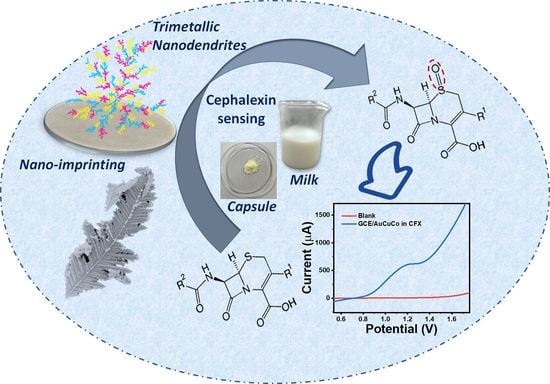
Electrochemical Nano-Imprinting of Trimetallic Dendritic Surface for Ultrasensitive Detection of Cephalexin in Pharmaceutical Formulations
Abstract
1. Introduction
2. Experiments
2.1. Chemicals and Instruments
2.2. Preparation of GCE/AuCoCu Dendritic Sensing Probe
2.3. Real Sample Preparation
2.4. Electrochemical Characterisations of the GCE/AuCoCu Dendritic Sensing Probe
3. Results and Discussions
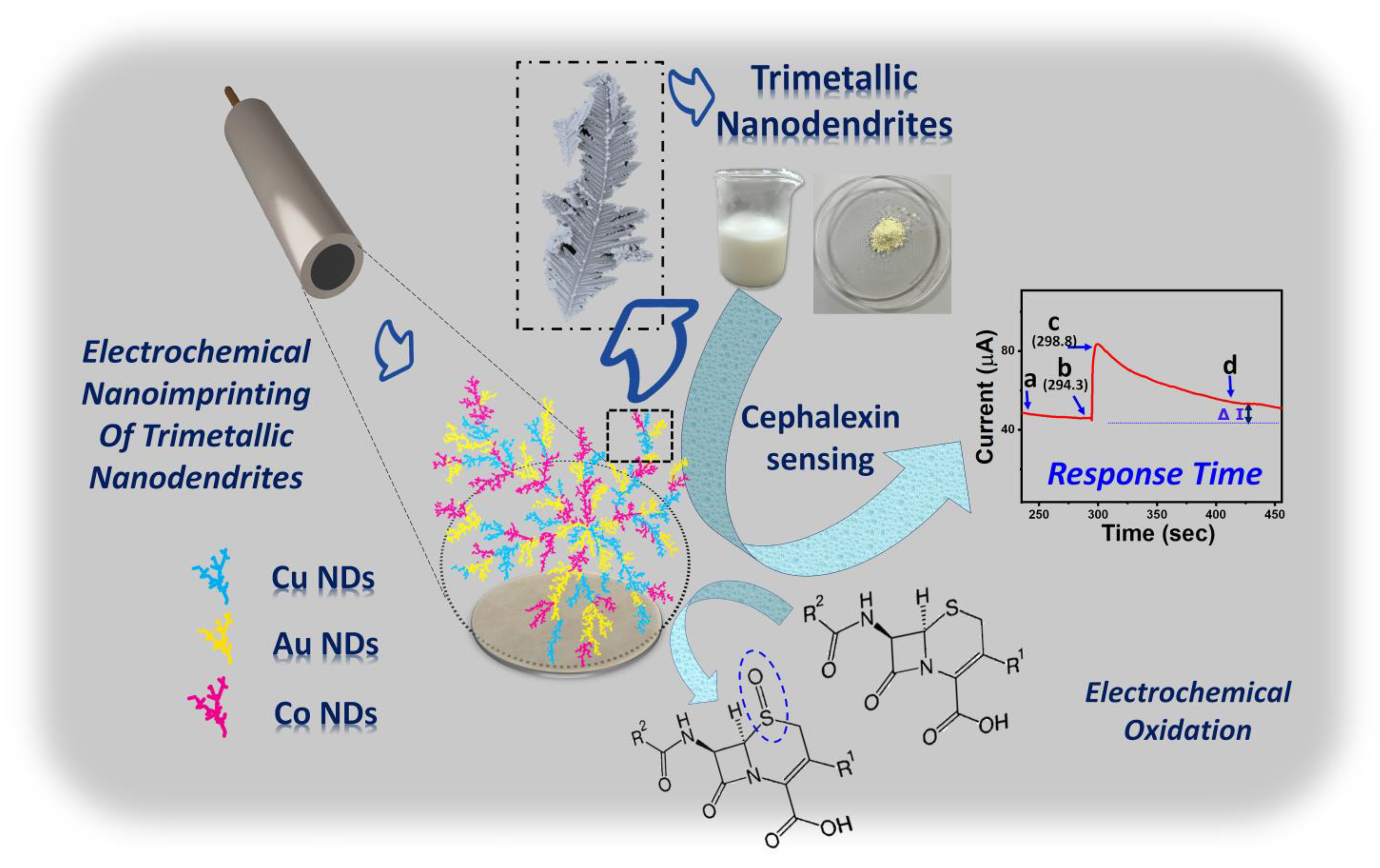
