Effect of Inhalation Profile on Delivery of Treprostinil Palmitil Inhalation Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. DPI Device
2.2. Compounds and Materials
2.3. Aerosol Characterization Using the Next Generation Impactor (NGI) and Breathing Simulator
2.4. Quantitation of TP
2.5. Determination of ED, MMAD, GSD, FPF and FPD
2.6. Data Analyses and Statistics
3. Results
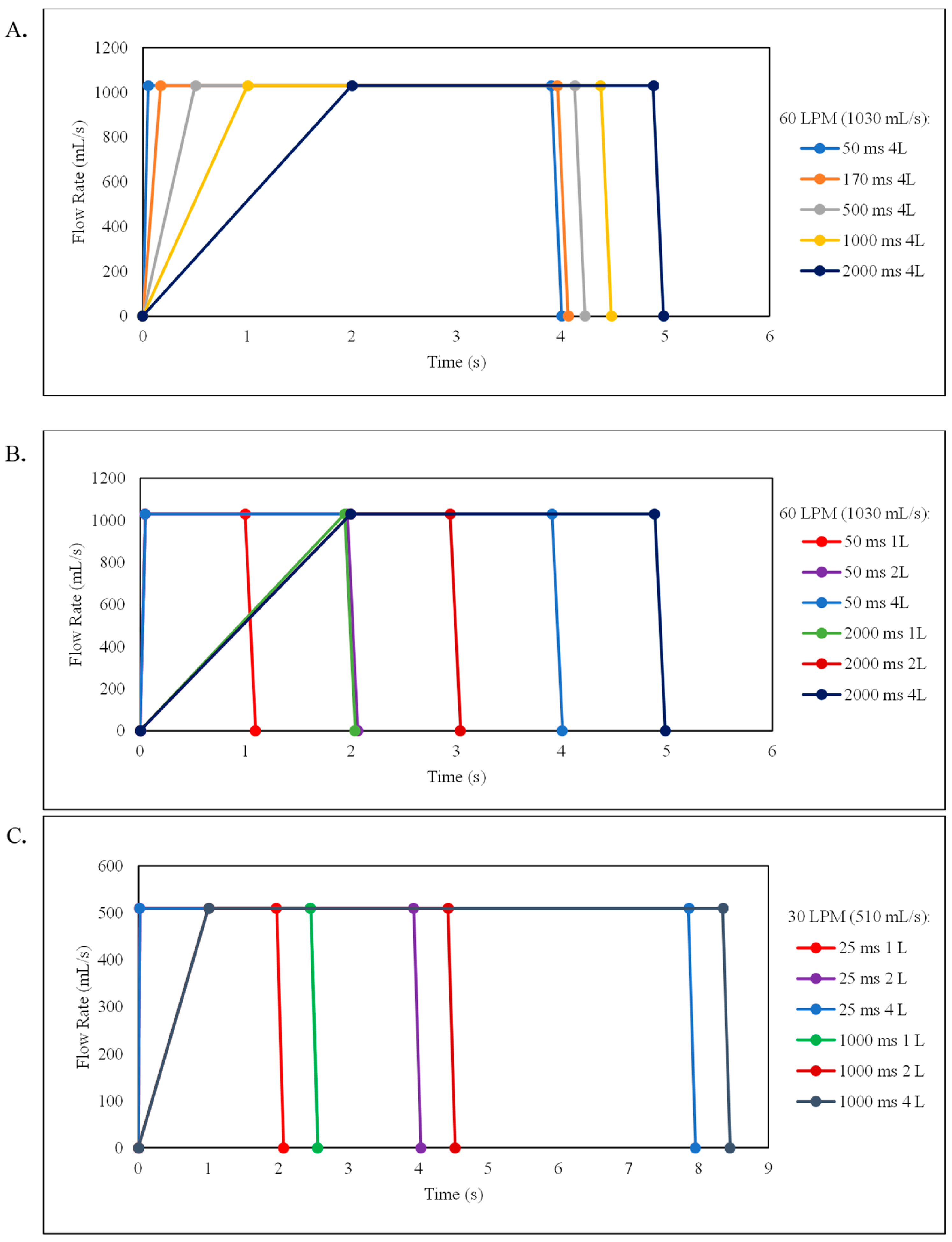
3.1. Evaluation of the Inhalation Acceleration Rates for the 16 and 32 mg TPIP Capsule Doses at 60 LPM Inspiratory Flow Rate and 4 L Inhalation Volume
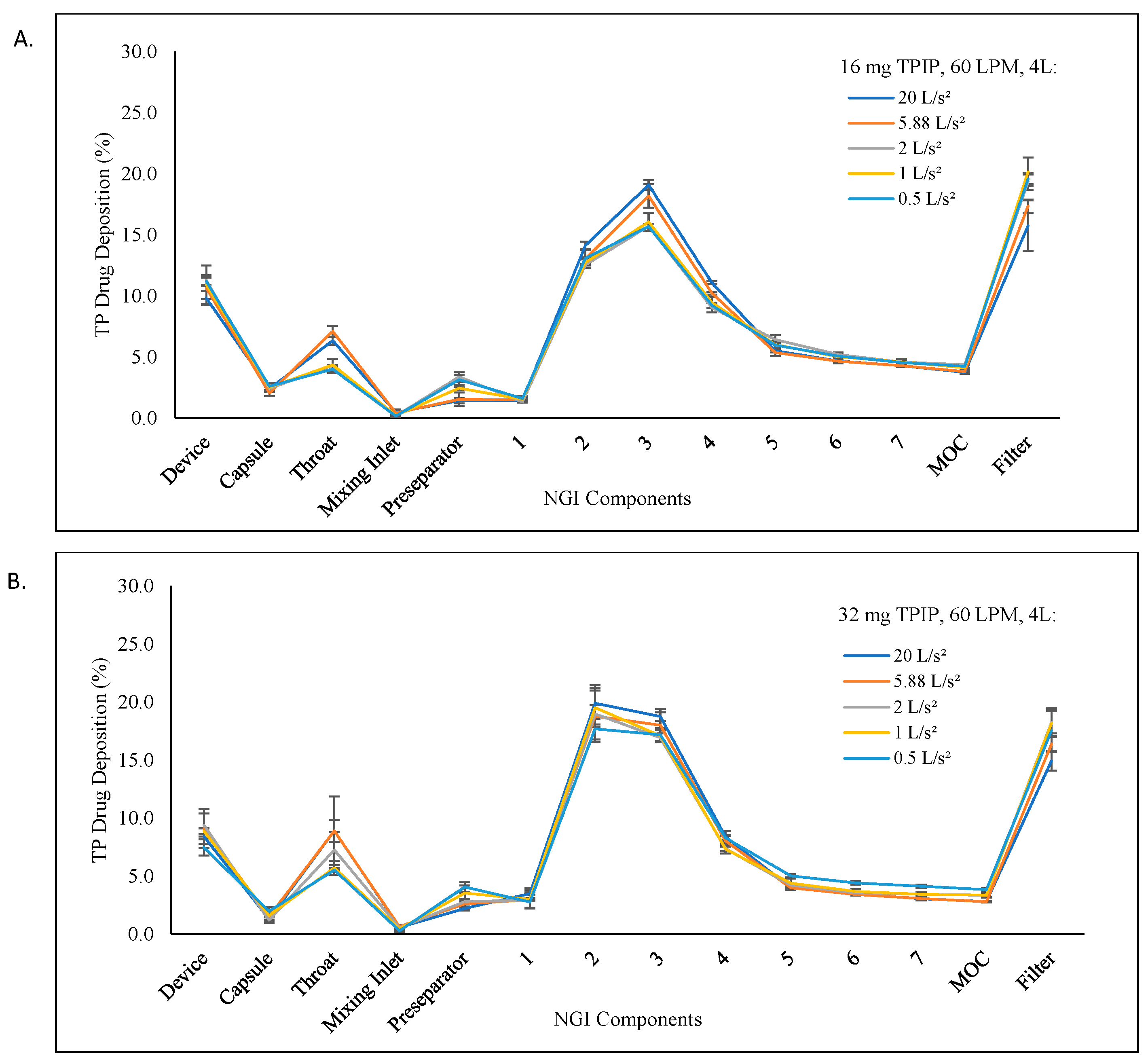
3.2. Evaluation of the Inhalation Volume for the 16 and 32 mg TPIP Capsule Doses at 60 LPM Inspiratory Flow Rate
3.3. Evaluation of the Inhalation Volume for the 16 mg TPIP Capsule Doss at 30 LPM Inspiratory Flow Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Therapeutics Corporation. Tyvaso® (Treprostinil) Inhalation Solution [Prescribing Information, Rev. 5/2022]; United Therapeutics Corporation: Research Triangle Park, NC, USA, 2002. [Google Scholar]
- United Therapeutics Corporation. Tyvaso DPI® (Treprostinil) Inhalation Powder [Prescribing Information, Rev. 05/2022]; United Therapeutics Corporation: Research Triangle Park, NC, USA, 2002. [Google Scholar]
- LeVarge, B.L. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther. Clin. Risk Manag. 2015, 11, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Corboz, M.R.; Malinin, V.S.; Plaunt, A.J.; Konicek, D.M.; Li, Z.; Perkins, W.R. An overview of the biology of a long-acting inhaled treprostinil prodrug. Pulm. Pharm. Ther. 2020, 65, 102002. [Google Scholar] [CrossRef] [PubMed]
