Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing
Abstract
1. Introduction
2. Wound Healing Process
2.1. Wound Types
2.2. Healing Process Phases
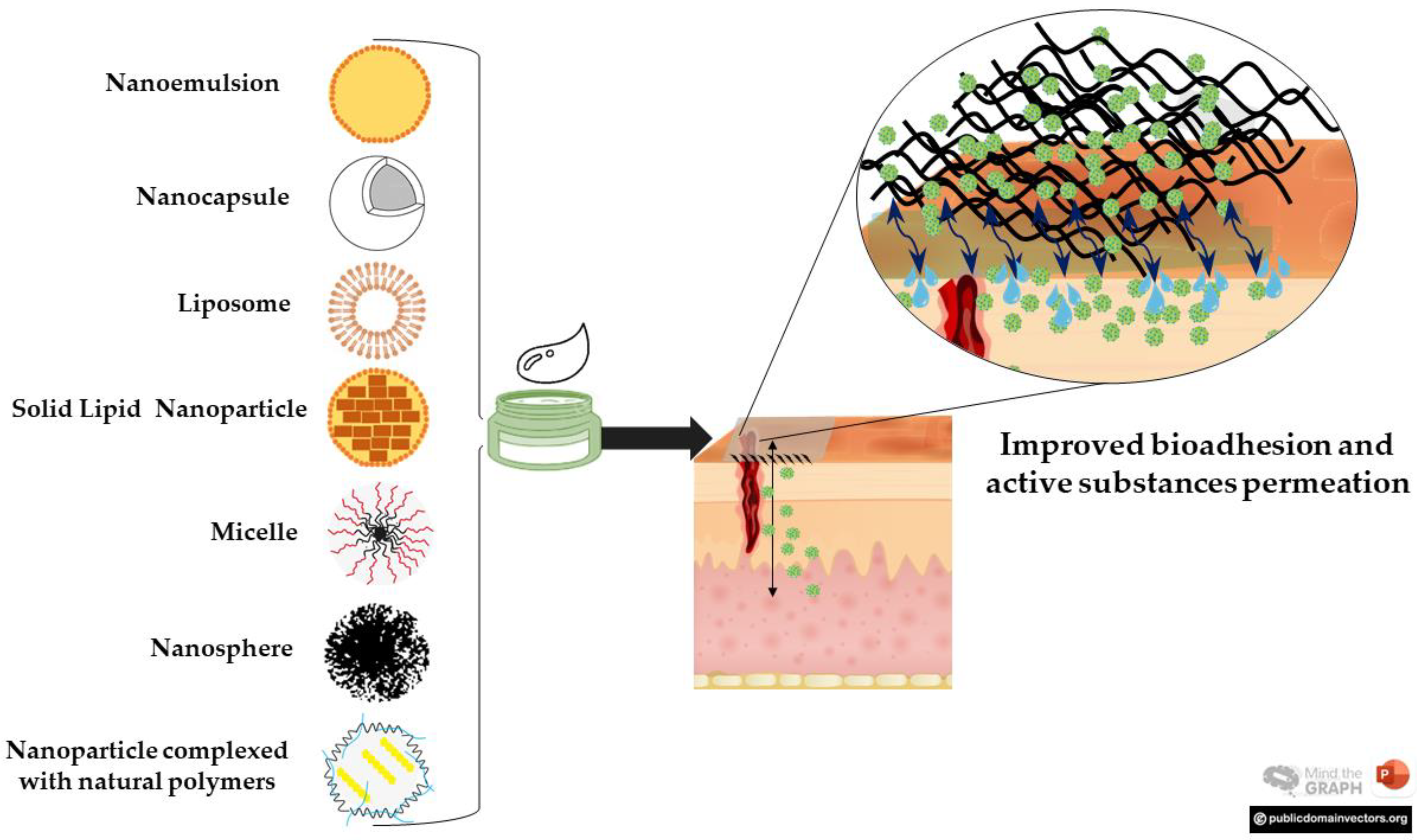
3. Soft Nanoparticles and Hydrogels for Wound Healing
3.1. Nanoemulsions
3.1.1. Natural Compounds-Based NE
3.1.2. Synthetic Drugs-Based Nanoemulsions
3.2. Solid Lipid Nanoparticles
3.2.1. Natural Products-Based Solid Lipid Nanoparticles
3.2.2. Synthetic Drugs-Based Solid Lipid Nanoparticles
3.3. Liposomes
3.4. Polymeric Systems
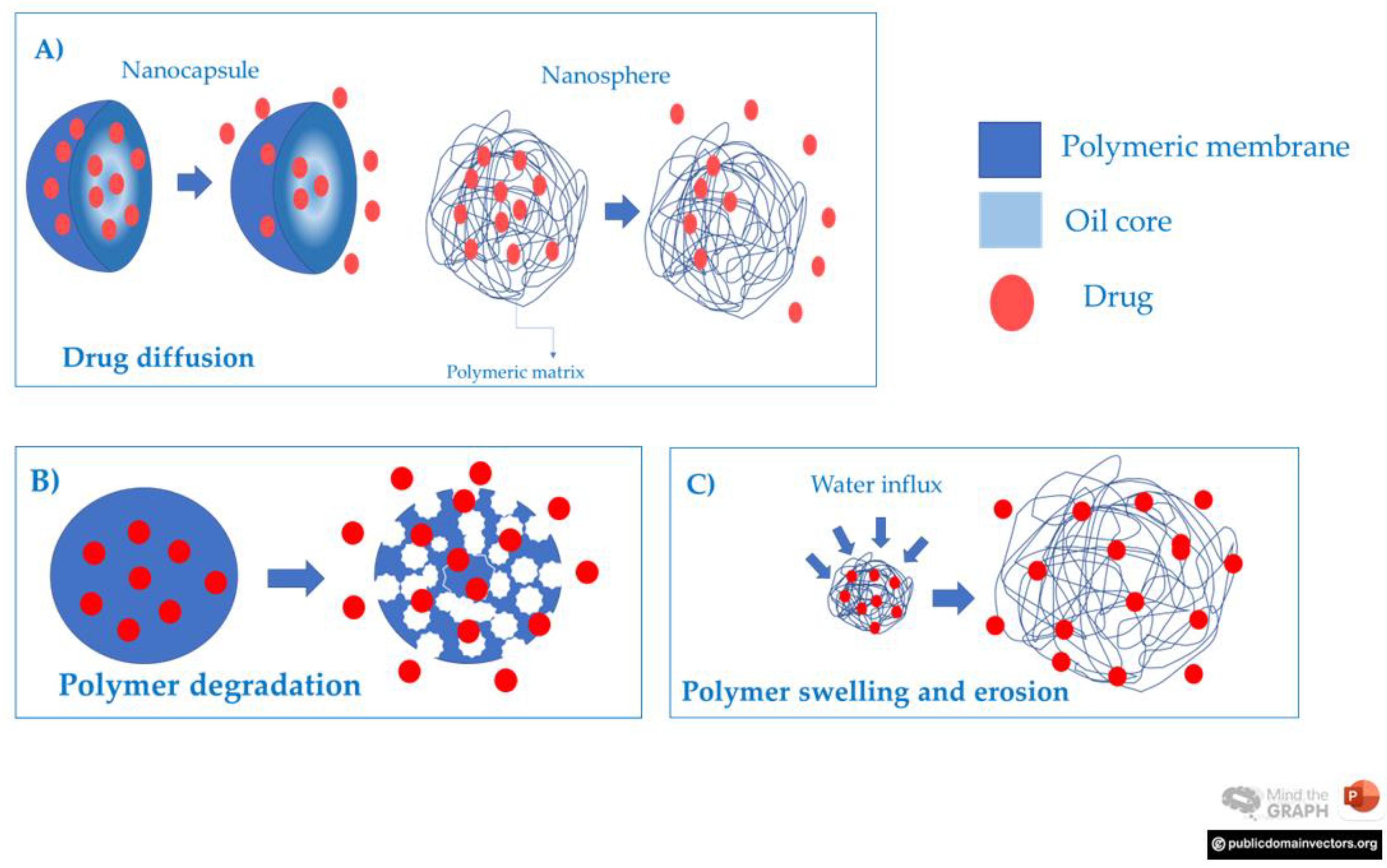
3.4.1. Nanocapsules and Nanospheres
3.4.2. Polymeric Micelles
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-Dependent Factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.; Mathieu, L.; Maibach, H.I. Acute Chemical Skin Injuries in the United States: A Review. Crit. Rev. Toxicol. 2018, 48, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Hosseinian-Far, M.; Kavoussi, H.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Rasoulpoor, S.; Mohammadi, M.; Shabani, S. Evaluation of Skin Lesions in Diabetic Patients: A Systematic Review and Meta-Analysis. J. Diabetes Metab. Disord. 2020, 19, 1909–1916. [Google Scholar] [CrossRef]
- Peralta, M.F.; Usseglio, N.A.; Bracamonte, M.E.; Guzmán, M.L.; Olivera, M.E.; Marco, J.D.; Barroso, P.A.; Carrer, D.C. Efficacy of Topical Miltefosine Formulations in an Experimental Model of Cutaneous Leishmaniasis. Drug Deliv. Transl. Res. 2022, 12, 180–196. [Google Scholar] [CrossRef]
- Miró, E.M.; Sánchez, N.P. Cutaneous Manifestations of Infectious Diseases. In Atlas of Dermatology in Internal Medicine; Springer: New York, NY, USA, 2012; Volume 40, pp. 77–119. [Google Scholar]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Raščan, I.M.; Smrke, D.M. Advanced Therapies of Skin Injuries. Wien. Klin. Wochenschr. 2015, 127, 187–198. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ye, H.; Wu, D. Recent Advances on Polymeric Hydrogels as Wound Dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective Are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Nayak, S.; Andrew Lyon, L. Soft Nanotechnology with Soft Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. [Google Scholar] [CrossRef]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite Hydrogels for Biomedical Applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. 2022, 61, e202107960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, P.; Yang, Z.; Liu, Y.; Yang, K.; Cheng, Y.; Yao, H.; Zhang, Z. Current Progress and Outlook of Nano-Based Hydrogel Dressings for Wound Healing. Pharmaceutics 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The Humanistic and Economic Burden of Chronic Wounds: A Protocol for a Systematic Review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Morbidelli, L.; Genah, S.; Cialdai, F. Effect of Microgravity on Endothelial Cell Function, Angiogenesis, and Vessel Remodeling During Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 720091. [Google Scholar] [CrossRef]
- Herman, T.F.; Bordoni, B. Wound Classification. In Principles of Surgery Vivas for the MRCS; Cambridge University Press: Cambridge, UK, 2022; pp. 323–328. [Google Scholar] [CrossRef]
- Quinn, J.V.; Polevoi, S.K.; Kohn, M.A. Traumatic Lacerations: What Are the Risks for Infection and Has the “golden Period” of Laceration Care Disappeared? Emerg. Med. J. 2014, 31, 96–100. [Google Scholar] [CrossRef]
- Leaper, D.J. Traumatic and Surgical Wounds. BMJ 2006, 332, 532–535. [Google Scholar] [CrossRef]
- Smith, N.; Overland, J.; Greenwood, J.E. Local Management of Deep Cavity Wounds – Current and Emerging Therapies. Chronic Wound Care Manag. Res. 2015, 2, 159–170. [Google Scholar] [CrossRef]
- Warby, R.; Maani, C.V. Burn Classification; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mokni, M. Cutaneous Leishmaniasis. Ann. Dermatol. Venereol. 2019, 146, 232–246. [Google Scholar] [CrossRef]
- Kaye, P.M.; Cruz, I.; Picado, A.; Van Bocxlaer, K.; Croft, S.L. Leishmaniasis Immunopathology—Impact on Design and Use of Vaccines, Diagnostics and Drugs. Semin. Immunopathol. 2020, 42, 247–264. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-Quality Health Systems in the Sustainable Development Goals Era: Time for a Revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.D.B.; de Araújo, T.M.E. Prevalence and Factors Associated with Chronic Wounds in Older Adults in Primary Care. Rev. Esc. Enferm. USP 2018, 52, e03415. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef]
- Chen, C.S.; Su, W.H.; Cheng, M.H.; Lee, W.L.; Tsou, T.S.; Chang, W.H.; Wang, P.H. Nonsteroidal Anti-Inflammatory Drugs for Wounds: Pain Relief or Excessive Scar Formation? Mediat. Inflamm. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Krischak, G.D.; Augat, P.; Claes, L.; Kinzl, L.; Beck, A. The Effects of Non-Steroidal Anti-Inflammatory Drug Application on Incisional Wound Healing in Rats. J. Wound Care 2007, 16, 76–78. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Nyangoga, H.; Aguado, E.; Goyenvalle, E.; Baslé, M.F.; Chappard, D. A Non-Steroidal Anti-Inflammatory Drug (Ketoprofen) Does Not Delay β-TCP Bone Graft Healing. Acta Biomater. 2010, 6, 3310–3317. [Google Scholar] [CrossRef]
