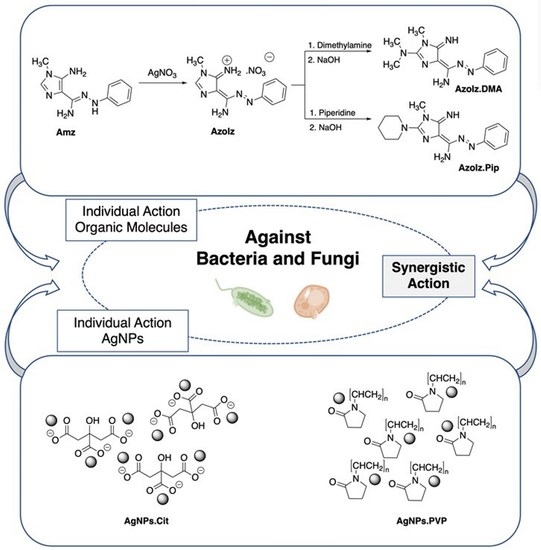
Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
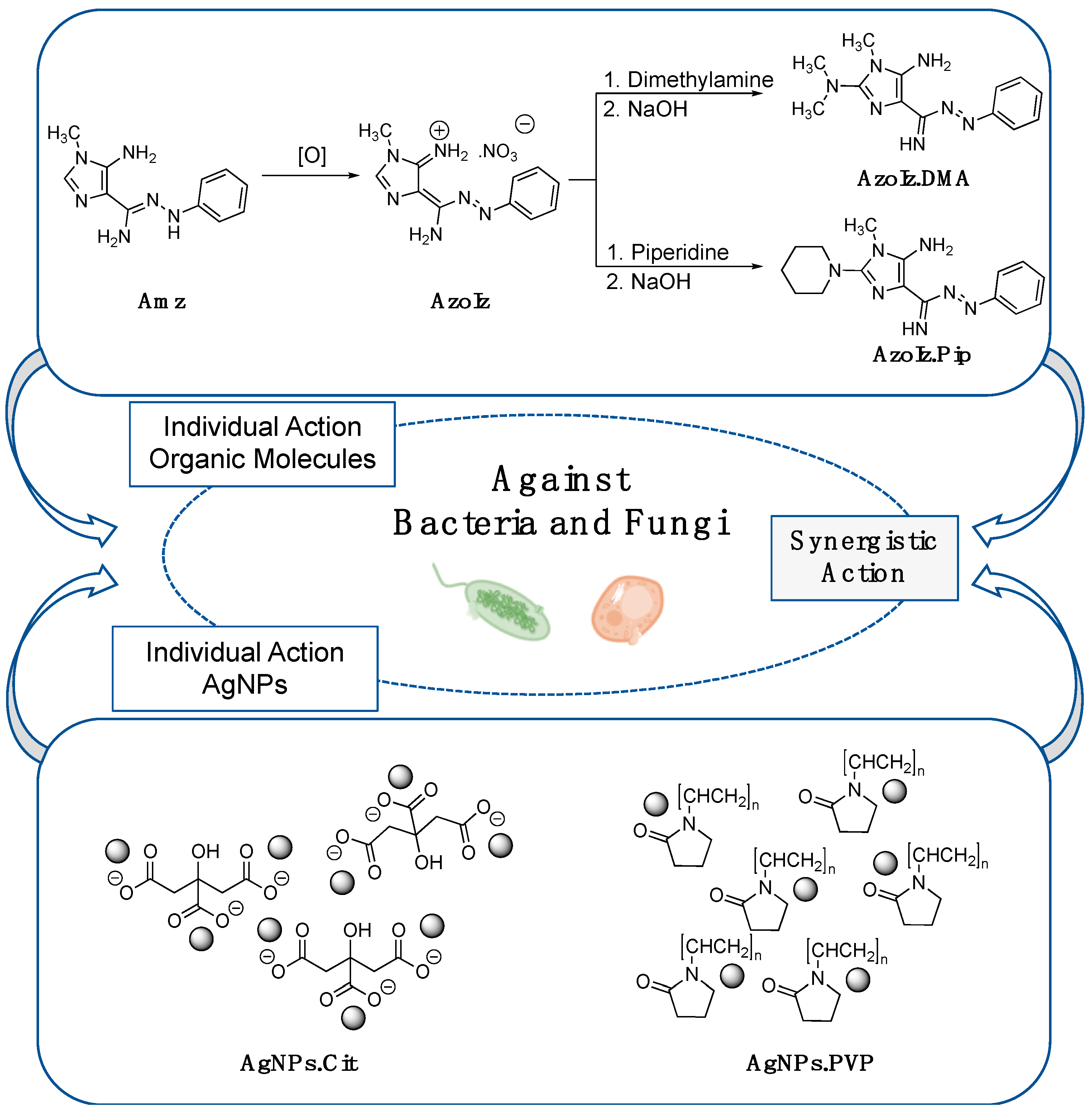
2.2. Synthesis and Characterization of Amidrazone (Amz) and Azoimidazoles (AzoIz, AzoIz.DMA and AzoIz.Pip) Compounds
2.3. Preparation of AgNPs Dispersions and Its Combination with Organic Molecules
2.4. UV–Vis Spectrophotometry
2.5. Scanning Transmission Electron Microscopy (STEM)
2.6. Dynamic Light Scattering (DLS) Analysis
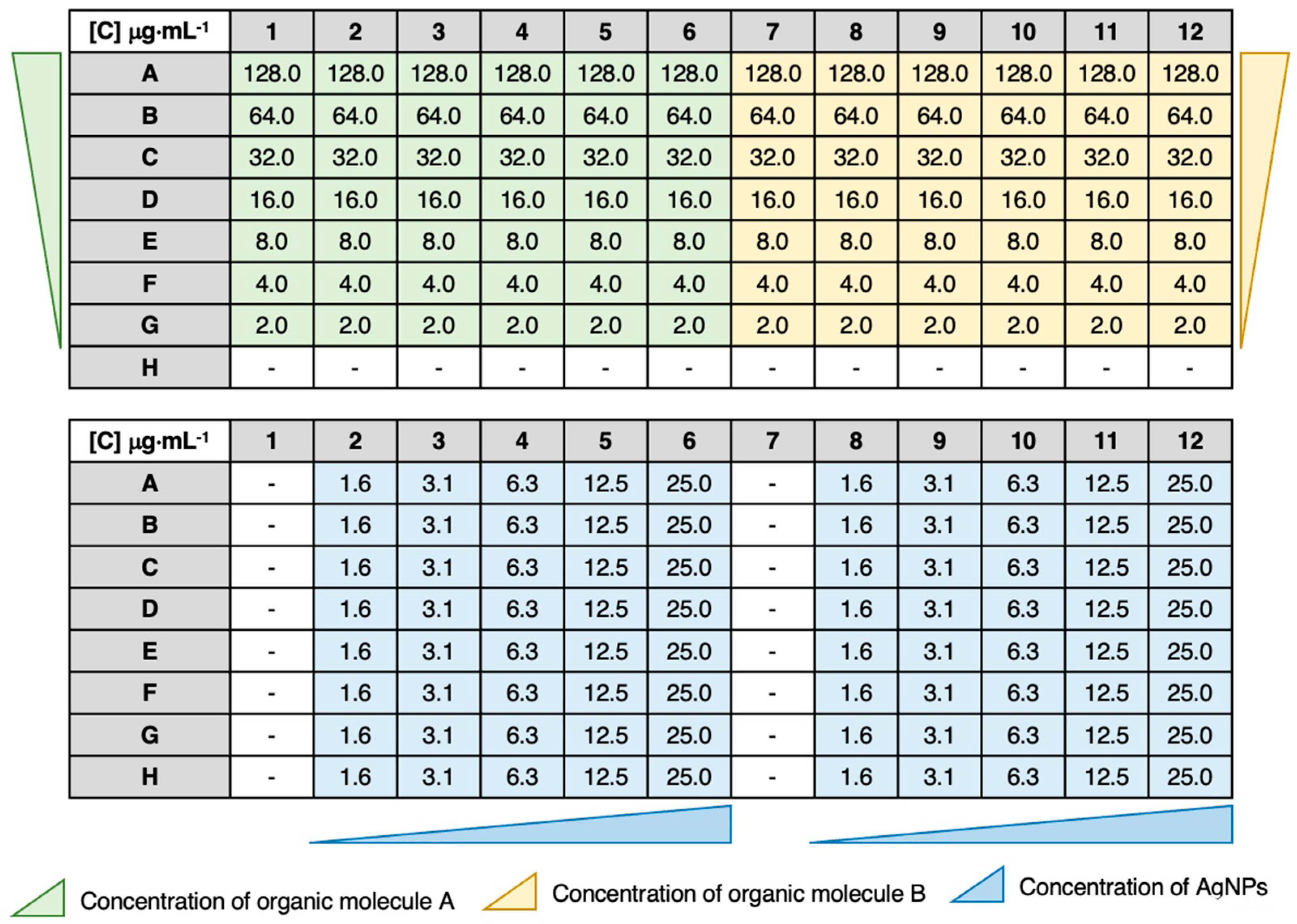
2.7. Synergy Testing by Checkerboard Assay
2.8. Cytotoxicity
2.8.1. HaCaT Cell Culture
2.8.2. Compound Cytotoxicity
2.8.3. Neutral Red Uptake Assay
2.8.4. Resazurin Reduction Assay
2.8.5. Sulforhodamine B Assay
2.8.6. Statistical Analysis
3. Results and Discussion
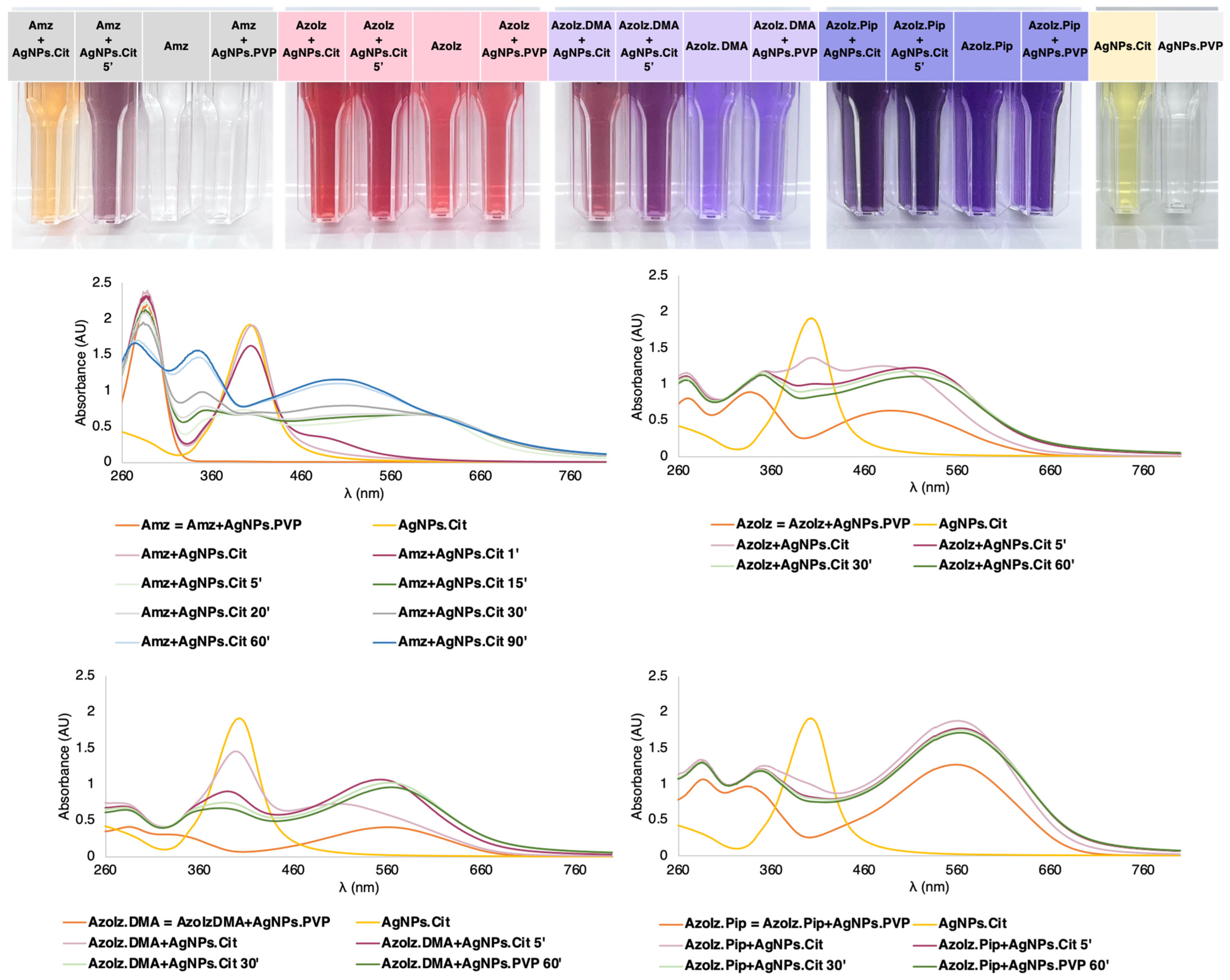
3.1. Conjugation of Organic Molecules with AgNPs and UV–Vis Characterization
3.2. Morphological Analysis of AgNPs Alone and Conjugated with Organic Molecules by STEM
3.3. DLS Measurements of AgNPs Alone and Conjugated with Organic Molecules
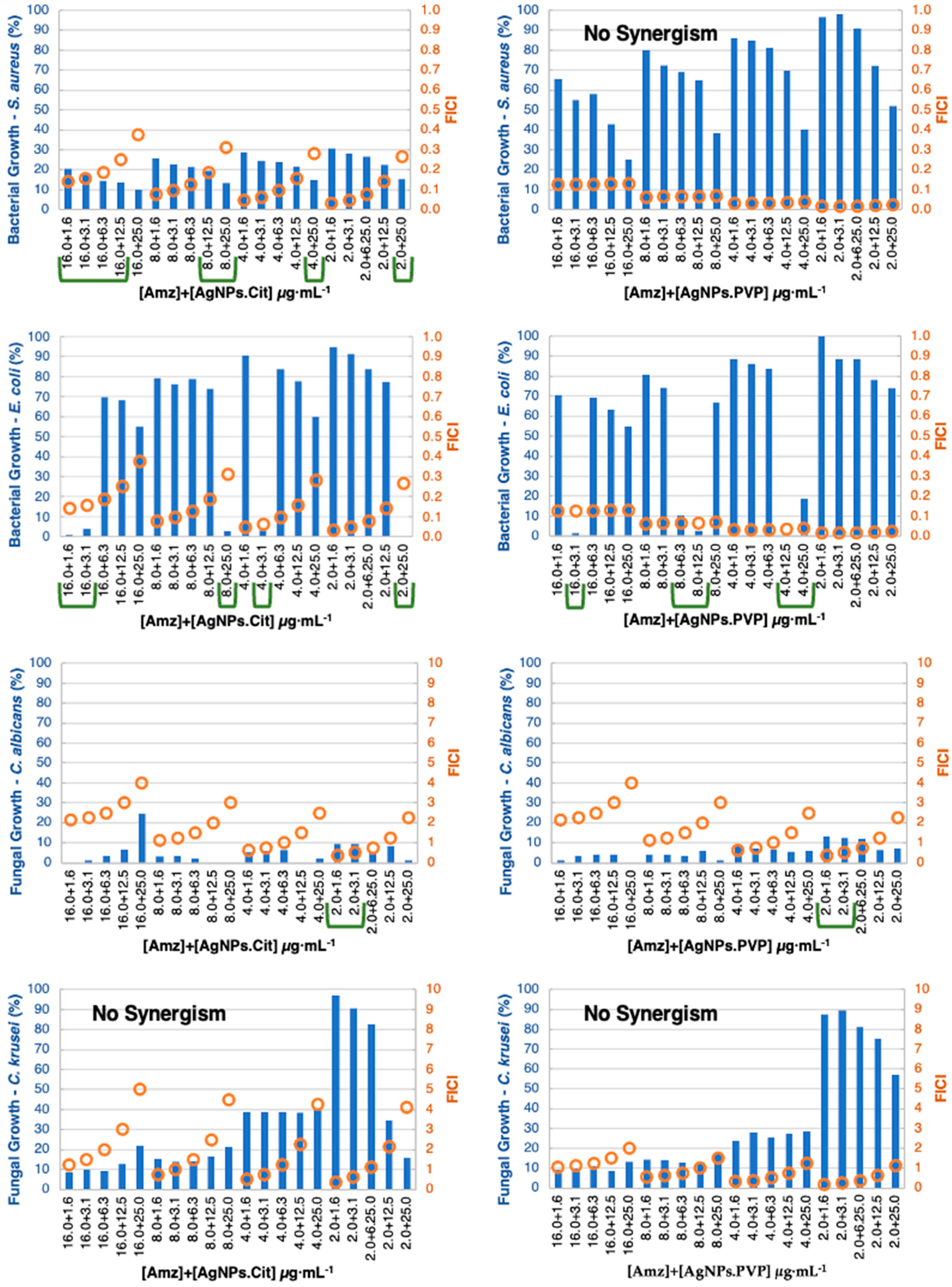
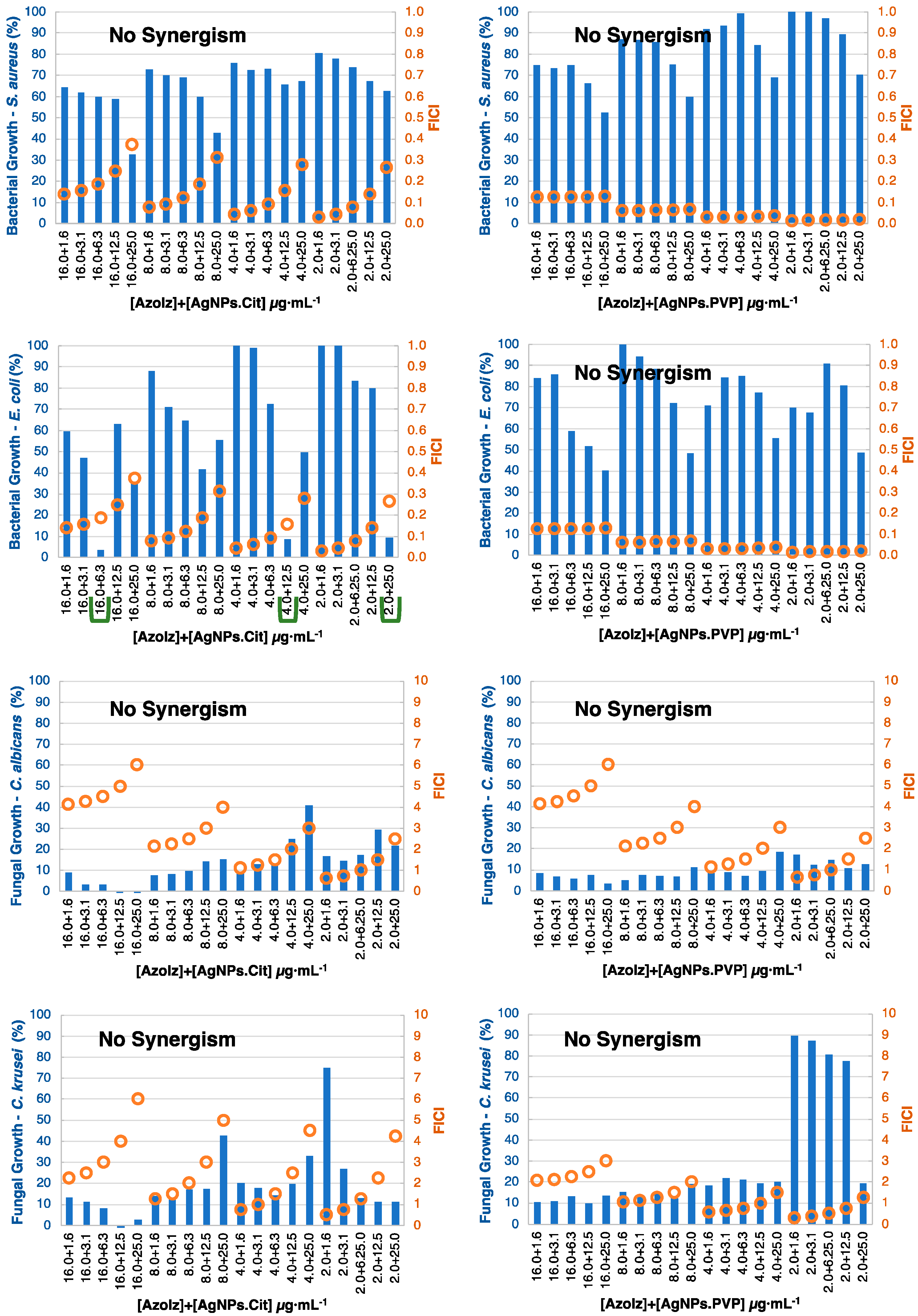
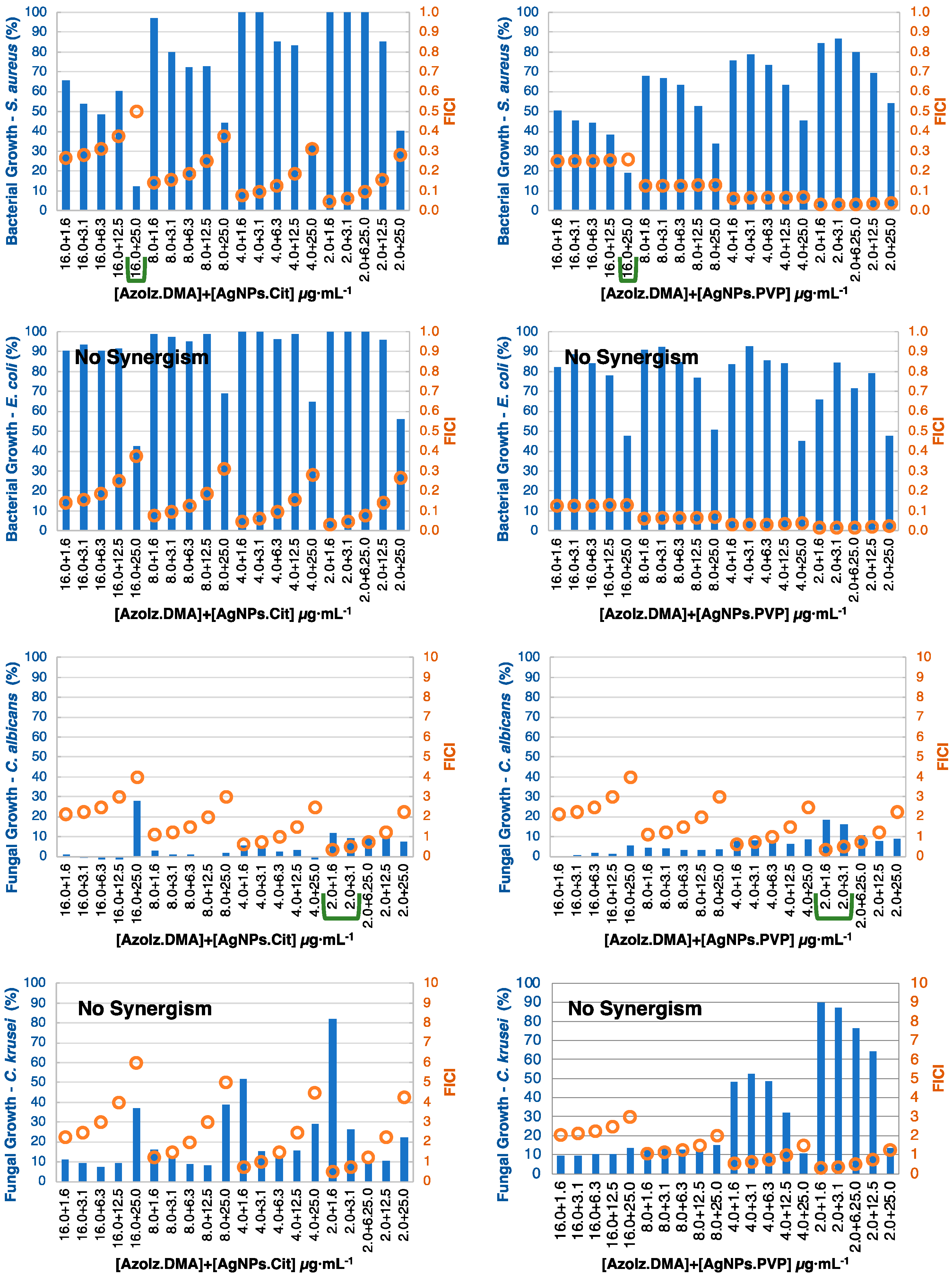
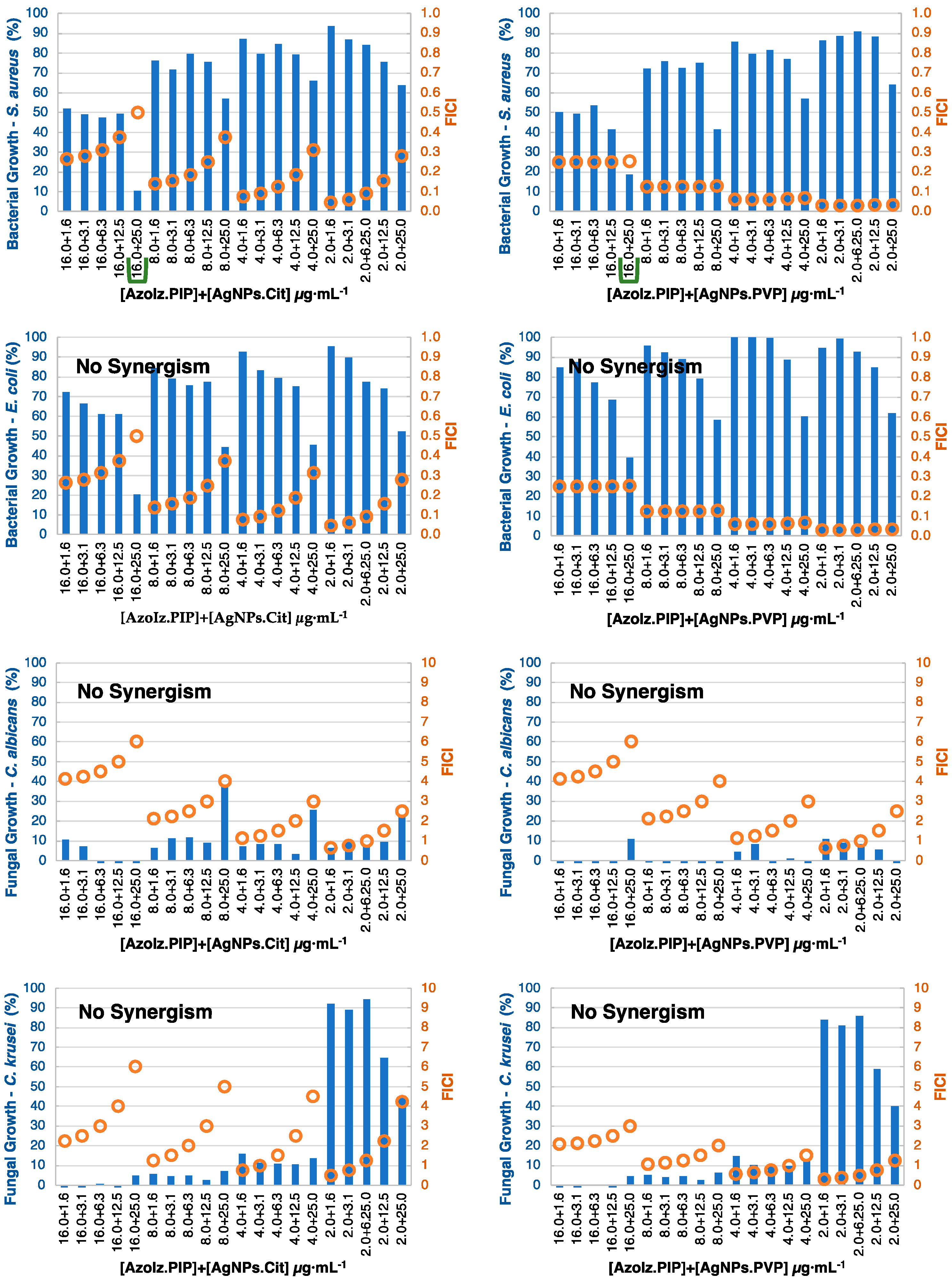
3.4. MIC Determination and Antibacterial Combination Assay (Checkerboard Results)
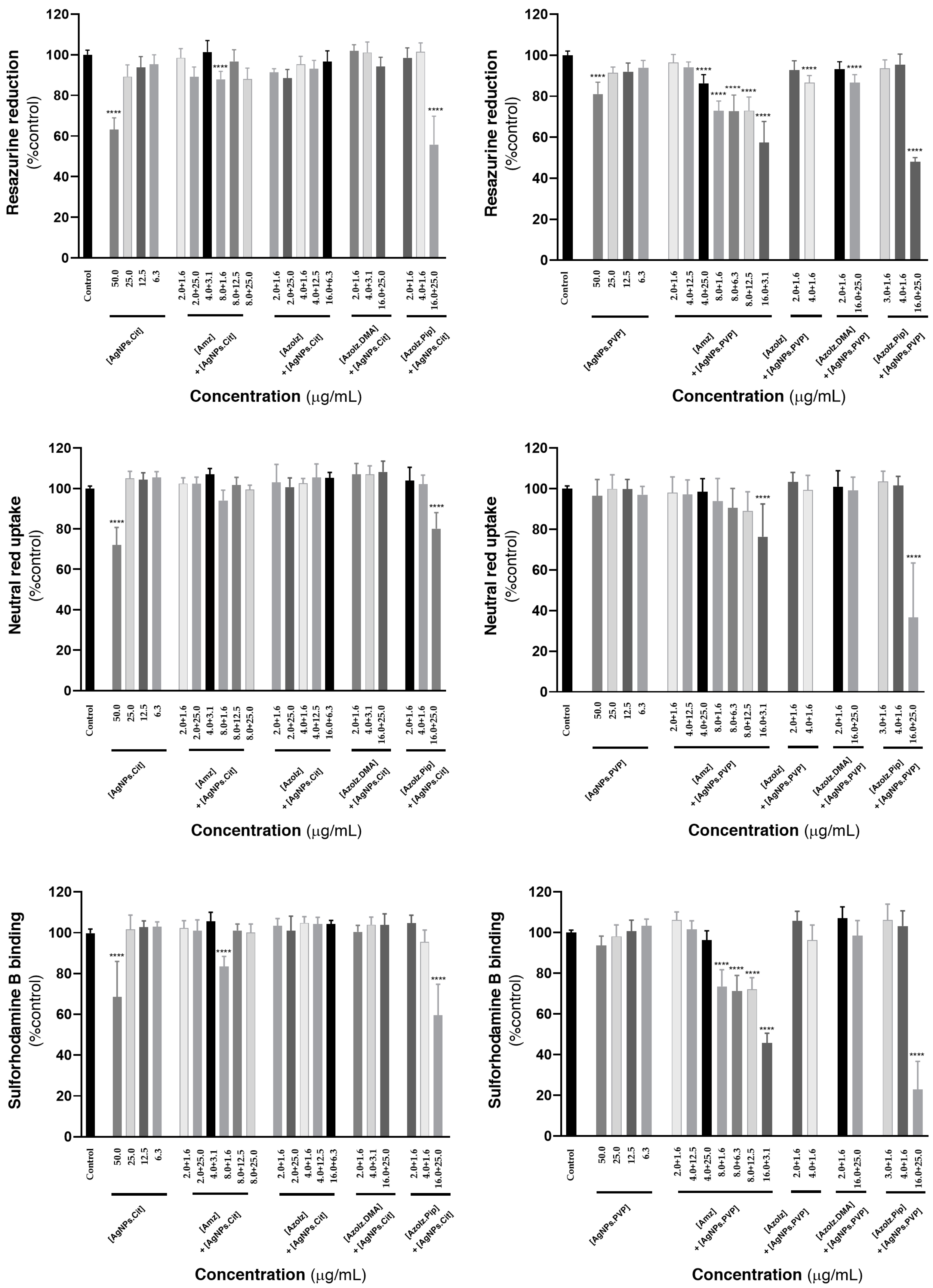