- Corboz, M.R.; Li, Z.; Malinin, V.; Plaunt, A.J.; Konicek, D.M.; Leifer, F.G.; Chen, K.-J.; Laurent, C.E.; Yin, H.; Biernat, M.C.; et al. Preclinical Pharmacology and Pharmacokinetics of Inhaled Hexadecyl-Treprostinil (C16TR), a Pulmonary Vasodilator Prodrug. J. Pharm. Exp. Ther. 2017, 363, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Li, Z.; Corboz, M.R.; Gauani, H.; Plaunt, A.J.; Konicek, D.M.; Leifer, F.G.; Laurent, C.E.; Yin, H.; Salvail, D.; et al. Inhaled hexadecyl-treprostinil provides pulmonary vasodilator activity at significantly lower plasma concentrations than infused treprostinil. Pulm. Pharm. Ther. 2018, 49, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Plaunt, A.J.; Islam, S.; Macaluso, T.; Gauani, H.; Baker, T.; Chun, D.; Viramontes, V.; Chang, C.; Corboz, M.R.; Chapman, R.W.; et al. Development and Characterization of Treprostinil Palmitil Inhalation Aerosol for the Investigational Treatment of Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Leifer, F.G.; Konicek, D.M.; Chen, K.-J.; Plaunt, A.J.; Salvail, D.; Laurent, C.E.; Corboz, M.R.; Li, Z.; Chapman, R.W.; Perkins, W.R.; et al. Inhaled Treprostinil-Prodrug Lipid Nanoparticle Formulations Provide Long-Acting Pulmonary Vasodilation. Drug Res. 2018, 68, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Corboz, M.R.; Fernandez, C.; Sullivan, E.; Stautberg, A.; Plaunt, A.J.; Konicek, D.M.; Malinin, V.; Li, Z.; Cipolla, D.; et al. Characterisation of cough evoked by inhaled treprostinil and treprostinil palmitil. ERJ Open Res. 2021, 7, 00592-2020. [Google Scholar] [CrossRef] [PubMed]
- Gauani, H.; Baker, T.; Li, Z.; Malinin, V.S.; Perkins, W.; Sullivan, E.; Cipolla, D. Evaluation and Selection of the Inhaler Device for Treprostinil Palmitil Inhalation Powder. Front. Drug. Deliv. 2020, 2, 864922. [Google Scholar] [CrossRef]
- United States Pharmacopeia and National Formulary (USP 43-NF 38), General Chapters <601> Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders-Performance Quality Tests. Available online: https://online.uspnf.com/uspnf/document/1_GUID-FA5F788A-4449-4F16-8435-9B8D5EECB5C9_5_en-US?source=Quick%20Search&highlight=%3C601%3E (accessed on 9 March 2023).
