Anti-Inflammatory Chilean Endemic Plants
Abstract
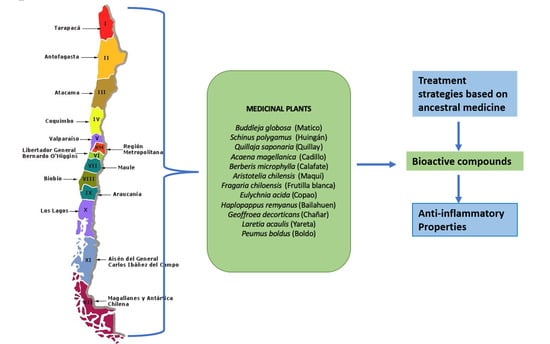
1. Introduction
2. Native Plants and Their Metabolites
2.1. Ugni molinae Turcz (murta)
2.2. Buddleja globosa Hoppe (matico)
2.3. Schinus polygamus (Cav.) Cabrera (huingán)
2.4. Quillaja saponaria Mol. (quillay)
2.5. Acaena magellanica (Lam.) Vahl (cadillo)
2.6. Berberis microphylla (calafate)
2.7. Aristotelia chilensis (Mol.) Stuntz (maqui)
2.8. Fragaria chiloensis spp. Chiloensis (Frutilla blanca)
2.9. Eulychnia acida Phil., Cactaceae (copao)
2.10. Haplopappus remyanus (bailahuén)
2.11. Geoffroea decorticans (chañar)
2.12. Laretia acaulis (yareta or llareta)
2.13. Peumus boldus (boldo)
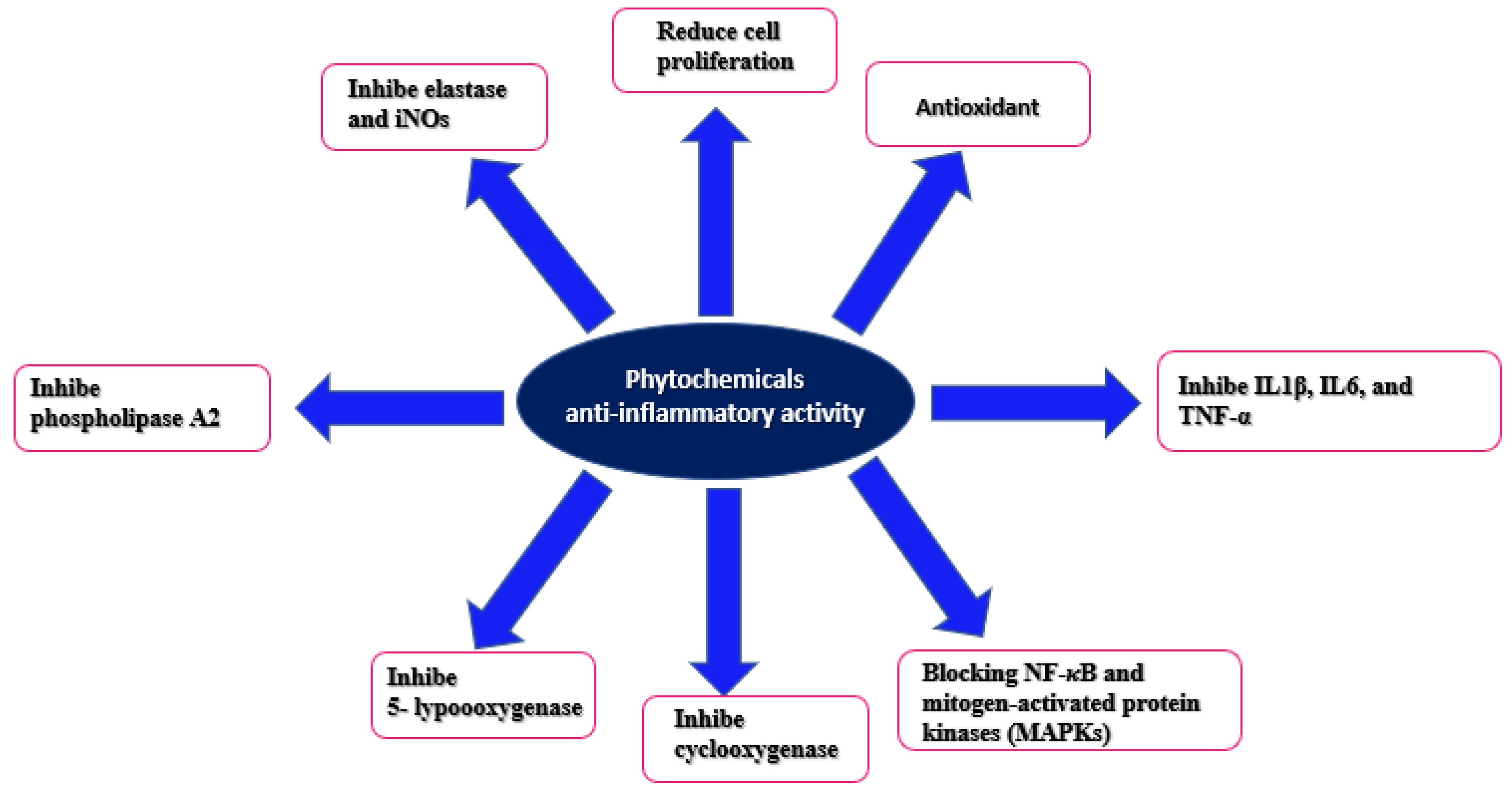
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Bao, Y.M.; Jiang, B.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S.J.M. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- O’Neill, L.A.J. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006, 5, 549–563. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Gomez-Guillen, M.C.; Ihl, M.; Bifani, V.; Silva, A.; Montero, P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocoll. 2007, 21, 1133–1143. [Google Scholar] [CrossRef]
- Bralley, E.E.; Greenspan, P.; Hargrove, J.L.; Wicker, L.; Hartle, D.K. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J. Inflamm. 2008, 5, 1–7. [Google Scholar] [CrossRef]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef]
- Goity, L.E.; Queupil, M.J.; Jara, D.; Alegria, S.E.; Peña, M.; Barriga, A.; Delporte, C. An HPLC-UV and HPLC-ESI-MS based method for identification of anti-inflammatory triterpenoids from the extracts of Ugni molinae. Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 108–116. [Google Scholar]
- Sabando, C.; Ide, W.; Rodríguez-Díaz, M.; Cabrera-Barjas, G.; Castaño, J.; Bouza, R.; Müller, N.; Gutiérrez, C.; Barral, L.; Rojas, J.; et al. A novel hydrocolloid film based on pectin, starch and Gunnera tinctoria and Ugni molinae plant extracts for wound dressing applications. Curr. Top Med. Chem. 2020, 20, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Zamorano-Aguilar, P.; Morales, M.; Rivillas, Y.; López, J.; Rojano, B. Antioxidant activity and cytotoxic effect of Chilean Buddleja globosa (matico) and Ribes magellanicum (zarzaparrilla) flower extracts. Acta Sci. Pol. Hortorum Cultos 2020, 19, 59–70. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Apablaza, C.; Farías, M.; Goïty, L.; Arrau, S.; Negrete, R.; Castro, C.; Miranda, H. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J. Ethnopharmacol. 2008, 119, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, B.; Marino, G.; Assef, Y.; Silva, F.; Catalán, C.; González, S. Nutraceutical properties of herbal infusions from six native plants of Argentine Patagonia. Plant Food Human Nutr. 2018, 73, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, G.; El-Nashar, H.; Mostafa, N.; Eldahshan, O.; Boiangiu, R.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Jimenez, P.; Garcia-Diaz, D.F. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Parveen, Z.; Deng, Y.; Saeed, M.K.; Dai, R.; Ahamad, W.; Yu, Y.H. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-O-glucoside. Yakugaku Zasshi 2007, 127, 1275–1279. [Google Scholar] [CrossRef]
- Muñoz, O.; Christen, P.; Cretton, S.; Backhouse, N.; Torres, V.; Correa, O.; Costa, E.; Miranda, H.; Delporte, C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011, 63, 849–859. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Ozaki, Y. Anti-inflammatory effect of tetramethylpyrazine and ferulic acid. Chem. Pharmaceut. Bull. 1992, 40, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The Chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, M.; Delporte, C.; Cartagena, C.; Cassels, B.K.; González, P.; Silva, X.; León, F.; Wessjohann, L.A. Topical anti-inflammatory activity of quillaic acid from Quillaja saponaria Mol. and some derivatives. J. Pharm. Pharmacol. 