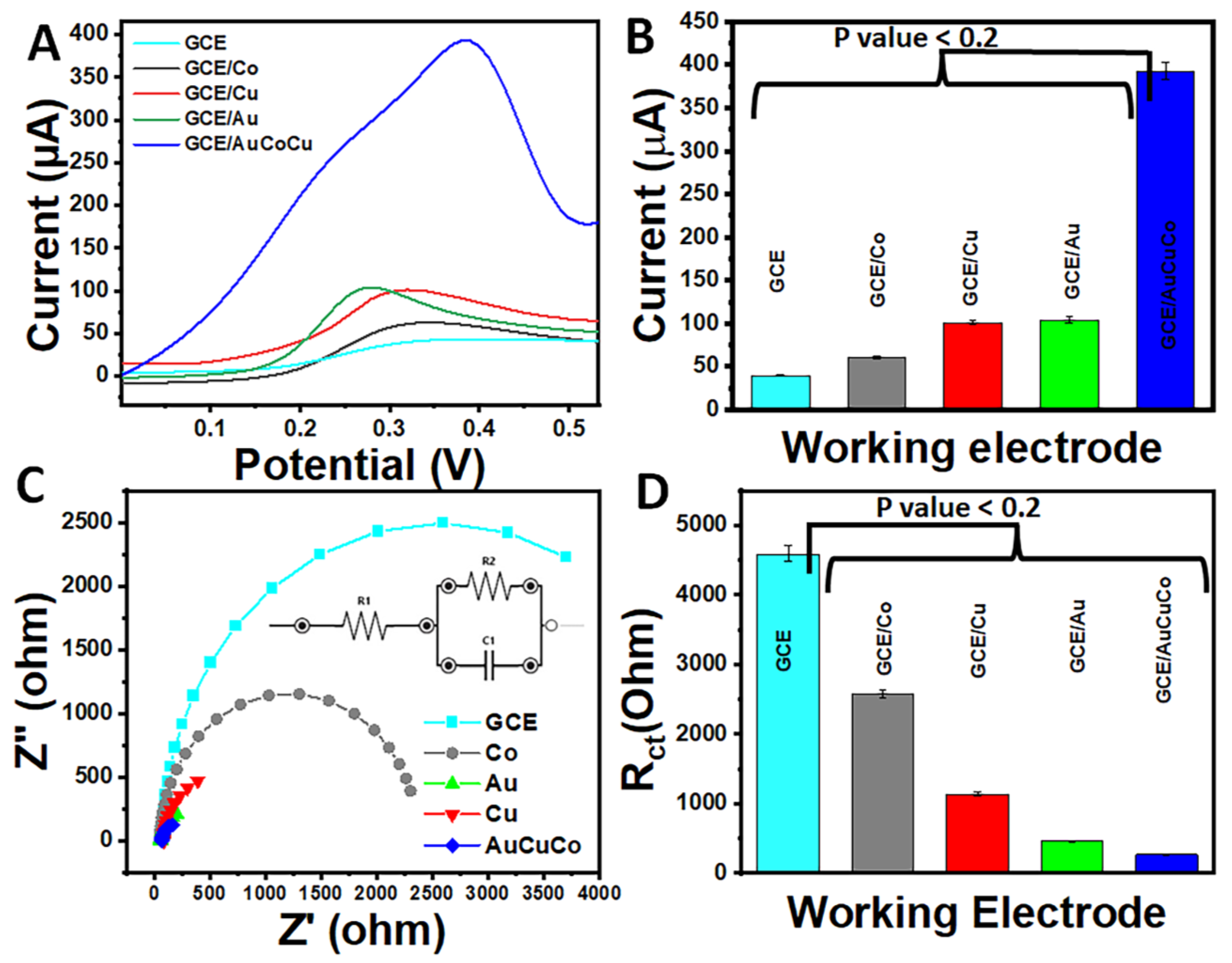
3.1. Electrochemical Nanoimprinting of 3D AuCoCu Dendritic Sensing Probe
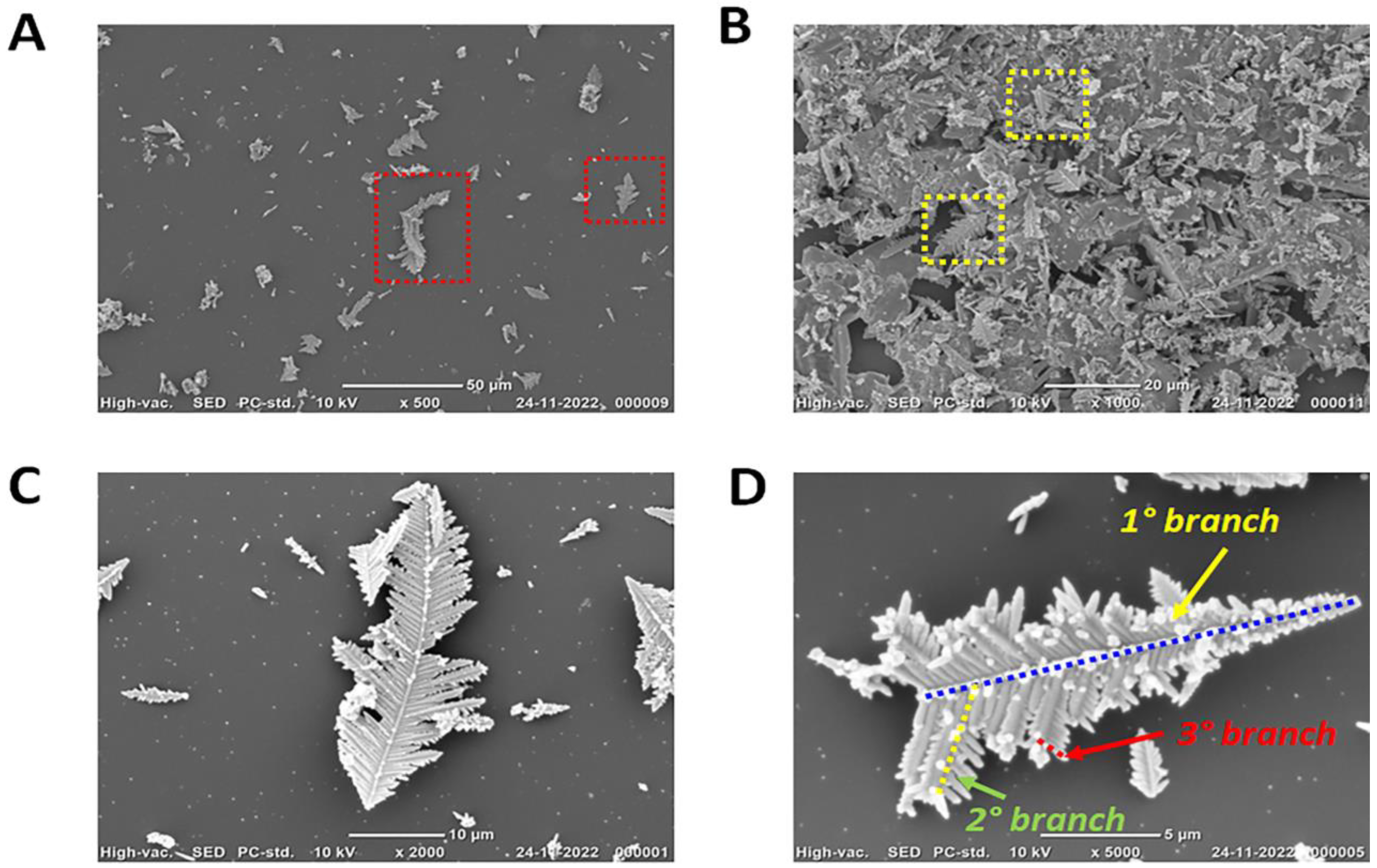
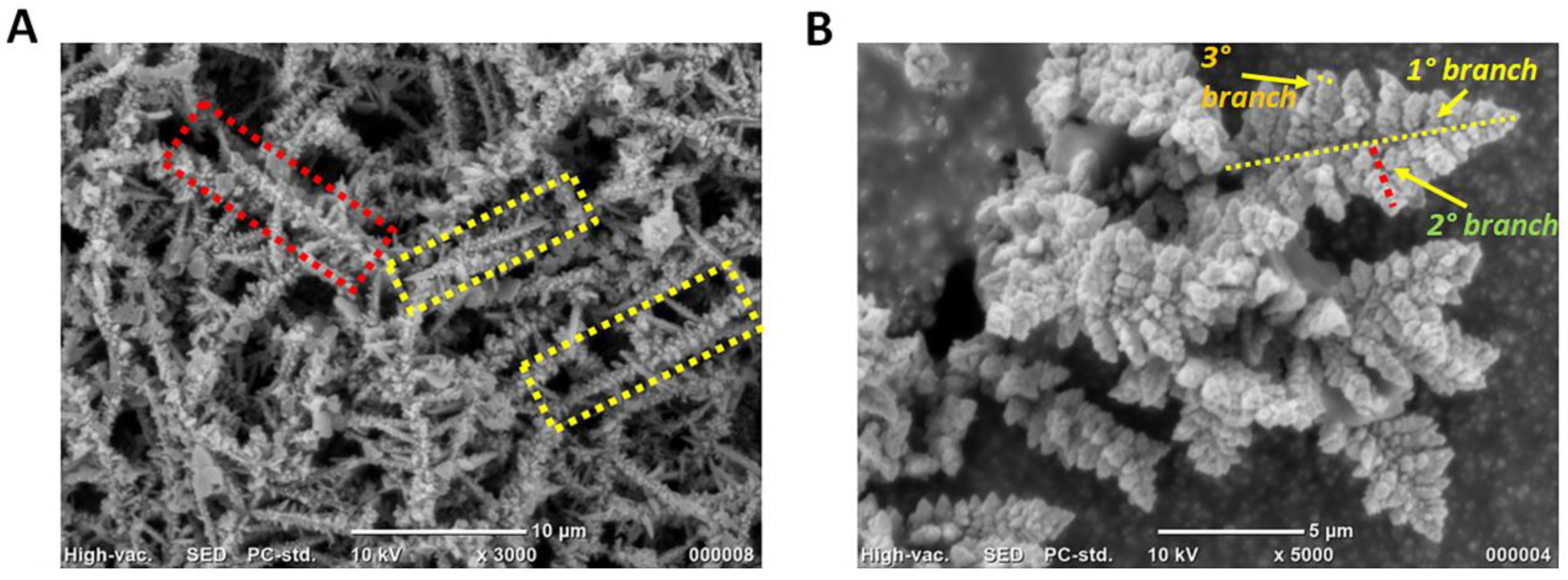
3.2. Physical Characterisation of 3D AuCoCu Dendritic Sensing Probe