3.5. Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Gerace, E.; Mancuso, G.; Midiri, A.; Poidomani, S.; Zummo, S.; Biondo, C. Recent Advances in the Use of Molecular Methods for the Diagnosis of Bacterial Infections. Pathogens 2022, 11, 663. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, L.; Bergström, R.; Dustdar, S.; Meulien, P.; Draghia-Akli, R. Increased momentum in antimicrobial resistance research. Lancet 2016, 388, 865. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Chang, P.H.; Chen, I.L.; Lai, W.H.; Chen, Y.J.; Li, W.F.; Lee, I.K.; Wang, C.C. Risk factors and mortality associated with multi-drug-resistant Gram-negative bacterial infection in adult patients following abdominal surgery. J. Hosp. Infect. 2022, 119, 22–32. [Google Scholar] [CrossRef]
- Kadam, S.; Nadkarni, S.; Lele, J.; Sakhalkar, S.; Mokashi, P.; Kaushik, K.S. Bioengineered Platforms for Chronic Wound Infection Studies: How Can We Make Them More Human-Relevant? Front. Bioeng. Biotechnol. 2019, 7, 418. [Google Scholar] [CrossRef] [Green Version]
- Caplin, J.D.; García, A.J. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef]
- Ge, X. Antimicrobial biomaterials with non-antibiotic strategy. Biosurface Biotribol. 2019, 5, 71–82. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Healthc. Mater. 2020, 10, e2001199. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 12, 21159–21182. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Hall, L.; White, N.; Barnett, A.G.; Halton, K.; Paterson, D.L.; Riley, T.V.; Gardner, A.; Page, K.; Farrington, A.; et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): A multicentre, randomised trial. Lancet Infect. Dis. 2019, 19, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- MacAlpine, J.; Robbins, N.; Cowen, L.E. Bacterial-fungal interactions and their impact on microbial pathogenesis. Mol. Ecol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Ahmed, A.; Getti, G.; Boateng, J. Medicated multi-targeted alginate-based dressings for potential treatment of mixed bacterial-fungal infections in diabetic foot ulcers. Int. J. Pharm. 2021, 606, 120903. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Mahira, S.; Jain, A.; Khan, W.; Domb, A.J. Chapter 1. Antimicrobial Materials—An Overview. In Antimicrobial Materials for Biomedical Applications; Royal Society of Chemistry: London, UK, 2019; pp. 1–37. [Google Scholar]
- Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; Decaens, J.; Vermeersch, O. Antimicrobial Agents for Textiles: Types, Mechanisms and Analysis Standards. In Textiles for Functional Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Talapko, J.; Matijević, T.; Juzbašić, M.; Antolović-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Teli, P.; Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, S. Imidazole derivatives: Impact and prospects in antiviral drug discovery. In Imidazole-Based Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 167–193. [Google Scholar] [CrossRef]