- European Pharmacopoeia. 2.9.18. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles, Monograph 20918, 7th ed.; European Pharmacopoeia: London, UK, 2008. [Google Scholar]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.; Wilken, L.; Hart, M.K. Pulmonary Disease Aerosol Delivery Devices: A Guide for Physicians, Nurses, Pharmacists, and Other Health Care Professionals, 3rd ed.; American Association for Respiratory Care: Irving, TX, USA, 2017. [Google Scholar]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, F.; Jeanneret, A.; Couture, J. Sex differences in thoracic dimensions and configuration. Am. J. Respir. Crit. Care Med. 2003, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. The physiological basis and clinical significance of lung volume measurements. Multidiscip. Respir. Med. 2017, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, T.; Bozic-Krstic, V.; Rakic, R. Correlation of Vital Lung Capacity with Body Weight, Longitudinal and Circumference Dimensions. Biotechnol. Biotechnol. Equip. 2010, 24, 325–328. [Google Scholar] [CrossRef]
- Gea, J.; Pascual, S.; Casadevall, C.; Orozco-Levi, M.; Barreiro, E. Muscle dysfunction in chronic obstructive pulmonary disease: Update on causes and biological findings. J. Thorac. Dis. 2015, 7, E418–E438. [Google Scholar] [PubMed]
- Cipolla, D.; Chan, H.K.; Schuster, J.; Farina, D. Personalized Medicine: Development of Inhalation Systems Tailored to the Individual. Ther. Deliv. 2010, 1, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Mylan Pharmaceuticals Inc. Tobi® Podhaler™ (Tobramycin Inhalation Powder) [Patient Product Information Insert]; Mylan Pharmaceuticals Inc.: San Carlos, CA, USA, 2020. [Google Scholar]