2011, 63, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Arrau, S.; Delporte, C.; Cartagena, C.; Rodríguez-Díaz, M.; González, P.; Silva, X.; Cassels, B.K.; Miranda, H.F. Antinociceptive activity of Quillaja saponaria Mol. saponin extract, quillaic acid and derivatives in mice. J. Ethnopharmacol. 2011, 133, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B. Vaccination of older adults: Influenza, pneumococcal disease, herpes zoster, COVID-19 and beyond. Immun. Ageing 2021, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, M.; Ordoñez, R.; Zampini, I.; Sayago, J.; Isla, M. Nutritional and antioxidant properties of Geoffroea decorticans, an Argentinean fruit, and derived products (flour, arrope, decoction and hydroalcoholic beverage). Food Res. Int. 2013, 54, 160–168. [Google Scholar] [CrossRef]
- Reynoso, M.; Vera, N.; Aristimuño, M.; Daud, A.; Riera, A.S. Antinociceptive activity of fruits extracts and “arrope” of Geoffroea decorticans (chañar). J. Ethnopharmacol. 2013, 145, 355–362. [Google Scholar] [CrossRef]
- Costamagna, M.; Zampini, I.; Alberto, M.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Givernau, M.; Cassels, B.; Valenzuela, A.; Speisky, H. Anti-inflammatory and antipyretic effects of boldine. Inflamm. Res. 1994, 42, 114–117. [Google Scholar] [CrossRef]
- Santanam, N.; Penumetcha, M.; Speisky, H.; Parthasarathy, S. A novel alkaloid antioxidant, Boldine and synthetic antioxidant, reduced form of RU486, inhibit the oxidation of LDL in-vitro and atherosclerosis in vivo in LDLR−/− mice. Atherosclerosis 2004, 173, 203–210. [Google Scholar] [CrossRef]
- Degenhardt, R.T.; Farias, I.V.; Grassi, L.T.; Franchi, G.C.; Nowill, A.E.; Bittencourt, C.M.; Wagner, T.M.; de Souza, M.M.; Cruz, A.B.; Malheiros, A. Characterization and evaluation of the cytotoxic potential of the essential oil of Chenopodium ambrosioides. Rev. Bras. Farmacogn. 2016, 26, 56–61. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharmaceut. Sci. 2011, 1, 7–15. [Google Scholar]
- Molinett, S.; Nuñez, F.; Moya-León, M.A.; Zúñiga-Hernández, J. Chilean strawberry consumption protects against LPS-induced liver injury by anti-inflammatory and antioxidant capability in Sprague-Dawley rats. Evid. Based Complement. Alt. Med. 2015, 2015, 320136. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Alberto, M.R.; Quispe, C.; Caramantin-Soriano, M.P.; Theoduloz, C.; Zampini, I.C.; Isla, M.I.; Schmeda-Hirschmann, G. Anti-inflammatory activity of copao (Eulychnia acida Phil., Cactaceae) fruits. Plant Foods Human Nutr. 2015, 70, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Harlev, E.; Nevo, E.; Solowey, E.; Bishayee, A. Cancer preventive and curative attributes of plants of the Cactaceae family: A review. Planta Med. 2013, 79, 713–722. [Google Scholar] [CrossRef]
- Delporte, C.; Backhouse, N.; Salinas, P.; San-Martȷn, A.; Bórquez, J.; Loyola, A. Pharmaco-toxicological study of diterpenoids. Bioorg. Med. Chem. 2003, 11, 1187–1190. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Bórquez, J.; Ardiles, A.; Said-Fernández, S.; Loyola, L.A.; San-Martín, A.; Peña-Rodríguez, L.M. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Fitoterapia 2010, 81, 50–54. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; Angel, G.R.; Delporte, C.; Erazo, N.B.; Schmeda-Hirschmann, G. Free radical scavengers, anti-inflammatory, and analgesic activity of Acaena magellanica. J. Pharm. Pharmacol. 2002, 54, 835–844. [Google Scholar] [CrossRef]
- Faini, F.; Torres, R.; Rodilla, J.M.; Labbé, C.; Delporte, C.; Jaña, F. Chemistry and bioactivity of Haplopappus remyanus (bailahuen), a Chilean medicinal plant. J. Braz. Chem. Soc. 2011, 22, 2344–2349. [Google Scholar] [CrossRef]
- Dillehay, T.D. Monte Verde: Un asentamiento humano del pleistoceno tardío en el sur de Chile. Chungara 2005, 37, 275–276. [Google Scholar] [CrossRef]
- Farga, C.; Lastra, J.; Hoffmann, A. Plantas Medicinales de uso común en Chile; Paesmi, E., Ed.; Ediciones PAESMI: Santiago, Chile, 1988; Volume I, pp. 11–19. [Google Scholar]