- Gujjarappa, R.; Kabi, A.K.; Sravani, S.; Garg, A.; Vodnala, N.; Tyagi, U.; Kaldhi, D.; Velayutham, R.; Singh, V.; Gupta, S.; et al. Overview on Biological Activities of Imidazole Derivatives. In Nanostructured Biomaterials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 135–227. [Google Scholar] [CrossRef]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef] [PubMed]
- Sahiba, N.; Sethiya, A.; Agarwal, S. Imidazole heterocycles: Therapeutically potent lead compounds as antimicrobials. In Imidazole-Based Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–261. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Soni, J.; Sethiya, A.; Sahiba, N.; Teli, P.; Agarwal, S. Recent advancements on imidazole containing heterocycles as antitubercular agents. In Imidazole-Based Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 133–166. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Gabriel, C.; Cerqueira, F.; Maia, M.; Pinto, E.; Sousa, J.C.; Medeiros, R.; Proença, M.F.; Dias, A.M. Synthesis and antimicrobial activity of novel 5-aminoimidazole-4-carboxamidrazones. Bioorg. Med. Chem. Lett. 2014, 24, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Dantas, D.; Ribeiro, A.I.; Carvalho, F.; Gil-Martins, E.; Silva, R.; Remião, F.; Zille, A.; Cerqueira, F.; Pinto, E.; Dias, A.M. Red-shifted and pH-Responsive Imidazole-based Azo Dyes with Potent Antimicrobial Activity. Chem. Commun. 2023, 59, 2791–2794. [Google Scholar] [CrossRef]
