1. Introduction
Bone tissue engineering combines the principles and methods of engineering and life sciences in order to develop biological substitutes that will restore, preserve, and improve hard tissue function. This can be accomplished by integrating 3D porous scaffolds made of suitable biomaterials, cells, and biological compounds, including growth factors and active ingredients [
1]. In order to reproduce the complex morphology of osteal tissue, osteoinductive materials that can integrate with the biological environment and promote bone regeneration are used in the fabrication of composite biodegradable and bioactive 3D porous scaffolds. The disadvantages of autologous grafts, as well as the prolonged healing process and elevated risk of infection, are all eliminated by the use of composite scaffolds [
2].
The use of various artificial three-dimensional (3D) scaffolds for the regeneration and restoration of bone tissue has been the subject of extensive research in recent years. The biggest problem is the inability to create neovascularization in the area of bone damage, which prevents the normal homeostasis of bone tissue and leads to defective bone. The creation of scaffolds aimed at enhancing angiogenesis could be a reliable solution to the problem [
3]. The restoration of bone damage is a challenge, especially in osteoporotic patients, where the loss of bone mass coexists with the degradation of the structural architecture of the bones [
4].
The most important requirement for an ideal scaffold for bone tissue engineering applications is a highly interconnected porous structure with pores in the range of 200–500 μm, which is essential for bone formation, nutrient delivery, and neovascularization. Pores in the range 0–200 µm play an important role in cell adhesion, enhancing the flow of biological fluids [
5].
The foam replica technique was the first scaffold synthesis method established to produce ceramic materials with controlled porosity [
6] and, in 2006, it was proposed to develop scaffolds made of bioactive glass [
7]. Composite scaffolds are a positive replica of a porous template that is usually a foam based on polyurethane (PU) [
7]. After several coatings with a bioactive glass paste or a sol–gel solution of a glass [
8], the porous template matrix is burned while the bioactive glass is heated at a high temperature. The final 3D structures own a highly open and interconnected porous network that mimics the actual architecture of the cancellous bone [
7]. Silica-based nanoparticles or mesoporous particles, biodegradable polymers, and polymeric microspheres can be used to coat bioactive glass-based scaffolds developed using the foam replica technique, turning them into local drug delivery systems and enhancing their mechanical properties [
9].
Bioceramic scaffolds, despite their important biological properties, exhibit limited mechanical integrity. Their fragility could be addressed by combining synthetic/biodegradable polymers. The most sought-after candidates for tissue engineering applications are biodegradable polymers with high processing flexibility [
10]. In tissue engineering, synthetic biodegradable polymers with clearly defined structures and no immunological reactions are frequently used [
11]. The most widely used synthetic biodegradable polymers in tissue regeneration, aliphatic polyesters, can form stable porous materials that do not dissolve or melt in vitro and are used as 3D scaffolds. The degradation of such polymers is achieved through the hydrolysis of esters [
12,
13]. Some of the most popular polyesters include poly(lactic acid) (PLA), polyglycolic acid (PGA), and their copolymer, poly(lactic-co-glycolic) acid (PLGA) [
14].
The US Food and Drug Administration approved the synthetic copolymer of poly(lactic acid) (PLA) and polyglycolic acid (PGA) for biomedical applications. The ratio of PLA to PGA in the copolymer structure can be changed to control the degradation rate of PLGA, which can range from weeks to months [
15]. Consequently, the optimum PLA/PGA ratio could give the scaffolds a desired rate of degradation and improved mechanical properties [
14].
Ceramic nanocomposites constitute a biomimetic approach to bone regeneration as they mimic the structure of healthy bone and combine unique biocompatible and stimulating properties with excellent mechanical performance [
16]. Silica-based nanoparticles used in biomedical applications typically have diameters between 10 and 100 nm, and their biological response is affected by their size and specific surface area [
17]. These properties are also important for the application of nanobioceramics as fillers in composite materials and polymeric coatings for ceramic scaffolds [
18]. Nanobioceramic–polymeric structures mimic the organic–inorganic natural structure of bone, providing nanosized crystalline materials with biomineralization capacity acting as nanoscale hydroxyapatite crystals embedded in a polymeric matrix, similar to the collagen phase of bone [
19].