- Parada, M. Legislación en Chile sobre fitofármacos y plantas medicinales. Rev. Farmacol. Chile 2012, 5, 711. [Google Scholar]
- Eyzaguirre, J. Historia de Chile. Bol. Acad. Chil. Hist. 1965, 32, 165. [Google Scholar]
- Baxter, H.; Puri, B.; Harborne, J.B.; Hall, A.; Moss, G.P. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; CRC Press: London, UK, 1998; 976p. [Google Scholar] [CrossRef]
- Cespedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Russell, W.; Duthie, G. Plant secondary metabolites and gut health: The case for phenolic acids. Proceed. Nutr. Soc. 2011, 70, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Avila, J.G.; Marin, J.C.; Dominguez, M.; Torres, P.; Aranda, E. Natural compounds as antioxidant and molting inhibitors can play a role as a model for the search of new botanical pesticides. In Natural Antioxidants and Biocides from Wild Medicinal Plants; Cespedes, C.L., Sampietro, D.A., Seigler, D.S., Rai, M., Eds.; CABI Publishing: Wallingford, UK, 2013. [Google Scholar]
- Bhakuni, D.S.; Bittner, M.; Marticorena, C.; Silva, M.; Weldt, E.; Hoeneisen, M. Screening of Chilean plants for anticancer activity. Lloydia 1976, 39, 225–243. [Google Scholar] [PubMed]
- Cappillino, P.; Kleiman, R.; Botti, C. Composition of Chilean jojoba seeds. Ind. Crops Prod. 2003, 17, 177–182. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeon, M.E.; Raskin, I. Innovations in health value and functional food development of quinoa (Chenopodium quinoa Willd.). Comprehensive Rev. Food Sci. Food Safety 2015, 14, 431–445. [Google Scholar] [CrossRef]
- Jofre, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and vasodilator activity of Ugni molinae Turcz. (Murtilla) and its modulatory mechanism in hypotensive response. Oxid. Med. Cell. Long. 2016, 2016, 6513416. [Google Scholar] [CrossRef]
- Houghton, P.; Manby, J. Medicinal plants of the Mapuche. J. Ethnopharmacol. 1985, 13, 89–103. [Google Scholar] [CrossRef]
- Hoffmann, A.; Farga, C.; Lastra, J.; Veghazi, E. Plantas Medicinales de uso Común en Chile; Fundación Claudio Gay: Santiago, Chile, 1992. [Google Scholar]
- Higuchi, R.; Tokimitsu, Y.; Fujioka, T.; Komori, T.; Kawasaki, T.; Oakenful, D.G. Structure of desacylsaponins obtained from the bark of Quillaja saponaria. Phytochemistry 1986, 26, 229–235. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Theoduloz, C.; Caligari, P.D.; Schmeda-Hirschmann, G. Comparison of phenolic composition and antioxidant properties of two native Chilean and one domestic strawberry genotypes. Food Chem. 2009, 113, 377–385. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.S.; Salvatierra, M.A.G.; Robert, P.C.; Encina, C.A.; Camilo, C.M. Chemical and nutritional composition of copao fruit (Eulychnia acida Phil.) under three environmental conditions in the Coquimbo region. Chil. J. Agric. Res. 2011, 71, 521–529. [Google Scholar] [CrossRef]
- Quispe, C.; Viveros-Valdez, E.; Schmeda-Hirschmann, G. Phenolic constituents of the Chilean herbal tea Fabiana imbricata R. et P. Plant Foods Human Nutr. 2012, 67, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants. Macmillan: New York, NY, USA, 2005. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Hartmann, T.J.P. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Jones, W.P.; Kinghorn, A.D. Extraction of plant secondary metabolites. In Natural Products Isolation; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principles, strengths and limitations. Med. Aromat. Plants. 2015, 4, 2167–2412. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef]
- Tolstikov, V.V.; Lommen, A.; Nakanishi, K.; Tanaka, N.; Fiehn, O. Monolithic silica-based capillary reversed-phase liquid chromatography/electrospray mass spectrometry for plant metabolomics. Anal. Chem. 2003, 75, 6737–6740. [Google Scholar] [CrossRef]