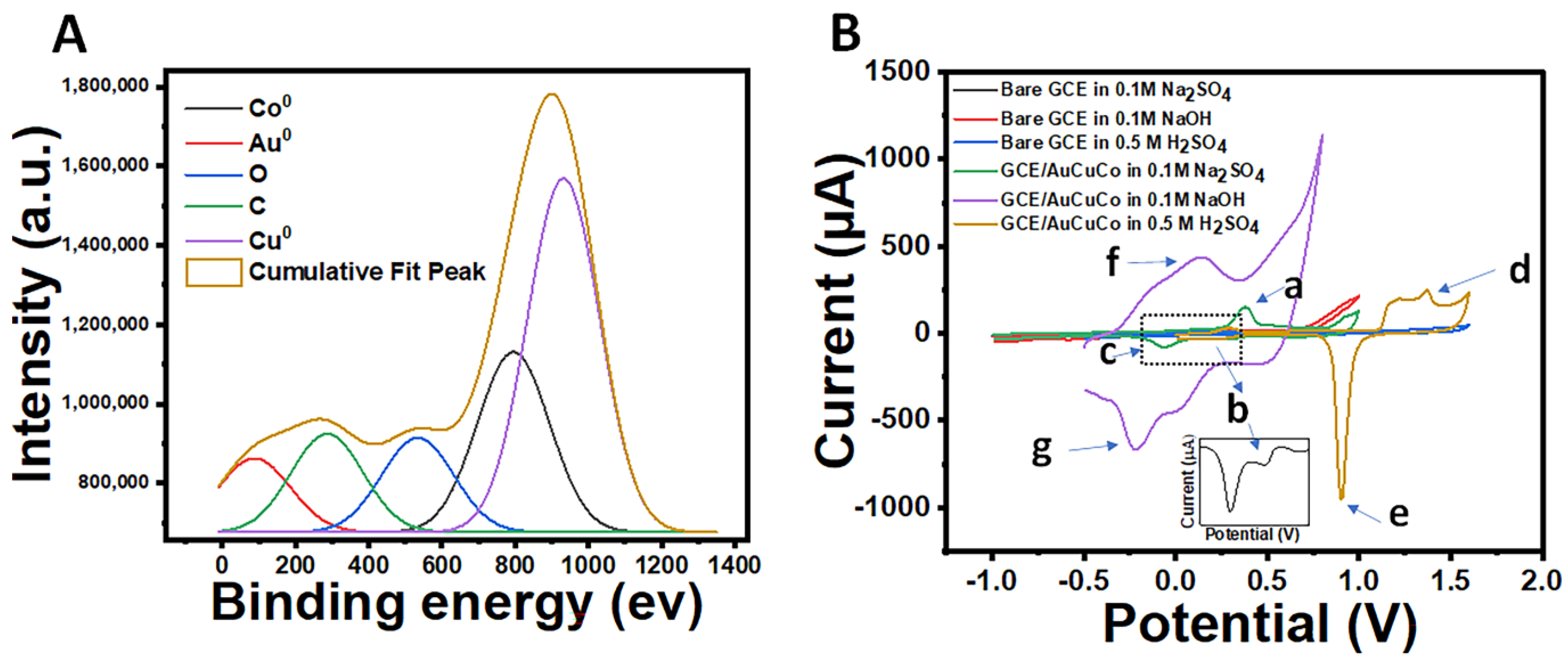
3.3. Validation of Au, Co, and Cu Trimetallic System Imprinted at the Electrode Surface
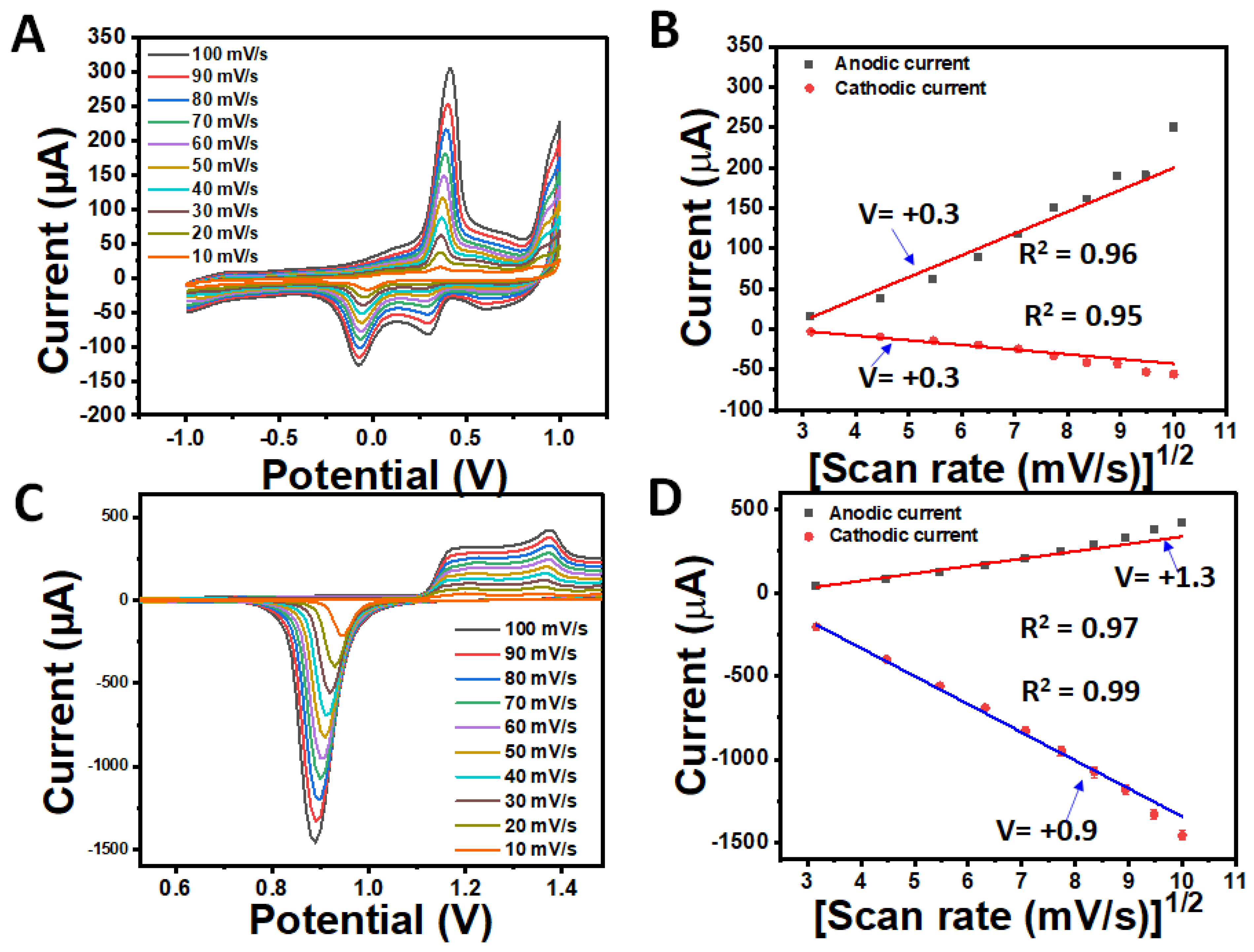
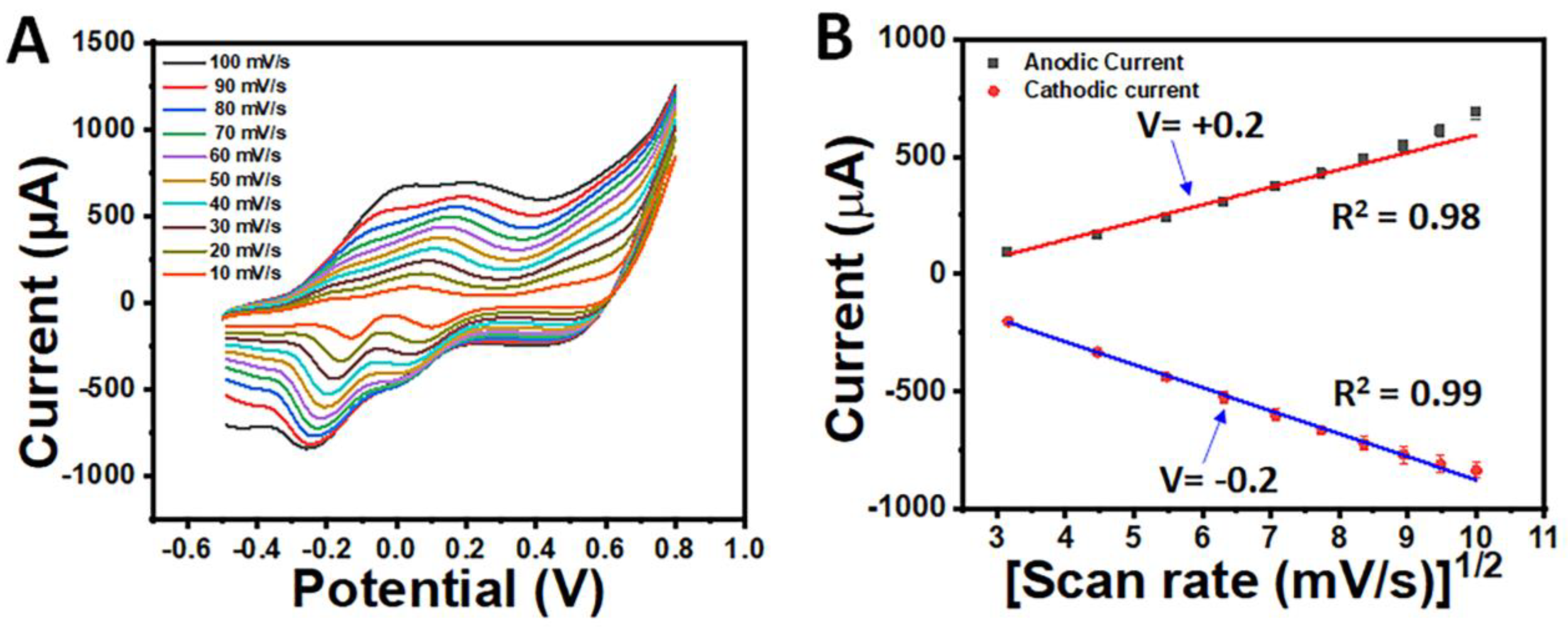
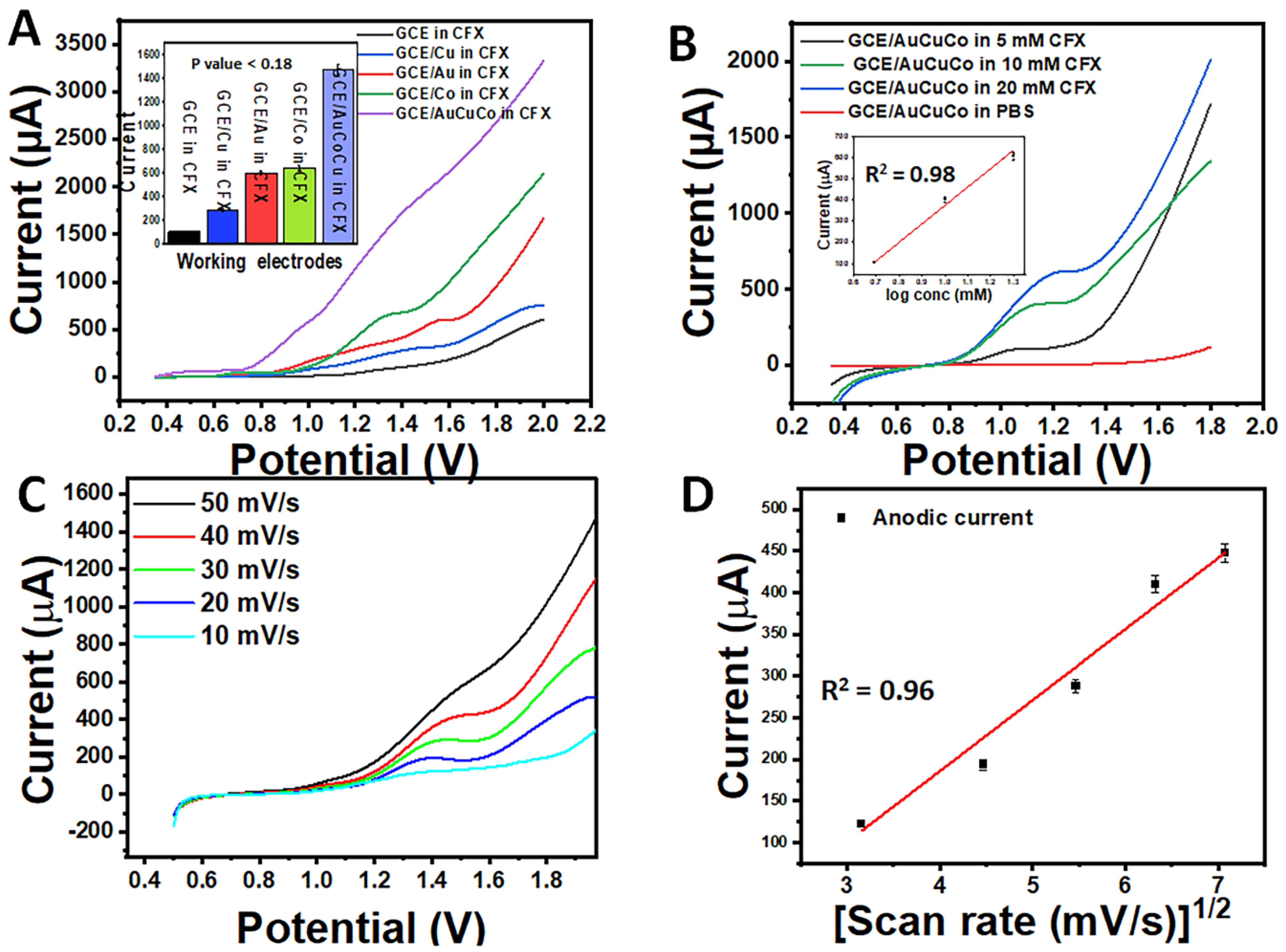
