Identification of Blood Transport Proteins to Carry Temoporfin: A Domino Approach from Virtual Screening to Synthesis and In Vitro PDT Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Details
2.1.1. Blood Transport Proteins Structural Database
2.1.2. Docking
2.1.3. MD Simulations
2.1.4. Molecular Mechanics-Generalized Born Surface Area (MM-GBSA) Analysis
2.2. Synthesis and Characterization of the mTHPC@apoMb Complex
2.2.1. Materials
2.2.2. Synthesis of the Reconstituted Myoglobin-Temoporfin Adduct
2.2.3. Characterization of mTHPC@apoMb
2.2.4. Detection of Reactive Oxygen Species
2.3. Cytotoxicity and Phototoxicity of mTHPC@apoMb Complex in CAL27 Cells
2.3.1. Cell Line
2.3.2. Cell Viability
3. Results and Discussion
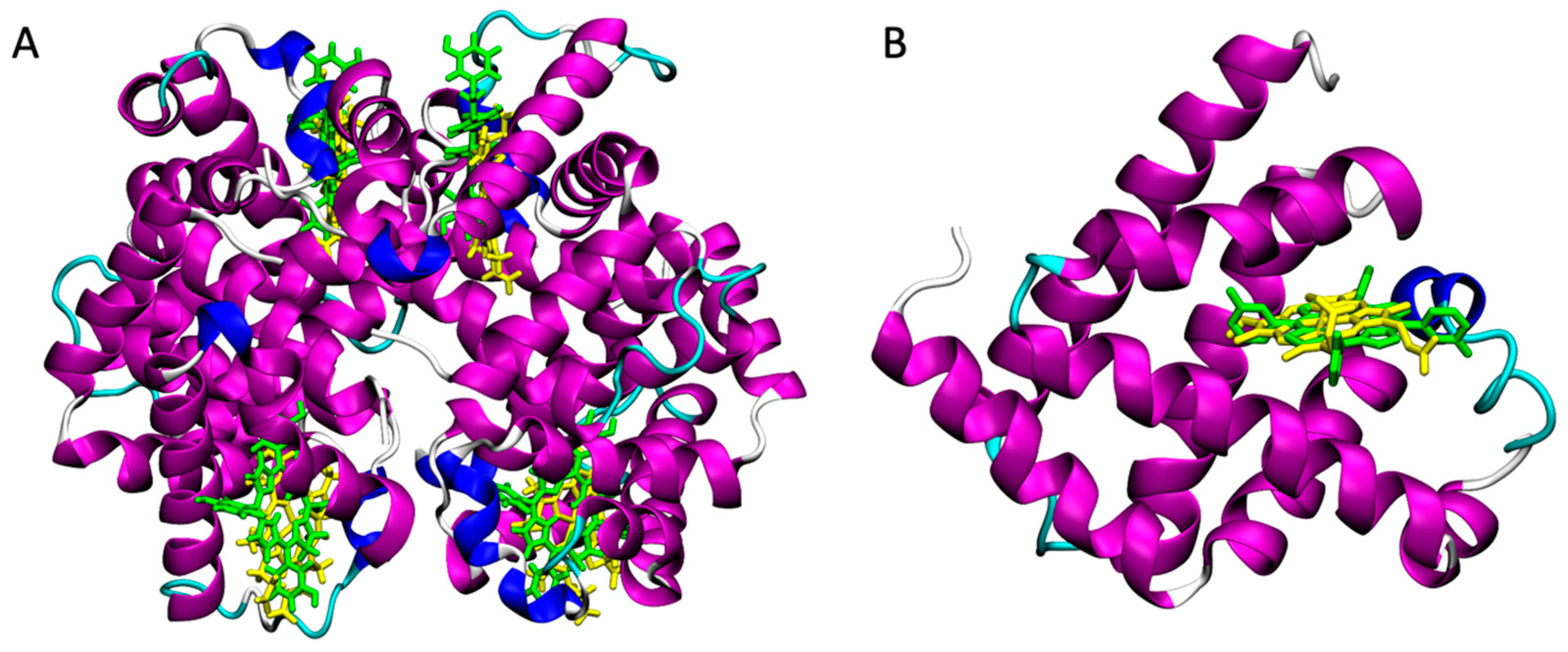
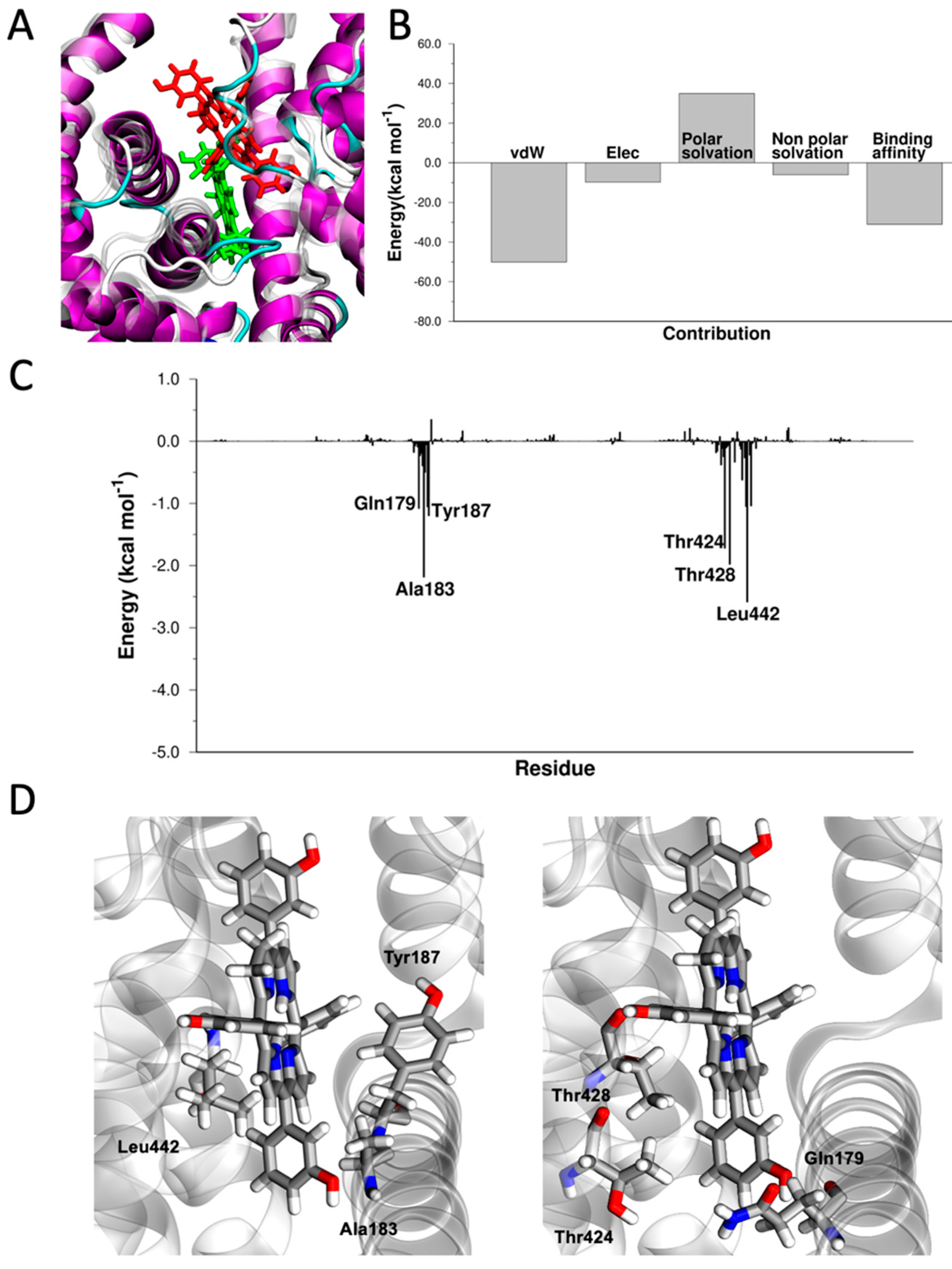
3.1. Identification of Blood Proteins as Carriers for mTHPC by Virtual Screening
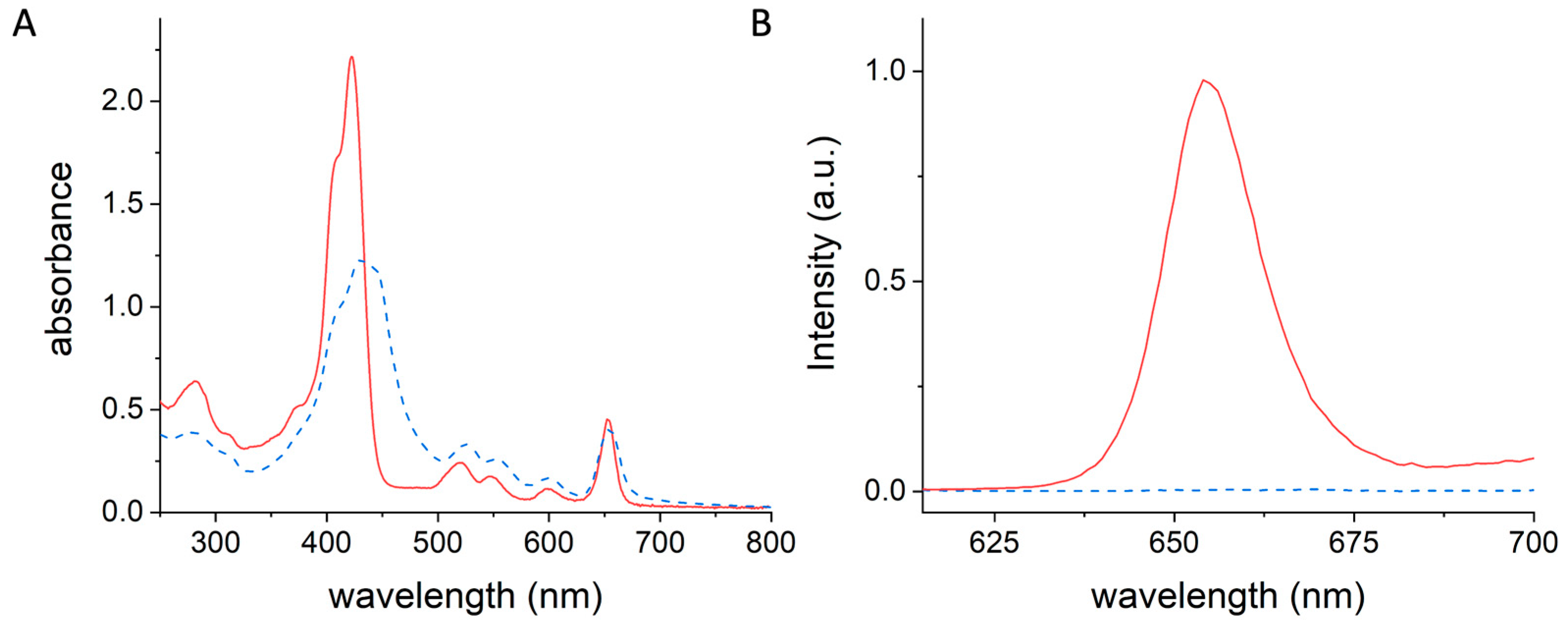
3.2. Synthesis and Characterization of the mTHPC@apoMb Complex
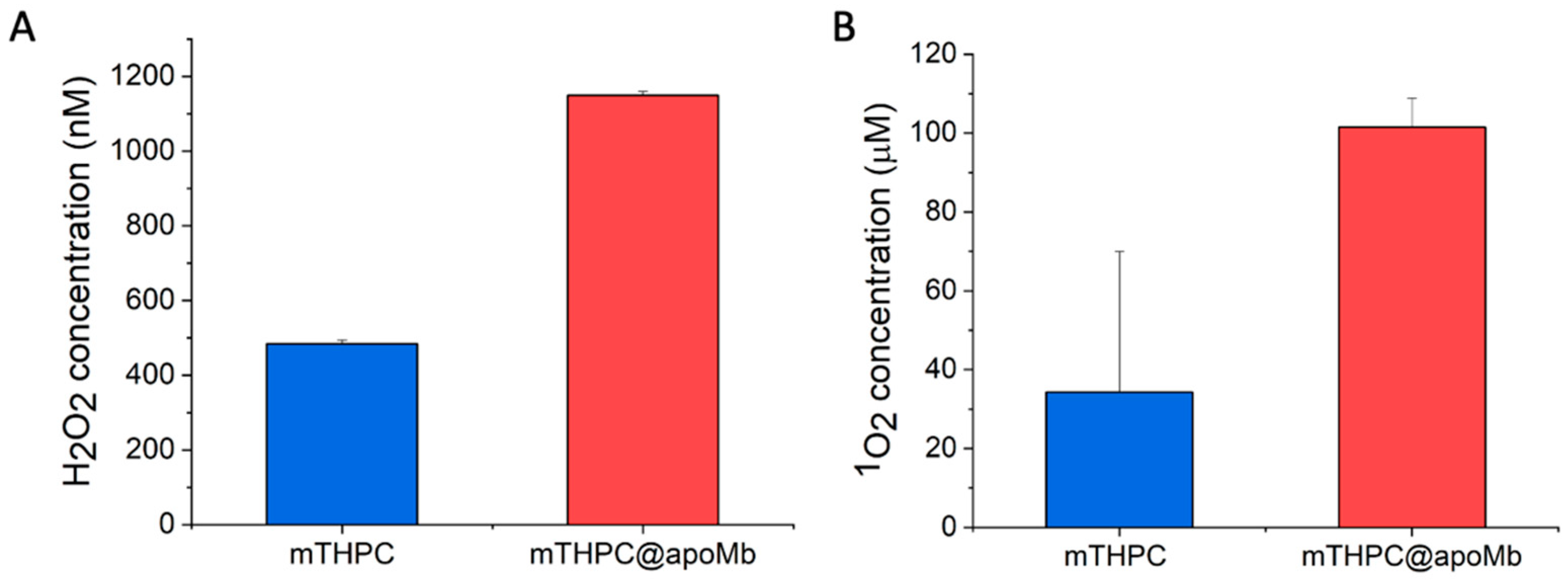
3.3. Evaluation of the PDT Performances of the mTHPC@apoMb Complex
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Ulfo, L.; Costantini, P.E.; di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Kundu, B.K.; Han, G.; Sun, Y. Derivatized Benzothiazoles as Two-Photon-Absorbing Organic Photosensitizers Active under Near Infrared Light Irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-Hydroxyphenyl)Chlorin)—A Second-Generation Photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. MTHPC—A Drug on Its Way from Second to Third Generation Photosensitizer? Photodiagn. Photodyn. 2012, 9, 170–179. [Google Scholar] [CrossRef]
- Wiehe, A.; Senge, M.O. The Photosensitizer Temoporfin (MTHPC)—Chemical, Pre-Clinical and Clinical Developments in the Last Decade. Photochem. Photobiol. 2022; early view. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Foscan. Available online: https://www.ema.europa.eu/en/documents/product-information/foscan-epar-product-information_en.pdf (accessed on 6 March 2023).
