Electrically Triggered Quercetin Release from Polycaprolactone/Bismuth Ferrite Microfibrous Scaffold for Skeletal Muscle Tissue
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of BFO Nanoparticles
2.3. Preparation of the Solutions
2.4. Electrospinning
2.5. Characterisation of the Microfibrous Scaffolds
3. Results and Discussions
3.1. Morphological Examinations
3.2. Characterisation of the Microfibrous Scaffolds
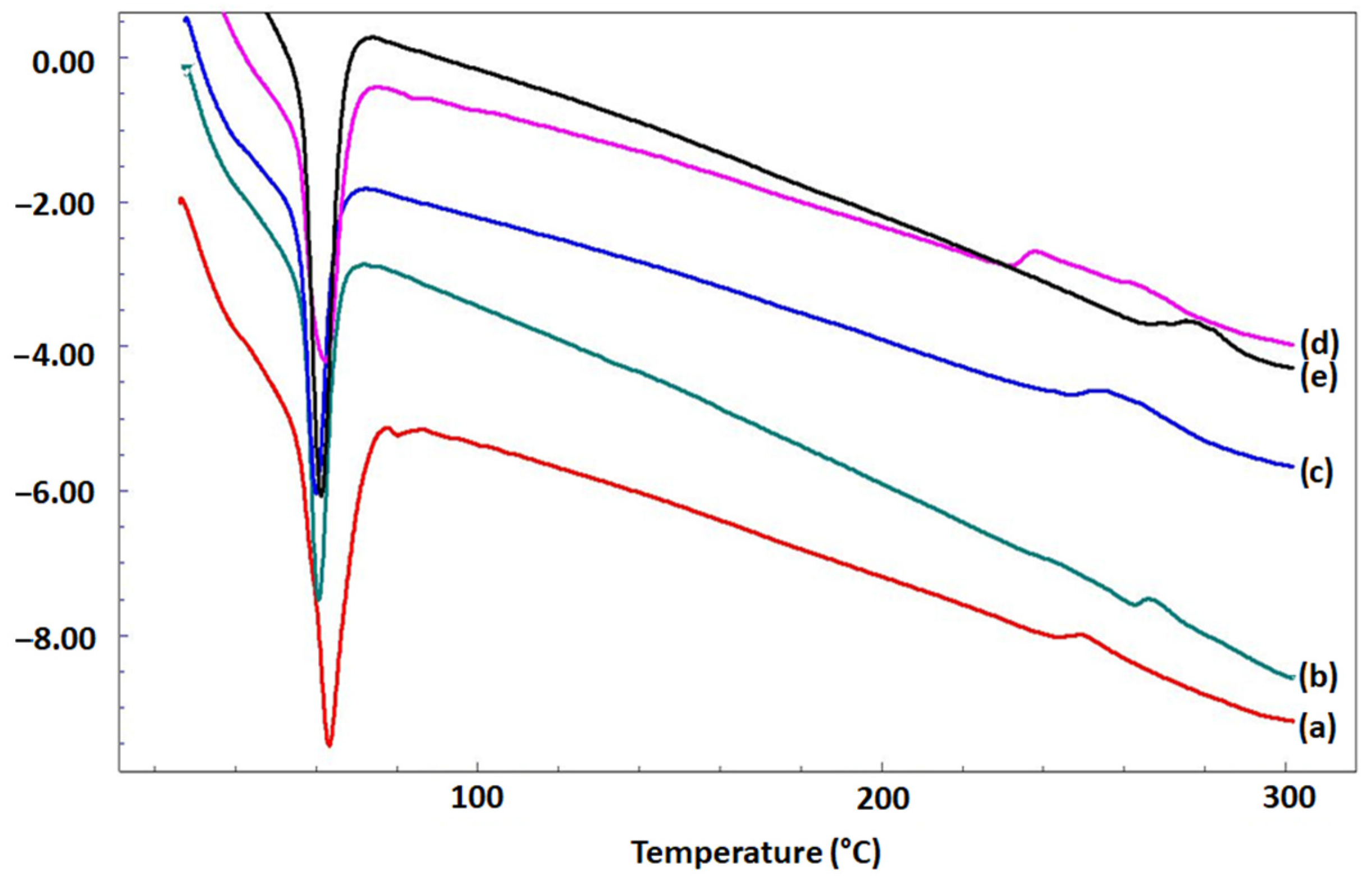
3.3. Thermal Properties of Microfibrous Scaffolds
3.4. The Effect of Q on Mechanical Properties
3.5. In Vitro Drug Release
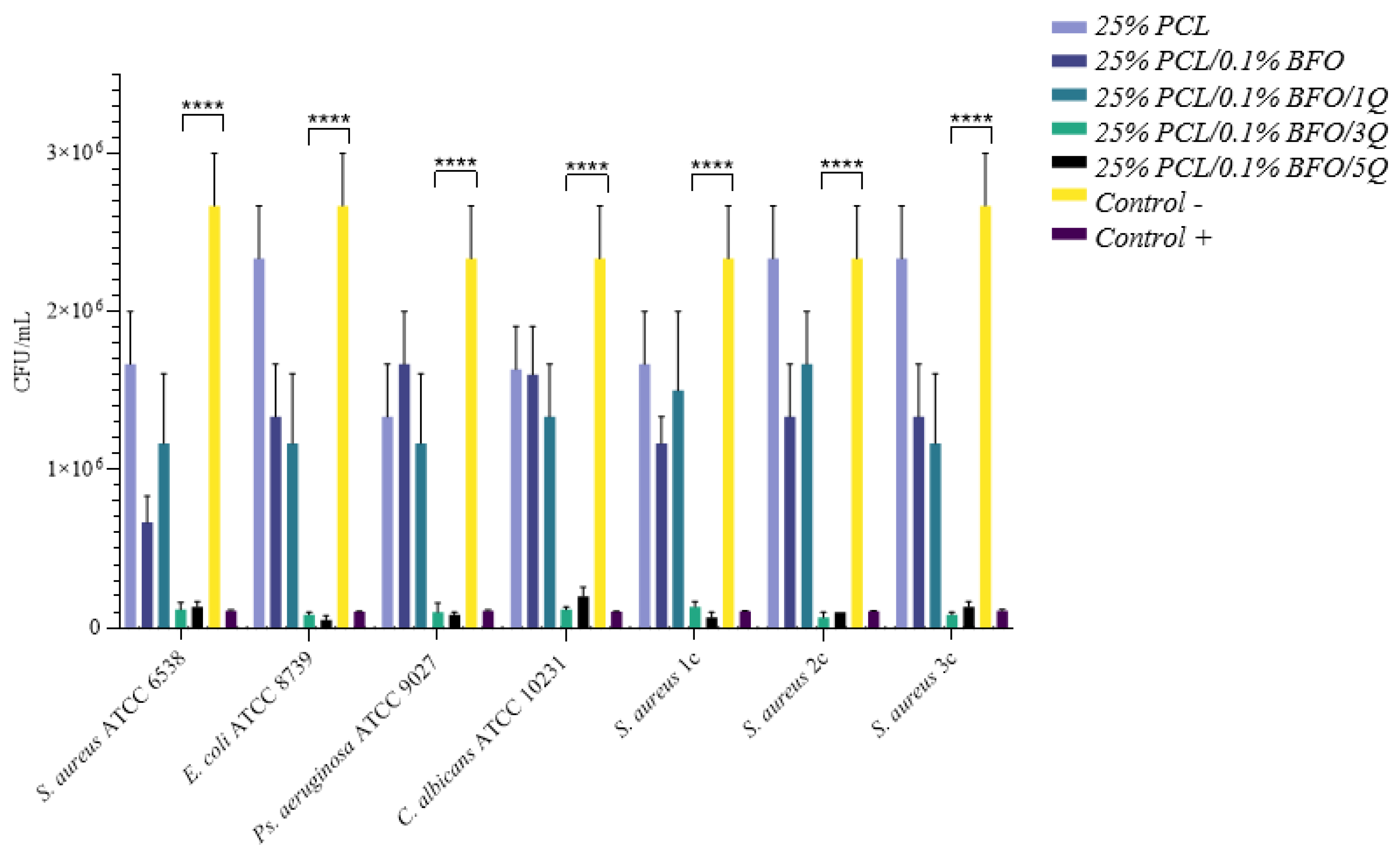
3.6. Antibacterial Activity
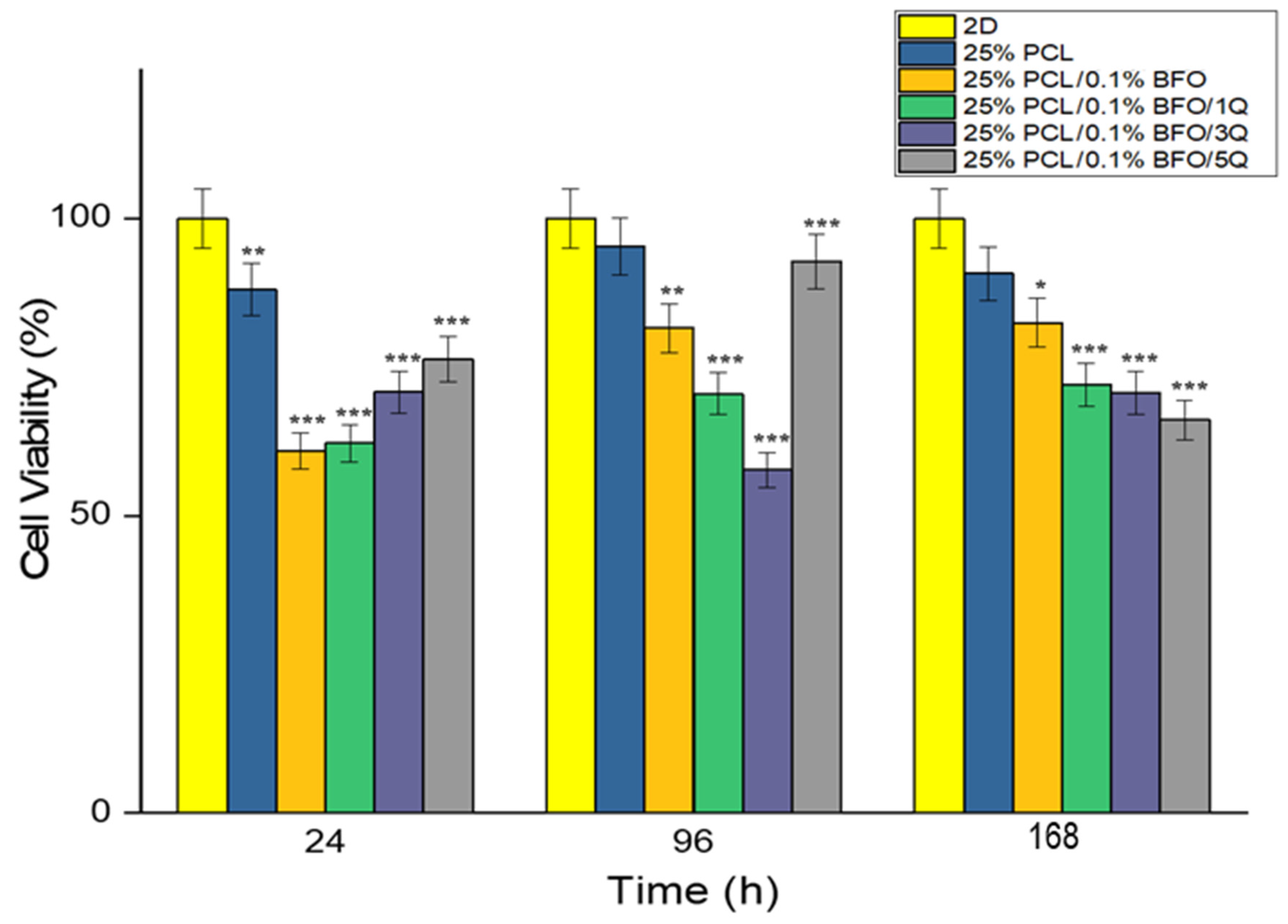
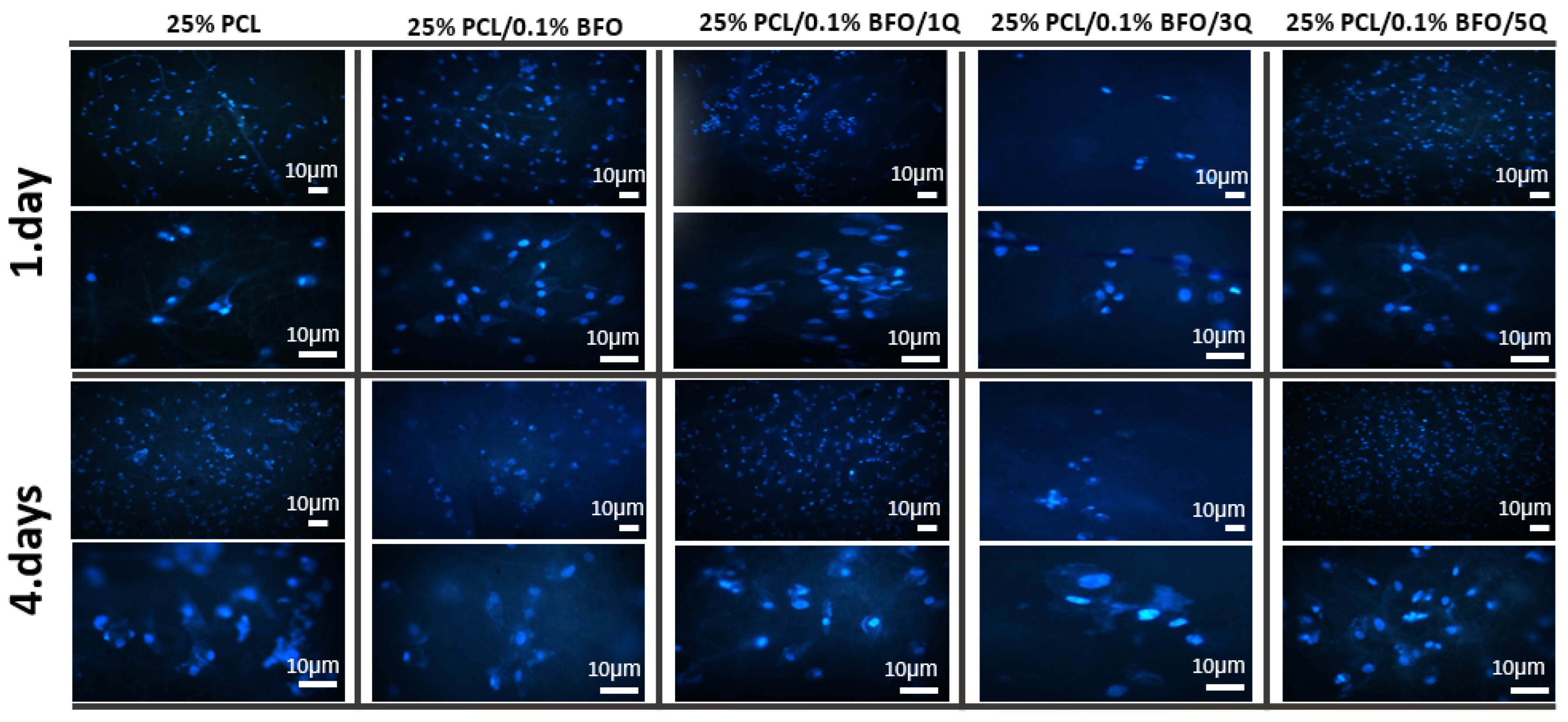
3.7. Biocompatibility Properties of the Microfibrous Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H.; Lehto, M.; Järvinen, M. Healing of skeletal muscle injury: An ultrastructural and immunohistochemical study. Med. Sci. Sports Exerc. 1991, 23, 801–810. [Google Scholar] [CrossRef]
- Campion, D.R. The muscle satellite cell: A review. Int. Rev. Cytol. 1984, 87, 225–251. [Google Scholar] [PubMed]
- Papy-Garcia, D.; Barbosa, I.; Duchesnay, A.; Saadi, S.; Caruelle, J.P.; Barritault, D.; Martelly, I. Glycosaminoglycan mimetics (RGTA) modulate adult skeletal muscle satellite cell proliferation in vitro. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 62, 46–55. [Google Scholar]
- Wozniak, A.C.; Pilipowicz, O.; Yablonka-Reuveni, Z.; Greenway, S.; Craven, S.; Scott, E.; Anderson, J.E. C-Met Expression and Mechanical Activation of Satellite Cells on Cultured Muscle Fibers. J. Histochem. Cytochem. 2003, 51, 1437–1445. [Google Scholar] [CrossRef]
- Saxena, A.K.; Marler, J.; Benvenuto, M.; Willital, G.H.; Vacanti, J.P. Skeletal Muscle Tissue Engineering Using Isolated Myoblasts on Synthetic Biodegradable Polymers: Preliminary Studies. Tissue Eng. 1999, 5, 525–531. [Google Scholar] [CrossRef]
