery, 5 mL of water:methanol (4:1, v:v) eluent is added with stirring; residual impurities are washed, and the material is recovered; 5 mL of the eluent methanol:acetic acid (4:1, v:v) is added to the treated MMIP with oscillation of the eluent for 60 min; after magnetic separation, the liquid is poured off, dried and redissolved in 500 µL of methanol solution and filtered. | HPLC-UV | 12.51 and 50 ng/mL | 50–250,000 ng/mL | [80] |
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| MSPE |
|
|
| SPDE |
|
|
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| MIPs |
|
|
| TFME |
|
|
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Quetiapine, Clozapine | 200 μL of plasma and 500 μL of urine | The sample is diluted in 2 mL of water, and the pH value of the samples is adjusted to 9.5 and again diluted to volume with water for the next procedure. Magnetic ODS-PAN TFME (silica-coated magnetic nanoparticles (SiO2@Fe3O4)): a piece of thin film is preconditioned with methanol and water, added to the previously prepared sample, and the adsorption is performed by mechanical shaking for 50 min; the thin film is collected and rinsed with 3 mL of water; desorption is processed by mechanically shaking the magnetic thin film in 1 mL of methanol for 5 min; the obtained solution is evaporated, and the residue is redissolved in 100 μL of methanol. | HPLC-UV | 0.013 and 0.05 μg/mL (Quetiapine), 0.015 and 0.054 μg/mL (Clozapine) for plasma samples; 0.003 and 0.01 μg/mL (Quetiapine, Clozapine) for urine samples | 0.070–9 μg/mL (Quetiapine, Clozapine) for plasma samples; 0.012–9 μg/mL (Quetiapine, Clozapine) for urine samples | [91] |
| Sorafenib, Dasatinib, Erlotinib hydrochloride | 2 mL of plasma, serum and n.a. of urine | 100 μL of hydrochloric acid (12 mol/L) and 100 μL of trifluoracetic acid is mixed with 2 mL of plasma which is agitated, centrifuged and its supernatant separated and diluted with water (2:8, v:v), and the acid solution (pH 1) is neutralized with sodium hydroxide (0.01 mol/L) and filtered through a PVDF membrane. A total of 2 mL of acetonitrile is added to 2 mL of serum which is centrifuged and its supernatant separated, filtered and diluted with water (2:8, v:v) before extraction. The urine sample is centrifuged, filtered and diluted with water (5:5, v:v) before extraction. TF-SPME (polyfam/Co-MOF-74 composite nanofibers): the piece of sorbent (1 cm2) is cut from the nanofiber sheet and submerged in 10 mL of acetonitrile for 10 min for conditioning; it is immersed in 20 mL of the sample solution (optimum pH 10) for adsorption under agitation for 10 min for extraction; the sorbent is transferred to a vial to which 500 µL of alkaline methanol is added, with stirring for 7 min for the desorption process. | HPLC-UV | 0.03 and 0.1 μg/L (Sorafenib), 0.15 and 0.5 μg/L (Dasatinib), 0.2 and 0.5 μg/L (Erlotinib hydrochloride) for all the samples | 0.1–1500 μg/L (Sorafenib), 0.5–1500 μg/L (Dasatinib, Erlotinib hydro-chloride) for all the samples | [38] |
| Topiramate | n.a. of serum and 10 mL of urine | The serum is diluted 50 times in 0.1 M acetate buffer (pH 5); 100 µL of diluted serum sample is mixed with 5 mL of acetate buffer and transferred to an electrochemical cell. Urine is filtered and diluted in 3 mL of acetate buffer (pH 5). MIP/GO/GCE sensor and MIP/PVC/GCE sensor. | Voltammetry for MIP/GO/GCE sensor and Potentiometry for MIP/PVC/GCE sensor | 5 × 10−11 and 2.7 × 10−10 mol/L (MIP/GO/GCE), 2.4 × 10−10 and 1 × 10−9 mol/L (MIP/PVC/GCE) | 2.7 × 10−10–4.9 × 10−3 mol/L (MIP/GO/GCE), 1 × 10−9–3.4 × 10−3 mol/L (MIP/PVC/GCE) | [92] |
| Amitriptyline, Imipramine, Clomipramine, Desipramine, Doxepin, Trimipramine, Nortriptyline | 700 μL of plasma | TF-MIP: the thin film is inserted into a vial containing 700 µL of plasma with 1% tri-ethylamine; the batch extraction process is carried out by agitation for 60 min; the thin film is washed by immersion in 1% aqueous triethylamine for 8 s; the thin film is dried and desorbed with 700 μL of 0.1% formic acid in 50% of aqueous acetonitrile for 20 min, then filtered. | UHPLC-MS/MS (ESI) | n.a. and 2.5 ng/mL (Amitriptyline), n.a. and 1 ng/mL (Imipramine, Clomipramine, Doxepin, Trimipramine, Nortriptyline), n.a. and 5 ng/mL (Desipramine) | 2.5–500 ng/mL (Amitriptyline), 1–500 ng/mL (Imipramine, Clomipramine, Doxepin, Trimipramine, Nortriptyline), 5–500 ng/mL (Desipramine) | [93] |