- Salvat, A.; Antonacci, L.; Fortunato, R.H.; Suarez, E.Y. Godoy, HM Anti-microbial activity in methanolic extracts of several plant species from northern Argentina. Phytomedicine 2004, 11, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Molgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbaek, L.B.; Jebjerg, C.P.; Jorgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Anti-microbial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sewlikar, S.; D’Souza, D.H. Anti-microbial effects of Quillaja saponaria extract against Escherichia coli O157:H7 and the emerging non-O157 Shiga toxin-producing E. coli. J. Food Sci. 2017, 82, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Lim, Y.; Wong, L.; Lianto, F.; Wong, S.; Lim, K.; Joe, C.; Lim, T. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Lim, T.; Lim, Y.; Yule, C. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Taguchi, Y.; Akazawa, H.; Ukiya, M.; Kimura, Y.; Suzuki, T.; Nishino, H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharmaceut. Bull. 2005, 28, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Alarcon, J.; Avila, J.G.; Nieto, A. Anti-inflammatory activity of Aristotelia chilensis Mol. (Stuntz)(Elaeocarpaceae). Bol. Latinoam. Caribe Plant. Med. Aromat. 2010, 9, 127–135. [Google Scholar]
- López, J.; Vera, C.; Bustos, R.; Florez-Mendez, J. Native berries of Chile: A comprehensive review on nutritional aspects, functional properties, and potential health benefits. J. Food Measurement Charact. 2021, 15, 1139–1160. [Google Scholar] [CrossRef]
- Seguel, I.; Peñaloza, E.; Gaete, N.; Montenegro, A.; Torres, A. Colecta y caracterización molecular de germoplasma de murta (Ugni molinae Turcz.) en Chile. Agro Sur 2000, 28, 32–41. [Google Scholar] [CrossRef]
- Barreau, A.; Ibarra, J.T.; Wyndham, F.S.; Rojas, A.; Kozak, R.A. How can we teach our children if we cannot access the forest? Generational change in Mapuche knowledge of wild edible plants in Andean temperate ecosystems of Chile. J. Ethnobiol. 2016, 36, 412–432. [Google Scholar] [CrossRef]
- Avello, M.; Torres, E.; Carvajal, R.; Pastene, E. Effect of in vitro digestion gastrointestinal of the extract aqueous of leaves of Ugni molinae, on the viability of colorectal cancer cells. J. Chil. Chem. Soc. 2021, 66, 5268–5272. [Google Scholar] [CrossRef]
- Puente-Díaz, L.; Ah-Hen, K.; Vega-Gálvez, A.; Lemus-Mondaca, R.; Scala, K.D. Combined infrared-convective drying of murta (Ugni molinae Turcz) berries: Kinetic modeling and quality assessment. Drying Technol. 2013, 31, 329–338. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.; Vega-Gálvez, A.; López, J.; Quispe-Fuentes, I.; Lemus-Mondaca, R.; Gálvez-Ranilla, L. Changes in bioactive compounds and antioxidant activity during convective drying of murta (Ugni molinae T.) berries. Int. J. Food Sci. Technol. 2014, 49, 990–1000. [Google Scholar] [CrossRef]
- Morales, M.A.; Mendibure, R.; Vergara, F. Fundamentación básica al uso etnomédico de matico (Buddleja globosa Hope). Rev. Fitoter. 2014, 15, 37–51. [Google Scholar]
- Fernández, A.; Simian, D.; Quera, R.; Flores, L.; Ibáñez, P.; Lubascher, J.; Figueroa, C.; Kronberg, U.; Pizarro, G.; Fluxá, D. Complementary and alternative medicine in patients with inflammatory bowel disease: A survey performed in a tertiary center in Chile. Complement. Ther. Med. 2018, 40, 77–82. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, E. Polyphenolic compounds extracted and purified from Buddleja Globosa Hope (Buddlejaceae) leaves using natural deep eutectic solvents and centrifugal partition chromatography. Molecules 2021, 26, 2192. [Google Scholar] [CrossRef]
- Navas, L. Flora de la cuenca de Santiago de Chile: Tomo 2. In Dicotyledoneae, Archichlamydeae; Universidad de Chile: Santiago, Chile, 1976. [Google Scholar]
- El-Nashar, H.; Mostafa, N.; El-Brady, M.; Eldahshan, O.; Singab, A. Chemical composition, anti-microbial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) Cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021, 35, 5369–5372. [Google Scholar] [CrossRef]