- Payumo, F.C.; Kim, H.D.; Sherling, M.A.; Smith, L.P.; Powell, C.; Wang, X.; Keeping, H.S.; Valentini, R.F.; Vandenburgh, H.H. Tissue engineering skeletal muscle for orthopaedic applications. Clin. Orthop. Relat. Res. 2002, 403, S228–S242. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Smiley, B.L.; Mills, J.; VanDenburgh, H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Physiol. 2002, 283, C1557–C1565. [Google Scholar] [CrossRef]
- Negroni, E.; Butler-Browne, G.; Mouly, V. Myogenic stem cells: Regeneration and cell therapy in human skeletal muscle. Pathol. Biol. 2006, 54, 100–108. [Google Scholar] [CrossRef]
- Sampaolesi, M.; Torrente, Y.; Innocenzi, A.; Tonlorenzi, R.; D’Antona, G.; Pellegrino, M.A.; Barresi, R.; Bresolin, N.; De Angelis, M.G.C.; Campbell, K.P.; et al. Cell Therapy of α-Sarcoglycan Null Dystrophic Mice Through Intra-Arterial Delivery of Mesoangioblasts. Science 2003, 301, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly (epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Dennis, R.G.; Larkin, L.M.; Baar, K. Rapid formation of functional muscle in vitro using fibrin gels. J. Appl. Physiol. 2005, 98, 706–713. [Google Scholar] [CrossRef]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef]
- Bian, W.; Bursac, N. Tissue engineering of functional skeletal muscle: Challenges and recent advances. IEEE Eng. Med. Biol. Mag. 2008, 27, 109–113. [Google Scholar] [PubMed]
- Mueller, C.; Trujillo-Miranda, M.; Maier, M.; Heath, D.E.; O’Connor, A.J.; Salehi, S. Effects of External Stimulators on Engineered Skeletal Muscle Tissue Maturation. Adv. Mater. Interfaces 2020, 8, 2001167. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Boonen, K.J.M.; Rosaria-Chak, K.Y.; van der Schaft, D.W.J.; Post, M.J.; Baaijens, F.P.T. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2010, 5, 529–539. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ostrovidov, S.; Salehi, S.; Kim, S.B.; Bae, H.; Khademhosseini, A. Enhanced skeletal muscle formation on microfluidic spun gelatin methacryloyl (GelMA) fibres using surface patterning and agrin treatment. J. Tissue Eng. Regen. Med. 2018, 12, 2151–2163. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, H.Q.; Shi, L.Y.; Ng, S.Y.; Stanton, L.W.; Chew, S.Y. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012, 8, 1290–1302. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef]
- Montero, R.B.; Vial, X.; Nguyen, D.T.; Farhand, S.; Reardon, M.; Pham, S.M.; Tsechpenakis, G.; Andreopoulos, F.M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012, 8, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.J.; Patel, S.; Wang, A.; Chu, J.; Li, S. In Vitro Regulation of Neural Differentiation and Axon Growth by Growth Factors and Bioactive Nanofibers. Tissue Eng. Part A 2010, 16, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Kathryn, F.A.C.; John, G.H. Gene delivery with organic electronic biomaterials. Curr. Pharm. Des. 2017, 23, 3614–3625. [Google Scholar]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Mushtaq, F.; Torlakcik, H.; Vallmajo-Martin, Q.; Siringil, E.C.; Zhang, J.; Röhrig, C.; Shen, Y.; Yu, Y.; Chen, X.-Z.; Müller, R.; et al. Magnetoelectric 3D scaffolds for enhanced bone cell proliferation. Appl. Mater. Today 2019, 16, 290–300. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning Nanofibers as Uniaxially Aligned Arrays and Layer-by-Layer Stacked Films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; Del Romero, A.; Peláez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-derived mesenchymal stem cells: A promising tool in the treatment of musculoskeletal diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef]
- Alvarez-Perez, M.A.; Guarino, V.; Cirillo, V.; Ambrosio, L. Influence of Gelatin Cues in PCL Electrospun Membranes on Nerve Outgrowth. Biomacromolecules 2010, 11, 2238–2246. [Google Scholar] [CrossRef]