| Favipiravir | 10 μL of urine | The sample is mixed with 200 µL of acetonitrile and centrifuged; the supernatant is filtered and dried; the residue is dissolved and diluted with acetate buffer solution (pH 5) to a volume of 5 mL; 100 µL of the solution is added to an electrolytic cell for testing. MoS2@ MIP core-shell nanocomposite: the modified electrode is immersed into a blank acetate buffer solution (0.1 M, pH 5) for 5 min; the modified electrode is incubated with the sample for 5 min. | DPV | 0.002 and 0.01 nM (3.14 × 10−7 and 1.57 × 10−6 μg/mL) | 0.01–100 nM (1.57 × 10−6~1.57 × 10−2 μg/mL) | [94] |
| Methotrexatein | 50 µL of serum | MMIP-MSPE: 10 mL of methanol is added to the sample, the mixture is carried out by ultrasound, centrifugation and collection of the supernatant; 10 mL of methanol is added to MMIP (100 mg) with stirring; after activation the liquid is separated, discarded by magnetic separation and the supernatant is added to 100 mg of MMIP; the sample is extracted and loaded by stirring at room temperature for 240 min; for magnetic separation and MMIP recovery, 5 mL of water:methanol (4:1, v:v) eluent is added with stirring; residual impurities are washed, and the material is recovered; 5 mL of the eluent methanol:acetic acid (4:1, v:v) is added to the treated MMIP with oscillation of the eluent for 60 min; after magnetic separation, the liquid is poured off, dried and redissolved in 500 µL of methanol solution and filtered. | HPLC-UV | 12.51 and 50 ng/mL | 50–250,000 ng/mL | [80] |
| Ceftazidime, Avibactam | n.a. of serum | Protein precipitation with three times the volume of methanol, centrifugation and collection of the supernatant which is diluted with the same volume of phosphate buffer saline (0.1 M, pH 7) for subsequent detection. MIP (N-Mo2C/SPE). | SWV | 35 and 50 μM (Ceftazidime), 0.5 and 1 μM (Avibactam) | 50–1000 μM (Ceftazidime), 1–1000 μM (Avibactam) | [95] |
| Imipenem, Piperacillin | n.a. of plasma and bronchoalveolar lavage (without validation) | TFME (96 DVB blades): sample extraction time is 30 min; as desorption solvent, a mixture of methanol:water (1:1, v:v) is used; the desorption time is 45 min. | LC-MS/MS (ESI) | n.a. and 0.01 mg/L for plasma samples | 0.01–1 mg/L for plasma samples | [96] |
| Extraction Technique | Advantages | Disadvantages |
|---|---|---|
| SBSE |
|
|
| FPSE |
|
|
| Analytes | Sample (Amount) | Sample Pretreatment and Extraction Procedure | Analytical Technique | LOD; LOQ | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Rifampicin | 200 μL of plasma | SBSE (magnetic PDMS coated stir bar): the bar is conditioned for 24 h in a vial containing acetonitrile; plasma is placed in a vial to which 4 mL of 0.25 mol/L sodium acetate buffer (pH 5) is added; the vial is sealed, the stir bar is immersed in the sample, and the extraction is performed under magnetic stirring for 50 min; for desorption stir bar is removed, rinsed with water, dried and placed in a vial containing 1 mL of acetonitrile ensuring total immersion for magnetic agitation at 24 °C for 20 min; the stir bar is removed, the solvent is evaporated, and the residue is redissolved in 100 µL of mobile phase and 50 µL of hexane. | HPLC-UV | 0.09 and 0.125 μg/mL | 0.125–50 μg/mL | [107] |
| Fluoxetine, Sertraline, Citalopram, Paroxetine | 800 μL of plasma | MEPS and SBSE (magnetic PDMS coated stir bar): the bar is conditioned for 24 h under stirring in a solution of acetonitrile:methanol (80:20, v:v); plasma is placed in a vial to which 4 mL of buffer solution is added; the vial is sealed, heated up to 50 °C, the stir bar is immersed into the sample, and the extraction is performed under magnetic stirring for 45 min; for desorption stir bar is removed, rinsed with water, dried and placed in a vial containing 1 mL of acetonitrile ensuring total immersion at 50 °C for 15 min; the stir bar is removed, the solvent is evaporated, and the residue is redissolved in 50 µL of acetonitrile. | NACE-DAD | n.a. and 20 ng/mL (Fluoxetine, Paroxetine), n.a. and 10 ng/mL (Sertraline), n.a. and 25 ng/mL (Citalopram) for SBSE technique | 20–500 ng/mL (Fluoxetine, Paroxetine), 10–500 ng/mL (Sertraline), 25–500 ng/mL (Citalopram) for SBSE technique | [108] |