- Sevimli, R.; Üzel, M.; Sayar, H.; Kalender, A.M.; Dökmeci, Ö. The Effect of Dexketoprofen Trometamol on the Healing of Diaphysis Fractures of Rat Tibia. Acta Orthop. Traumatol. Turc. 2013, 47, 423–429. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Espinosa-Cano, E.; Rojo, L.; Boulmedais, F.; Aguilar, M.R.; Hernández, R. Injectable Tripeptide/Polymer Nanoparticles Supramolecular Hydrogel: A Candidate for the Treatment of Inflammatory Pathologies. ACS Appl. Mater. Interfaces 2022, 14, 10068–10080. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.; Peneva, P.; Georgiev, G.S.; Toncheva, V.T.; Apostolova, E.; Peychev, Z.; Dimitrova, S.; Katsarova, M.; Petrova, N.; Kassarova, M. Ketoprofen-Loaded Polymer Carriers in Bigel Formulation: An Approach to Enhancing Drug Photostability in Topical Application Forms. Int. J. Nanomed. 2017, 12, 6221–6238. [Google Scholar] [CrossRef] [PubMed]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Turkoglu Sasmazel, H. Novel Poly(ε-Caprolactone)/Gelatin Wound Dressings Prepared by Emulsion Electrospinning with Controlled Release Capacity of Ketoprofen Anti-Inflammatory Drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Yenilmez, E.; Yazan, Y. Dexketoprofen Trometamol-Loaded Eudragit® Rl 100 Nanoparticle Formulation, Characterization and Release Kinetics. ACTA Pharm. Sci. 2019, 57, 69. [Google Scholar] [CrossRef]
- de Souza, M.L.; dos Santos, W.M.; de Sousa, A.L.M.D.; de Albuquerque Wanderley Sales, V.; Nóbrega, F.P.; de Oliveira, M.V.G.; Rolim-Neto, P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020, 26, 4536–4550. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2022, 8, 1198. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound Dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-Dimensional Nanomaterials: Fascinating Materials in Biomedical Field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef]
- Elkhoury, K.; Russell, C.S.; Sanchez-Gonzalez, L.; Mostafavi, A.; Williams, T.J.; Kahn, C.; Peppas, N.A.; Arab-Tehrany, E.; Tamayol, A. Soft-Nanoparticle Functionalization of Natural Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2019, 8, 1900506. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological Status of Nanoparticles: What We Know and What We Don’t Know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174. [Google Scholar] [CrossRef]
- Reimer, K.; Vogt, P.M.; Broegmann, B.; Hauser, J.; Rossbach, O.; Kramer, A.; Rudolph, P.; Bosse, B.; Schreier, H.; Fleischer, W. An Innovative Topical Drug Formulation for Wound Healing and Infection Treatment: In Vitro and in Vivo Investigations of a Povidone-Iodine Liposome Hydrogel. Dermatology 2000, 201, 235–241. [Google Scholar] [CrossRef]
- Hurler, J.; Berg, O.A.; Skar, M.; Conradi, A.H.; Johnsen, P.J.; Škalko-Basnet, N. Improved Burns Therapy: Liposomes-in-Hydrogel Delivery System for Mupirocin. J. Pharm. Sci. 2012, 101, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Škalko-Basnet, N. Liposomes-in-Hydrogel Delivery System with Mupirocin: In Vitro Antibiofilm Studies and In Vivo Evaluation in Mice Burn Model. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.H.; Shan, Y.H.; Li, N.; Wei, W.; Yu, L.; Li, Q.M.; Liang, W.Q.; Gao, J.Q. Astragaloside IV-Loaded Nanoparticle-Enriched Hydrogel Induces Wound Healing and Anti-Scar Activity through Topical Delivery. Int. J. Pharm. 2013, 447, 171–181. [Google Scholar] [CrossRef]
- Flores, F.C.; De Lima, J.A.; Da Silva, C.R.; Benvegnú, D.; Ferreira, J.; Burger, M.E.; Beck, R.C.R.; Rolim, C.M.B.; Rocha, M.I.U.M.; Da Veiga, M.L.; et al. Hydrogels Containing Nanocapsules and Nanoemulsions of Tea Tree Oil Provide Antiedematogenic Effect and Improved Skin Wound Healing. J. Nanosci. Nanotechnol. 2015, 15, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Synthesis and Preparation of Biodegradable Hybrid Dextran Hydrogel Incorporated with Biodegradable Curcumin Nanomicelles for Full Thickness Wound Healing. Int. J. Pharm. 2017, 532, 466–477. [Google Scholar] [CrossRef]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In Vitro and In Vivo Characterization of Quercetin Loaded Multiphase Hydrogel for Wound Healing Application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical Delivery of Tetrahydrocurcumin Lipid Nanoparticles Effectively Inhibits Skin Inflammation: In Vitro and in Vivo Study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, Characterization, and in Vivo Evaluation of Nano Formulations of Ferulic Acid in Diabetic Wound Healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef]
- Dawoud, M.H.S.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Insulin Mucoadhesive Liposomal Gel for Wound Healing: A Formulation with Sustained Release and Extended Stability Using Quality by Design Approach. AAPS PharmSciTech 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; de Oliveira, E.G.; Coradini, K.; Bruinsmann, F.A.; Aguirre, T.; Lorenzoni, R.; Barcelos, R.C.S.; Roversi, K.; Rossato, D.R.; Pohlmann, A.R.; et al. Chitosan Hydrogels Containing Nanoencapsulated Phenytoin for Cutaneous Use: Skin Permeation/Penetration and Efficacy in Wound Healing. Mater. Sci. Eng. C 2019, 96, 205–217. [Google Scholar] [CrossRef]
- Aly, U.F.; Abou-Taleb, H.A.; Abdellatif, A.A.H.; Tolba, N.S. Formulation and Evaluation of Simvastatin Polymeric Nanoparticles Loaded in Hydrogel for Optimum Wound Healing Purpose. Drug Des. Dev. Ther. 2019, 13, 1567–1580. [Google Scholar] [CrossRef]
- Oveissi, F.; Tavakoli, N.; Minaiyan, M.; Mofid, M.R.; Taheri, A. Alginate Hydrogel Enriched with Ambystoma Mexicanum Epidermal Lipoxygenase-Loaded Pectin Nanoparticles for Enhanced Wound Healing. J. Biomater. Appl. 2020, 34, 1171–1187. [Google Scholar] [CrossRef]