- Muñoz, M.; Barrera, E.; Meza, I. El uso medicinal y alimenticio de plantas nativas y naturalizadas en Chile. Bol. Museo Nac. Hist. Nat.- 1981, 33, 37. [Google Scholar]
- Abdelghffar, E.; Mostafa, N.; El-Nashar, H.; Eldahshan, O.; Singab, A.N. Chilean pepper (Shinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high-fat diet/streptozotocin-induced type 2 diabetic rat model. Ind. Crops Prod. 2022, 183, 114953. [Google Scholar] [CrossRef]
- Erazo, S.; Delporte, C.; Negrete, R.; Garcia, R.; Zaldivar, M.; Iturra, G.; Caballero, E.; Lopez, J.L.; Backhouse, N. Constituents and biological activities of Schinus polygamus. J. Ethnopharmacol. 2006, 107, 395–400. [Google Scholar] [CrossRef]
- Kubitzki, K.; Rohwer, J.; Bittrich, V. 1990. The Families and Genera of Vascular Plants. Springer 1. La Leyenda del Calafate. 2020. Available online: https://www.patagonia.com.ar/El+Calafate/527_La+leyenda+del+calafate.html (accessed on 18 January 2023).
- Zin, J.; Weiss, C. La Salud por Medio de las Plantas Medicinales; Editorial Salesiana: Santiago, Chile, 1980. [Google Scholar]
- Fleck, J.D.; Betti, A.H.; Da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef]
- Cañon-Jones, H.; Cortes, H.; Castillo-Ruiz, M.; Schlotterbeck, T.; San Martin, R. Quillaja saponaria (Molina) extracts inhibits in vitro Piscirickettsia salmonis infections. Animals 2020, 10, 2286. [Google Scholar] [CrossRef]
- Cañon-Jones, H.; Schlotterbeck, T.; Castillo-Ruiz, M.; Cortes, H.; Asencio, G.; Latuz, S.; San Martin, R. In vitro efficacy of Quillaja saponaria extracts on the infective life-stage of ectoparasite Caligus rogercresseyi. J. World Aquac. Soc. 2021, 52, 1234–1242. [Google Scholar] [CrossRef]
- Moore, D.M. The flora of the Fuego-Patagonian Cordilleras: Its origins and affinities. Rev. Chil. Hist. Nat. 1983, 56, 123–136. [Google Scholar]
- Orsi, M. Berberidaceae. Flora Patagónica IV 1984, 8, 325–348. [Google Scholar]
- Chamorro, M.; Ladio, A. Native and exotic plants with edible fleshy fruits utilized in Patagonia and their role as sources of local functional foods. BMC Complement. Med. Ther. 2020, 20, 155. [Google Scholar] [CrossRef]
- Armas-Ricard, M.; Quinán-Cárdenas, F.; Sanhueza, H.; Pérez-Vidal, R.; Mayorga-Lobos, C.; Ramírez-Rodríguez, O. Phytochemical screening and antioxidant activity of seven native species growing in the forests of southern Chilean Patagonia. Molecules 2021, 26, 6722. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N. Antioxidant Activities of Flavonoids as Bioactive Components of Food; Portland Press Limited: London, UK, 1996. [Google Scholar]
- Mariangel, E.; Reyes-Diaz, M.; Lobos, W.; Bensch, E.; Schalchli, H.; Ibarra, P. The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc. Investig. Agrar. 2013, 40, 161–170. [Google Scholar] [CrossRef]
- Serafim, T.L.; Oliveira, P.J.; Sardao, V.A.; Perkins, E.; Parke, D.; Holy, J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother. Pharmacol. 2008, 61, 1007–1018. [Google Scholar] [CrossRef]
- Chao, J.; Lu, T.C.; Liao, J.W.; Huang, T.H.; Lee, M.S.; Cheng, H.Y.; Ho, L.K.; Kuo, C.L.; Peng, W.H. Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J. Ethnopharmacol. 2009, 125, 297–303. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ha, Y.M.; Jin, Y.C.; Shi, L.Y.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; et al.; et al. Palmatine from Coptidis rhizoma reduces ischemia–reperfusion-mediated acute myocardial injury in the rat. Food Chem. Toxicol. 2009, 47, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Caro, L.; Radojkovic, C.; Chau, S.; Nova, D.; Bustamante, L.; Neira, J.; Perez, A.; Mardones, C. Berberis microphylla G. forst (Calafate) berry extract reduces oxidative stress and lipid peroxidation of human LDL. Antioxidants 2020, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Guzmán, C. Description of the antioxidant capacity of Calafate berries (Berberis microphylla) collected in southern Chile. Food Sci. Technol. 2021, 41, 864–869. [Google Scholar] [CrossRef]