3.4. Electrochemical Characterisation of AuCoCu Dendritic Sensing Probe
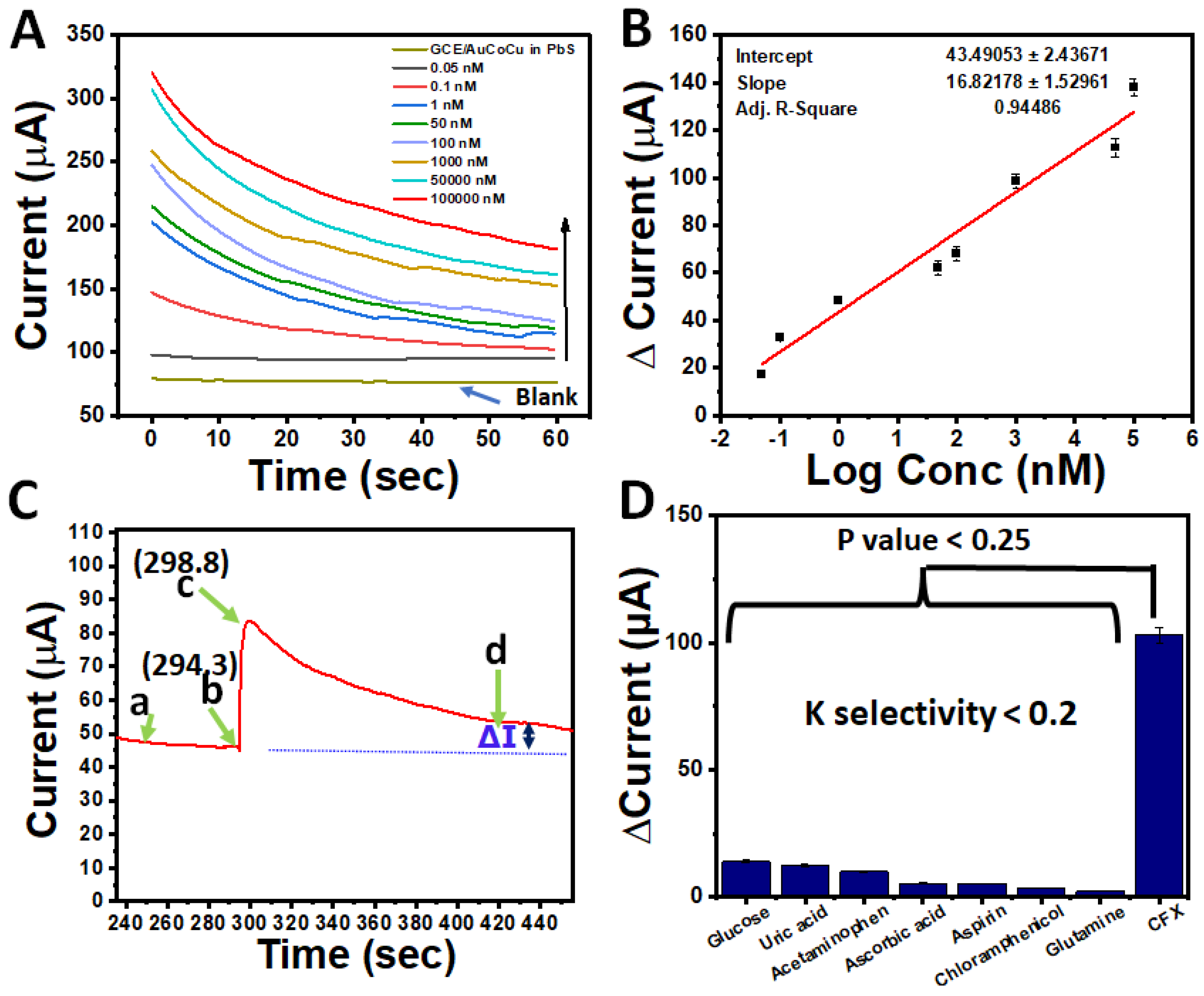
3.5. Analytical Investigations of AuCoCu Nanoimprinted Dendrites
3.6. Selectivity Assay
3.7. Real Sample Analysis
3.8. Reproducibility and Stability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, T.F.; Hashmi, M.F. Cephalexin; StatPearls Publishing: Tampa, FL, USA, 2022; pp. 1–6. Available online: www.ncbi.nlm.nih.gov/books/NBK549780 (accessed on 25 January 2023).