- Buttini, F.; Balducci, A.G.; Colombo, G.; Sonvico, F.; Montanari, S.; Pisi, G.; Rossi, A.; Colombo, P.; Bettini, R. Dose Administration Maneuvers and Patient Care in Tobramycin Dry Powder Inhalation Therapy. Int. J. Pharm. 2018, 548, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Malinin, V.S.; Cipolla, D.; Finlay, W.H. Modeling regional deposition and pharmacokinetics for inhaled prodrug treprostinil palmitil. J. Aer. Med. Pulm. Drug Del. 2021, 34, A-1–A-22, Abstract 45. [Google Scholar] [CrossRef] [PubMed]
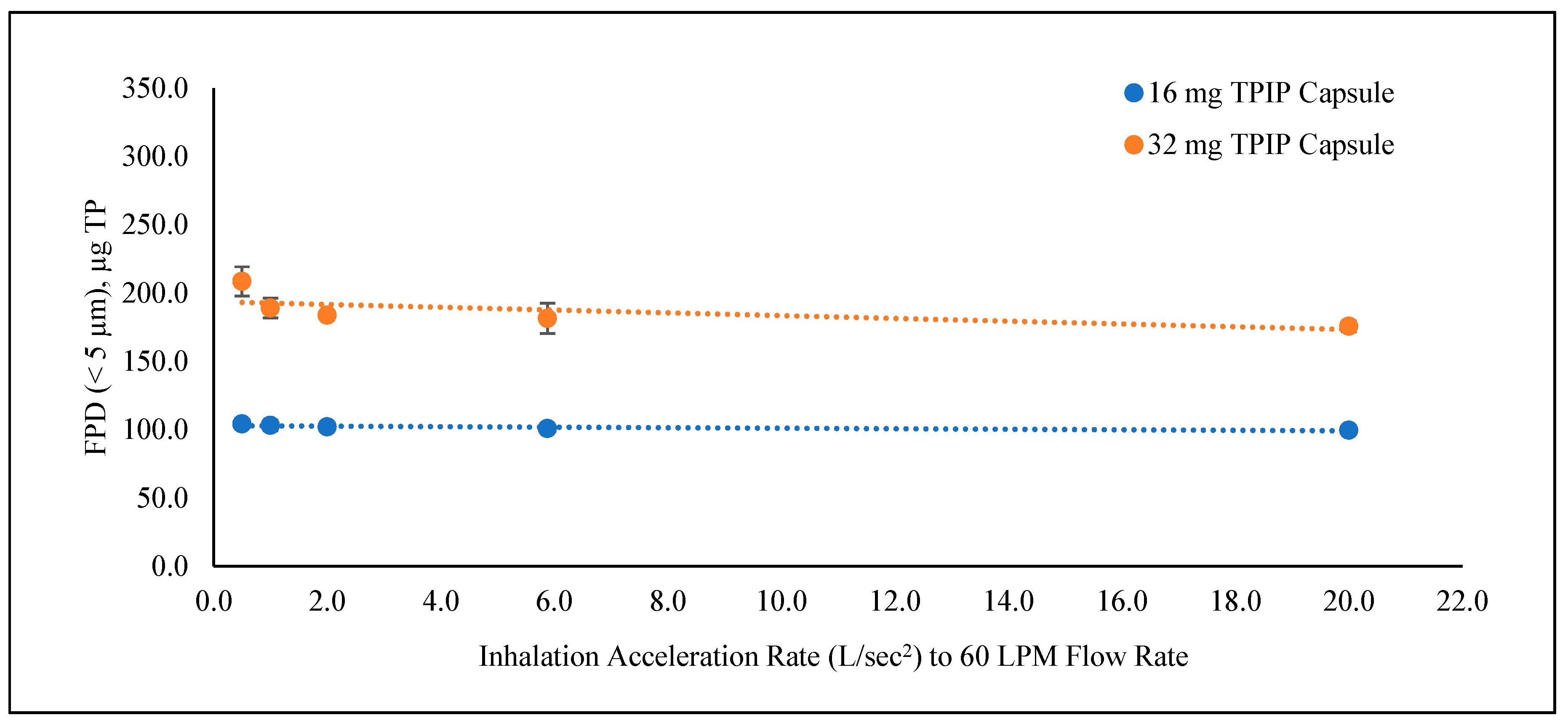

| Inhalation Ramp Time | n | Emitted Dose | MMAD | GSD | FPF (<5 µm) | FPD (<5 µm) | ||
|---|---|---|---|---|---|---|---|---|
| % | µg, TP | µm | % of Emitted Dose | % of Loaded Dose | µg, TP | |||
| 16 mg capsule containing 160 µg TP | ||||||||
| 50 ms | 3 | 79.8 ± 1.1 | 127.68 ± 1.70 | 1.27 ± 0.08 | 3.54 ± 0.11 | 78.1 ± 1.1 | 62.3 ± 1.7 | 99.71 ± 2.76 |
| 170 ms | 3 | 81.3 ± 1.2 | 130.11 ± 1.95 | 1.18 ± 0.03 | 3.71 ± 0.05 | 77.6 ± 1.1 | 63.1 ± 0.8 | 100.89 ± 1.30 |
| 500 ms | 3 | 79.8 ± 0.9 | 127.69 ± 1.46 | 1.04 ± 0.03 | 3.83. ± 0.05 | 80.0 ± 0.4 | 63.8 ± 1.0 | 102.11 ± 1.64 |
| 1000 ms | 3 | 80.3 ± 2.7 | 128.47 ± 4.24 | 1.05 ± 0.05 | 3.93 ± 0.10 | 80.4 ± 0.3 | 64.5 ± 2.4 | 103.23 ± 3.80 |
| 2000 ms | 3 | 81.8 ± 0.6 | 130.88 ± 1.00 | 1.06 ± 0.02 | 3.97 ± 0.10 | 79.6 ± 1.4 | 65.1 ± 1.6 | 104.23 ± 2.57 |
| 32 mg capsule containing 320 µg TP | ||||||||
| 50 ms | 3 | 81.3 ± 1.7 | 260.10 ± 5.55 | 1.66 ± 0.07 | 4.30 ± 0.21 | 67.6 ± 1.3 | 54.9 ± 1.3 | 175.81 ± 4.14 |
| 170 ms | 3 | 82.7 ± 1.3 | 264.76 ± 4.01 | 1.51 ± 0.02 | 4.35 ± 0.10 | 68.6 ± 3.3 | 56.8 ± 3.5 | 181.59 ± 11.15 |
| 500 ms | 3 | 81.3 ± 0.3 | 260.04 ± 1.07 | 1.38 ± 0.10 | 4.59 ± 0.26 | 70.7 ± 1.6 | 57.5 ± 1.1 | 183.93 ± 3.48 |
| 1000 ms | 3 | 82.9 ± 0.4 | 265.20 ± 1.12 | 1.40 ± 0.13 | 4.57 ±0.23 | 71.3 ± 2.4 | 59.1 ± 2.3 | 189.18 ± 7.19 |