Although various inorganic biomaterials have been used to repair damaged tissues, silicate biomaterials, such as bioactive glass, CaSiO
3, and Ca-Si-M ceramic systems (where M = Mg, Zn, Ti, Zr), exhibit the characteristic property of releasing Si ions in such a concentration that the growth of osteoblasts is stimulated, which makes these materials suitable for application in bone regeneration [
20]. That is, glass and glass–ceramic materials containing Si, Ca, and Mg have been proven to exhibit excellent bioactivity and can be applied in biomedical applications due to improved properties relating to tissue regeneration [
21]. The ternary system SiO
2-CaO-MgO can produce different bioactive phases, such as diopside (CaMgSi
2O
6), akermanite (Ca
2MgSi
2O
7), and bredigite (Ca
7MgSi
4O
16). It has been proven (also in vivo and in vitro) that such materials are characterized by excellent bioactivity, remarkable mechanical strength [
22], and their biodegradability, while the ionic derivatives of the materials mentioned above favor cell proliferation [
23].
Researchers have begun to investigate the use of metal ions as therapeutic agents in the field of tissue engineering. Certain metal ions, which are also necessary for the production of enzymes, enhance bone formation, which is attributed to their stimulatory effect on the mechanism of osteogenesis (Ca and Mg) and angiogenesis (Cu). The use of metal ions, such as copper, zinc, and silver, brings about further therapeutic effects due to their anti-inflammatory and antimicrobial properties [
24].
Although some ions are found in the body at trace concentrations, such as strontium, copper, zinc, and cobalt, in vitro studies with inorganic ions released from bioactive glass have shown that, although they increase ion concentration, these biomaterials exert stimulatory effects on cells and, in many cases, no other toxic effects. Copper ions stimulate angiogenesis and promote the formation and maturation of blood vessels. Sr increases osteogenesis both in vivo and in vitro, and Cu increases angiogenesis and increases the differentiation of human mesenchymal stem cells. Moreover, Cu also inhibits bone resorption [
24,
25].
Local delivery of active substances has advantages over oral administration for the treatment of bone infections as local antibiotics can be administered to treat bacterial adhesion during the early stages of tissue healing, thus avoiding the creation of inflammation [
26]. Alternative fluoroquinolone antibiotics of the fourth generation, such as moxifloxacin (MOX), exhibit antimicrobial activity against both aerobic and anaerobic bacteria, including S. aureus, the primary pathogen linked to osteomyelitis. As moxifloxacin is effective against Staphylococcus aureus, it may be used topically to eradicate bacteria [
20].
In conclusion, ion release from multifunctional ceramic-based scaffolds can create an ideal microenvironment for the formation of new bone aiming to accelerate osseointegration and promote angiogenesis. Combined with the capacity of local drug administration, this could be highly beneficial in certain dental and orthopedic applications, offering a proactive strategy for dealing with bone infections. The aim of this study was the fabrication of bioactive polymeric–ceramic composite scaffolds with prolonged and sustained MOX release using nanofillers for enhanced mechanical properties.
4. Discussion
This work investigated the fabrication of composite 3D bioceramic/polymer scaffolds based on an akermanite bioceramic structure. The studied materials were fabricated with the goal of developing scaffolds for bone tissue engineering, a significant biomedical application that presents numerous material challenges. Bioceramic scaffolds such as akermanite have limited mechanical integrity; however, by combining synthetic degradable polymers, their fragility can be reduced. Synthetic degradable polymer PLGA with a well-defined structure and no immunological reactions was used to reduce fragility. Seven silica-based NPs were synthesized to be used as nanofillers in the PLGA matrix to mimic natural bone and enhance the biocompatibility and mechanical performance of akermanite scaffolds [
45]. The nAkCuSr NPs were selected as fillers. As was mentioned in the results section, the seven nanobiomaterials did not present remarkable differences, remaining negatively charged and nanosized with high amorphous percentages and good hemocompatibility. However, the Sr and Cu co-doped NPs were selected for further study as they contain a unique composition that could enhance antibacterial capacity and angiogenesis, thus making them excellent candidates for tissue regeneration applications as functional fillers in composite polymeric scaffolds or coatings on the surface of implants.