- Lai, E.P.C.; Wu, S.G. Molecularly Imprinted Solid Phase Extraction for Rapid Screening of Cephalexin in Human Plasma and Serum. Anal. Chim. Acta 2003, 481, 165–174. [Google Scholar] [CrossRef]
- Wang, K.; Guan, F.; Li, H.; Li, M.; Feng, H.; Fan, H. One-Step Synthesis of Carbon Nanodots for Sensitive Detection of Cephalexin. RSC Adv. 2015, 5, 20511–20515. [Google Scholar] [CrossRef]
- Benarab, N.; Fangninou, F.F. The Issues of Antibiotics: Cephalexin Antibiotic as Emerging Environment Contaminant. Int. J. Sci. Res. Publ. 2020, 10, 306–318. [Google Scholar] [CrossRef]
- DaCunha, M.; Moore, S.; Kaplan, D. Cephalexin-Induced Acute Generalized Exanthematous Pustulosis. Dermatol. Rep. 2018, 10, 7686. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Based Biosensors for Antibiotics Detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zou, H.; Sun, C.; Li, Y. Recent Advances in Biosensors for Antibiotic Detection: Selectivity and Signal Amplification with Nanomaterials. Food Chem. 2021, 361, 130109. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, H.S.; Shetty, S.S.; Thomas, N.J.; Dhamu, V.N.; Bhide, A.; Prasad, S. Ultrasensitive and Rapid-Response Sensor for the Electrochemical Detection of Antibiotic Residues within Meat Samples. ACS Omega 2019, 4, 6324–6330. [Google Scholar] [CrossRef]
- Feier, B.; Blidar, A.; Pusta, A.; Carciuc, P.; Cristea, C. Electrochemical Sensor Based on Molecularly Imprinted Polymer for the Detection of Cefalexin. Biosensors 2019, 9, 31. [Google Scholar] [CrossRef]
- Percin-Ozkorucuklu, S.; Uka, B.; Yildirim-Bastemur, G. Voltammetric Analysis of Cephalexin and Cefazolin in Pharmaceutical Formulations and Biological Samples. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 217–224. [Google Scholar] [CrossRef]
- Won, S.Y.; Chandra, P.; Hee, T.S.; Shim, Y.B. Simultaneous Detection of Antibacterial Sulfonamides in a Microfluidic Device with Amperometry. Biosens. Bioelectron. 2013, 39, 204–209. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Lobnik, A.; Turel, M.; Urek, Š.K. Optical Chemical Sensors: Design and Applications. In Advances in Chemical Sensors; Wen, W., Ed.; BoD—Books on Demand: Norderstedt, Germany, 2012; pp. 4–28. [Google Scholar] [CrossRef]
- Feier, B.; Gui, A.; Cristea, C.; Săndulescu, R. Electrochemical Determination of Cephalosporins Using a Bare Boron-Doped Diamond Electrode. Anal. Chim. Acta 2017, 976, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Chandra, P.; Pandey, L.M. Design and Characterization of Novel Al-Doped ZnO Nanoassembly as an Effective Nanoantibiotic. Appl. Nanosci. 2018, 8, 1925–1941. [Google Scholar] [CrossRef]
- Akhtar, M.H.; Hussain, K.K.; Gurudatt, N.G.; Chandra, P.; Shim, Y.B. Ultrasensitive Dual Probe Immunosensor for the Monitoring of Nicotine Induced-Brain Derived Neurotrophic Factor Released from Cancer Cells. Biosens. Bioelectron. 2018, 116, 108–115. [Google Scholar] [CrossRef]
- Mahato, K.; Prasad, A.; Maurya, P.; Chandra, P. Nanobiosensors: Next Generation Point-of-Care Biomedical Devices for Personalized Diagnosis. J. Anal. Bioanal. Technol. 2016, 7, 1000e1. [Google Scholar] [CrossRef]
- Chandra, P.; Prakash, R. Nanobiomaterial Engineering: Concepts and Their Applications in Biomedicine and Diagnostics; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789813298408. [Google Scholar]
- Kumari, R.; Dkhar, D.S.; Mahapatra, S.; Divya; Singh, S.P.; Chandra, P. Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective. Biosensors 2022, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Electrodeposition of Metallic Nanostructures for Biosensing Applications in Health Care. J. Sci. Res. 2020, 64, 68–73. [Google Scholar] [CrossRef]
- Purohit, B.; Kumar, A.; Mahato, K.; Srivastava, A.; Chandra, P. Engineered Three-Dimensional Au-Cu Bimetallic Dendritic Nanosensor for Ultrasensitive Drug Detection in Urine Samples and in Vitro Human Embryonic Kidney Cells Model. Microchem. J. 2022, 176, 107239. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. Therapeutic Nanodendrites: Current Applications and Prospects. Nanoscale Adv. 2020, 2, 5152–5165. [Google Scholar] [CrossRef] [PubMed]
- Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Flow Injection Enzyme-Free Amperometric Uric Acid Sensor Consisting of Ordered Mesoporous Carbon Decorated with 3D Pd-Pt Alloy Nanodendrite Modified Screen-Printed Carbon Electrode. Microchem. J. 2020, 157, 104923. [Google Scholar] [CrossRef]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Novel Sensing Assembly Comprising Engineered Gold Dendrites and MWCNT-AuNPs Nanohybrid for Acetaminophen Detection in Human Urine. Electroanalysis 2020, 32, 561–570. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Feng, J.; Wang, P.; Dong, Y. The Label-Free Immunosensor Based on Rhodium@palladium Nanodendrites/Sulfo Group Functionalized Multi-Walled Carbon Nanotubes for the Sensitive Analysis of Carcino Embryonic Antigen. Anal. Chim. Acta 2018, 1007, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhang, Y.; Zhang, L.; Liang, L.; Liu, H.; Yan, M.; Huang, J.; Yu, J. Ultrasensitive Electrochemical Cancer Cells Sensor Based on Trimetallic Dendritic Au@PtPd Nanoparticles for Signal Amplification on Lab-on-Paper Device. Sens. Actuators B Chem. 2015, 220, 665–672. [Google Scholar] [CrossRef]
