Immunotherapy Resumption/Rechallenge in Melanoma Patients after Toxicity: Do We Have Another Chance?
Abstract
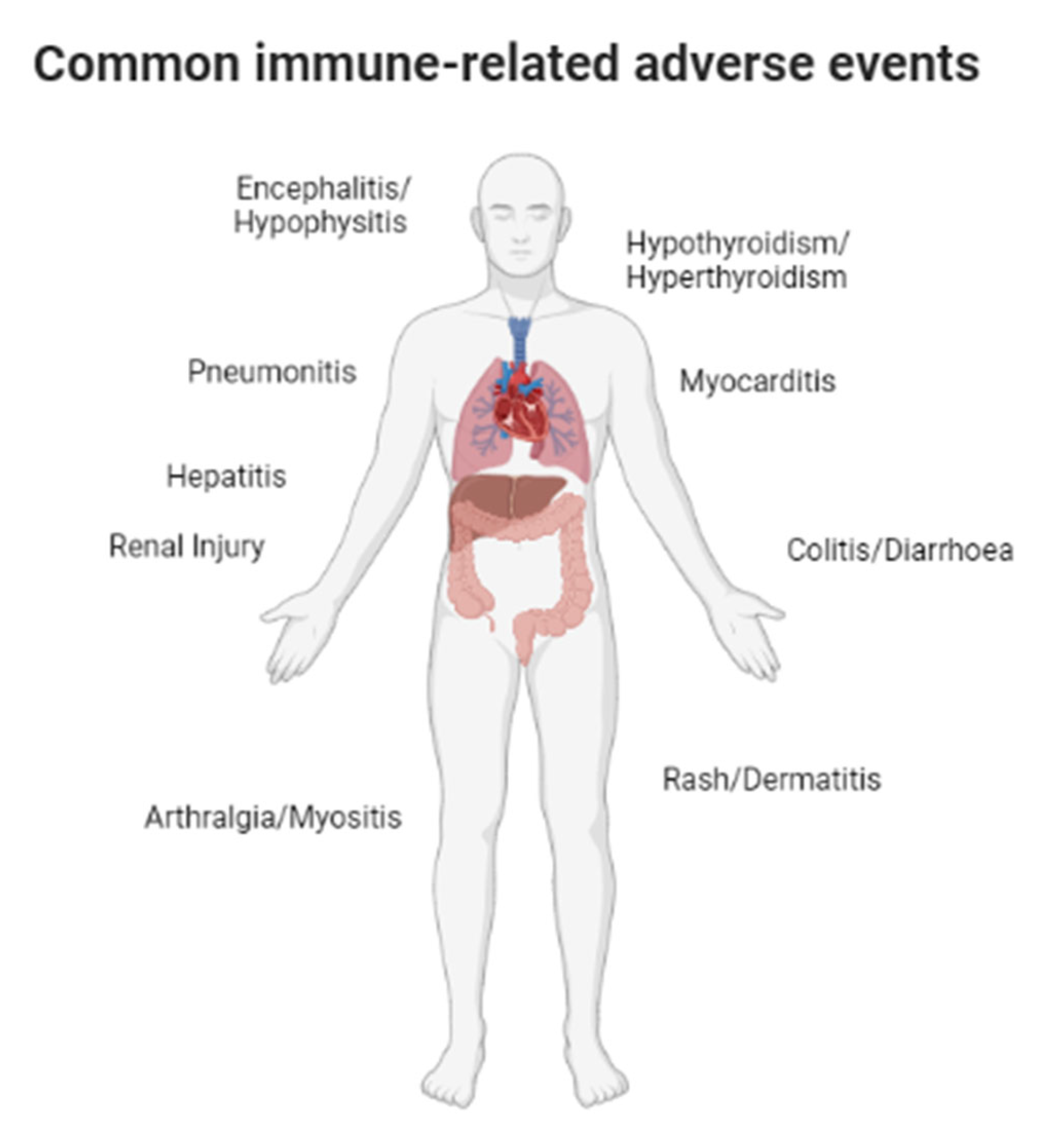
1. Introduction
2. Methods
3. Description of the Evidence
4. Discussion
- (a)
- Patient-specific. This group includes demographic and social history data. Age, race, or gender could be involved in the risk of suffering an irAE. Obviously, other patient characteristics like ECOG or physical status (sarcopenia, level of muscle mass) could also affect it. Medical history is important too; for instance, a previous history of autoimmune disease increased the irAE risk. Finally, some studies suggested that antibiotics or influenza vaccinations, for example, could be involved in immune toxicity.