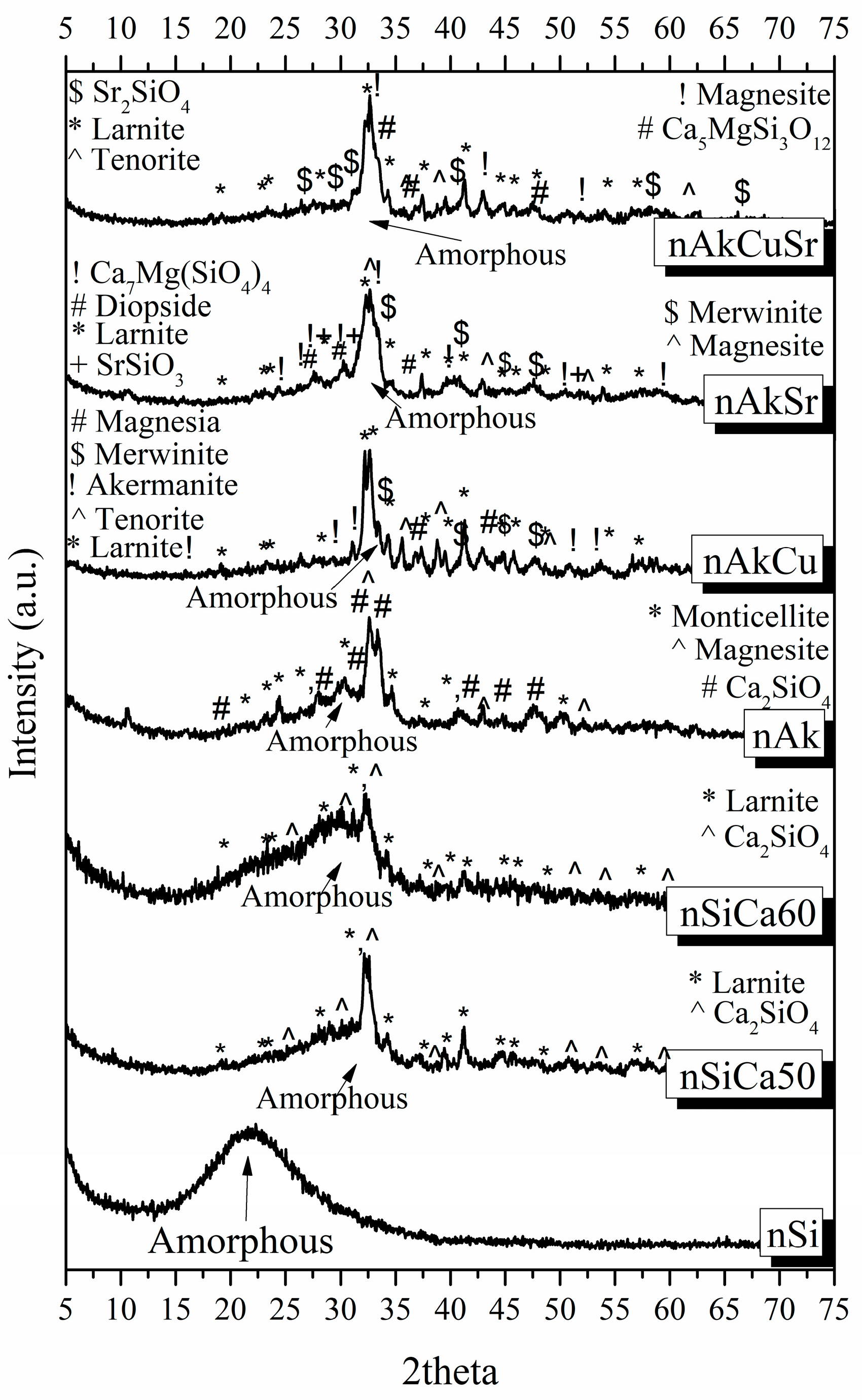
Different bioactive powders were created using the sol–gel technique in the composition of akermanite to investigate the optimal conditions for the synthesis of the 3D bioceramic porous scaffolds. Akermanite is a crystalline phase of the ternary system SiO
2CaOMgO, and, according to its phase diagram, presents a melting point at the temperature of 1454 °C, justifying the absence of a melting point in the studied temperature range [
46]. The XRD results, in combination with the TG/DSC measurements, revealed that the optimal temperature to synthesize akermanite-based scaffolds is higher than 1300 °C, verifying that increases in temperature increase the percentage of akermanite crystallinity. Chengtie Wu et al. studied the formation of akermanite after sintering at 1100, 1200, and 1300 °C [
30]. Study of the formation of crystalline phases using X-ray diffraction revealed the co-existence of merwinite and diopside, as well as the formation of the akermanite crystalline phase. However, the transformation of merwinite and diopside to akermanite was observed when the sintering temperature was increased. Furthermore, the same authors suggest 1300 °C as the temperature above which the merwinite and diopside crystalline phases can completely transform to akermanite. The transformation of merwinite and diopside into akermanite nanocrystals by increasing the temperature was also confirmed in a similar study by Choudhary et al. Those findings are in line with our findings [