| Propranolol | n.a. of urine | The sample is diluted with water (1:4, v:v), the pH is adjusted to 9 by diluted ammonia, and 10 mL of the diluted sample is subjected to the extraction process. SBSE (GO/MIP coated stir bar): in a vial, 10 mL of sample solution is added with a GO/MIP coated stir bar, the vial is sealed and placed on a magnetic stir for 40 min; the stir bar is taken out of the sample solution, washed with water and dried; the stir bar is placed in a vial containing 100 μL of desorption solution of methanol and 10 mmol/L of sodium hydroxide (60:40, v:v), sealed and ultrasonicated for 20 min; the desorption solution is filtered. | HPLC-UV | 0.37 and 1 μg/L | 1–1000 μg/L | [109] |
| Ciprofloxacin, Sulfasalazine, Cortisone | 180 μL of blood, 450 μL of plasma and 900 μL of urine | Blood sample is diluted with water (1:5, v:v). FPSE (sol-gel CW 20M (polyethylene glycol) coated): the membrane is cut into circular disks, cleaned with 2 mL of acetonitrile:methanol (50:50, v:v) for 5 min and rinsed 2/3 times in water; analytes extraction at TAAB rotator for 30 min; elution/back-extraction using 150 μL of methanol for 10 min and centrifugation. | HPLC-PDA | 0.02 and 0.05 μg/mL for blood samples for all the compounds, 0.1 and 0.25 μg/mL for plasma samples for all the compounds, 0.03 and 0.10 μg/mL for urine samples for all the compounds | 0.05–10 μg/mL for blood samples for all the compounds, 0.25–10 μg/mL for plasma samples for all the compounds, 0.10–10 μg/mL for urine samples for all the compounds | [110] |
| Fluoxetine | 240 μL of plasma | SBSE (PDMS stir bar): the bar is conditioned for 24 h under magnetic stirring in a solution of methanol:acetonitrile (20:80, v:v); plasma is placed in a vial to which the SBSE bar and 3750 μL of sodium borate buffer (pH 9) is added, which is shaken; for the desorption step, 4000 μL of methanol:acetonitrile (75:25, v:v) are used, stirring for 50 min at a temperature of 50 °C; the bar is removed from the desorption solution, the sample is evaporated, and the residue is redissolved in 250 µL of desorption solution. | HPLC-FD | 9.8 and 32.67 ng/mL | 25–250 ng/mL | [111] |
| Febuxostat, Montelukast | 20 μL of plasma | FPSE (sol-gel PCAP-PDMS-PCAP coated membrane): the membrane is cut into squares of 1 cm2 of surface, cleaned and activated by methanol:acetonitrile (40:60, v:v) for 5 min; it is rinsed in water, and 20 μL of plasma is diluted with 280 μL of isotonic solution (0.9% sodium chloride); the extraction is carried out under magnetic stirring at room temperature for 30 min; the back-extraction of the analytes from the membrane is carried out by means of 100 μL of methanol for 10 min. | HPLC-FLD | 0.1 and 0.3 ng/mL (Febuxostat), 1.5 and 5 ng/mL (Montelukast) | 0.3–10 ng/mL (Febuxostat), 5–100 ng/mL (Montelukast) | [112] |
| Favipiravir | 350 μL of plasma and breast milk | FPSE (sol-gel PCAP-PDMS-PCAP coated membrane): the membrane is cut into squares of 1 cm2 of surface, cleaned and activated by methanol:water (60:40, v:v) for 5 min; it is rinsed in water and immersed into dilution of 350 μL of plasma and breast milk solutions, with 200 μL of serum physiologic (0.9% sodium hydroxide); the extraction is carried out under magnetic stirring at room temperature for 30 min; the back-extraction is carried out by using 500 μL of methanol for 30 min. | HPLC-UV | 0.06 and 0.2 μg/mL for plasma samples, 0.15 and 0.5 μg/mL for breast milk samples | 0.2–50 μg/mL for plasma samples, 0.5–25 μg/mL for breast milk samples | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring. Pharmaceutics 2023, 15, 1055. https://doi.org/10.3390/pharmaceutics15041055
Soares S, Rosado T, Barroso M, Gallardo E. Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring. Pharmaceutics. 2023; 15(4):1055. https://doi.org/10.3390/pharmaceutics15041055
Chicago/Turabian StyleSoares, Sofia, Tiago Rosado, Mário Barroso, and Eugenia Gallardo. 2023. "Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring" Pharmaceutics 15, no. 4: 1055. https://doi.org/10.3390/pharmaceutics15041055
APA StyleSoares, S., Rosado, T., Barroso, M., & Gallardo, E. (2023). Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring. Pharmaceutics, 15(4), 1055. https://doi.org/10.3390/pharmaceutics15041055