- (b)
- Tumor-specific. There are characteristics such as disease burden or higher tumor mutational burden (TMB) that affect disease evolution and treatment evolution. It is worth highlighting that some tumors are more related to some specific toxicities. For instance, melanoma is more related to diarrhea, colitis, and skin toxicities than other tumors like non-small cell lung cancer.
- (c)
- Agent-specific. As we mentioned previously, the incidence and type of toxicity differ according to the use of anti-PD1 or anti-CTLA4 in monotherapy or in combination.
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef]
- Dobry, A.S.; Zogg, C.K.; Hodi, F.S.; Smith, T.R.; Ott, P.A.; Iorgulescu, J.B. Management of metastatic melanoma: Improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol. Immunother. 2018, 67, 1833–1844. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 23, 488–496. [Google Scholar]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of Melanoma: Facts and Hopes. Clin. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef]
- Sood, S.; Jayachandiran, R.; Pandey, S. Current Advancements and Novel Strategies in the Treatment of Metastatic Melanoma. Integr. Cancer Ther. 2021, 20, 1–20. [Google Scholar] [CrossRef]
- Kashiwabara, K.; Fujii, S.; Tsumura, S.; Sakamoto, K. Timing of resumption of immune checkpoint inhibitor therapy after successful control of immune-related adverse events in seven advanced non-small cell lung cancer patients. Anticancer Drugs 2020, 31, 872–875. [Google Scholar] [CrossRef]
- Pauken, K.E.; Dougan, M.; Rose, N.R.; Lichtman, A.H.; Sharpe, A.H. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019, 40, 511–523. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef]
- Rajha, E.; Chaftari, P.; Kamal, M.; Maamari, J.; Chaftari, C.; Yeung, S.J. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol. Rep. 2020, 8, 25–30. [Google Scholar] [CrossRef]
- Espi, M.; Teuma, C.; Novel-Catin, E.; Maillet, D.; Souquet, P.J.; Dalle, S.; Koppe, L.; Fouque, D. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur. J. Cancer 2021, 147, 29–39. [Google Scholar] [CrossRef]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 2016, 4, 50. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, Y.; Zhou, Y.; Xia, R.; Tang, L.; Liu, J. Neurological Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Perspectives and New Development. Clin. Med. Insights Oncol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- De Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.; Wang, Y.; Menzies, A.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.; Obeid, M. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: Review of the literature and suggested prophylactic strategy. J. Immunother. Cancer 2020, 8, e000604. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Miranda Poma, J.; Ostios Garcia, L.; Villamayor Sanchez, J.; D’Errico, G. What do we know about cancer immunotherapy? Long-term survival and immune-related adverse events. Allergol. Immunopathol. 2019, 47, 303–308. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Spain, L.; Walls, G.; Messiou, C.; Turajlic, S.; Gore, M.; Larkin, J. Efficacy and toxicity of rechallenge with combination immune checkpoint blockade in metastatic melanoma: A case series. Cancer Immunol. Immunother. 2017, 66, 113–117. [Google Scholar] [CrossRef]
- Pollack, M.H.; Betof, A.; Dearden, H.; Rapazzo, K.; Valentine, I.; Brohl, A.S.; Ancell, K.K.; Long, G.V.; Menzies, A.M.; Eroglu, Z.; et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 2018, 29, 250–255. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Allouchery, M.; Lombard, T.; Martin, M.; Rouby, F.; Sassier, M.; Bertin, C.; Atzenhoffer, M.; Miremont-Salame, G.; Perault-Pochat, M.C.; Puyade, M.; et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade >/=2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 2020, 8, e001622. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.H.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef]
- De Malet, A.; Antoni, G.; Collins, M.; Soularue, E.; Marthey, L.; Vaysse, T.; Coutzac, C.; Chaput, N.; Mateus, C.; Robert, C.; et al. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur. J. Cancer 2019, 106, 106–114. [Google Scholar] [CrossRef]