| 2000 ms | 3 | 89.4 ± 2.2 | 286.14 ± 7.08 | 1.33 ± 0.10 | 4.32 ± 0.09 | 72.9 ± 2.0 | 65.2 ± 3.3 | 208.75 ± 10.62 |
| Ramp Time | FPD (<5 µm) t-Test 2-Tailed p-Values | ||||
|---|---|---|---|---|---|
| vs. 50 ms | vs. 170 ms | vs. 500 ms | vs. 1000 ms | vs. 2000 ms | |
| 16 mg capsule containing 160 µg TP | |||||
| 50 ms | - | 0.540 | 0.265 | 0.265 | 0.106 |
| 170 ms | 0.540 | - | 0.370 | 0.371 | 0.115 |
| 500 ms | 0.265 | 0.370 | - | 0.664 | 0.294 |
| 1000 ms | 0.265 | 0.371 | 0.664 | - | 0.724 |
| 2000 ms | 0.106 | 0.115 | 0.294 | 0.724 | - |
| Overall p-value (ANOVA: single factor) | 0.282 | ||||
| 32 mg capsule containing 320 µg TP | |||||
| 50 ms | - | 0.447 | 0.060 | 0.049 | 0.007 |
| 170 ms | 0.447 | - | 0.747 | 0.378 | 0.038 |
| 500 ms | 0.060 | 0.747 | - | 0.318 | 0.018 |
| 1000 ms | 0.049 | 0.378 | 0.318 | - | 0.057 |
| 2000 ms | 0.007 | 0.038 | 0.018 | 0.057 | - |
| Overall p-value (ANOVA: single factor) | 0.005 | ||||
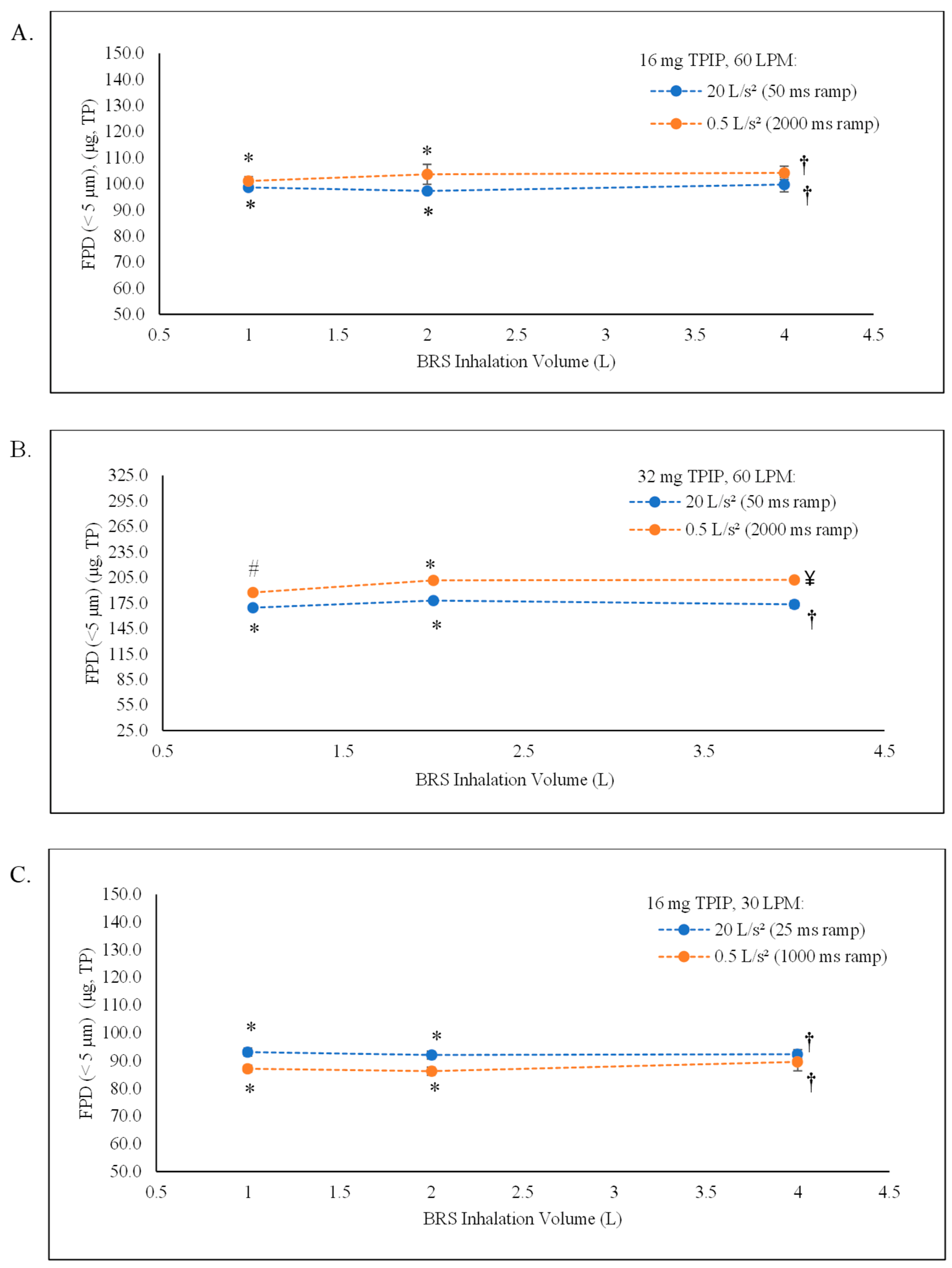
| Inhalation Parameters: Ramp Time, Inhaled Volume | n | Emitted Dose | MMAD | GSD | FPF (<5 µm) | FPD (<5 µm) | ||
|---|---|---|---|---|---|---|---|---|
| % | µg, TP | µm | % of Emitted Dose | % of Loaded Dose | µg, TP | |||
| 16 mg capsule containing 160 µg TP at 60 LPM (50 and 2000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||||
| 50 ms, 1 L | 3 | 81.1 ± 0.6 | 129.81 ± 0.96 | 1.24 ± 0.02 | 3.80 ± 0.07 | 76.0 ± 1.5 | 61.7 ± 0.7 | 98.70 ± 1.19 |
| 50 ms, 2 L | 3 | 79.1 ± 1.9 | 126.53 ± 3.07 | 1.28 ± 0.01 | 3.68 ± 0.13 | 76.9 ± 1.0 | 60.8 ± 0.7 | 97.28 ± 1.12 |
| * 50 ms, 4 L | 3 | 79.8 ± 1.1 | 127.68 ± 1.70 | 1.27 ± 0.08 | 3.54 ± 0.11 | 78.1 ± 1.1 | 62.3 ± 1.7 | 99.71 ± 2.76 |
| 2000 ms, 1 L | 3 | 82.3 ± 1.2 | 131.71 ± 1.92 | 1.19 ± 0.05 | 4.16 ± 0.05 | 76.8 ± 1.0 | 63.2 ± 1.0 | 101.13 ± 1.61 |
| 2000 ms, 2 L | 3 | 82.5 ± 2.3 | 131.94 ± 3.63 | 1.06 ± 0.03 | 3.95 ± 0.05 | 78.6 ± 0.7 | 64.8 ± 2.4 | 103.68 ± 3.81 |