- Patel, M.; Nakaji-Hirabayashi, T.; Matsumura, K. Effect of Dual-drug-releasing Micelle–Hydrogel Composite on Wound Healing in Vivo in Full-thickness Excision Wound Rat Model. J. Biomed. Mater. Res. Part A 2019, 107, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Parisotto-Peterle, J.; Bidone, J.; Lucca, L.G.; Araújo, G.d.M.S.; Falkembach, M.C.; da Silva Marques, M.; Horn, A.P.; dos Santos, M.K.; da Veiga, V.F.; Limberger, R.P.; et al. Healing Activity of Hydrogel Containing Nanoemulsified β-Caryophyllene. Eur. J. Pharm. Sci. 2020, 148, 105318. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Cargnin, S.T.; Costa, S.A.; Sinhorin, V.D.G.; Damazo, A.S.; Sinhorin, A.P.; Bicudo, R.d.C.; Cavalheiro, L.; Valladão, D.M.d.S.; Pohlmann, A.R.; et al. Healing of Dermal Wounds Property of Caryocar Brasiliense Oil Loaded Polymeric Lipid-Core Nanocapsules: Formulation and in Vivo Evaluation. Eur. J. Pharm. Sci. 2020, 150, 105356. [Google Scholar] [CrossRef] [PubMed]
- Back, P.I.; Balestrin, L.A.; Fachel, F.N.S.; Nemitz, M.C.; Falkembach, M.; Soares, G.; Marques, M.d.S.; Silveira, T.; Dal Prá, M.; Horn, A.P.; et al. Hydrogels Containing Soybean Isoflavone Aglycones-Rich Fraction-Loaded Nanoemulsions for Wound Healing Treatment—In Vitro and in Vivo Studies. Colloids Surf. B Biointerfaces 2020, 196, 111301. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kiick, K.L.; Sullivan, M.O. Encapsulation of Collagen Mimetic Peptide-Tethered Vancomycin Liposomes in Collagen-Based Scaffolds for Infection Control in Wounds. Acta Biomater. 2020, 103, 115–128. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of Green Tea Polyphenol Nanospheres in PVA/Alginate Hydrogel for Promoting Wound Healing of Diabetic Rats by Regulating PI3K/AKT Pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Correa, V.L.R.; da Silva, F.K.L.; Casas, A.A.; das Chagas, A.d.L.; de Oliveira, L.P.; Miguel, M.P.; Diniz, D.G.A.; Amaral, A.C.; de Menezes, L.B. Wound Healing Treatment Using Insulin within Polymeric Nanoparticles in the Diabetes Animal Model. Eur. J. Pharm. Sci. 2020, 150, 105330. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid Gelation of Oxidized Hyaluronic Acid and Succinyl Chitosan for Integration with Insulin-Loaded Micelles and Epidermal Growth Factor on Diabetic Wound Healing. Mater. Sci. Eng. C 2020, 117, 111273. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef]
- Thapa, R.K.; Winther-Larsen, H.C.; Ovchinnikov, K.; Carlsen, H.; Diep, D.B.; Tønnesen, H.H. Hybrid Hydrogels for Bacteriocin Delivery to Infected Wounds. Eur. J. Pharm. Sci. 2021, 166, 105990. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and in-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and in Vivo Evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef]
- Ansari, M.N.; Soliman, G.A.; Rehman, N.U.; Anwer, M.K. Crisaborole Loaded Nanoemulsion Based Chitosan Gel: Formulation, Physicochemical Characterization and Wound Healing Studies. Gels 2022, 8, 318. [Google Scholar] [CrossRef]
- Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y.; et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics 2022, 14, 1971. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lin, S.-J. Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation. Pharmaceutics 2022, 14, 537. [Google Scholar] [CrossRef]
- Gupta, B.; Sharma, G.; Sharma, P.; Sandhu, S.K.; Kaur, I.P. Self-Gelling Solid Lipid Nanoparticle Hydrogel Containing Simvastatin as Suitable Wound Dressing: An Investigative Study. Gels 2022, 8, 58. [Google Scholar] [CrossRef]
- Reczyńska-Kolman, K.; Hartman, K.; Kwiecień, K.; Brzychczy-Włoch, M.; Pamuła, E. Composites Based on Gellan Gum, Alginate and Nisin-Enriched Lipid Nanoparticles for the Treatment of Infected Wounds. Int. J. Mol. Sci. 2021, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Daodu, I.M.; Ilomuanya, M.O.; Azubuike, C.P. Development of Curcumin-Loaded Liposomes in Lysine–Collagen Hydrogel for Surgical Wound Healing. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 100. [Google Scholar] [CrossRef]
- Abou El-ezz, D.; Abdel-Rahman, L.H.; Al-Farhan, B.S.; Mostafa, D.A.; Ayad, E.G.; Basha, M.T.; Abdelaziz, M.; Abdalla, E.M. Enhanced In Vivo Wound Healing Efficacy of a Novel Hydrogel Loaded with Copper (II) Schiff Base Quinoline Complex (CuSQ) Solid Lipid Nanoparticles. Pharmaceuticals 2022, 15, 978. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; Sabra, S.A.; El-Hosseiny, L.S. Encapsulation of Thymus Vulgaris Essential Oil in Caseinate/Gelatin Nanocomposite Hydrogel: In Vitro Antibacterial Activity and in Vivo Wound Healing Potential. Int. J. Pharm. 2022, 628, 122280. [Google Scholar] [CrossRef]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process Selection and Application in Cosmetics—A Review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural Product-Based Nanomedicines for Wound Healing Purposes: Therapeutic Targets and Drug Delivery Systems. Int. J. Nanomed. 2018, 13, 5023. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.A.; Abou-Zekry, S.S.; Sami, D.G.; Abdellatif, A. Natural Products as Wound Healing Agents. In Wound Healing Research; Springer: Singapore, 2021; pp. 77–94. [Google Scholar] [CrossRef]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The Use of Nanoencapsulation to Decrease Human Skin Irritation Caused by Capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef]