- Spaldin, N.A. Multiferroics beyond electric-field control of magnetism. Proc. R. Soc. A 2020, 476, 20190542. [Google Scholar] [CrossRef]
- Hatzistergos, K.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef]
- Ulag, S.; Kalkandelen, C.; Bedir, T.; Erdemir, G.; Kuruca, S.E.; Dumludag, F.; Ustundag, C.B.; Rayaman, E.; Ekren, N.; Kilic, B.; et al. Fabrication of three-dimensional PCL/BiFeO3 scaffolds for biomedical applications. Mater. Sci. Eng. B 2020, 261, 114660. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.M.; et al. Electrically triggered drug delivery from novel electrospun poly (lactic acid)/graphene oxide/quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing: A CLSI Supplement for Global Application, 29th ed.; (CLSI Document M 100-S29); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Ege, Z.R.; Akan, A.; Oktar, F.N.; Lin, C.C.; Kuruca, D.S.; Karademir, B.; Sahin, Y.M.; Erdemir, G.; Gunduz, O. Indocyanine green based fluorescent polymeric nanoprobes for in vitro imaging. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, M.N.; Triyono, D. Structural properties of bismuth ferrite synthesized by sol-gel method with variation of calcination temperature. J. Physics Conf. Ser. 2019, 1245, 012087. [Google Scholar] [CrossRef]
- Aliakbari, A.; Seifi, M.; Mirzaee, S.; Hekmatara, H. Influence of different synthesis conditions on properties of oleic acid-coated-FeO nanoparticles. Mater. Sci.-Pol. 2015, 33, 100–106. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, J.; Yu, S.; Che, L.; Meng, Z. Hydrothermal synthesis of perovskite bismuth ferrite crystallites. J. Cryst. Growth 2006, 291, 135–139. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Chobot, A.N.; Mantytskaya, O.S.; Tereshko, N.V. Magnetic properties of Bi (Fe1−xMx)O3(M = Mn, Ti). Inorg. Mater. 2010, 46, 424–428. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.F.; Trotta, A.; Fossey, S.; Nagarajan, S.; Nagarajan, R.; Samuelson, L.A.; Kumar, J. Enzymatic Synthesis and Characterization of PolyQuercetin. J. Macromol. Sci. Part A 2010, 47, 1191–1196. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Tsivintzelis, I. On the Thermodynamic Thermal Properties of Quercetin and Similar Pharmaceuticals. Molecules 2022, 27, 6630. [Google Scholar] [CrossRef] [PubMed]
- Ayran, M.; Dirican, A.Y.; Saatcioglu, E.; Ulag, S.; Sahin, A.; Aksu, B.; Croitoru, A.-M.; Ficai, D.; Gunduz, O.; Ficai, A. 3D-Printed PCL Scaffolds Combined with Juglone for Skin Tissue Engineering. Bioengineering 2022, 9, 427. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014, 20, 255–264. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Ramalingam, R.; Khalith, S.M.; Musthafa, S.A.; Dowlath, M.J.H.; Munuswamy-Ramanujam, G.; Arunachalam, K.D. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dressing. Mater. Lett. 2021, 294, 129787. [Google Scholar] [CrossRef]
- Baghersad, S.; Hajir Bahrami, S.; Mohammadi, M.R.; Mojtahedi, M.R.M.; Milan, P.B. Development of biodegradable electrospun gelatin/aloe-vera/poly(ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Griaznova, O.Y.; Shevchenko, K.G.; Ivanov, A.V.; Baidyuk, E.V.; Serejnikova, N.B.; Volovetskiy, A.B.; Deyev, S.M.; Zvyagin, A.V. Flash drug release from nanoparticles accumulated in the targeted blood vessels facilitates the tumour treatment. Nat. Commun. 2022, 13, 6910. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hao, R.; Qin, J.; Song, J.; Chen, X.; Rao, F.; Zhai, J.; Zhao, Y.; Zhang, L.; Xue, J. Electrospun Fibers Control Drug Delivery for Tissue Regeneration and Cancer Therapy. Adv. Fiber Mater. 2022, 4, 1375–1413. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Cezar, C.A.; Mooney, D.J. Biomaterial-based delivery for skeletal muscle repair. Adv. Drug Deliv. Rev. 2014, 84, 188–197. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Shansky, J.; Wang, Z.; Mooney, D.; Vandenburgh, H. Minimally Invasive Approach to the Repair of Injured Skeletal Muscle With a Shape-memory Scaffold. Mol. Ther. 2014, 22, 1441–1449. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, W.; Hu, L.; Yi, X.; Tang, F. Application of Hydrogels as Sustained-Release Drug Carriers in Bone Defect Repair. Polymers 2022, 14, 4906. [Google Scholar] [CrossRef]
- Preethi, A.M.; Bellare, J.R. Concomitant Effect of Quercetin- and Magnesium-Doped Calcium Silicate on the Osteogenic and Antibacterial Activity of Scaffolds for Bone Regeneration. Antibiotics 2021, 10, 1170. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Meng, W.; Yuan, J.; Moon, S.; Jeong, Y.; Kang, I.-K. In Vitro Assessment of Antibacterial Activity and Cytocompatibility of Quercetin-Containing PLGA Nanofibrous Scaffolds for Tissue Engineering. J. Nanomater. 2012, 2012, 202608. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shayeh, J.S. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S.; Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K.; Ma, Z.; He, W.; Yong, T.; Kotaki, M.; et al. Biocompatible Nanofiber Matrices for the Engineering of a Dermal Substitute for Skin Regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.-I.; et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J. Biomed. Mater. Res. Part A 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, G.; Bonde, G.V.; Thokala, S.; Mittal, P.; Khan, G.; Singh, J.; Pandey, V.K.; Mishra, B. Ciprofloxacin HCl and quercetin functionalized electrospun nanofiber membrane: Fabrication and its evaluation in full thickness wound healing. Artif. Cells Nanomed. Biotechnol. 2019, 47, 228–240. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Ayaz, N.; Karthick, A.S.; Senthil, R.; Sastry, T.P. Quercetin impregnated chitosan–fibrin composite scaffolds as potential wound dressing materials—Fabrication, characterization and in vivo analysis. Eur. J. Pharm. Sci. 2017, 97, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]








