Polydopamine-Coated Cu-BTC Nanowires for Effective Magnetic Resonance Imaging and Photothermal Therapy
Abstract
1. Introduction
- We developed Cu-BTC@PDA NWs for effective PTT treatment of HCC.
- As-prepared NWs serve as an outstanding T1 MRI contrast agent.
- It was found that the PTT conversion efficiency of NWs was 13.32%.
- As a result of their high conversion efficiency, NWs are able to eliminate HCC in the presence of laser light.
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Characterization
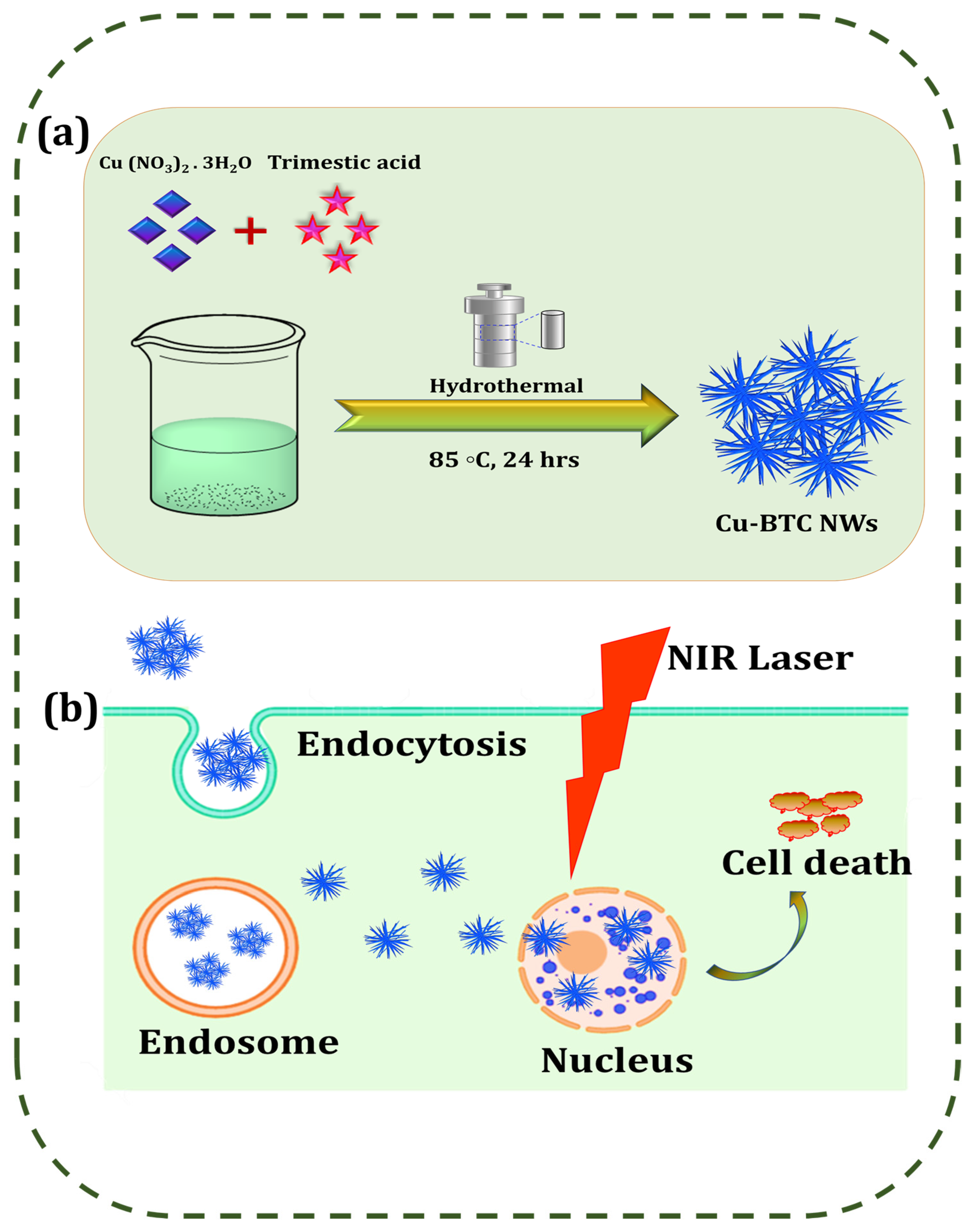
2.3. Preparation of Cu-BTC NWs
2.4. Preparation of PDA-Coated Cu-BTC NWs
2.5. Photothermal Effect of Cu-BTC@PDA
2.6. PTT Conversion Efficiency
2.7. In Vitro MRI