- Zuben, V.; Bergonzi, C.; Oliveira Eloy, J.; Destro Inácio, M.; Hugo Sousa Araujo, V.; Martins Baviera, A.; Palmira Daflon Gremião, M.; Chorilli, M. Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics 2022, 14, 2492. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; Akisin, C.J.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric Biomaterials for Wound Healing Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1998–2050. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.U.; Khan, H.M.S.; Rasool, F.; Saeed, T.; Murtaza, G. Penetration Enhancing Effect of Polysorbate 20 and 80 on the in Vitro Percutaneous Absorption of L-Ascorbic Acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation Enhancer Strategies in Transdermal Drug Delivery. Drug Deliv. 2014, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.X.; Affendi, M.M.; Chong, P.P.; Lee, S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer 2022, 74, 3058–3076. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; Ribeiro, R.F.; Ourique, A.F.; Rolim, C.M.B.; De Bona Da Silva, C.; Pohlmann, A.R.; Beck, R.C.R.; Guterres, S.S. Nanostructured Systems Containing an Essential Oil: Protection against Volatilization. Quim. Nova 2011, 34, 968–972. [Google Scholar] [CrossRef]
- Suhail, M.; Wu, P.-C.; Minhas, M.U. Using Carbomer-Based Hydrogels for Control the Release Rate of Diclofenac Sodium: Preparation and In Vitro Evaluation. Pharmaceuticals 2020, 13, 399. [Google Scholar] [CrossRef]
- Rossetti, A.; Pizzetti, F.; Rossi, F.; Mauri, E.; Borghi, E.; Ottaviano, E.; Sacchetti, A. Synthesis and Characterization of Carbomer-Based Hydrogels for Drug Delivery Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 743–753. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative Stress in Normal and Impaired Wound Repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Külkamp, I.C.; Rabelo, B.D.; Berlitz, S.J.; Isoppo, M.; Bianchin, M.D.; Schaffazick, S.R.; Pohlmann, A.R.; Guterres, S.S. Nanoencapsulation Improves the In Vitro Antioxidant Activity of Lipoic Acid. J. Biomed. Nanotechnol. 2011, 7, 598–607. [Google Scholar] [CrossRef]
- Paton, D.M. Crisaborole: Phosphodiesterase Inhibitor for Treatment of Atopic Dermatitis. Drugs Today 2017, 53, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kandregula, B.; Narisepalli, S.; Chitkara, D.; Mittal, A. Exploration of Lipid-Based Nanocarriers as Drug Delivery Systems in Diabetic Foot Ulcer. Mol. Pharm. 2022, 19, 1977–1998. [Google Scholar] [CrossRef] [PubMed]
- Rampaka, R.; Ommi, K.; Chella, N. Role of Solid Lipid Nanoparticles as Drug Delivery Vehicles on the Pharmacokinetic Variability of Erlotinib HCl. J. Drug Deliv. Sci. Technol. 2021, 66, 102886. [Google Scholar] [CrossRef]
- Seyithanoglu, M.H.; Abdallah, A.; Kitis, S.; Güler, E.M.; Koçyigit, A.; Dündar, T.T.; Papaker, M.G. Investigation of Cytotoxic, Genotoxic, and Apoptotic Effects of Curcumin on Glioma Cells. Cell. Mol. Biol. 2019, 65, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.R.; Patel, R.P.; Patel, M.M. Poloxamers: A Pharmaceutical Excipients with Therapeutic Behaviors. Int. J. PharmTech Res. 2009, 1, 299–303. [Google Scholar]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Spížek, J.; Sigler, K.; Řezanka, T.; Demain, A. Biogenesis of Antibiotics—Viewing Its History and Glimpses of the Future. Folia Microbiol. 2016, 61, 347–358. [Google Scholar] [CrossRef]
- Henrique Marcondes Sari, M.; Mota Ferreira, L.; Cruz, L. The Use of Natural Gums to Produce Nano-Based Hydrogels and Films for Topical Application. Int. J. Pharm. 2022, 626, 122166. [Google Scholar] [CrossRef]
- Rasool, A.; Al, B.K.; Skalko-Basnet, N.; Rasool, B.K.A.; Al Mahri, N.; Alburaimi, N.; Abdallah, F.; Saeed, A.; Shamma, B. A Narrative Review of the Potential Roles of Lipid-Based Vesicles (Vesiculosomes) in Burn Management. Sci. Pharm. 2022, 90, 39. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Norman, G.; Dumville, J.C.; Mohapatra, D.P.; Owens, G.L.; Crosbie, E.J. Antibiotics and Antiseptics for Surgical Wounds Healing by Secondary Intention. Cochrane Database Syst. Rev. 2016, 2022, CD011712. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Yu, C.; Huang, Q.; Du, Y.-Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable Hemostatic Hydrogels Comprising Composites of Sacrificial Templates and Honeycomb-like Nanofibrous Mats of Chitosan. Nat. Commun. 2019, 10, 2307. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Fasiku, V.O.; Omolo, C.A.; Devnarain, N.; Ibrahim, U.H.; Rambharose, S.; Faya, M.; Mocktar, C.; Singh, S.D.; Govender, T. Chitosan-Based Hydrogel for the Dual Delivery of Antimicrobial Agents Against Bacterial Methicillin-Resistant Staphylococcus Aureus Biofilm-Infected Wounds. ACS Omega 2021, 6, 21994–22010. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Patel, M.; Kaneko, T.; Matsumura, K. Switchable Release Nano-Reservoirs for Co-Delivery of Drugs via a Facile Micelle–Hydrogel Composite. J. Mater. Chem. B 2017, 5, 3488–3497. [Google Scholar] [CrossRef]



| Reference | Active | System | Gelling Agent | Wound Protocol |
|---|---|---|---|---|
| Reimer et al., 2000 [61] | Povidone-Iodine | LP | Polyacrylic acid | Burn and chronic wound |
| Hurler et al., 2012 [62] | Mupirocin | LP | Chitosan | Burn Wound |
| Hurler et al., 2013 [63] | Mupirocin | LP | Chitosan | Burn Wound |
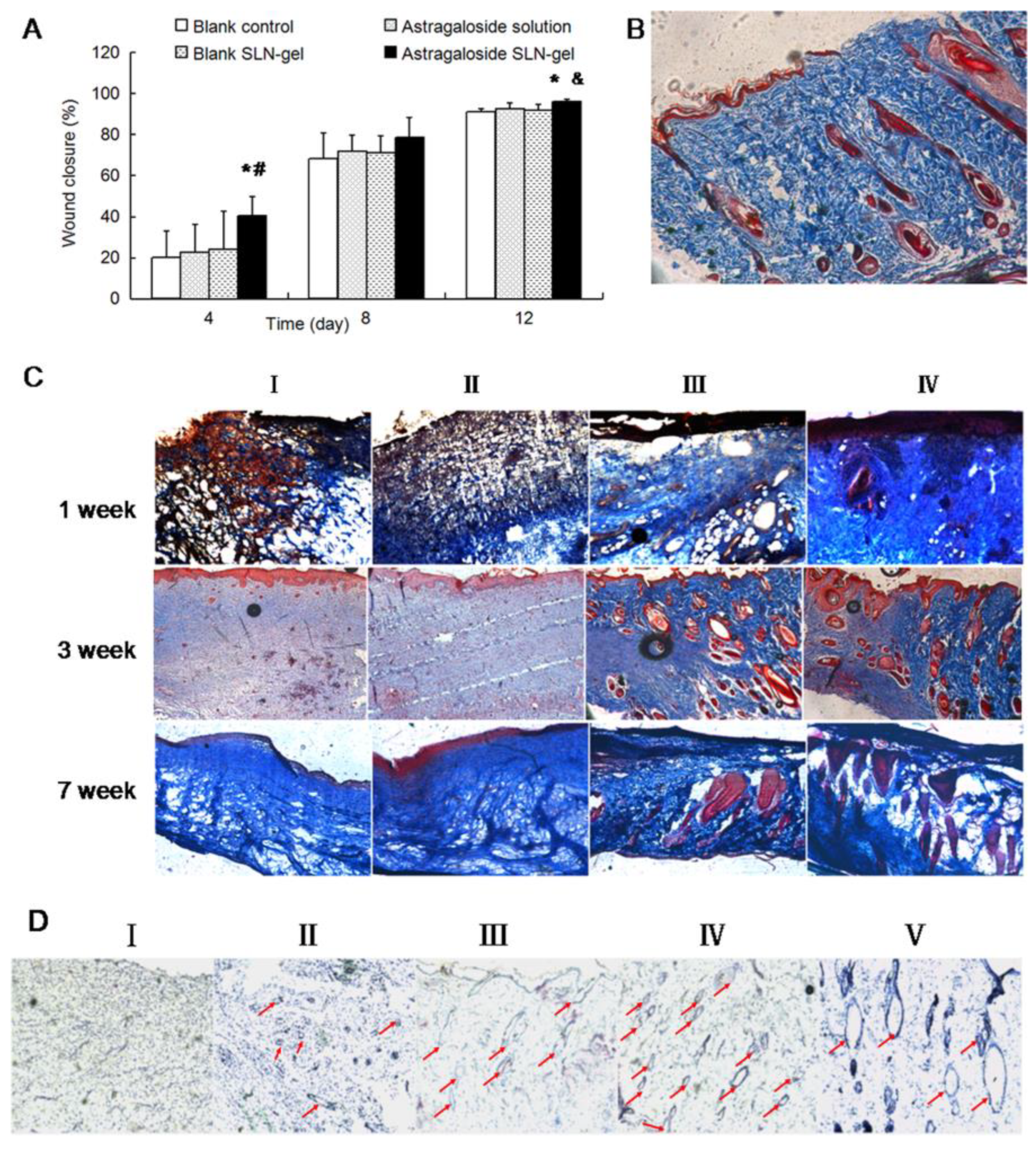
| Chen et al., 2013 [64] | Astragaloside IV | SLN | Carbomer 934 | Wound scratch assay and Excisional Wound |