- Guzman, C.; Sánches, R. Effect of calafate (Berberis microphylla) supplementation on lipid profile in rats with diet-induced obesity. Funct. Foods Health Dis. 2021, 11, 512–521. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Lotton, J.; Lila, M.A.; de Mejia, E.G. Berries from South America: A comprehensive review on chemistry, health potential, and commercialization. J. Med. Food 2010, 13, 233–246. [Google Scholar] [CrossRef] [PubMed]
- De Mosbach, E.W. Botánica Indígena de Chile; Museo chileno de Arte precolombino. Fundación Andes. Editorial Andrés Bello: Santiago, Chile, 1992; p. 91. [Google Scholar]
- Montes, M.; Wilkomirsky, T. Medicina Tradicional Chilena; Editorial de la Universidad de Concepción: Concepcion, Chile, 1987. [Google Scholar]
- Silva, M.; Alarcón, J.; Bittner, M.; Becerra, J.; Sanhueza, L.; Marticorena, C. Aristotelia chilensis (Mol.) Stuntz en. In 270 Plantas Medicinales Iberoamericanas; Gupta, M., Ed.; Cyted-Cecab: Bogotá, Colombia, 1995. [Google Scholar]
- Agulló, V.; González-Trujano, E.; Hernandez-Leon, A.; Estrada-Camarena, E.; Pellicer, F.; García-Viguera, C. Antinociceptive effects of maqui-berry (Aristotelia chilensis (Mol.) Stuntz). Int. J. Food Sci. Nutr. 2021, 72, 947–955. [Google Scholar] [CrossRef]
- Rodríguez, L.; Trostchansky, A.; Wood, I.; Mastrogiovanni, M.; Vogel, H.; González, B.; Maróstica, M.; Fuentes, E.; Palomo, I. Antiplatelet activity and chemical analysis of leaf and fruit extracts from Aristotelia chilensis. PLoS ONE 2021, 16, e0250852. [Google Scholar] [CrossRef]
- Bianchi, F.; Giuberti, G.; Cervini, M.; Simonato, B. Fortification of durum wheat fresh pasta with maqui (Aristotelia chilensis) and its effects on technological, nutritional, sensory properties, and predicted glycemic index. Food Bioproc. Technol. 2022, 15, 1563–1572. [Google Scholar] [CrossRef]
- Moon, H.D.; Kim, B.H. Inhibitory effects of Aristotelia chilensis water extract on 2,4-dinitrochlorobenzene induced atopic-like dermatitis in BALB/c Mice. Asian Pac. J. Allergy Immunol. 2019. [Google Scholar] [CrossRef]
- Crisóstomo-Ayala, K.; Hernández de la Torre, M.; Pedreño, M.; Hernández, J.; Pérez, C.; Bustos, E.; Sánchez-Olete, M.; Ríos, D. Seasonal changes in photosynthesis, phenolic content, antioxidant activity, and anatomy of apical and basal leaves of Aristotelia chilensis. Biol. Plant. 2021, 65, 342–350. [Google Scholar] [CrossRef]
- Yin, M.C.; Chan, K.C. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef]
- Finn, C.E.; Retamales, J.B.; Lobos, G.A.; Hancock, J.F. The Chilean strawberry (Fragaria chiloensis): Over 1000 years of domestication. HortScience 2013, 48, 418–421. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Composit. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Ávila, F.; Theoduloz, C.; López-Alarcón, C.; Dorta, E.; Schmeda-Hirschmann, G. Cytoprotective mechanisms mediated by polyphenols from Chilean native berries against free radical-induced damage on AGS cells. Oxid. Med. Cell. Longevit. 2017, 2017, 9808520. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol composition and (bio)activity of Berberis species and wild strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Quispe, C.; Caramantin-Soriano, M.P.; Gonzalez, J.F.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MS. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Vogel, H.; González, M.; Faini, F.; Razmilic, I.; Rodríguez, J.; San Martín, J.; Urbina, F. Antioxidant properties and TLC characterization of four Chilean Haplopappus-species known as bailahuen. J. Ethnopharmacol. 2005, 97, 97–100. [Google Scholar] [CrossRef]
- Scarpa, G.F. Etnobotánica médica de los indígenas chorote y su comparación con la de los criollos del Chaco semiárido (Argentina). Darwiniana 2009, 47, 92–101. [Google Scholar]
- Larraín, H. Chañar Árbol Frutal Imprescindible para Atacameños y Aimaras: Testimonios Históricos y Experiencias. Available online: http://eco-antropologia.blogspot.cl/2011/08/el-chanar-arbol-frutal-de-atacamenos-y.html (accessed on 18 January 2023).