2.8. Cellular Uptake of NWs
2.8.1. Preparation of Fluorescently Labelled Cu-BTC@PDA NWs
2.8.2. Fluorescence Microscopy (FM) Analysis
2.9. Cell Viability and PTT Effect Assay
2.10. Intracellular Detection of ROS
2.11. Statistical Analysis
3. Results
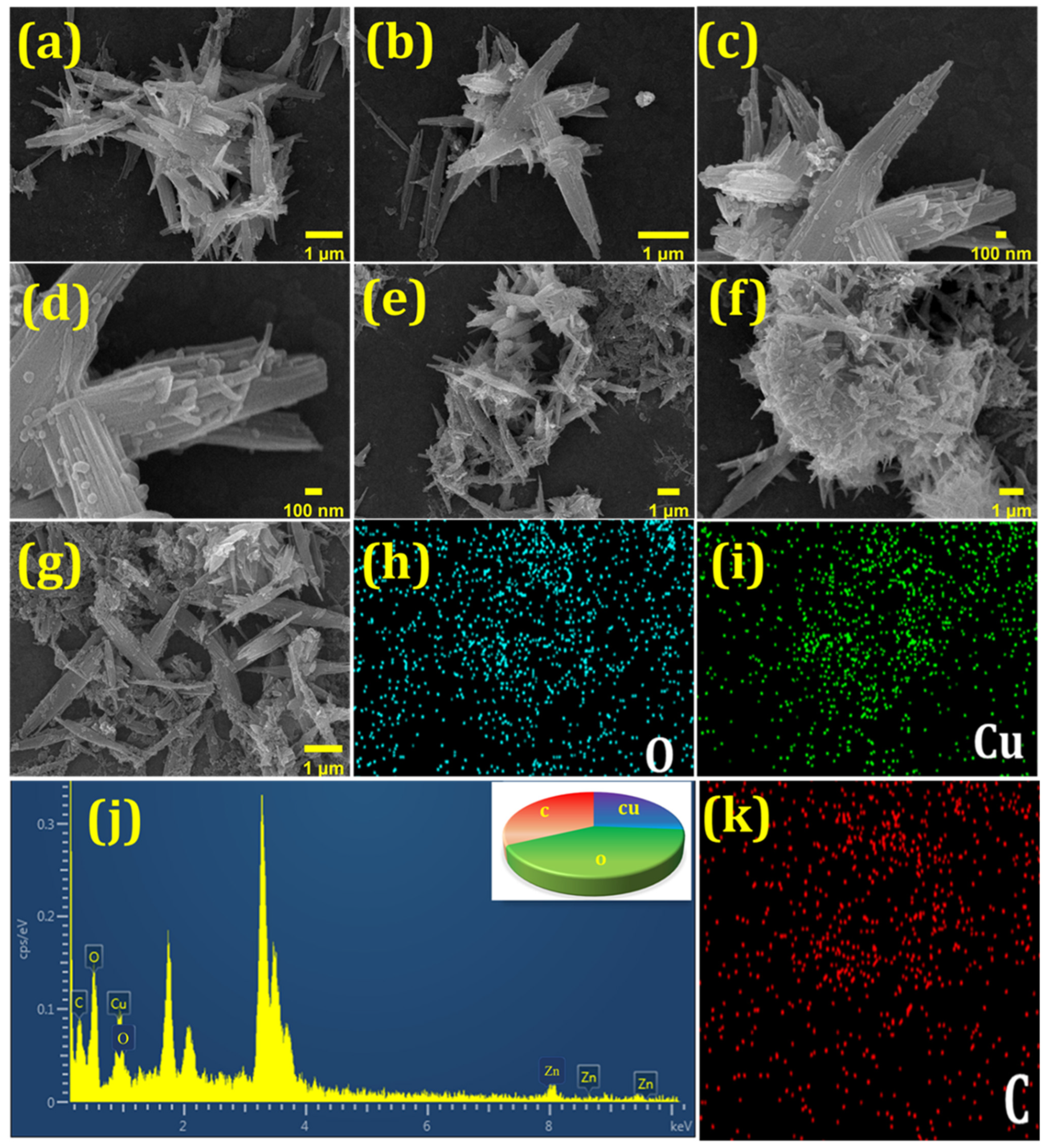
3.1. Characterization of Cu-BTC@PDA NWs
3.2. XPS Analysis of Cu-BTC@PDA NWs