- Gabriel, C.; Grenho, L.; Cerqueira, F.; Medeiros, R.; Dias, A.M.; Ribeiro, A.I.; Proença, M.F.; Fernandes, M.H.; Sousa, J.C.; Monteiro, F.J.; et al. Inhibitory Effect of 5-Aminoimidazole-4-Carbohydrazonamides Derivatives Against Candida spp. Biofilm on Nanohydroxyapatite Substrate. Mycopathologia 2019, 184, 775–786. [Google Scholar] [CrossRef]
- Cerqueira, F.; Maia, M.; Gabriel, C.; Medeiros, R.; Cravo, S.; Ribeiro, A.I.; Dantas, D.; Dias, A.M.; Saraiva, L.; Raimundo, L.; et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics 2021, 10, 183. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Alves, M.J.; Booth, B.L.; Proenç, M.F.J.R.P. Synthesis of 5-amino-4-(cyanoformimidoyl)-1H-imidazole: A reactive intermediate for the synthesis of 6-carbamoyl-1,2-dihydropurines and 6-carbamoylpurines. J. Chem. Soc. Perkin Trans. 1990, 1705–1712. [Google Scholar] [CrossRef]
- Wan, Y.; Guo, Z.; Jiang, X.; Fang, K.; Lu, X.; Zhang, Y.; Gu, N. Quasi-spherical silver nanoparticles: Aqueous synthesis and size control by the seed-mediated Lee–Meisel method. J. Colloid Interface Sci. 2013, 394, 263–268. [Google Scholar] [CrossRef]