47]. In previous studies, it was also reported that it is possible for merwinite and diopside crystalline phases to form akermanite crystalline phase at a temperature above 800 °C, which is required for the formation to start according to the following equation [
40]:
Gibbs free energy (ΔG) could help to predict the thermodynamic behaviors of chemical changes. The Gibbs free energy in the akermanite system at 800°C was determined by Myat Myat-Htun et al. (ΔG
800°C = −18.06 kJ mol
−1K
−1), which was estimated by fitting the Gibbs equation [
40,
48]. If the ΔG value is negative, the reaction is spontaneous (the reaction product is favored). If ΔG is positive, the reaction is nonspontaneous (reactant favored), and if ΔG is zero, the system is at equilibrium. The reaction presented a negative ΔG value, indicating the spontaneous formation of akermanite from a thermodynamic point of view. In full agreement with previous studies, the presence of a small percentage of merwinite and diopside, which are calcium magnesium silicate crystalline phases, is an expected finding. X-ray diffraction patterns also confirmed the transformation of these crystalline phases into akermanite with the increase in temperature [
40,
48]. Lower sintering temperatures can be used to improve the bioactivity of HAp bone grafts or scaffolds [
49]. Keeping this in mind, we selected 1300 °C as a sintering temperature, the XRD results of which presented a high percentage of akermanite.