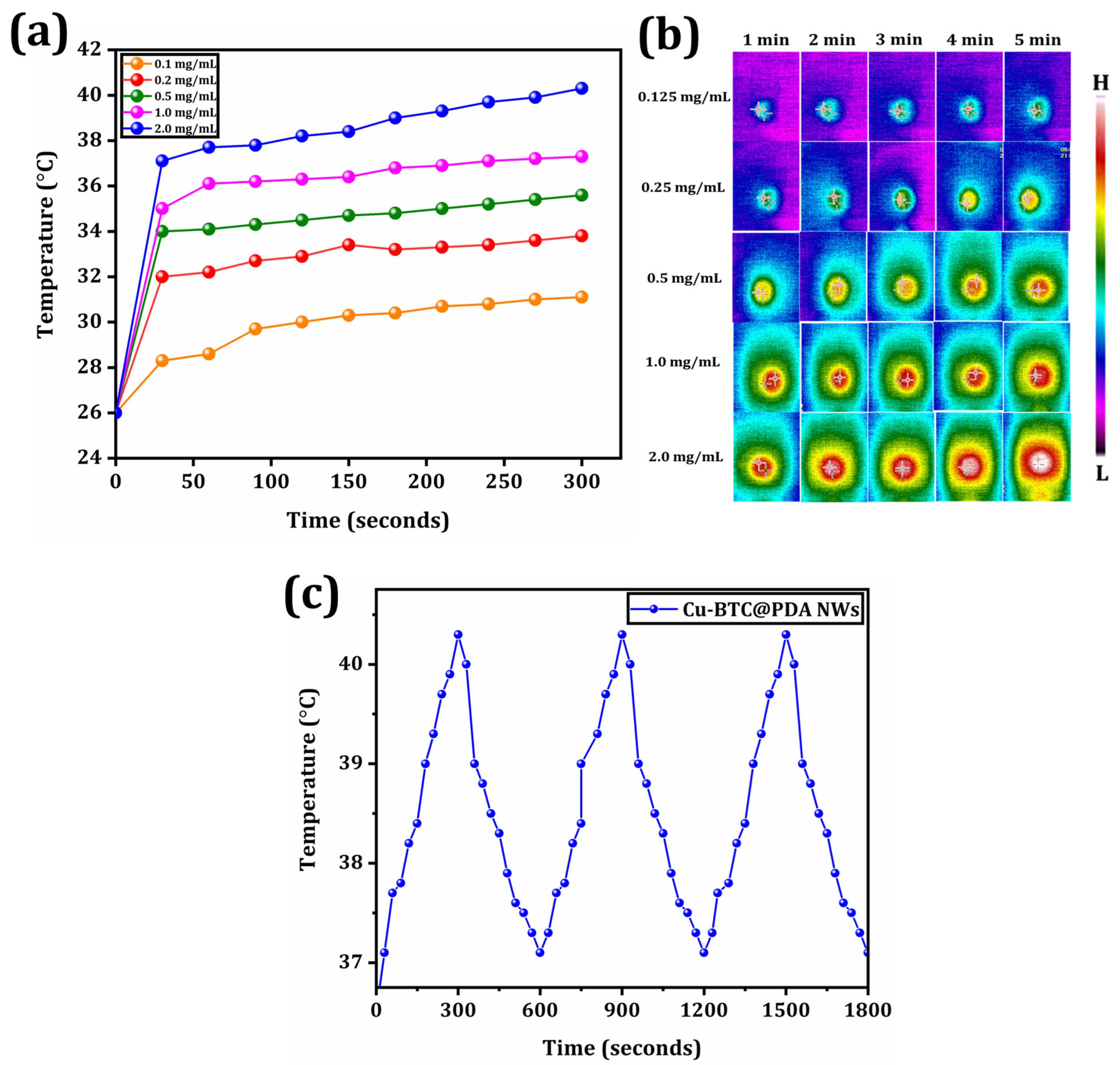
3.3. Photothermal Therapy
3.4. MRI
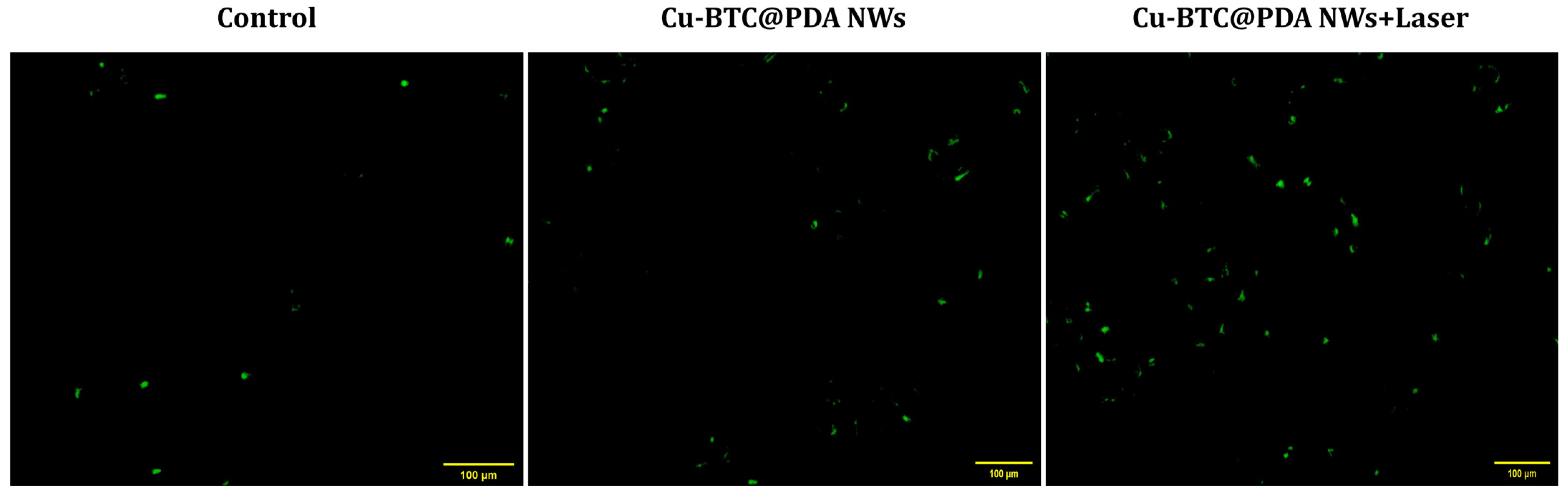
3.5. Cytotoxicity
3.6. Detection of ROS
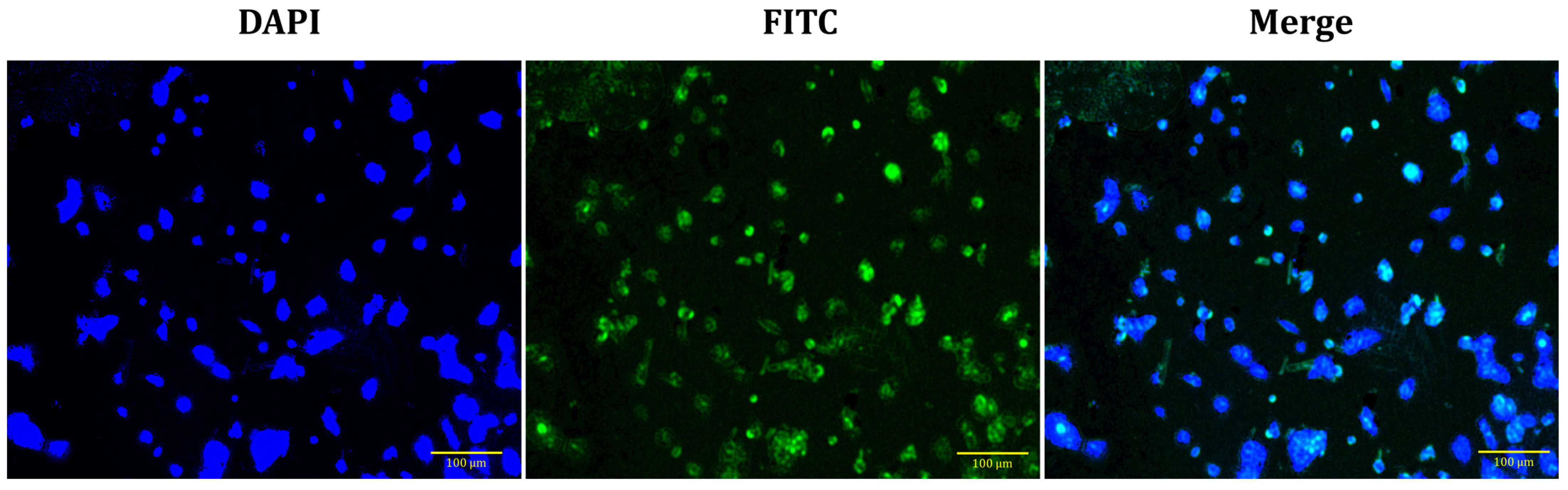
3.7. Cellular Uptake of NWs
4. Discussions
5. Conclusions
- The Cu-BTC@PDA NWs exhibited good PTT conversion efficiency and thermal stability.