| Fibers | Tensile Strength (MPa) | Strain at Break (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| 25% PCL | 7.67 ± 0.52 | 25.42 ± 8.76 | 35.19 ± 4.24 |
| 25% PCL/0.1% BFO | 7.09 ± 0.42 | 27.74 ± 7.86 | 28.45 ± 5.53 |
| 25% PCL/0.1% BFO/1Q | 2.11 ± 0.51 | 8.57 ± 1.65 | 25.48 ± 7.04 |
| 25% PCL/0.1% BFO/3Q | 4.24 ± 0.84 | 17.92 ± 3.90 | 25.43 ± 7.06 |
| 25% PCL/0.1% BFO/5Q | 4.52 ± 0.64 | 20.80 ± 3.49 | 23.79 ± 5.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayran, M.; Karabulut, H.; Deniz, K.I.; Akcanli, G.C.; Ulag, S.; Croitoru, A.-M.; Tihăuan, B.-M.; Sahin, A.; Ficai, D.; Gunduz, O.; et al. Electrically Triggered Quercetin Release from Polycaprolactone/Bismuth Ferrite Microfibrous Scaffold for Skeletal Muscle Tissue. Pharmaceutics 2023, 15, 920. https://doi.org/10.3390/pharmaceutics15030920
Ayran M, Karabulut H, Deniz KI, Akcanli GC, Ulag S, Croitoru A-M, Tihăuan B-M, Sahin A, Ficai D, Gunduz O, et al. Electrically Triggered Quercetin Release from Polycaprolactone/Bismuth Ferrite Microfibrous Scaffold for Skeletal Muscle Tissue. Pharmaceutics. 2023; 15(3):920. https://doi.org/10.3390/pharmaceutics15030920
Chicago/Turabian StyleAyran, Musa, Hatice Karabulut, Kudret Irem Deniz, Gamze Ceren Akcanli, Songul Ulag, Alexa-Maria Croitoru, Bianca-Maria Tihăuan, Ali Sahin, Denisa Ficai, Oguzhan Gunduz, and et al. 2023. "Electrically Triggered Quercetin Release from Polycaprolactone/Bismuth Ferrite Microfibrous Scaffold for Skeletal Muscle Tissue" Pharmaceutics 15, no. 3: 920. https://doi.org/10.3390/pharmaceutics15030920
APA StyleAyran, M., Karabulut, H., Deniz, K. I., Akcanli, G. C., Ulag, S., Croitoru, A.-M., Tihăuan, B.-M., Sahin, A., Ficai, D., Gunduz, O., & Ficai, A. (2023). Electrically Triggered Quercetin Release from Polycaprolactone/Bismuth Ferrite Microfibrous Scaffold for Skeletal Muscle Tissue. Pharmaceutics, 15(3), 920. https://doi.org/10.3390/pharmaceutics15030920