| Flores et al., 2015 [65] | Tea tree oil | NC and NE | Carbopol® Ultrez 10 NF | Excisional Wound |
| Alibolandi et al., 2017 [66] | Curcumin | MC | Dextran | Excisional Wound |
| Jangde et al., 2018 [67] | Quercetin | LP | Carbopol® | Excisional Wound |
| Kakkar et al., 2018 [68] | Tetrahydrocurcumin | SLN | Carbopol® 934 | Excisional Wound |
| Bairagi et al., 2018 [69] | Ferulic acid | NS | Carbopol® 980 | Diabetic wound |
| Dawoud et al., 2019 [70] | Insulin | LP | Chitosan | Diabetic wound |
| Cardoso et al., 2019 [71] | Phenytoin | NC and NE | Chitosan | Excisional Wound |
| Aly et al., 2019 [72] | Simvastatin | PN | Carbopol® 934 | Excisional Wound |
| Oveissi et al., 2019 [73] | Ambystoma mexicanum epidermal lipoxygenase | PN | Alginate | Excisional Wound |
| Patel et al., 2019 [74] | Curcumin and amphotericin | MC | Genipin | Excisional Wound |
| Parisotto-Peterle et al., 2020 [75] | β-caryophyllene | NE | Hydroxyethylcellulose | Excisional Wound |
| Pires et al., 2020 [76] | Caryocar brasiliense oil | NC | HPMC | Excisional Wound |
| Back et al., 2020 [77] | Soybean isoflavone | NE | Carbopol® Ultrez-21 | Excisional Wound |
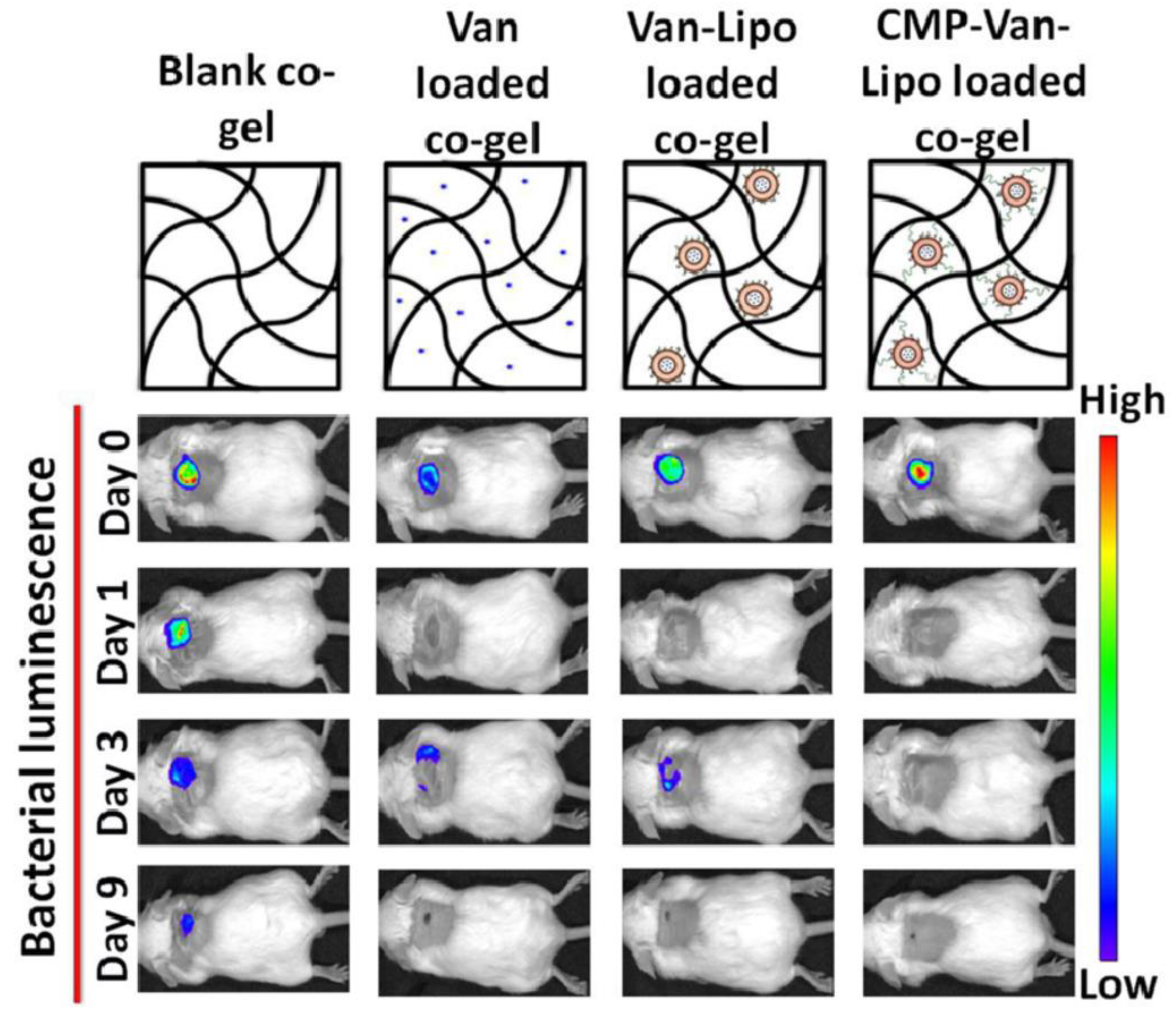
| Thapa et al., 2020 [78] | Vancomycin | LP | Collagen | Infected wound |
| Chen et al., 2020 [79] | Green tea polyphenol | NS | PVA/Alginate | Diabetic Wound |
| Ribeiro et al., 2020 [80] | Insulin | PN | Sepigel | Diabetic Wound |
| Zhu et al., 2020 [81] | Insulin | MC | Hyaluronic acid and chitosan | Diabetic Wound |
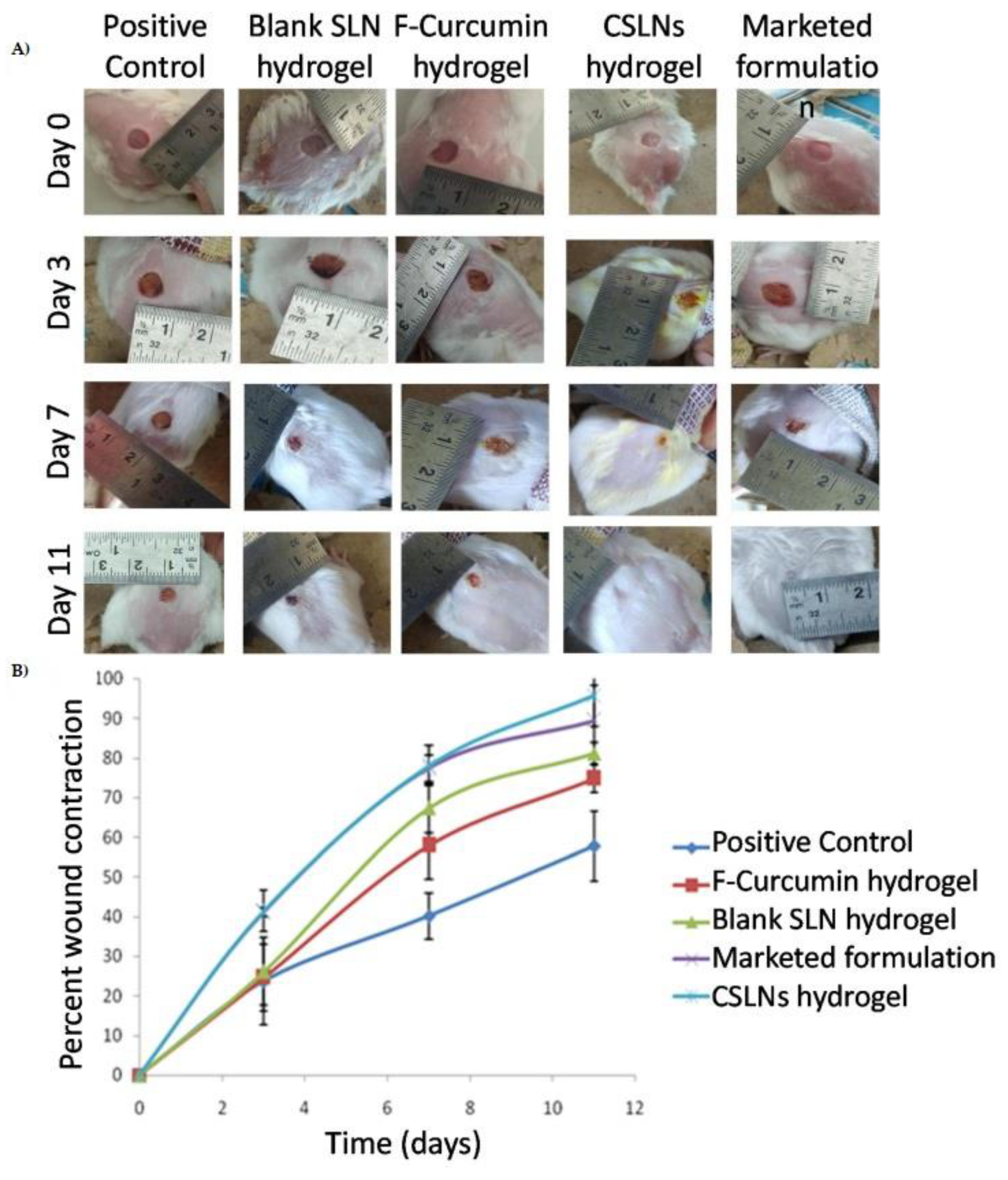
| Sandhu et al., 2021 [82] | Curcumin extract | SLN | Carbopol® 934P | Burn Wound |
| Tahpa et al., 2021 [83] | Ethylenediaminetetraacetic | LP | Pluronic F127 and bacteriocin Garvicin KS (GarKS) | Infected wound |
| Algahtani et al., 2021 [84] | Thymoquinone | NE | Carbopol® 940 | Excisional Wound |
| Algahtani et al., 2021 [85] | Curcumin | NE | Carbopol® 940 | Excisional Wound |
| Ansari et al., 2022 [86] | Crisaborole | NE | Chitosan | Excisional Wound |
| Rehman et al., 2022 [87] | Eucalyptol | NE | Carbopol® 940 | Excisional Wound |
| Lee and Lin, 2022 [88] | Perfluorocarbon bromide and epidermal growth factor | NE and NP | Chitosan and PVA | Diabetic wound |
| Gupta et al., 2022 [89] | Simvastatin | SLN | Carbopol® 934 | Excisional Wound |
| Reczyńska-Kolman et al., 2022 [90] | Peptide—nisin | SLN | Gellan gum and Sodium Alginate | Cell migration and wound healing in vitro |
| Peralta et al., 2022 [7] | Miltefosine | LP | Carbomer 934-P NF | Cutaneous Leishmaniosis |
| Cardoso-Daodu et al., 2022 [91] | Curcumim | LP | Carbopol® Ultrez | Excisional Wound |
| El-ezz et al., 2022 [92] | Copper (II) Schiff Base Quinoline | SLN | Carbopol® 940 | Excisional Wound |
| Alsakhawy et al., 2022 [93] | Thymus vulgaris essential oil | MC | Gelatin | Excisional Wound |
| Reference | Main Findings | Formulations’ Hallmark Features |
|---|---|---|
| Reimer et al., 2000 [61] | A hydrogel of polyacrylic acid containing povidone-iodine-loaded phosphatidylcholine LP for achieving both antiseptic and moist treatment was prepared. The nanocarriers presented a multilamellar structure and particle size between 0.2 and 4.5 µm. An improved interaction with microorganisms (Candida albicans and Staphylococcus aureus) was achieved, which enhanced the antimicrobial action. The nano-based semisolid spreads easily and had significantly better tolerability in comparison with the conventional povidone-iodine ointment | Rate of neo-epithelization: NH > 90% × CP < 80% |
| Hurler et al., 2012; 2013 [62,63] | The liposomal suspensions showed an entrapment efficiency of mupirocin of 62.4% (±8.8%). The formulation was stable at different storage conditions (cold and accelerated studies) and presented a prolonged and controlled-release of mupirocin from the nano-based hydrogel. A satisfactory antimicrobial potential was obtained for chitosan hydrogel containing mupirocin-loaded phosphatidylcholine LP and an antibiofilm effect. The formulation presented promising effects in a mouse model of burn | Retention of formulation on the skin surface: NH~50% × CP < 20% Wound size after 20 days: NH~4 nm × H 4–6 nm × CP~6 nm |
| Chen et al., 2013 [64] | Astragaloside IV was incorporated into SLN, using glycerol tristearate as a lipid component, and further thickened in a Carbopol® 934 hydrogel. Astragaloside IV-based SLNs enhanced the proliferation and migration of keratinocytes, indicating its capability to accelerate wound re-epithelialization | Wound scratch assay—wound closure (%): SLN > drug solution > C In vivo model—wound closure (%): NH > BH > drug solution > C |
| Flores et al., 2015 [65] | Melaleuca alternifolia essential oil was incorporated into NC and NE to prepared Carbopol® Ultrez hydrogel for investigating its potential application in wound healing. NC-based semisolid was more promising in comparison to the NE. The formulation reduced the wound area and accelerated re-epithelization and angiogenesis to a greater extent than NE-based hydrogel | Spreadability factor (mm2/g): NCH 4.53 × NEH 4.78 × FH 2.85 × BH~3.70 Wound area reduction (%): NCH >50% × NEH < 40% × FH~40% × BH~25% |