- The Cu-BTC@PDA NWs exhibited an excellent T1 MRI contrast agent.
- The results in vitro demonstrated the efficacy of PTT therapeutic effects and their biocompatibility.
- Cu-BTC@PDA NWs effectively scavenged the large amount of ROS generated during PPT, thus improving the toxic side effects caused by these ROS on healthy tissues.
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary Liver Cancer: Worldwide Incidence and Trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef]
- Tang, Y.H.; Wen, T.F.; Chen, X. Anatomic versus Non-Anatomic Liver Resection for Hepatocellular Carcinoma: A Systematic Review. Hepatogastroenterology 2013, 60, 2019–2025. [Google Scholar] [PubMed]
- Abdel-Wahab, M.; Sultan, A.M.; Fathy, O.M.; Salah, T.; Elshobary, M.M.; Elghawalby, N.A.; Yassen, A.M.; Elsarraf, W.M.R.; Elsaadany, M.F.; Zalatah, K. Factors Affecting Recurrence and Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma. Hepatogastroenterology 2013, 60, 1847–1853. [Google Scholar] [CrossRef]
- Imamura, H.; Kawasaki, S.; Miyagawa, S.I.; Ikegami, T.; Kitamura, H.; Shimada, R. Aggressive Surgical Approach to Recurrent Tumors after Hepatectomy for Metastatic Spread of Colorectal Cancer to the Liver. Surgery 2000, 127, 528–535. [Google Scholar] [CrossRef]
- Lai, Q.; Nudo, F.; Mennini, G. Expanded Criteria for Hepatocellular Carcinoma after Liver Transplantation: A 20-Year Evolution. Hepatogastroenterology 2013, 60, 2039–2041. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Fuster, J.; Bruix, J. The Barcelona Approach: Diagnosis, Staging, and Treatment of Hepatocellular Carcinoma. Liver Transplant. 2004, 10, S115–S120. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Golba, K.; Coy, E.; Synoradzki, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Magnetite Nanoparticles and Spheres for Chemo-and Photothermal Therapy of Hepatocellular Carcinoma in Vitro. Int. J. Nanomed. 2020, 15, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, Q.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-Loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; Zhang, Y.; Xu, J.; Ji, J.; Ye, L.; Yi, F.; Zhai, G. Photo-Triggered Self-Destructive ROS-Responsive Nanoparticles of High Paclitaxel/Chlorin E6 Co-Loading Capacity for Synergetic Chemo-Photodynamic Therapy. J. Control. Release 2020, 323, 333–349. [Google Scholar] [CrossRef]
- Pan, Y.T.; Ding, Y.F.; Han, Z.H.; Yuwen, L.; Ye, Z.; Mok, G.S.P.; Li, S.; Wang, L.H. Hyaluronic Acid-Based Nanogels Derived from Multicomponent Self-Assembly for Imaging-Guided Chemo-Photodynamic Cancer Therapy. Carbohydr. Polym. 2021, 268, 118257. [Google Scholar] [CrossRef]
- Luo, L.; Qi, Y.; Zhong, H.; Jiang, S.; Zhang, H.; Cai, H.; Wu, Y.; Gu, Z.; Gong, Q.; Luo, K. GSH-Sensitive Polymeric Prodrug: Synthesis and Loading with Photosensitizers as Nanoscale Chemo-Photodynamic Anti-Cancer Nanomedicine. Acta Pharm. Sin. B 2022, 12, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liang, Y.; Wang, M.; Xu, X.; Zhao, F.; Wang, X.; Sun, Y.; Chen, W. Multifunctional Nanoplatforms as Cascade-Responsive Drug-Delivery Carriers for Effective Synergistic Chemo-Photodynamic Cancer Treatment. J. Nanobiotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Shen, Q.; Zhang, T.; Fan, Y.; Xu, X.; Shao, J.; Yang, D.; Zhao, W.; Dong, X.; Mou, X. Fluorescence Resonance Energy Transfer Enhanced Photothermal and Photodynamic Antibacterial Therapy Post a Single Injection. Mater. Chem. Front. 2021, 5, 6061–6070. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, Y.; Qiao, Z.; Xin, N.; Chen, S.; Sun, J.; Jin, R.; Nie, Y.; Fan, H. A Gd-Doped Polydopamine (PDA)-Based Theranostic Nanoplatform as a Strong MR/PA Dual-Modal Imaging Agent for PTT/PDT Synergistic Therapy. J. Mater. Chem. B 2021, 9, 1846–1857. [Google Scholar] [CrossRef]