- Padrão, J.; Pinheiro, I.; Silva, C.; Ribeiro, A.; Bouça, V.; Melro, L.; Fernandes, R.D.V.; Ribeiro, A.I.; Felgueiras, H.; Zille, A. Distinct Antimicrobial Analysis to Evaluate Multi-Component Wound Dressing Performance. J. Biomim. Biomater. Biomed. Eng. 2022, 57, 9–16. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; de Paula e Silva, A.C.A.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl azo dyes as molecular photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A.; Almahri, A.; Alzahrani, E.; Pashameah, R.A.; Abu-Hassan, A.A.; El Hamd, M.A. Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method. Chemosensors 2022, 10, 358. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
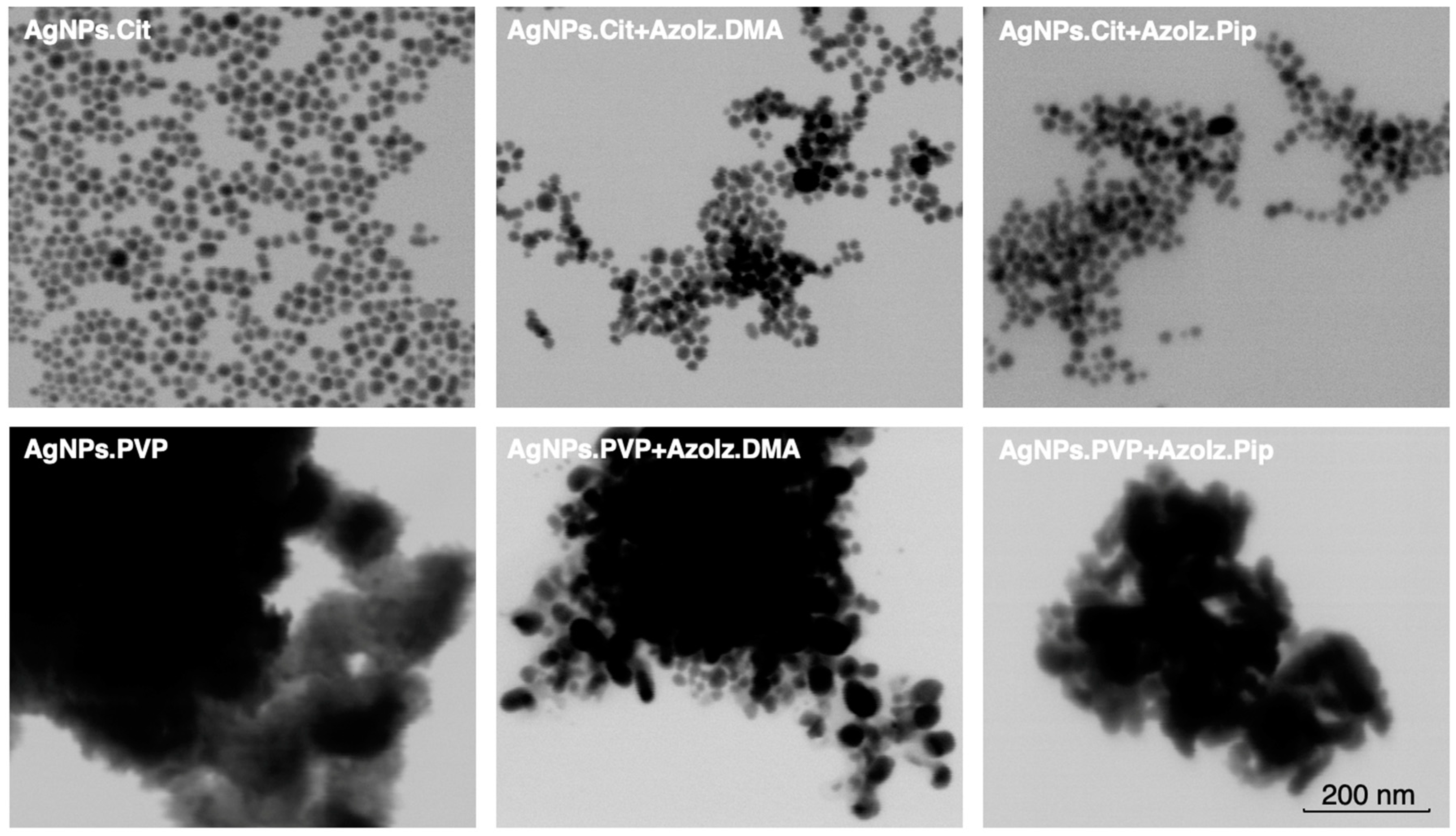
| AgNPs.Cit | AgNPs.PVP | ||||||
|---|---|---|---|---|---|---|---|
| [μg·mL−1] | Size (nm) | PdI | Zeta Potential (mV) | Size (nm) | PdI | Zeta Potential (mV) | |
| AgNPs | 25.0 | 34.89 ± 1.3 | 0.3 | −30.5 ± 0.1 | 273.2 ± 1.6 | 0.4 | −22.6 ± 0.1 |
| Amz | 16.0 | 1104.0 ± 94.6 | 0.4 | 0.8 ± 0.2 | 258.7 ± 23.8 | 0.3 | −8.6 ± 0.3 |
| 32.0 | 713.7 ± 187.6 | 0.3 | −13.3 ± 0.6 | 369.0 ± 53.3 | 0.5 | −7.5 ± 0.2 | |
| AzoIz | 16.0 | 426.6 ± 35.9 | 0.5 | −22.8 ± 0.4 | 252.6 ± 16.3 | 0.4 | −19.4 ± 0.3 |
| 32.0 | 748.2 ± 1.1 | 0.3 | −12.7 ± 0.1 | 345.8 ± 65.8 | 0.5 | −18.0 ± 0.6 | |
| AzoIz.DMA | 16.0 | 40.85 ± 3.5 | 0.3 | −27.2 ± 0.5 | 438.0 ± 54.9 | 0.5 | −13.1 ± 0.5 |
| 32.0 | 39.31 ± 1.2 | 0.3 | −25.3 ± 0.7 | 527.6 ± 4.7 | 0.5 | −3.4 ± 0.2 | |
| AzoIz.Pip | 16.0 | 21,740.0 ± 234.6 | 0.5 | −7.5 ± 0.4 | 16,590.0 ± 174.3 | 0.7 | −1.7 ± 0.6 |