- Latcham, R. La Agricultura en Chile y en los Países Vecinos; Ediciones de la Universidad de Chile: Santiago, Chile, 1936; p. 336. [Google Scholar]
- Hidalgo, J. Prehistoria. Desde sus Orígenes Hasta los Albores de la Conquista; Series culturas de Chile; Editorial Andrés Bello: Santiago, Chile, 1989; 460p. [Google Scholar]
- Gleisner, C.; Montt, S. Serie introducción histórica y relatos de los pueblos originarios de Chile; Unidad de Cultura FUCO: Colla, Santiago de Chile, 2014; 140p. [Google Scholar]
- Jiménez-Aspee, F.; Theoduloz, C.; Soriano, M.; Ugalde-Arbizu, M.; Alberto, M.; Zampini, I.; Isla, M.; Simirgiotis, M.; Schmeda-Hirschmann, G. The native fruit Geoffroea decorticans from arid northern Chile: Phenolic composition, antioxidant activities and in vitro inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Molecules 2017, 22, 1565. [Google Scholar] [CrossRef]
- Alliende, M.; Hoffmann, A. Laretia acaulis, a cushion plant of the Andes: Ethnobotanical aspects and the impact of Its harvesting. Mount. Res. Develop. 1983, 3, 45–51. [Google Scholar] [CrossRef]
- Wickens, G.E. Llareta (Azorella compacta, Umbelliferae): A review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; San-Martȷn, A. Mulinane-type diterpenoids from Laretia acaulis. Phytochemistry 2000, 53, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Borquez, J.; Morales, G.; Araya, J.; Gonzalez, J.; Neira, I.; Sagua, H.; San-Martín, A. Azorellane diterpenoids from Laretia acaulis, and its toxoplasmacidal activity. Bol. Soc. Chil. Quím. 2001, 46, 9–13. [Google Scholar] [CrossRef]
- Borquez, J.; Loyola, L.A.; Morales, G.; San-Martín, A.; Roldan, R.; Marquez, N.; Muñoz, E. Azorellane diterpenoids from Laretia acaulis inhibit nuclear factor-kappa B activity. Phytother. Res. 2007, 21, 1082–1086. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem.-Biol. Interactions 2006, 159, 1–17. [Google Scholar] [CrossRef]
- Rodriguez, V. Boldo, Naturaleza Chilena. 2020. Available online: https://virginiarodriguezvet.es/boldo-naturaleza-chilena (accessed on 10 November 2020).