- Bian, H.; Ma, D.; Zhang, X.; Xin, K.; Yang, Y.; Peng, X.; Xiao, Y.; Bian, H.; Ma, D.; Zhang, X.; et al. Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 Nm Laser toward Deep-Seated Tumors. Small 2021, 17, 2100398. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, D.; Liu, Y.; Jiang, B.; Jiang, W.; Yan, X.; Fan, K. Platinum-Carbon-Integrated Nanozymes for Enhanced Tumor Photodynamic and Photothermal Therapy. Nanoscale 2020, 12, 13548–13557. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, Y.; Liang, M.; Ma, Y.; Sun, N.; Gao, X.; Li, J. Pt@polydopamine Nanoparticles as Nanozymes for Enhanced Photodynamic and Photothermal Therapy. Chem. Commun. 2021, 57, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, J.; He, M.; Hou, X.; Wang, Y.; Cai, X.; Xin, H.; Gao, F.; Chen, Y. Combined Cancer Chemo-Photodynamic and Photothermal Therapy Based on ICG/PDA/TPZ-Loaded Nanoparticles. Mol. Pharm. 2019, 16, 2172–2183. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Wu, Y.P.; Yang, X.; Kuang, X.P.; Li, W.X.; Li, Y.F.; He, R.R.; Liu, M. Multifunctional HNT@Fe3O4@PPy@DOX Nanoplatform for Effective Chemo-Photothermal Combination Therapy of Breast Cancer with MR Imaging. ACS Biomater. Sci. Eng. 2020, 6, 3361–3374. [Google Scholar] [CrossRef]
- Wang, H.; Williams, G.R.; Xie, X.; Wu, M.; Wu, J.; Zhu, L.M. Stealth Polydopamine-Based Nanoparticles with Red Blood Cell Membrane for the Chemo-Photothermal Therapy of Cancer. ACS Appl. Biol. Mater. 2020, 3, 2350–2359. [Google Scholar] [CrossRef]
- Li, Z.; Ni, J.; Liu, L.; Gu, L.; Wu, Z.; Li, T.; Ivanovich, K.I.; Zhao, W.; Sun, T.; Wang, T. Imaging-Guided Chemo-Photothermal Polydopamine Carbon Dots for EpCAM-Targeted Delivery toward Liver Tumor. ACS Appl. Mater. Interfaces 2021, 13, 29340–29348. [Google Scholar] [CrossRef]
- Dibaba, S.T.; Caputo, R.; Xi, W.; Zhang, J.Z.; Wei, R.; Zhang, Q.; Zhang, J.; Ren, W.; Sun, L. NIR Light-Degradable Antimony Nanoparticle-Based Drug-Delivery Nanosystem for Synergistic Chemo-Photothermal Therapy in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 48290–48299. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, L.; Zhang, Y.; Timashev, P.S.; Huang, Y.; Liang, X.J. Polymer-Based Nanomaterials for Noninvasive Cancer Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4289–4305. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-Photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Huang, Z.; Duan, Y.; Fu, Z.; Liu, B. Reprogramming Tumor Microenvironment with Photothermal Therapy. Bioconjug. Chem. 2020, 31, 1268–1278. [Google Scholar] [CrossRef]
- Bian, W.; Wang, Y.; Pan, Z.; Chen, N.; Li, X.; Wong, W.L.; Liu, X.; He, Y.; Zhang, K.; Lu, Y.J. Review of Functionalized Nanomaterials for Photothermal Therapy of Cancers. ACS Appl. Nano Mater. 2021, 4, 11. [Google Scholar] [CrossRef]
- He, J.; Ramachandraiah, K.; Huang, T.; Yuan, T.; Liu, X.; Zhang, H.; Ke, F. Core−shell Structured Hollow Copper Sulfide@metal−organic Framework for Magnetic Resonance Imaging Guided Photothermal Therapy in Second Near-Infrared Biological Window. Biochem. Biophys. Res. Commun. 2023, 638, 51–57. [Google Scholar] [CrossRef]