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.-P.; Bezdetnaya, L. Current State of the Nanoscale Delivery Systems for Temoporfin-Based Photodynamic Therapy: Advanced Delivery Strategies. J. Control. Release 2019, 304, 268–287. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagn. Photodyn. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Chen, X.; Dobrovolskaia, M.A.; Lammers, T. Cancer Nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef]
- Calvaresi, M. The Route towards Nanoparticle Shape Metrology. Nat. Nanotechnol. 2020, 15, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Chen, H. Protein-Based Nanoplatforms for Tumor Imaging and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1616. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Miao, D.; Zhou, M.; Wang, L.; Zhou, H.; Su, G. Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Front. Pharm. 2018, 9, 421. [Google Scholar] [CrossRef]
- Zhang, N.; Mei, K.; Guan, P.; Hu, X.; Zhao, Y. Protein-Based Artificial Nanosystems in Cancer Therapy. Small 2020, 16, 1907256. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- di Giosia, M.; Bomans, P.H.H.; Bottoni, A.; Cantelli, A.; Falini, G.; Franchi, P.; Guarracino, G.; Friedrich, H.; Lucarini, M.; Paolucci, F.; et al. Proteins as Supramolecular Hosts for C60: A True Solution of C60 in Water. Nanoscale 2018, 10, 9908–9916. [Google Scholar] [CrossRef]
- di Giosia, M.; Zerbetto, F.; Calvaresi, M. Incorporation of Molecular Nanoparticles Inside Proteins: The Trojan Horse Approach in Theranostics. Acc. Mater. Res. 2021, 2, 594–605. [Google Scholar] [CrossRef]
- Calvaresi, M.; Arnesano, F.; Bonacchi, S.; Bottoni, A.; Calò, V.; Conte, S.; Falini, G.; Fermani, S.; Losacco, M.; Montalti, M.; et al. C60@Lysozyme: Direct Observation by Nuclear Magnetic Resonance of a 1:1 Fullerene Protein Adduct. ACS Nano 2014, 8, 1871–1877. [Google Scholar] [CrossRef]
- Pezzuoli, D.; Cozzolino, M.; Montali, C.; Brancaleon, L.; Bianchini, P.; Zantedeschi, M.; Bonardi, S.; Viappiani, C.; Abbruzzetti, S. Serum Albumins Are Efficient Delivery Systems for the Photosensitizer Hypericin in Photosensitization-Based Treatments against Staphylococcus Aureus. Food Control 2018, 94, 254–262. [Google Scholar] [CrossRef]
- Hu, J.; Hernandez Soraiz, E.; Johnson, C.N.; Demeler, B.; Brancaleon, L. Novel Combinations of Experimental and Computational Analysis Tested on the Binding of Metalloprotoporphyrins to Albumin. Int. J. Biol. Macromol. 2019, 134, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Allen, R.; Rozinek, S.; Brancaleon, L. Experimental and Computational Characterization of Photosensitized Conformational Effects Mediated by Protoporphyrin Ligands on Human Serum Albumin. Photochem. Photobiol. Sci. 2017, 16, 694–710. [Google Scholar] [CrossRef]
- Cantelli, A.; Malferrari, M.; Mattioli, E.J.; Marconi, A.; Mirra, G.; Soldà, A.; Marforio, T.D.; Zerbetto, F.; Rapino, S.; di Giosia, M.; et al. Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation. Nanomaterials 2022, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Delcanale, P.; Montali, C.; Tognolini, M.; Giorgio, C.; Corrado, M.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. Enhanced Photosensitizing Properties of Protein Bound Curcumin. Life Sci. 2019, 233, 116710. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jeong, K.; Lee, Y.; Guo, T.; Lee, D.; Park, J.; Kwon, N.; Na, J.H.; Hong, S.K.; Cha, S.S.; et al. Water-Soluble Phthalocyanines Selectively Bind to Albumin Dimers: A Green Approach toward Enhancing Tumor-Targeted Photodynamic Therapy. Theranostics 2019, 9, 6412–6423. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, C.; Lyu, M.; Lyu, S.; Hu, L.; Xiao, E.; Xu, P. Novel Albumin-Binding Photodynamic Agent EB-Ppa for Targeted Fluorescent Imaging Guided Tumour Photodynamic Therapy. RSC Adv. 2023, 13, 3534–3540. [Google Scholar] [CrossRef]