| Alibolandi et al., 2017 [66] | Curcumin-loaded polylactic acid–polyethylene glycol (PLA–PEG) nanomicelles were prepared and converted into a dextran hydrogel. The water absorption property of dextran hydrogel lasted until eight days after its preparation. Curcumin was slower released when nanomicelles were incorporated into hydrogel than the suspension form. Curcumin nanomicelle/dextran hydrogel presented a sustained drug release from a hydrated device, reducing inflammatory responses, promoting fibroblast proliferation and collagen production, and improving angiogenesis in wound healing | Collagen deposition: NH > BH > C Wound area (cm2): NH < BH < C |
| Jangde et al., 2018 [67] | The nano-based formulations had a water vapor transmission rate suitable for assisting in maintaining the humectants’ properties of the skin. The quercetin-loaded LP hydrogel was found to be stable under both normal and accelerated conditions, hemocompatible, and presented an adequate swelling ratio to the management of wounds | Wound contraction after 12 days: NH 98.93% × CP 92.97% × control 76.83% Epithelization period (days): NH 16 × CP 18 × control 26 |
| Kakkar et al., 2018 [68] | The tetrahydrocurcumin-loaded SLN (Compritol® 888 ATO and Phospholipon 90G as a lipid matrix) hydrogel formed an intact lipid film on the filter paper surface, suggesting a possible occlusion effect on the formulation, which could be attributed to the solid nature of the lipid components. Otherwise, vehicle Carbopol® 934 hydrogels had the lowest occlusion factor due to the absence of any lipids in the formulation | Occlusion factor after 48 h (%): NH~83 × BH~82 × H~23 × CP~2 |
| Bairagi et al., 2018 [69] | Ferulic acid-loaded polymeric nanoparticles dispersion (oral administration) and ferulic acid-loaded polymeric nanoparticles based hydrogel (topical administration) treated wounds were found to epithelize faster as compared with the diabetic wound control group. Both oral and topical treatments showed faster healing in comparison to diabetic control groups. The group that received both oral and topical treatments showed the best pharmacological effect. Nanoparticles presented a biphasic drug release profile, consisting of an initial burst release followed by sustained release up to 48 h | Wound area (cm2): NH < CP < FH |
| Dawoud et al., 2019 [70] | The LP (egg phosphatidylcholine and cholesterol) presented particle size around 250 nm, negative zeta potential values, and 87.379% entrapment efficiency of insulin. The nano-based hydrogel presented high physicochemical stability, suitable viscosity, and sustained the insulin release for up to 24 h. In a diabetic wound healing model, an improvement of 16-fold in the healing rate in comparison to the control group was observed, suggesting a highly stable and therapeutically promising drug delivery system for diabetic wound management | In vitro drug release: FH 6 h × NH 24 h Healing rate: NH 36.67 ± 12.179 mm2/day × H 36.67 ± 12.179 mm2/day |
| Cardoso et al., 2019 [71] | Phenytoin was incorporated into an NC (poly-epsilon-caprolactone and grape seed oil) or NE system (grape seed oil). A superior content of phenytoin reached the dermis layer from the liquid form of NE in comparison to NC. As expected, the drug penetration increased using injured skin, reaching the epidermis and dermis layers | Consistency index (Pasn): NCH 24.23 × NEH 24.53 × FH 55.25 BH~17 Work of adhesion (mN.mm): NCH 9.5 × NEH 10.3 × FH 1.1 Wound area contraction (%) after day 6: NCH~0.6 × NEH > 0.6 × FH < 0.6 |
| Aly et al., 2019 [72] | The simvastatin-loaded NS (PEG 4000) Carbopol® 940 hydrogel showed significant results in the healing process, presenting complete epithelialization, minimal inflammatory cell infiltration, mature collagen fiber formation, and more activated hair follicle growth after 11 days of the protocol | Wound contraction (%): NH~90 × C~80 |
| Oveissi et al., 2019 [73] | The alginate hydrogel containing epidermal lipoxygenase enzyme-loaded pectin nanoparticles had a swelling ratio higher than empty alginate hydrogel. Nano-based formulation demonstrated better mechanical properties than empty hydrogel. A sustained delivery of drug from nano-based hydrogel was achieved in comparison to drug solution and hydrogel containing non-encapsulated pectin | The score of wound re-epithelialization: NH 1.66 × BH 0.66 × C 0.33 |
| Patel et al., 2019 [74] | The study applied a dual-drug release system composed of polylactic acid–polyethylene glycol to deliver an anti-bacterial drug (amphotericin B) during the early stages of healing, followed by a slow release rate of the healing enhancer drug (curcumin). Better healing was observed in rats treated with the drug-loaded hydrogels than in the blank and control groups. Wounds showed up to 80% closure in the treated group, with high collagen deposition. Re-epithelialization and granulation were also better in the treated group than in the non-treated control and blank groups | Wound closure (%): NH~87 × BH~33 × C19 |
| Parisotto-Peterle et al., 2020 [75] | The hydroxyethylcellulose hydrogels containing β-caryophyllene-loaded NE presented 892.40 ± 81.53 mN and 444.37 ± 78.82 mN of maximum force for detachment in intact skin and injured skin (stratum corneum removal), respectively. In vivo healing study revealed that treatment with the NE-based hydrogel accelerated wound closure (with reduced surface area and wound contraction) and that the effect may be related to a decrease in the acute inflammatory process as evidenced by a decrease in IL-1, TNF-α, and MPO markers | Drug permeation: Stratum corneum: drug solution > NE > NH Epidermis: NE = NH > drug solution Dermis: NE > NH > drug solution |