- Fan, R.; Chen, C.; Hou, H.; Chuan, D.; Mu, M.; Liu, Z.; Liang, R.; Guo, G.; Xu, J.; Fan, R.; et al. Tumor Acidity and Near-Infrared Light Responsive Dual Drug Delivery Polydopamine-Based Nanoparticles for Chemo-Photothermal Therapy. Adv. Funct. Mater. 2021, 31, 2009733. [Google Scholar] [CrossRef]
- Ruan, J.; Liu, H.; Chen, B.; Wang, F.; Wang, W.; Zha, Z.; Qian, H.; Miao, Z.; Sun, J.; Tian, T.; et al. Interfacially Engineered ZnxMn1- XS@Polydopamine Hollow Nanospheres for Glutathione Depleting Photothermally Enhanced Chemodynamic Therapy. ACS Nano 2021, 15, 11428–11440. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and Their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, L.; Guo, Q.; Zuo, Y.; Wang, N.; Wang, H.; Kong, D.; Zhu, D.; Zhang, L. Robust Nanovaccine Based on Polydopamine-Coated Mesoporous Silica Nanoparticles for Effective Photothermal-Immunotherapy Against Melanoma. Adv. Funct. Mater. 2021, 31, 2010637. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W.; Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous Metal–Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Cai, W.; Chu, C.C.; Liu, G.; Wáng, Y.X.J. Metal–Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, W.; Li, J.; Yang, L.; Su, Y.; Peng, J.; Chen, R.; Tham, H.P.; Chen, H.; Lim, W.Q.; et al. Independent of EPR Effect: A Smart Delivery Nanosystem for Tracking and Treatment of Nonvascularized Intra-Abdominal Metastases. Adv. Funct. Mater. 2018, 28, 1806162. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Bui, N.Q.; Lee, K.D.; Oh, J. Chlorin E6 Conjugated Copper Sulfide Nanoparticles for Photodynamic Combined Photothermal Therapy. Photodiagnosis. Photodyn. Ther. 2017, 19, 128–134. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal Organic Framework (MOF) Porous Octahedral Nanocrystals of Cu-BTC: Synthesis, Properties, and Enhanced Adsorption Properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Xiangjun, C.; Manjie, Z.; Shengnan, L.; Lu, L.; Lingyu, Z.; Tingting, W.; Min, Y.; Zhongcheng, M.; Chungang, W. Facile synthesis of polypyrrole@metal–organic framework core–shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells. J. Mater. Chem. B 2017, 5, 1772–1778. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, K.; Fu, C.; Liu, H.; Lei, M.; Bao, J.; Fu, A.; Yu, Y.; Zhang, W. Interfacial Engineered Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging Guided Microenvironment-Mediated Synergetic Chemodynamic/Photothermal Therapy. Biomaterials 2019, 219, 119379. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hao, J.; Wu, K. Triethylamine-Controlled Cu-BTC Frameworks for Electrochemical Sensing Fish Freshness. Anal. Chim. Acta 2019, 1085, 68–74. [Google Scholar] [CrossRef]
- Uysal, Ş.; Kurşunlu, A.N. The Synthesis and Characterization of Star Shaped Metal Complexes of Triazine Cored Schiff Bases: Their Thermal Decompositions and Magnetic Moment Values. J. Inorg. Organomet. Polym. Mater. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Jia, X.; Li, G.; An, T.; Gao, Y. Novel Approach for Removing Brominated Flame Retardant from Aquatic Environments Using Cu/Fe-Based Metal-Organic Frameworks: A Case of Hexabromocyclododecane (HBCD). Sci. Total Environ. 