- Rapozzi, V.; Moret, F.; Menilli, L.; Guerrini, A.; Tedesco, D.; Naldi, M.; Bartolini, M.; Gani, M.; Zorzet, S.; Columbaro, M.; et al. HSA-Binding Prodrugs-Based Nanoparticles Endowed with Chemo and Photo-Toxicity against Breast Cancer. Cancers 2022, 14, 877. [Google Scholar] [CrossRef]
- Soldà, A.; Cantelli, A.; di Giosia, M.; Montalti, M.; Zerbetto, F.; Rapino, S.; Calvaresi, M. C60@lysozyme: A New Photosensitizing Agent for Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 6608–6615. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, M.; Bottoni, A.; Zerbetto, F. Thermodynamics of Binding between Proteins and Carbon Nanoparticles: The Case of C60@Lysozyme. J. Phys. Chem. C 2015, 119, 28077–28082. [Google Scholar] [CrossRef]
- Rodríguez-Amigo, B.; Delcanale, P.; Rotger, G.; Juárez-Jiménez, J.; Abbruzzetti, S.; Summer, A.; Agut, M.; Luque, F.J.; Nonell, S.; Viappiani, C. The Complex of Hypericin with β-Lactoglobulin Has Antimicrobial Activity with Potential Applications in Dairy Industry. J. Dairy Sci. 2015, 98, 89–94. [Google Scholar] [CrossRef]
- Pröll, S.; Wilhelm, B.; Robert, B.; Scheer, H. Myoglobin with Modified Tetrapyrrole Chromophores: Binding Specificity and Photochemistry. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Delcanale, P.; Pennacchietti, F.; Maestrini, G.; Rodríguez-Amigo, B.; Bianchini, P.; Diaspro, A.; Iagatti, A.; Patrizi, B.; Foggi, P.; Agut, M.; et al. Subdiffraction Localization of a Nanostructured Photosensitizer in Bacterial Cells. Sci. Rep. 2015, 5, 15564. [Google Scholar] [CrossRef] [PubMed]
- Delcanale, P.; Montali, C.; Rodríguez-Amigo, B.; Abbruzzetti, S.; Bruno, S.; Bianchini, P.; Diaspro, A.; Agut, M.; Nonell, S.; Viappiani, C. Zinc-Substituted Myoglobin Is a Naturally Occurring Photo-Antimicrobial Agent with Potential Applications in Food Decontamination. J. Agric. Food Chem. 2016, 64, 8633–8639. [Google Scholar] [CrossRef]
- Cozzolino, M.; Pesce, L.; Pezzuoli, D.; Montali, C.; Brancaleon, L.; Cavanna, L.; Abbruzzetti, S.; Diaspro, A.; Bianchini, P.; Viappiani, C. Apomyoglobin Is an Efficient Carrier for Zinc Phthalocyanine in Photodynamic Therapy of Tumors. Biophys. Chem. 2019, 253, 106228. [Google Scholar] [CrossRef]
- Bianchini, P.; Cozzolino, M.; Oneto, M.; Pesce, L.; Pennacchietti, F.; Tognolini, M.; Giorgio, C.; Nonell, S.; Cavanna, L.; Delcanale, P.; et al. Hypericin–Apomyoglobin: An Enhanced Photosensitizer Complex for the Treatment of Tumor Cells. Biomacromolecules 2019, 20, 2024–2033. [Google Scholar] [CrossRef]
- di Giosia, M.; Genovese, D.; Cantelli, A.; Cingolani, M.; Rampazzo, E.; Strever, G.; Tavoni, M.; Zaccheroni, N.; Calvaresi, M.; Prodi, L. Synthesis and Characterization of a Reconstituted Myoglobin-Chlorin E6 Adduct for Theranostic Applications. J. Porphyr. Phthalocyanines 2020, 24, 887–893. [Google Scholar] [CrossRef]
- Bruno, S.; Margiotta, M.; Cozzolino, M.; Bianchini, P.; Diaspro, A.; Cavanna, L.; Tognolini, M.; Abbruzzetti, S.; Viappiani, C. A Photosensitizing Fusion Protein with Targeting Capabilities. Biomol. Concepts 2022, 13, 175–182. [Google Scholar] [CrossRef]
- Yamada, T.; Morita, Y.; Takada, R.; Funamoto, M.; Okamoto, W.; Kohno, M.; Komatsu, T. Zinc Substituted Myoglobin—Albumin Fusion Protein: A Photosensitizer for Cancer Therapy. Chem. Eur. J. 2023, e20220395. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, J.; Hu, X.; Xia, X.; Dong, Q.; Liu, Z.; Chen, Z.; Tan, W. Zinc-Substituted Hemoglobin with Specific Drug Binding Sites and Fatty Acid Resistance Ability for Enhanced Photodynamic Therapy. Nano Res. 2019, 12, 1880–1887. [Google Scholar] [CrossRef]