The ideal scaffold for bone engineering applications should have a porous structure with pores in the 200–500 μm range, which is essential for bone formation, neovascularization, and nutrient delivery [
50]. In full agreement with the literature, the pore size of akermanite scaffolds was around 307 μm, while their mechanical properties were limited. As was observed in a previous study, PLGA scaffolds enhanced with hydroxyapatite (HAp) nanoparticles at 10–20% wt., possess higher compressive strength than neat PLGA scaffolds [
51]. Previous studies have also shown that nanoparticles own high surface energy. Thus, the connection between the particles and the linear chains of the polymer is strengthened when nanofillers are added to the polymeric matrix [
52]. In addition, the nanoparticles are wrapped by the linear chains of the polymer, which could lead to higher strength and cross-link junctions. Therefore, compressive strength increases with an increasing percentage of nanofillers in the matrix of PLGA [
52]. This is also confirmed by our findings, as mechanical strength increased by up to almost 190% after application of the PLGA/NPs coating. Although compressive strength was increased, it is limited compared to the compressive strength of human cancellous bone, which is between 1.5 and 45 MPa [
53]. This was expected as foam replica fabricated scaffolds present low compressive strength values. Similar findings were also observed by Wu et al. They fabricated akermanite scaffolds using the foam replica technique that presented compressive strength between 1.13 and 0.53 MPa [
31]. The limited mechanical properties of foam replica scaffolds has driven attention to the development of ceramic scaffolds, which can be fabricated through the use of innovative alternative methods such as 3D printing [
54]; 3D printing is one of the most promising methods for the development of biomimetic scaffolds for bone tissue engineering, but their mechanical strength is still limited. Advanced additive manufacturing techniques can be used to enhance the mechanical performance of ceramic scaffolds, such as using glass ceramics as a secondary filler to “heal” cracks and increase mechanical strength [
55,
56].
The successful regeneration of tissue depends in large part on the degradation of biomaterial scaffolds. Many biomedical applications, such as replacing hard tissues, require a scaffold to have the proper mechanical properties, which are typically on the same order of magnitude as those of the tissue it replaces. Additionally, the scaffold must degrade while retaining a particular minimal mechanical strength to support the formation of the new tissue. For example, in order to support bone regeneration, the material must be able to maintain its strength during the healing process, which, from a clinical standpoint, is typically at least 12 weeks [
57,
58]. In our study, the addition of nanofillers into the polymer coating (AkP30N scaffolds) increased the degradation rate, which was 10% after 4 weeks of study. In full agreement with these findings, previous studies revealed that the incorporation of bioactive nanofillers into a polymer matrix can prevent the collapse of the 3D porous structure of the composite scaffolds and increase the degradation rate. This fact is due to the percentage of added particles and the enhanced bioactivity due to their excellent bioactive behavior [
45,
59].
Τhe systemic toxicity of akermanite bioceramics was already investigated following well-accepted ISO standard methods in healthy adult rats. The injection of akermanite extracts in Wistar rats did not cause pathological changes to important organs, thus indicating the biosafety of akermanite bioceramics in clinical applications [
60].
In a previous in vivo study, animals implanted with akermanite and composite akermanite/PCL scaffolds experienced severe hypothermia and weakness 48 h after implantation [
61,
62]. Hemolysis was then observed, and chemical analysis of the hemolytic blood revealed severe hypophosphatemia (<0.3 mg/dL) and phosphorus deficiency. Akermanite is a biocompatible ceramic that has been shown to improve stem cell adhesion, proliferation, and the maintenance of the osteogenic phenotype. However, in vivo implantation of two types of scaffolds resulted in acute toxicity and the disturbance of phosphorus homeostasis. This is due to the binding of phosphorus from the blood serum, thus creating hypophosphatemia and hemolysis [
60]. To address the dose-dependent toxicity of akermanite, however, more research is required [
61,
62]. In our study, the addition of nanofillers to the polymeric coating of the composite scaffolds (AkP30N) did not induce hemolytic interactions and instead improved the hemolytic behavior of the scaffolds by creating a hemocompatible profile. PLGA is a highly hemocompatible biodegradable polymer [
63]. Nevertheless, nanoparticles of the SiO
2CaOMgO system doped with strontium and copper showed higher hemocompatibility, suggesting that this combination of ions is the most ideal for their use as nanofillers. This is confirmed by the increased hemocompatibility of the PLGA-coated akermanite scaffolds in which nanofillers from the nAkCuSr group were used within the polymeric coating. Our results are in accordance with other similar studies. Specifically, Nam et al. also used copper and strontium in TiO
2 nanotubes to improve the hemocompatibility and the cytocompatibility of TiO
2 implants for cardiovascular devices [
64]. Additionally, strontium ion doping of mesoporous glass appeared to improve the hemocompatible profile of such materials for multifunctional biomedical applications [
65]. In combination with the above, even if the high bioactivity of akermanite scaffolds can cause hypophosphatemia due to the rapid uptake of phosphorus from blood serum in the formation of an apatite layer, ultimately leading to hemolysis, it seems that the polymeric coating and the doping of NPs with copper and strontium improves hemocompatibility and prolongs the release of ions from the bioceramic structure (akermanite scaffold) upon interaction with the surrounding tissues and blood serum, thus reducing the severity of hemolytic effects.