2018, 621, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Ma, Y.; Cao, Y.; Huang, J.; Zhang, M.; Zhang, Z. PEGylated Carbon Nanoparticles for Efficient in Vitro Photothermal Cancer Therapy. J. Mater. Chem. B 2014, 2, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring Reactive Oxygen and Nitrogen Species with Fluorescent Probes: Challenges and Limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Kollenda, S.; Kopp, M.; Wens, J.; Koch, J.; Schulze, N.; Papadopoulos, C.; Pöhler, R.; Meyer, H.; Epple, M. A PH-Sensitive Fluorescent Protein Sensor to Follow the Pathway of Calcium Phosphate Nanoparticles into Cells. Acta Biomater. 2020, 111, 406–417. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Ozmen, M.; Güler, E. A Novel Fluorescent Chemosensor for Cu (II) Ion: Click Synthesis of Dual-Bodipy Including the Triazole Groups and Bioimaging of Yeast Cells. J. Fluoresc. 2019, 29, 1321–1329. [Google Scholar] [CrossRef]
- Karlsson, M.; Kurz, T.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C.I. What Does the Commonly Used DCF Test for Oxidative Stress Really Show? Biochem. J. 2010, 428, 183–190. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, P.; Du, K.; Zhang, M.; Wen, D.; Lu, Y.; Feng, J.; Zhang, H. Near-Infrared-Light-Responsive Copper Oxide Nanoparticles as Efficient Theranostic Nanoagents for Photothermal Tumor Ablation. ACS Appl. Biol. Mater. 2021, 4, 5266–5275. [Google Scholar] [CrossRef]
- Vieira, P.; Jesus, V.; Cândido, M.A.; Pacheco-Soares, C.; Castilho, M.; Raniero, L. Specific Nanomarkers Fluorescence: In Vitro Analysis for EGFR Overexpressed Cells in Triple-Negative Breast Cancer and Malignant Glioblastoma. Photodiagnosis. Photodyn. Ther. 2022, 39, 102997. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, X.; Yin, C. A Study on Improving the Efficacy of Nanoparticle-based Photothermal Therapy: From Nanoscale to Micron Scale to Millimeter Scale. Materials 2021, 14, 2407. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sun, F.; Zhang, Y.; Yang, Z.; Liu, P.; Zou, Y.; Yu, Y.; Tong, F.; Yi, C.; Yang, S.; et al. Polydopamine-Mediated Bio-Inspired Synthesis of Copper Sulfide Nanoparticles for T1-Weighted Magnetic Resonance Imaging Guided Photothermal Cancer Therapy. Colloids Surf. B Biointerfaces 2019, 173, 607–615. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirumurugan, S.; Samuvel Muthiah, K.; Sakthivel, R.; Liao, M.-Y.; Kasai, H.; Chung, R.-J. Polydopamine-Coated Cu-BTC Nanowires for Effective Magnetic Resonance Imaging and Photothermal Therapy. Pharmaceutics 2023, 15, 822. https://doi.org/10.3390/pharmaceutics15030822
Thirumurugan S, Samuvel Muthiah K, Sakthivel R, Liao M-Y, Kasai H, Chung R-J. Polydopamine-Coated Cu-BTC Nanowires for Effective Magnetic Resonance Imaging and Photothermal Therapy. Pharmaceutics. 2023; 15(3):822. https://doi.org/10.3390/pharmaceutics15030822
Chicago/Turabian StyleThirumurugan, Senthilkumar, Kayalvizhi Samuvel Muthiah, Rajalakshmi Sakthivel, Mei-Yi Liao, Hitoshi Kasai, and Ren-Jei Chung. 2023. "Polydopamine-Coated Cu-BTC Nanowires for Effective Magnetic Resonance Imaging and Photothermal Therapy" Pharmaceutics 15, no. 3: 822. https://doi.org/10.3390/pharmaceutics15030822
APA StyleThirumurugan, S., Samuvel Muthiah, K., Sakthivel, R., Liao, M.-Y., Kasai, H., & Chung, R.-J. (2023). Polydopamine-Coated Cu-BTC Nanowires for Effective Magnetic Resonance Imaging and Photothermal Therapy. Pharmaceutics, 15(3), 822. https://doi.org/10.3390/pharmaceutics15030822