- Pires, I.S.; O’boyle, Q.T.; Munoz, C.J.; Savla, C.; Cabrales, P.; Palmer, A.F. Enhanced Photodynamic Therapy Using the Apohemoglobin-Haptoglobin Complex as a Carrier of Aluminum Phthalocyanine. ACS Appl. Bio Mater. 2020, 3, 4495–4506. [Google Scholar] [CrossRef]
- di Giosia, M.; Valle, F.; Cantelli, A.; Bottoni, A.; Zerbetto, F.; Calvaresi, M. C60 Bioconjugation with Proteins: Towards a Palette of Carriers for All PH Ranges. Materials 2018, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Shisaka, Y.; Sakakibara, E.; Suzuki, K.; Stanfield, J.K.; Onoda, H.; Ueda, G.; Hatano, M.; Sugimoto, H.; Shoji, O. Tetraphenylporphyrin Enters the Ring: First Example of a Complex between Highly Bulky Porphyrins and a Protein. Chembiochem 2022, 23, e202200095. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z. Albumin Carriers for Cancer Theranostics: A Conventional Platform with New Promise. Adv. Mater. 2016, 28, 10557–10566. [Google Scholar] [CrossRef]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A. Albumin Nanovectors in Cancer Therapy and Imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef]
- Kratz, F. A Clinical Update of Using Albumin as a Drug Vehicle—A Commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Gerber, S.; Kämpfer, U.; Lejon, S.; Trachsel, C. Human Blood Plasma Proteins: Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Schneidman-Duhovny, D.; Inbar, Y.; Polak, V.; Shatsky, M.; Halperin, I.; Benyamini, H.; Barzilai, A.; Dror, O.; Haspel, N.; Nussinov, R.; et al. Taking Geometry to Its Edge: Fast Unbound Rigid (and Hinge-Bent) Docking. Proteins 2003, 52, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: Fast Interaction Refinement in Molecular Docking. Proteins 2007, 69, 139–159. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.Py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Teale, F.W.J. Cleavage of the Haem-Protein Link by Acid Methylethylketone. BBA Biochim. Biophys. Acta 1959, 35, 543. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal Bioconjugate for Targeted Gram-Negative Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B 2020, 206, 111852. [Google Scholar] [CrossRef] [PubMed]
- Ulfo, L.; Cantelli, A.; Petrosino, A.; Costantini, P.E.; Nigro, M.; Starinieri, F.; Turrini, E.; Zadran, S.K.; Zuccheri, G.; Saporetti, R.; et al. Orthogonal Nanoarchitectonics of M13 Phage for Receptor Targeted Anticancer Photodynamic Therapy. Nanoscale 2022, 14, 632–641. [Google Scholar] [CrossRef]
- Cantelli, A.; Malferrari, M.; Soldà, A.; Simonetti, G.; Forni, S.; Toscanella, E.; Mattioli, E.J.; Zerbetto, F.; Zanelli, A.; di Giosia, M.; et al. Human Serum Albumin–Oligothiophene Bioconjugate: A Phototheranostic Platform for Localized Killing of Cancer Cells by Precise Light Activation. JACS Au 2021, 1, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Ulfo, L.; Turrini, E.; Marconi, A.; Costantini, P.E.; Marforio, T.D.; Mattioli, E.J.; di Giosia, M.; Danielli, A.; Fimognari, C.; et al. Light-Enhanced Cytotoxicity of Doxorubicin by Photoactivation. Cells 2023, 12, 392. [Google Scholar] [CrossRef]
- di Giosia, M.; Nicolini, F.; Ferrazzano, L.; Soldà, A.; Valle, F.; Cantelli, A.; Marforio, T.D.; Bottoni, A.; Zerbetto, F.; Montalti, M.; et al. Stable and Biocompatible Monodispersion of C60 in Water by Peptides. Bioconjug. Chem. 2019, 30, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Bortot, B.; Apollonio, M.; Baj, G.; Andolfi, L.; Zupin, L.; Crovella, S.; di Giosia, M.; Cantelli, A.; Saporetti, R.; Ulfo, L.; et al. Advanced Photodynamic Therapy with an Engineered M13 Phage Targeting EGFR: Mitochondrial Localization and Autophagy Induction in Ovarian Cancer Cell Lines. Free Radic. Biol. Med. 2022, 179, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, E.J.; Ulfo, L.; Marconi, A.; Pellicioni, V.; Costantini, P.E.; Marforio, T.D.; di Giosia, M.; Danielli, A.; Fimognari, C.; Turrini, E.; et al. Carrying Temoporfin with Human Serum Albumin: A New Perspective for Photodynamic Application in Head and Neck Cancer. Biomolecules 2023, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Cocchi, V.; Novaković, A.; Karaman, M.; Sakač, M.; Mandić, A.; Pojić, M.; Barbalace, M.C.; Angeloni, C.; Hrelia, P.; et al. Meripilus Giganteus Ethanolic Extract Exhibits Pro-Apoptotic and Anti-Proliferative Effects in Leukemic Cell Lines. BMC Complement. Altern. Med. 2018, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.K.; Pragti; Mobin, S.M.; Mukhopadhyay, S. Studies on the Influence of the Nuclearity of Zinc(Ii) Hemi-Salen Complexes on Some Pivotal Biological Applications. Dalton Trans. 2020, 49, 15481–15503. [Google Scholar] [CrossRef]
- Marconi, A.; Mattioli, E.J.; Marconi, A.; Mattioli, J.; Ingargiola, F.; Giugliano, G.; Marforio, D.; Prodi, L.; di Giosia, M.; Calvaresi, M. Dissecting the Interactions between Chlorin E6 and Human Serum Albumin. Molecules 2023, 28, 2348. [Google Scholar] [CrossRef]
- Winterhalter, K.H.; Deranleauf, D.A. The Structure of a Hemoglobin Carrying Only Two Hemes. Biochemistry 1967, 6, 3136–3143. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tolosano, E.; Altruda, F. Hemopexin: Structure, Function, and Regulation. DNA Cell Biol. 2002, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Seery, V.L.; Morgan, W.T.; Muller Eberhard, U. Interaction of Rabbit Hemopexin with Rose Bengal and Photooxidation of the Rose Bengal Hemopexin Complex. J. Biol. Chem. 1975, 250, 6439–6444. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.T.; Smith, A.; Koskelo, P. The Interaction of Human Serum Albumin and Hemopexin with Porphyrins. Biochim. Biophys. Acta 1980, 624, 271–285. [Google Scholar] [CrossRef]
- Bartošová, J.; Kalousek, I.; Hrkal, Z. Binding of Meso-Tetra(4-Sulfonatophenyl)Porphine to Haemopexin and Albumin Studied by Spectroscopy Methods. Int. J. Biochem. 1994, 26, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Moriyama, T.; Hayashi, H.; Sakata, I.; Nakae, Y.; Takemura, T. Hemopexin as a Carrier Protein of Tumor-Localizing Ga-Metalloporphyrin-ATN-2. Cancer Lett. 2000, 149, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, L.; Voegele, A.F.; Chwatal, S.; Kronenberg, F.; Radcliffe, C.M.; Wormald, M.R.; Lobentanz, E.M.; Ezeh, B.; Eller, P.; Dejori, N.; et al. Afamin Is a Novel Human Vitamin E-Binding Glycoprotein Characterization and In Vitro Expression. J. Proteome Res. 2005, 4, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.S.; Belcher, D.A.; Hickey, R.; Miller, C.; Badu-Tawiah, A.K.; Baek, J.H.; Buehler, P.W.; Palmer, A.F. Novel Manufacturing Method for Producing Apohemoglobin and Its Biophysical Properties. Biotechnol. Bioeng. 2020, 117, 125–145. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, X.; Hu, D.; Wang, K.; Zhi, F.; Chen, X.I.; Gong, G.; Wu, J.; Hu, Y. Replacing Heme with Paclitaxel to Prepare Drug-Loaded Globin Nanoassembles for CD163 Targeting. J. Pharm. Sci. 2015, 104, 1045–1055. [Google Scholar] [CrossRef]
- Key, H.M.; Dydio, P.; Clark, D.S.; Hartwig, J.F. Abiological Catalysis by Artificial Haem Proteins Containing Noble Metals in Place of Iron. Nature 2016, 534, 534–537. [Google Scholar] [CrossRef]
- Oohora, K.; Onoda, A.; Hayashi, T. Hemoproteins Reconstituted with Artificial Metal Complexes as Biohybrid Catalysts. Acc. Chem. Res. 2019, 52, 945–954. [Google Scholar] [CrossRef]