| 32.0 | 795.6 ± 112.9 | 0.3 | 2.3 ± 0.1 | 496.6 ± 6.7 | 0.5 | 0.8 ± 0.4 | |
| MIC [μg·mL−1] | ||||||
|---|---|---|---|---|---|---|
| Amz+ | AzoIz+ | AzoIz.DMA+ | AzoIz.Pip+ | AgNPs.Cit | AgNPs.PVP | |
| S. aureus | 128.0 | 128.0 | 64.0 | 64.0 | 100.0 [44] | 4000.0 [45] |
| E. coli | 128.0 | 128.0 | 128.0 | 64.0 | 100.0 [44] | 4000.0 [45] |
| C. albicans | 8.0 | 4.0 | 8.0 | 4.0 | 12.5 | 12.5 |
| C. krusei | 16.0 | 8.0 | 8.0 | 8.0 | 6.3 | 25.0 |
| Amz | |||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | C. krusei | ||||
| AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP |
| 16.0 + 1.6 16.0 + 3.1 16.0 + 6.3 16.0 + 12.5 16.0 + 25.0 8.0 + 12.5 8.0 + 25.0 4.0 + 25.0 2.0 + 25.0 | No synergism | 16.0 + 1.6 16.0 + 3.1 8.0 + 25 4.0 + 3.1 2.0 + 25.0 | 16.0 + 3.1 8.0 + 6.3 8.0 + 12.5 4.0 + 12.5 4.0 + 25.0 | 2.0 + 1.6 2.0 + 3.1 | 2.0 + 1.6 2.0 + 3.1 | No synergism | No synergism |
| AzoIz | |||||||
| S. aureus | E. coli | C. albicans | C. krusei | ||||
| AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP |
| No synergism | No synergism | 16.0 + 6.3 4.0 + 12.5 2.0 + 25.0 | No synergism | No synergism | No synergism | No synergism | No synergism |
| AzoIz.DMA | |||||||
| S. aureus | E. coli | C. albicans | C. krusei | ||||
| AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP |
| 16.0 + 25.0 | 16.0 + 25.0 | No synergism | No synergism | 2.0 + 1.6 2.0 + 3.1 | 2.0 + 1.6 2.0 + 3.1 | No synergism | No synergism |
| AzoIz.Pip | |||||||