| * 2000 ms, 4 L | 3 | 81.8 ± 0.6 | 130.88 ± 1.00 | 1.06 ± 0.02 | 3.97 ± 0.10 | 79.6 ± 1.4 | 65.1 ± 1.6 | 104.23 ± 2.57 |
| 32 mg capsule containing 320 µg TP at 60 LPM (50 and 2000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||||
| 50 ms, 1 L | 3 | 84.1 ± 0.5 | 269.01 ± 1.62 | 1.68 ± 0.03 | 4.51 ± 0.07 | 63.0 ± 1.0 | 53.0 ± 0.8 | 169.43 ± 2.45 |
| 50 ms, 2 L | 3 | 84.7 ± 1.3 | 271.12 ± 3.99 | 1.65 ± 0.02 | 4.30 ± 0.14 | 65.6 ± 1.0 | 55.6 ± 0.9 | 177.94 ± 2.73 |
| * 50 ms, 4 L | 3 | 81.3 ± 1.7 | 260.10 ± 5.55 | 1.66 ± 0.07 | 4.30 ± 0.21 | 67.6 ± 1.3 | 54.9 ± 1.3 | 175.81 ± 4.14 |
| 50 ms, 4 L | 3 | 83.9 ± 0.5 | 268.37 ± 1.53 | 1.69 ± 0.07 | 4.35 ± 0.08 | 64.6 ± 1.5 | 54.2 ± 1.3 | 173.38 ± 4.28 |
| 2000 ms, 1 L | 3 | 81.2 ± 1.3 | 259.99 ± 4.08 | 1.27 ± 0.08 | 4.37 ± 0.16 | 72.1 ± 1.4 | 58.6 ± 0.3 | 187.40 ± 0.88 |
| 2000 ms, 2 L | 3 | 86.8 ± 0.6 | 277.59 ± 2.00 | 1.34 ± 0.03 | 4.25 ± 0.10 | 72.6 ± 1.4 | 63.0 ± 1.1 | 201.61 ± 3.49 |
| * 2000 ms, 4 L | 3 | 89.4 ± 2.2 | 286.14 ± 7.08 | 1.33 ± 0.10 | 4.32 ± 0.09 | 72.9 ± 2.0 | 65.2 ± 3.3 | 208.75 ± 10.62 |
| 2000 ms, 4 L | 3 | 87.6 ± 0.9 | 280.22 ± 2.81 | 1.35 ± 0.03 | 4.33 ± 0.01 | 72.2 ± 0.2 | 63.2 ± 0.6 | 202.20 ± 1.96 |
| Ramp Time, Volume | FPD (<5 µm) t-Test 2-Tailed p-Values | |||||
|---|---|---|---|---|---|---|
| vs. 50 ms 1 L | vs. 50 ms 2 L | vs. 50 ms 4 L | vs. 2000 ms 1 L | vs. 2000 ms 2 L | vs. 2000 ms 4 L | |
| 16 mg capsule containing 160 µg TP at 60 LPM (50 and 2000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||
| 50 ms, 1 L | - | 0.206 | 0.588 | 0.103 | 0.097 | 0.028 |
| 50 ms, 2 L | 0.206 | - | 0.229 | 0.027 | 0.049 | 0.013 |
| 50 ms, 4 L | 0.588 | 0.229 | - | 0.485 | 0.218 | 0.106 |
| 2000 ms, 1 L | 0.103 | 0.027 | 0.485 | - | 0.345 | 0.151 |
| 2000 ms, 2 L | 0.097 | 0.049 | 0.218 | 0.345 | - | 0.846 |