- Fruk, L.; Kuo, C.H.; Torres, E.; Niemeyer, C.M. Apoenzyme Reconstitution as a Chemical Tool for Structural Enzymology and Biotechnology. Angew. Chem. Int. Ed. 2009, 48, 1550–1574. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Han, M.; Chen, Z.; Zou, G. Neutral Porphyrin J-Aggregates in Premicellar SDS Solution. Colloids Surf. A Phys. Eng. Asp. 2005, 256, 151–156. [Google Scholar] [CrossRef]
- Chen, J.Y.; Mak, N.K.; Yow, C.M.N.; Fung, M.C.; Chiu, L.C.; Leung, W.N.; Cheung, N.H. The Binding Characteristics and Intracellular Localization of Temoporfin (MTHPC) in Myeloid Leukemia Cells: Phototoxicity and Mitochondrial Damage¶. Photochem. Photobiol. 2000, 72, 541–547. [Google Scholar] [CrossRef]
- Ma, L.; Moan, J.; Berg, K. Evaluation of a New Photosensitizer, Meso-tetra-hydroxyphenyl-chlorin, for Use in Photodynamic Therapy: A Comparison of Its Photobiological Properties with Those of Two Other Photosensitizers. Int. J. Cancer 1994, 57, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Belitchenko, I.; Melnikova, V.; Bezdetnaya, L.; Rezzoug, H.; Merlin, J.L.; Potapenko, A.; Guillemin, F. Characterization of Photodegradation of Meta-Tetra (Hydroxyphenyl)Chlorin (MTHPC) in Solution: Biological Consequences in Human Tumor Cells. Photochem. Photobiol. 1998, 67, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Yogo, T.; Urano, Y.; Mizushima, A.; Sunahara, H.; Inoue, T.; Hirose, K.; Iino, M.; Kikuchi, K.; Nagano, T. Selective Photoinactivation of Protein Function through Environment-Sensitive Switching of Singlet Oxygen Generation by Photosensitizer. Proc. Natl. Acad. Sci. USA 2008, 105, 28–32. [Google Scholar] [CrossRef]





| Protein | PDB/UniProt ID | FireDock Score |
|---|---|---|
| Hemoglobin | 2m6z | −70.1 |
| Human myoglobin | 3rgk | −67.4 |
| Hemopexin | P02790 | −65.2 |
| Afamin | 6fak | −59.9 |
| Serum Albumin | 6a7p | −53.2 |
| Lactotransferrin | 1lfg | −53.2 |
| Plasma-retinol-binding protein | 1qab | −51.5 |
| Thyroxine-binding globulin | 2ceo | −50.6 |
| α-fetoprotein | J3KMX3 | −50.3 |
| Serotransferrin | 6soy | −48.4 |
| Vitamin-D-binding protein | 1ma9 | −47.7 |
| Haptoglobin | 4x0l | −44.4 |
| Transthyretin | 1g1o | −42.7 |
| Ceruloplasmin | 4ejx | −36.7 |
| Sex-hormone-binding protein | 6pyb | −34.3 |
| Corticosteroid-binding globulin | 2vdy | −27.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, A.; Giugliano, G.; Di Giosia, M.; Marforio, T.D.; Trivini, M.; Turrini, E.; Fimognari, C.; Zerbetto, F.; Mattioli, E.J.; Calvaresi, M. Identification of Blood Transport Proteins to Carry Temoporfin: A Domino Approach from Virtual Screening to Synthesis and In Vitro PDT Testing. Pharmaceutics 2023, 15, 919. https://doi.org/10.3390/pharmaceutics15030919
Marconi A, Giugliano G, Di Giosia M, Marforio TD, Trivini M, Turrini E, Fimognari C, Zerbetto F, Mattioli EJ, Calvaresi M. Identification of Blood Transport Proteins to Carry Temoporfin: A Domino Approach from Virtual Screening to Synthesis and In Vitro PDT Testing. Pharmaceutics. 2023; 15(3):919. https://doi.org/10.3390/pharmaceutics15030919
Chicago/Turabian StyleMarconi, Alessia, Giulia Giugliano, Matteo Di Giosia, Tainah Dorina Marforio, Michele Trivini, Eleonora Turrini, Carmela Fimognari, Francesco Zerbetto, Edoardo Jun Mattioli, and Matteo Calvaresi. 2023. "Identification of Blood Transport Proteins to Carry Temoporfin: A Domino Approach from Virtual Screening to Synthesis and In Vitro PDT Testing" Pharmaceutics 15, no. 3: 919. https://doi.org/10.3390/pharmaceutics15030919
APA StyleMarconi, A., Giugliano, G., Di Giosia, M., Marforio, T. D., Trivini, M., Turrini, E., Fimognari, C., Zerbetto, F., Mattioli, E. J., & Calvaresi, M. (2023). Identification of Blood Transport Proteins to Carry Temoporfin: A Domino Approach from Virtual Screening to Synthesis and In Vitro PDT Testing. Pharmaceutics, 15(3), 919. https://doi.org/10.3390/pharmaceutics15030919