| S. aureus | E. coli | C. albicans | C. krusei | ||||
| AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP | AgNPs.Cit | AgNPs.PVP |
| 16.0 + 25.0 | 16.0 + 25.0 | No synergism | No synergism | No synergism | No synergism | No synergism | No synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.I.; Vieira, B.; Dantas, D.; Silva, B.; Pinto, E.; Cerqueira, F.; Silva, R.; Remião, F.; Padrão, J.; Dias, A.M.; et al. Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles. Pharmaceutics 2023, 15, 926. https://doi.org/10.3390/pharmaceutics15030926
Ribeiro AI, Vieira B, Dantas D, Silva B, Pinto E, Cerqueira F, Silva R, Remião F, Padrão J, Dias AM, et al. Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles. Pharmaceutics. 2023; 15(3):926. https://doi.org/10.3390/pharmaceutics15030926
Chicago/Turabian StyleRibeiro, Ana Isabel, Bárbara Vieira, Daniela Dantas, Bárbara Silva, Eugénia Pinto, Fátima Cerqueira, Renata Silva, Fernando Remião, Jorge Padrão, Alice Maria Dias, and et al. 2023. "Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles" Pharmaceutics 15, no. 3: 926. https://doi.org/10.3390/pharmaceutics15030926
APA StyleRibeiro, A. I., Vieira, B., Dantas, D., Silva, B., Pinto, E., Cerqueira, F., Silva, R., Remião, F., Padrão, J., Dias, A. M., & Zille, A. (2023). Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles. Pharmaceutics, 15(3), 926. https://doi.org/10.3390/pharmaceutics15030926