- Speisky, H.; Cassels, B.K. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. 1994, 29, 1–12. [Google Scholar] [CrossRef]
- Quezada, N.; Asencio, M.; Valle, J.D.; Aguilera, J.; Gómez, B. Antioxidant activity of crude extract, alkaloid fraction, and flavonoid fraction from Boldo (Peumus boldus Molina) leaves. J. Food Sci. 2004, 69, C371–C376. [Google Scholar] [CrossRef]
- De Lima, N.M.R.; Ferreira, E.D.O.; Fernandes, M.Y.S.; Lima, F.A.V.; Neves, K.R.T.; do Carmo, M.R.S.; de Andrade, G.M. Neuroinflammatory response to experimental stroke is inhibited by boldine. Behav. Pharmacol. 2017, 28, 223–237. [Google Scholar] [CrossRef]
- Muñoz, O. Plantas Medicinales de uso en Chile: Química y Farmacología; Editorial Universitaria: Santiago, Chile, 2001. [Google Scholar]
- Muñoz, O.M.; Maya, J.D.; Ferreira, J.; Christen, P.; San Martin, J.; López-Muñoz, R.; Kemmerling, U. Medicinal plants of Chile: Evaluation of their anti-Trypanosoma cruzi activity. Z. Für Nat. C 2013, 68, 198–202. [Google Scholar] [CrossRef]
- Ngabire, D.; Seong, Y.; Patil, M.P.; Niyonizigiye, I.; Seo, Y.B.; Kim, G.D. Anti-inflammatory effects of aster incisus through the inhibition of NF-κB, MAPK, and Akt pathways in LPS-stimulated RAW 264.7 Macrophages. Mediat. Inflamm. 2018, 2018, 4675204. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chi, J.; Zhou, M.; Li, R.; Li, Y.; Luo, J.; Kong, L. Anti-inflammatory lindenane sesquiterpeniods and dimers from Sarcandra glabra and its upregulating AKT/Nrf2/HO-1 signaling mechanism. Ind. Crops Prod. 2019, 137, 367–376. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Roy, D.N.; Goswami, R. IL-9 Signaling Pathway: An Update. In Th9 Cells. Methods in Molecular Biology; Goswami, R., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1585. [Google Scholar] [CrossRef]
- Bacigalupo, A.M. La voz del Kultrún en la Modernidad: Tradición y Cambio en la Terapéutica de Siete Machi Mapuche; Universidad Católica de Chile: Santiago, Chile, 2001; 271p. [Google Scholar] [CrossRef]
- Bacigalupo, A.M. Las prácticas espirituales de poder de los machi y su relación con la resistencia mapuche y el estado chileno. Rev. Chil. Antropol. 2010, 7. [Google Scholar] [CrossRef]












| Plant Species | Traditional Uses | Phytochemical Bioactives | In Vitro/In Vivo Studies | Clinical Studies |
|---|---|---|---|---|
| Ugni molinae Turcz | edible fruits, pain relief, diarrhea | phenolic compounds, pentacyclic triterpene acids such as corosolic acid | [7,8,9,10] | [11] |
| Buddleja globosa Hoppe | healing wounds, dysentery, stomach ulcers, scabies, syphilis | saponins, sesquiterpenes, triterpenes, diterpenes, phenylethanoids, flavonoids | [12,13,14] | NR |
| Schinus polygamus (Cav.) Cabrera | wound cleansing, arthritis | α- and β-pinene terpenoids, essential oil | [15] | NR |
| Berberis microphylla | edible fruits, pain relief, diarrhea | polyphenols, anthocyanins | [16] | NR |
| Aristotelia chilensis (Mol.) Stuntz | astringent and febrifuge properties, anti-diarrheal, anti-inflammatory, analgesic, anti-hemorrhagic | flavonoids, caffeic and ferulic acids | [17,18,19,20,21,22] | NR |
| Quillaja saponaria Mol. | pain relief | pentacycle triterpenoids | [23,24] | [25] |
| Geoffroea decorticans | edible fruit | sugar, fiber, and a complex mixture of polyphenols | [26,27,28] | NR |
| Peumus boldus Mol. | headaches, rheumatism, dyspepsia, menstrual pain, urinary tract inflammation | alkaloids, flavonoids, essential oil | [29,30,31] | NR |
| Fragaria Chiloensis spp. chiloensis | edible fruit | polyphenols, anthocyanins | [32,33] | NR |
| Eulychnia ACIDA Phil | edible fruit | flavonoids | [34,35] | NR |
| Laretia acaulis | gastric stimulus, diuretics, analgesics, rheumatism, diabetes treatment | mulinane diterpenoid | [36,37] | NR |
| Acaena magellanica (Lam.) Vahl | pain, gallbladder, allergies | triterpenes and steroids | [38] | NR |
| Haplopappus remyanus | Antispasmodic, antiseptic | flavonoids and coumarins | [39] | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, C.; Klagges, C.; Morales, B.; Sotomayor, P.; Escobar, J.; Fuentes, J.A.; Moreno, A.A.; Llancalahuen, F.M.; Arratia-Perez, R.; Gordillo-Fuenzalida, F.; et al. Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics 2023, 15, 897. https://doi.org/10.3390/pharmaceutics15030897
Otero C, Klagges C, Morales B, Sotomayor P, Escobar J, Fuentes JA, Moreno AA, Llancalahuen FM, Arratia-Perez R, Gordillo-Fuenzalida F, et al. Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics. 2023; 15(3):897. https://doi.org/10.3390/pharmaceutics15030897
Chicago/Turabian StyleOtero, Carolina, Carolina Klagges, Bernardo Morales, Paula Sotomayor, Jorge Escobar, Juan A. Fuentes, Adrian A. Moreno, Felipe M. Llancalahuen, Ramiro Arratia-Perez, Felipe Gordillo-Fuenzalida, and et al. 2023. "Anti-Inflammatory Chilean Endemic Plants" Pharmaceutics 15, no. 3: 897. https://doi.org/10.3390/pharmaceutics15030897
APA StyleOtero, C., Klagges, C., Morales, B., Sotomayor, P., Escobar, J., Fuentes, J. A., Moreno, A. A., Llancalahuen, F. M., Arratia-Perez, R., Gordillo-Fuenzalida, F., Herrera, M., Martínez, J. L., & Rodríguez-Díaz, M. (2023). Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics, 15(3), 897. https://doi.org/10.3390/pharmaceutics15030897