| Pires et al., 2020 [76] | The obtained hydrogels were homogeneous after associating the nanosystems (poly-episolon-caprolactone lipid core NC), presenting suitable topical applicability and spreadability, with a viscosity ranging from 8130 and 11,740 cp. The lesions’ average area of the nano-based hydrogel was 0.043 cm2, which was 400-fold lower than the other groups. Notably, no lesions were observed after 14 days of treatment | Lesion area (cm2): NH 0.043 × BH 0.454 × FH 0.634 × CP 0.131 |
| Back et al., 2020 [77] | Soybean isoflavone aglycones(IAF)-loaded NE dispersed in acrylic-acid hydrogels have been investigated as potential wound healing compounds for cutaneous application. The findings demonstrated the potential of nano-based hydrogels to promote wound healing by increasing angiogenesis, reducing lipid oxidation, and inflammation. | Drug permeation in intact skin: Stratum corneum: NH > NE > drug solution Epidermis: NH > NE > drug solution Dermis: NH > NE > drug solution Drug permeation in skin without stratum corneum: Epidermis: NE > NH > drug solution Dermis: NE > NH > drug solution |
| Thapa et al., 2020 [78] | The LP was composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC):Cholesterol:1,2-distearoyl-snglycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000-maleimide] at a molar ratio of 73:24:3 and had 150 nm of diameter with a low PDI (0.05) and negative zeta potential (−3.0 mV). The LP-based semisolid formulation exhibited desirable viscosity and rheological properties intending topical applications along with the controlled release of the peptide (up to nine days). Additionally, potent in vitro antibacterial and anti-biofilm effects devoid of fibroblast toxicity were assigned to the nano-based hydrogel gel. The mouse model of infected wounds demonstrated potent antibacterial effects of nano-based hydrogel | Antibacterial effects in wound site: NH up to 9 days × FH < 2 days |
| Chen et al., 2020 [79] | The PVA-alginate hydrogel containing green tea polyphenol NS enhanced the mobility of epidermal cells in vitro. In the diabetic wound model, hydrogel containing green tea polyphenol-loaded NS provided wound closure faster than control and vehicle hydrogel groups. Molecular investigations indicated that nano-based hydrogel containing green tea polyphenols may reduce inflammation and enhance diabetic wound healing by activating the PI3K/AKT pathway | Wound size (µm2)—in vitro: NH < FH < C |
| Ribeiro et al., 2020 [80] | Insulin-loaded chitosan nanoparticles were prepared by the ionotropic gelation method. Healing evaluations showed that polymorph nuclear infiltrate was more significant for blank- and insulin-chitosan nanoparticles, demonstrating that chitosan exerted chemotaxis of these cells, stimulating their migration to the wound | Degree of wound: CP > NH > FH > BH |
| Zhu et al., 2020 [81] | The hydrogels containing insulin-loaded micelles and epidermal growth factor showed an excellent wound healing performance for the promotion of fibroblast proliferation and tissue internal structure integrity, as well as the deposition of collagen and myofibrils | Wound area (%): NH < BH < Gauze |
| Sandhu et al., 2021 [82] | The curcumin-loaded SLN (Compritol® 888 ATO and Phospholipon 90G) association with hydrogel extended the phytochemical release for up to 120 h. In the excisional model, on day 11, hydrogel resulted in complete wound closure, which was superior to the positive control, blank SLN hydrogel, and non-encapsulated curcumin hydrogel | Wound contraction (%): NH~96 × CP~58 × BH~81 × FH~75 |
| Thapa et al., 2021 [83] | The phosphatidylcholine LP containing antibacterial peptides presented homogenous size distributions with an average particle size of ≈180 nm and PDI below 0.2. The Pluronic F127 hydrogel exhibited suitable viscosity and rheological properties along with controlled release behavior (up to nine days) for effective peptide delivery following topical application. Potent in vitro antibacterial and anti-biofilm effects of bacteriocin Garvicin KS (GarKS) gel were evident against the Gram-positive bacterium Staphylococcus aureus. The in vivo treatment of methicillin-resistant S. aureus infected mouse wounds suggested potent antibacterial effects of the GarKS gel following multiple applications of once-a-day application for three consecutive days | Anti-biofilm effects: FH~75% × NH~82% × BH~18% |
| Algahtani et al., 2021 [84] | The nanoemulgel system of thymoquinone-loaded black seed oil NE exhibited significant enhancement in skin penetrability and deposition characteristics after topical administration compared to the conventional hydrogel system. The developed nanoemulgel system of thymoquinone exhibited quicker and early healing in wounded Wistar rats compared to the conventional hydrogel of thymoquinone | Complete epithelialization (days): C~16 × CP~11 × FH~14 × NH~10 |
| Algahtani et al., 2021 [85] | The developed curcumin nanoemulgel (Labrafac PG and PEG 400) exhibited thixotropic rheological behavior and a significant increase in skin penetrability characteristics compared to curcumin dispersed in a conventional hydrogel system | Cumulative Amount of Drug Permeated (µg/cm2): NH 773.82 × FH 156.90 Drug Deposited in Skin (µg/cm2): NH 1161.54 × FH 179.47 Permeability (K Coefficient × 10−3): NH 5.49 × FH 0.876 |