| 2000 ms, 4 L | 0.028 | 0.013 | 0.106 | 0.151 | 0.846 | - |
| (ANOVA: single factor) | 0.336 | 0.409 | ||||
| Overall p-value (ANOVA: single factor) | 0.022 | |||||
| 32 mg capsule containing 320 µg TP at 60 LPM (50 and 2000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||
| 50 ms, 1 L | - | 0.016 | 0.237 | 0.000 | 0.000 | 0.000 |
| 50 ms, 2 L | 0.016 | - | 0.194 | 0.005 | 0.001 | 0.000 |
| 50 ms, 4 L | 0.237 | 0.194 | - | 0.005 | 0.001 | 0.000 |
| 2000 ms, 1 L | 0.000 | 0.005 | 0.005 | - | 0.002 | 0.000 |
| 2000 ms, 2 L | 0.000 | 0.001 | 0.001 | 0.002 | - | 0.811 |
| 2000 ms, 4 L | 0.000 | 0.000 | 0.000 | 0.000 | 0.811 | - |
| p-value (ANOVA: single factor) | 0.050 | 0.004 | ||||
| Overall p-value (ANOVA: single factor) | 0.000 | |||||
| Inhalation Parameters: Ramp Time, Inhaled Volume | n | Emitted Dose | MMAD | GSD | FPF (<5 µm) | FPD (<5 µm) | ||
|---|---|---|---|---|---|---|---|---|
| % | µg, TP | µm | % of Emitted Dose | % of Loaded Dose | µg, TP | |||
| 16 mg capsule containing 160 µg TP at 30 LPM (25 and 1000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||||
| 25 ms, 1 L | 3 | 76.4 ± 0.7 | 122.30 ± 1.04 | 2.19 ± 0.11 | 2.34 ± 0.05 | 76.2 ± 1.7 | 58.2 ± 0.9 | 93.13 ± 1.45 |
| 25 ms, 2 L | 3 | 76.1 ± 2.3 | 121.79 ± 3.61 | 2.25 ± 0.08 | 2.31 ± 0.10 | 75.6 ± 1.4 | 57.5 ± 0.9 | 92.08 ± 1.40 |
| 25 ms, 4 L | 3 | 76.1 ± 1.8 | 121.83 ± 2.95 | 2.16 ± 0.08 | 2.40 ± 0.06 | 75.9 ± 1.8 | 57.7 ± 1.0 | 92.39 ± 1.60 |
| 1000 ms, 1 L | 3 | 71.7 ± 1.8 | 114.73 ± 2.83 | 1.90 ± 0.11 | 2.58 ± 0.07 | 76.0 ± 1.3 | 54.5 ± 0.8 | 87.16 ± 1.27 |
| 1000 ms, 2 L | 3 | 72.7 ± 1.1 | 116.30 ± 1.71 | 1.88 ± 0.08 | 2.67 ± 0.03 | 74.2 ± 1.1 | 53.9 ± 0.9 | 86.26 ± 1.44 |
| 1000 ms, 4 L | 3 | 74.8 ± 2.3 | 119.67 ± 3.75 | 1.95 ± 0.11 | 2.54 ± 0.09 | 74.9 ± 0.6 | 56.0 ± 2.1 | 89.61 ± 3.29 |
| Ramp Time, Volume | FPD (< 5 µm) t-Test 2-Tailed p-Values | |||||