- Li, M.; Sack, J.S.; Rahma, O.E.; Hodi, F.S.; Zucker, S.D.; Grover, S. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer 2020, 126, 5088–5097. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef]
- Gupta, S.; Short, S.A.P.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, S.M.; Abudayyeh, A.; Anand, S.; et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Tetu, P.; Guegan, S.; Ursu, R.; Belin, C.; Sirven Villaros, L.; Mazoyer, J.; Lheure, C.; Lebbe, C.; Baroudjian, B.; et al. Reintroduction of immune-checkpoint inhibitors after immune-related meningitis: A case series of melanoma patients. J. Immunother. Cancer 2020, 8, e001034. [Google Scholar] [CrossRef]
- Weill, A.; Delyon, J.; Descamps, V.; Deschamps, L.; Dinulescu, M.; Dupuy, A.; Celerier, P.; Nardin, C.; Aubin, F.; Le Corre, Y.; et al. Treatment strategies and safety of rechallenge in the setting of immune checkpoint inhibitors-related myositis: A national multicentre study. Rheumatology 2021, 60, 5753–5764. [Google Scholar] [CrossRef]
- Rodriguez, L.M.; Jimenez-Rodriguez, B.; Llácer, C.; Trigo Perez, J.M.M. Rechallenge with immune checkpoint inhibitors beyond severe toxicities, an utopia? J. Clin. Oncol. 2022, 40, e14601. [Google Scholar] [CrossRef]
- Plazy, C.; Hannani, D.; Gobbini, E. Immune Checkpoint Inhibitor Rechallenge and Resumption: A Systematic Review. Curr. Oncol. Rep. 2022, 24, 1095–1106. [Google Scholar] [CrossRef]
- Zaremba, A.; Eggermont, A.M.M.; Robert, C.; Dummer, R.; Ugurel, S.; Livingstone, E.; Ascierto, P.A.; Long, G.V.; Schadendorf, D.; Zimmer, L. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur. J. Cancer 2021, 155, 268–280. [Google Scholar] [CrossRef]
- Allouchery, M.; Beuvon, C.; Perault-Pochat, M.C.; Roblot, P.; Puyade, M.; Martin, M. Safety of Immune Checkpoint Inhibitor Resumption after Interruption for Immune-Related Adverse Events, a Narrative Review. Cancers 2022, 14, 955. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov (accessed on 25 June 2022).
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chretien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Luo, W.; Qiao, W.; Raju, G.S.; Wang, Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2018, 6, 95. [Google Scholar] [CrossRef]
- Zou, F.; Wang, X.; Glitza Oliva, I.C.; McQuade, J.L.; Wang, J.; Zhang, H.C.; Thompson, J.A.; Thomas, A.S.; Wang, Y. Fecal calprotectin concentration to assess endoscopic and histologic remission in patients with cancer with immune-mediated diarrhea and colitis. J. Immunother. Cancer 2021, 9, e002058. [Google Scholar] [CrossRef]
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405–418. [Google Scholar] [CrossRef]
- Peleg Hasson, S.; Salwen, B.; Sivan, A.; Shamai, S.; Geva, R.; Merimsky, O.; Raphael, A.; Shmilovich, H.; Moshkovits, Y.; Kapusta, L.; et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin. Res. Cardiol. 2021, 110, 50–60. [Google Scholar] [CrossRef]
- Richter, M.D.; Crowson, C.; Kottschade, L.A.; Finnes, H.D.; Markovic, S.N.; Thanarajasingam, U. Rheumatic Syndromes Associated With Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis Rheumatol. 2019, 71, 468–475. [Google Scholar] [CrossRef]
- Nguyen, T.; Maria, A.T.J.; Ladhari, C.; Palassin, P.; Quantin, X.; Lesage, C.; Taieb, G.; Ayrignac, X.; Rullier, P.; Hillaire-Buys, D.; et al. Rheumatic disorders associated with immune checkpoint inhibitors: What about myositis? An analysis of the WHO’s adverse drug reactions database. Ann. Rheum. Dis. 2022, 81, e32. [Google Scholar] [CrossRef]
- Watson, A.S.; Goutam, S.; Stukalin, I.; Ewanchuk, B.W.; Sander, M.; Meyers, D.E.; Pabani, A.; Cheung, W.Y.; Heng, D.Y.C.; Cheng, T.; et al. Association of Immune-Related Adverse Events, Hospitalization, and Therapy Resumption With Survival Among Patients With Metastatic Melanoma Receiving Single-Agent or Combination Immunotherapy. JAMA Netw. Open 2022, 5, e2245596. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Inno, A.; Roviello, G.; Ghidini, A.; Luciani, A.; Catalano, M.; Gori, S.; Petrelli, F. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 165, 103434. [Google Scholar] [CrossRef]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Vauchier, C.; Auclin, E.; Barthelemy, P.; Carril-Ajuria, L.; Ryckewaert, T.; Borchiellini, D.; Castel-Ajgal, Z.; Bennamoun, M.; Campedel, L.; Thiery-Vuillemin, A.; et al. REchallenge of NIVOlumab (RENIVO) or Nivolumab-Ipilimumab in Metastatic Renal Cell Carcinoma: An Ambispective Multicenter Study. J. Oncol. 2022, 2022, 3449660. [Google Scholar] [CrossRef]