| Ansari et al., 2022 [86] | The chitosan gel containing crisaborole-loaded NE (Lauroglycol-90®) exhibited a flux of 0.211 mg/cm2/h, a drug release of 74.45 ± 5.4% in 24 h with a Korsmeyer-Peppas mechanism release behavior. The nano-based hydrogel presented promising wound healing and anti-inflammatory actions | Epithelialization period (days): C~28 × CP~20 × NH~21 |
| Rehman et al., 2022 [87] | Carbopol 940 hydrogel containing eucalyptol-loaded NE (black seed oil) exhibited pH values between five and six, and acceptable homogeneity and spreadability | Wound contraction (%) after 15 days: NH 100.00 × CP 98.17 × C 70.84 |
| Lee and Lin, 2022 [88] | Chitosan-based hydrogel associating perfluorocarbon (PFC)-loaded NE, epidermal growth factor (EGF)-loaded chitosan nanoparticles, and polyhexamethylene biguanide was prepared for treating diabetic wounds. The formulation could sustainably release the actives in an ion-rich environment to exert antibacterial effects and promote cell growth for wound repair | Degree of wound closure after 15 days: NE + NPH > NPH > NEH |
| Gupta et al., 2022 [89] | Significant loading of simvastatin (10% w/w) was achieved in spherical nanoparticle Carbopol 934 hydrogel (0.3 nm (nanoparticles) to 2 µm (gelled-matrix)) that exhibited good spreadability and mechanical properties and slow release up to 72 h. Complete healing of excision wounds observed in rats within 11 days was 10 times better than the commercial povidone-iodine product | Wound closure (%) after day 11: NH > 90 × FH < 50 × CP < 30 |
| Reczyńska-Kolman et al., 2022 [90] | Encapsulation of nisin into stearic acid-based SLN slowed its release from gellan gum-based hydrogels for up to 24 h. The most effective antimicrobial activity against Gram-positive Streptococcus pyogenes was observed for the nanoformulation. All materials were cytocompatible with L929 fibroblasts and did not cause an observable delay in wound healing | Drug release after 24 h: NH~70 × FH~90% |
| Peralta et al., 2022 [7] | The Carbomer 934 hydrogel containing Miltefosine-loaded LP eliminated 99% of the parasites and cured the lesions with a complete re-epithelisation, no visible scar, and re-growth of hair. Fluid liposomes decreased the time to heal the lesion and the time needed to eliminate viable amastigotes from the lesion site | Lesion size (mm2) after 3 weeks: NH~0 × FH < 10 × C > 40 Parasitic inhibition (%): NH 99.66 × FH 99.80 |
| Cardoso-Daodu et al., 2022 [91] | The in vivo wound healing evaluation showed that curcumin-loaded LP (phosphatidylcholine) + lysine and collagen in Carbopol hydrogel had the highest percentage of wound contraction at 79.25% by day three post-surgical operation. The histomorphometric values show the highest percentage of collagen, lowest inflammatory rates, highest presence of microvessels, and re-epithelization rates at the wound site | Wound contraction: NH 79.25% day 3 × BH 23.70% day 7 × C < 60% day 3 Microvessels in granulation tissue (vessels/mm2): NH~4 × BH~2 × C~1 |
| El-ezz et al., 2022 [92] | Carbopol 940 hydrogel loaded with Cu (II) Schiff base 8-hydroxy quinoline complex (CuSQ) SLN (soy lecithin and cholesterol) showed good homogeneity and stability, a pH of 6.4, and had no cytotoxicity on the human skin fibroblast cell line. The nano-based formulation showed significantly faster healing rates compared to standard and control rats | Wound healing (%): NH > BH > CP = C |
| Alsakhawy et al., 2022 [93] | The release profile of thyme essential oil-loaded nanocomposite hydrogel revealed a sustained release pattern compared to thyme essential oil-loaded micelles and free oil. The thyme essential oil-loaded nanomicelles exhibited a significantly higher antibacterial effect than the non-encapsulated compound. Furthermore, the nano-based hydrogel significantly promoted wound contraction, reduced interleukin-6, and increased transforming growth factor-β1 and vascular endothelial growth factor levels, versus control or blank hydrogel group | Inflammation: NH < BH < C Formation of blood vessels: NH > BH > C |
| Days | Control | Nano-Based Hydrogel | Commercial Product |
|---|---|---|---|
| 3rd day | 05.106% ± 0.110 | 25.633% ± 0.549 | 15.146% ± 0.254 |
| 5th day | 20.050% ± 0.055 | 40.483% ± 0.422 | 30.236% ± 0.409 |
| 7th day | 25.433% ± 0.388 | 55.536% ± 0.474 | 46.590% ± 0.510 |
| 9th day | 40.233% ± 0.245 | 60.583% ± 0.514 | 60.326% ± 0.473 |
| 11th day | 55.313% ± 0.419 | 80.443% ± 0.403 | 78.026% ± 0.380 |
| 13th day | 60.420% ± 0.435 | 90.430% ± 0.603 | 89.253% ± 1.090 |
| 15th day | 70.846% ± 0.830 | 100.000% ± 0.015 | 98.170% ± 0.749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, M.H.M.; Cobre, A.d.F.; Pontarolo, R.; Ferreira, L.M. Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing. Pharmaceutics 2023, 15, 874. https://doi.org/10.3390/pharmaceutics15030874
Sari MHM, Cobre AdF, Pontarolo R, Ferreira LM. Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing. Pharmaceutics. 2023; 15(3):874. https://doi.org/10.3390/pharmaceutics15030874
Chicago/Turabian StyleSari, Marcel Henrique Marcondes, Alexandre de Fátima Cobre, Roberto Pontarolo, and Luana Mota Ferreira. 2023. "Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing" Pharmaceutics 15, no. 3: 874. https://doi.org/10.3390/pharmaceutics15030874
APA StyleSari, M. H. M., Cobre, A. d. F., Pontarolo, R., & Ferreira, L. M. (2023). Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing. Pharmaceutics, 15(3), 874. https://doi.org/10.3390/pharmaceutics15030874