|---|---|---|---|---|---|---|
| vs. 25 ms 1 L | vs. 25 ms 2 L | vs. 25 ms 4 L | vs. 1000 ms 1 L | vs. 1000 ms 2 L | vs. 1000 ms 4 L | |
| 16 mg capsule containing 160 µg TP at 30 LPM (25 and 1000 ramp times corresponding to 20 and 0.5 L/s2 acceleration rates, respectively) | ||||||
| 25 ms, 1 L | - | 0.415 | 0.581 | 0.006 | 0.004 | 0.165 |
| 25 ms, 2 L | 0.415 | - | 0.813 | 0.011 | 0.007 | 0.298 |
| 25 ms, 4 L | 0.581 | 0.813 | - | 0.011 | 0.008 | 0.259 |
| 1000 ms, 1 L | 0.006 | 0.011 | 0.011 | - | 0.463 | 0.296 |
| 1000 ms, 2 L | 0.004 | 0.007 | 0.008 | 0.463 | - | 0.182 |
| 1000 ms, 4 L | 0.165 | 0.298 | 0.259 | 0.296 | 0.182 | - |
| (ANOVA: single factor) | 0.686 | 0.235 | ||||
| Overall p-value (ANOVA: single factor) | 0.003 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauani, H.; Baker, T.; Li, Z.; Malinin, V.S.; Perkins, W.R.; Sullivan, E.J.; Cipolla, D. Effect of Inhalation Profile on Delivery of Treprostinil Palmitil Inhalation Powder. Pharmaceutics 2023, 15, 934. https://doi.org/10.3390/pharmaceutics15030934
Gauani H, Baker T, Li Z, Malinin VS, Perkins WR, Sullivan EJ, Cipolla D. Effect of Inhalation Profile on Delivery of Treprostinil Palmitil Inhalation Powder. Pharmaceutics. 2023; 15(3):934. https://doi.org/10.3390/pharmaceutics15030934
Chicago/Turabian StyleGauani, Helena, Thomas Baker, Zhili Li, Vladimir S. Malinin, Walter R. Perkins, Eugene J. Sullivan, and David Cipolla. 2023. "Effect of Inhalation Profile on Delivery of Treprostinil Palmitil Inhalation Powder" Pharmaceutics 15, no. 3: 934. https://doi.org/10.3390/pharmaceutics15030934
APA StyleGauani, H., Baker, T., Li, Z., Malinin, V. S., Perkins, W. R., Sullivan, E. J., & Cipolla, D. (2023). Effect of Inhalation Profile on Delivery of Treprostinil Palmitil Inhalation Powder. Pharmaceutics, 15(3), 934. https://doi.org/10.3390/pharmaceutics15030934