- Santini, F.C.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Lacouture, M.E.; Gambarin-Gelwan, M.; Wilkins, O.; Panora, E.; Halpenny, D.F.; Long, N.M.; et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res. 2018, 6, 1093–1099. [Google Scholar] [CrossRef]
- Lebbe, C.; Weber, J.S.; Maio, M.; Neyns, B.; Harmankaya, K.; Hamid, O.; O’Day, S.J.; Konto, C.; Cykowski, L.; McHenry, M.B.; et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann. Oncol. 2014, 25, 2277–2284. [Google Scholar] [CrossRef]
- Nomura, M.; Otsuka, A.; Kondo, T.; Nagai, H.; Nonomura, Y.; Kaku, Y.; Matsumoto, S.; Muto, M. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother. Pharmacol. 2017, 80, 999–1004. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Xu, L.; Yang, H.; Liang, N.; Zhang, L.; Zhang, F.; Zhang, X. Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer: A Systemic Review and Meta-Analysis. Front. Immunol. 2021, 12, 730320. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.A.S.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–625. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshino, K.; Otsuka, A.; Funakoshi, T.; Fujimura, T.; Yamamoto, Y.; Hata, H.; Gosho, M.; Tanaka, R.; Yamaguchi, K.; et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J. Dermatol. Sci. 2017, 88, 225–231. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef]
- Akbulut, U.E.; Emeksiz, H.C.; Citli, S.; Cebi, A.H.; Korkmaz, H.A.A.; Baki, G. IL-17A, MCP-1, CCR-2, and ABCA1 polymorphisms in children with non-alcoholic fatty liver disease. J. Pediatr. 2019, 95, 350–357. [Google Scholar] [CrossRef]
- Pistillo, M.P.; Fontana, V.; Morabito, A.; Dozin, B.; Laurent, S.; Carosio, R.; Banelli, B.; Ferrero, F.; Spano, L.; Tanda, E.; et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: An Italian melanoma intergroup study. Cancer Immunol. Immunother. 2019, 68, 97–107. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
| Number of Patients (n) | Melanoma Patients (n) | Number of Patients on Resumption/Rechallenge * | % irAE Recurrence, Any Grade | % irAE Recurrence, Grade 3–4 | |
|---|---|---|---|---|---|
| Spain et al. [30] | 3 | 3 | 3 | 100 | 66 |
| Pollack et al. [31] | 80 | 80 | 80 | 50 | 18 |
| Simonaggio et al. [32] | 93 | 31 | 40 | 100 | 62 |
| Allouchery et al. [33] | 180 | 79 | 159 | NA | 38.9 |
| Abu-Sbeih et al. [34] | 167 | 90 | 167 | 34 | 82 |
| Malet et al. [35] | 80 | 60 | 26 | 23 | 33 |
| Li et al. [36] | 102 | 102 | 31 | 48 | 19.4 |
| Cortazar et al. [37] | 138 | 49 | 31 | 23 | NA |
| Gupta et al. [38] | 429 | 104 | 121 | 16.5 | 40 |
| Cuzzubbo et al. [39] | 7 | 7 | 4 | NA | 14.2 |
| Weill et al. [40] | 20 | 15 | 9 | NA | 1.1 |
| Medina et al. [41] | 220 | 32 | 17 | NA | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
España Fernandez, S.; Sun, C.; Solé-Blanch, C.; Boada, A.; Martínez-Cardús, A.; Manzano, J.L. Immunotherapy Resumption/Rechallenge in Melanoma Patients after Toxicity: Do We Have Another Chance? Pharmaceutics 2023, 15, 823. https://doi.org/10.3390/pharmaceutics15030823
España Fernandez S, Sun C, Solé-Blanch C, Boada A, Martínez-Cardús A, Manzano JL. Immunotherapy Resumption/Rechallenge in Melanoma Patients after Toxicity: Do We Have Another Chance? Pharmaceutics. 2023; 15(3):823. https://doi.org/10.3390/pharmaceutics15030823
Chicago/Turabian StyleEspaña Fernandez, Sofia, Chen Sun, Carme Solé-Blanch, Aram Boada, Anna Martínez-Cardús, and José Luis Manzano. 2023. "Immunotherapy Resumption/Rechallenge in Melanoma Patients after Toxicity: Do We Have Another Chance?" Pharmaceutics 15, no. 3: 823. https://doi.org/10.3390/pharmaceutics15030823
APA StyleEspaña Fernandez, S., Sun, C., Solé-Blanch, C., Boada, A., Martínez-Cardús, A., & Manzano, J. L. (2023). Immunotherapy Resumption/Rechallenge in Melanoma Patients after Toxicity: Do We Have Another Chance? Pharmaceutics, 15(3), 823. https://doi.org/10.3390/pharmaceutics15030823