Jadidi et al. investigated the encapsulation capacity and release rate of vancomycin from bredigite (Ca
7Mg(SiO
4)
4) scaffolds before and after PLGA coating [
43]. In their study, they found that the bredigite scaffolds without the PLGA coating showed the highest drug release rate (about 94.7 ± 2.5% of the encapsulated vancomycin was released within 9 h). However, they observed that the drug release rate after coating the scaffolds with PLGA was reduced beneficially in the first 6 h of release. It is important to mention that release of the drug in the first 6 h of implantation is necessary to prevent bacterial attachment and thus inhibit infections [
66,
67]. The authors observed that, after the initial burst of drug release, the rate of vancomycin release decreased, which was followed by a sustained release for the polymer-coated scaffolds. The akermanite scaffolds without the PLGA coating showed the highest drug release rate due to low degradation of the PLGA in composite scaffolds. Tailoring polymer blend/NPs percentage can help achieve more effective drug release. Staphylococcus aureus infections in the bone are a major issue in the biomedical field, and a new class of antibiotics must be investigated due to growing bacterial resistance [
68]. Moxifloxacin is one of the most effective drugs against gram-positive bacteria, making it suitable for the treatment of bone infections [
68,
69]. It is administered orally in the form of 400 mg tablets and is recommended for patients before and after hip replacement surgery, as blood flow across the operated leg is constrained during knee replacement surgery [
70]. Additionally, studies have been conducted to administer moxifloxacin or vancomycin to rat models for osteomyelitis. The experiment included treatment with 10 mg/kg BW moxifloxacin or vancomycin 10 mg/kg seven days after the experimental contamination. The authors concluded that, even if moxifloxacin was more sufficient against bacteria compared to vancomycin, the results of monotherapy were not as adequate as required [
68].
An initial burst release is necessary to reduce the risk of infection and should be followed by a high enough drug concentration for an antibacterial effect in the recovery stage. Controlled and gradual release behavior was achieved in all cases for the scaffolds studied in this work [
71]. In the present work, the loading capacity of ceramic structures at two different concentrations for local drug delivery was examined. The higher concentration (3 mg/mL) resulted in a higher loading percentage (~27.24%). This fact indicates the limited encapsulation capacity of the drug, which in absolute terms reveals that an increase in concentration leads to an increase in the encapsulation of MOX. For the Ak ceramic scaffolds, the quantity of drug released at the concentration of 1 mg/mL was 1.37% in the first 24 h and 3.31% at the concentration of 3 mg/mL, which can be translated into 0.092 mg and 0.544 mg of drug, respectively. Moreover, for the composite scaffolds (AkP30N), the quantity of drug released at the concentration of 1 mg/mL was 0.55% in the first 24 h and 1.37% at the concentration of 3 mg/mL, which can be translated into 0.024 mg and 0.21 mg of drug, respectively. However, according to the literature [
72], the daily dose of antibiotics against osteomyelitis for a set of bacteria such as
S. aureus (which grows during the early stages of implantation) is 0.06–128 µg/mL [
72]. A minimum inhibitory concentration (MIC
90) of 0.125 μg/mL has been reported for moxifloxacin against
S. aureus [
73]. This fact makes the composite scaffolds promising for use in bone tissue regeneration.
Additional research should be conducted in order to provide insights into the effects of the synthesized scaffolds on human mesenchymal stem cell (hMSC) attachment, proliferation, and multilineage differentiation, as well as the effects of coatings on the scaffolds.