- Protich, Z.; Santhanam, K.S.V.; Jaikumar, A.; Kandlikar, S.G.; Wong, P. Electrochemical Deposition of Copper in Graphene Quantum Dot Bath: Pool Boiling Enhancement. J. Electrochem. Soc. 2016, 163, E166. [Google Scholar] [CrossRef]
- Ma, W.; Ying, Y.L.; Qin, L.X.; Gu, Z.; Zhou, H.; Li, D.W.; Sutherland, T.C.; Chen, H.Y.; Long, Y.T. Investigating Electron-Transfer Processes Using a Biomimetic Hybrid Bilayer Membrane System. Nat. Protoc. 2013, 8, 439–450. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Quan, Z.L.; Song, G.; Kim, C.W.; Cha, H.G.; Kim, T.W.; Shin, W.; Lee, K.J.; Jung, M.H.; Kang, Y.S. Vertical Cobalt Dendrite Array Films: Electrochemical Deposition and Characterization, Glucose Oxidation and Magnetic Properties. J. Mater. Chem. 2012, 22, 12296–12304. [Google Scholar] [CrossRef]
- Allen, J.; Bard Larry, R. Faulkner ELECTROCHEMICAL METHODS Fundamentals and Applications Allen; Wiley: Hoboken, NJ, USA, 2019; ISBN 0471043729. [Google Scholar]
- Rodríguez Presa, M.J.; Gassa, L.M.; Azzaroni, O.; Gervasi, C.A. Estimating Diffusion Coefficients of Probe Molecules into Polyelectrolyte Brushes by Electrochemical Impedance Spectroscopy. Anal. Chem. 2009, 81, 7936–7943. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Huang, P.; Wang, H.; Zhang, L.; Zhao, Y. Self-Assembly of Gold Nanoparticles for the Voltammetric Sensing of Epinephrine. Electrochem. Commun. 2006, 8, 1035–1040. [Google Scholar] [CrossRef]
- Dillon, P.P.; Daly, S.J.; Browne, J.G.; Manning, B.M.; Loomans, E.; Van Amerongen, A.; O’Kennedy, R. Application of an Immunosensor for the Detection of the β-Lactam Antibiotic, Cephalexin. Food Agric. Immunol. 2003, 15, 225–234. [Google Scholar] [CrossRef]
- Sahu, S.; Karuppusamy, M.; Easwaramoorthi, S. Water-Dispersible Polymer Coated Silica Nanoparticle for Turn-on Fluorometric Detection of Cephalexin. Biosens. Bioelectron. X 2022, 12, 100231. [Google Scholar] [CrossRef]
- Kassa, A.; Amare, M.; Benor, A.; Tigineh, G.T.; Beyene, Y.; Tefera, M.; Abebe, A. Potentiodynamic Poly(Resorcinol)-Modified Glassy Carbon Electrode as a Voltammetric Sensor for Determining Cephalexin and Cefadroxil Simultaneously in Pharmaceutical Formulation and Biological Fluid Samples. ACS Omega 2022, 7, 34599–34607. [Google Scholar] [CrossRef] [PubMed]


| S. No. | Electrode Fabrication | LDR | LOD | Response Time | Real Sample | Reference |
|---|---|---|---|---|---|---|
| 1 | BDDE/MIP | 10–1000 nM | 4.9 nM | NR | River water and pharmaceutical samples | [9] |
| 2 | Sensor chip/CM/Dextran/Cephalexin | 0.667–10.689 nm | - | NR | Milk sample | [33] |
| 3 | MIP-coated silica nanoparticle | 50,000–6 × 105 nM | 1600 nM | NR | Urine | [34] |
| 4 | Carbon nanodots | 500–9500 nM | 311 nM | 6 min | NR | [3] |
| 5 | Poly(resorcinol)/GCE | 100–3 × 105 nM | 3.12 nM | NR | Blood, urine, And tablet samples | [35] |
| 6 | Bare BDDE | 500–7 × 105 nM, | 95 nM | NR | Capsules, river water, and human urine | [14] |
| 7 | GCE/AuCoCu NDs | 0.05–1 × 105 nM | 0.04 ± 0.01 nM | 4.5 ± 0.2 s | Pharmaceutical capsules and milk | This work |
| Co-Existing Molecules | Current Signal | Ksel |
|---|---|---|
| Glucose | 14 | 0.14 |
| Uric acid | 12.23 | 0.11 |
| Acetaminophen | 10.04 | 0.10 |
| Ascorbic acid | 5.35 | 0.05 |
| Aspirin | 5.20 | 0.05 |
| Chloramphenicol | 3.4 | 0.03 |
| Glutamine | 2.30 | 0.02 |
| CFX | 102.9 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, R.; Chandra, P. Electrochemical Nano-Imprinting of Trimetallic Dendritic Surface for Ultrasensitive Detection of Cephalexin in Pharmaceutical Formulations. Pharmaceutics 2023, 15, 876. https://doi.org/10.3390/pharmaceutics15030876
Kumari R, Chandra P. Electrochemical Nano-Imprinting of Trimetallic Dendritic Surface for Ultrasensitive Detection of Cephalexin in Pharmaceutical Formulations. Pharmaceutics. 2023; 15(3):876. https://doi.org/10.3390/pharmaceutics15030876
Chicago/Turabian StyleKumari, Rohini, and Pranjal Chandra. 2023. "Electrochemical Nano-Imprinting of Trimetallic Dendritic Surface for Ultrasensitive Detection of Cephalexin in Pharmaceutical Formulations" Pharmaceutics 15, no. 3: 876. https://doi.org/10.3390/pharmaceutics15030876
APA StyleKumari, R., & Chandra, P. (2023). Electrochemical Nano-Imprinting of Trimetallic Dendritic Surface for Ultrasensitive Detection of Cephalexin in Pharmaceutical Formulations. Pharmaceutics, 15(3), 876. https://doi.org/10.3390/pharmaceutics15030876