System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
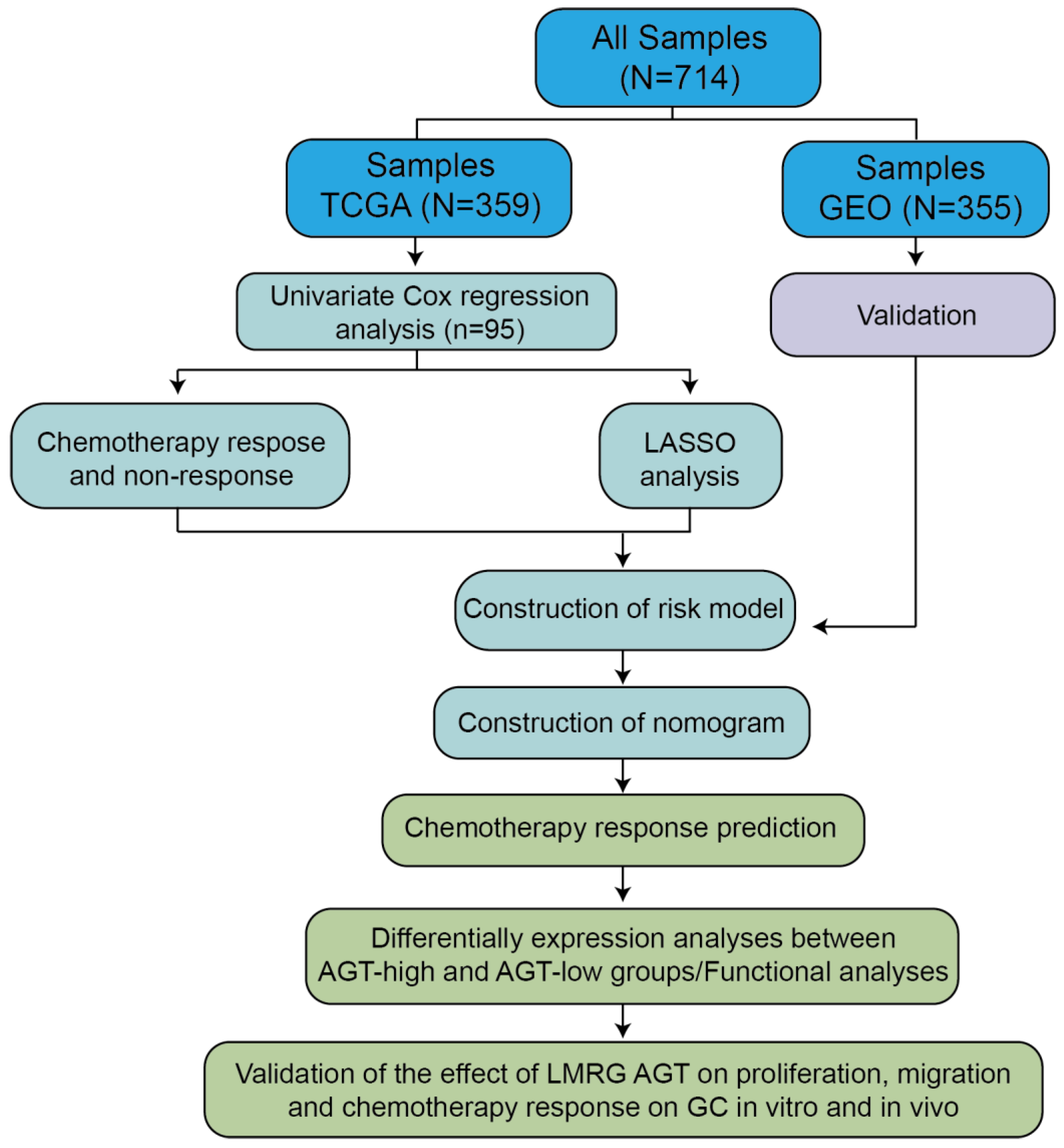
2.1. Patients and Cohorts
2.2. Extraction of Genes in Lipid Metabolism
2.3. Functional Analyses
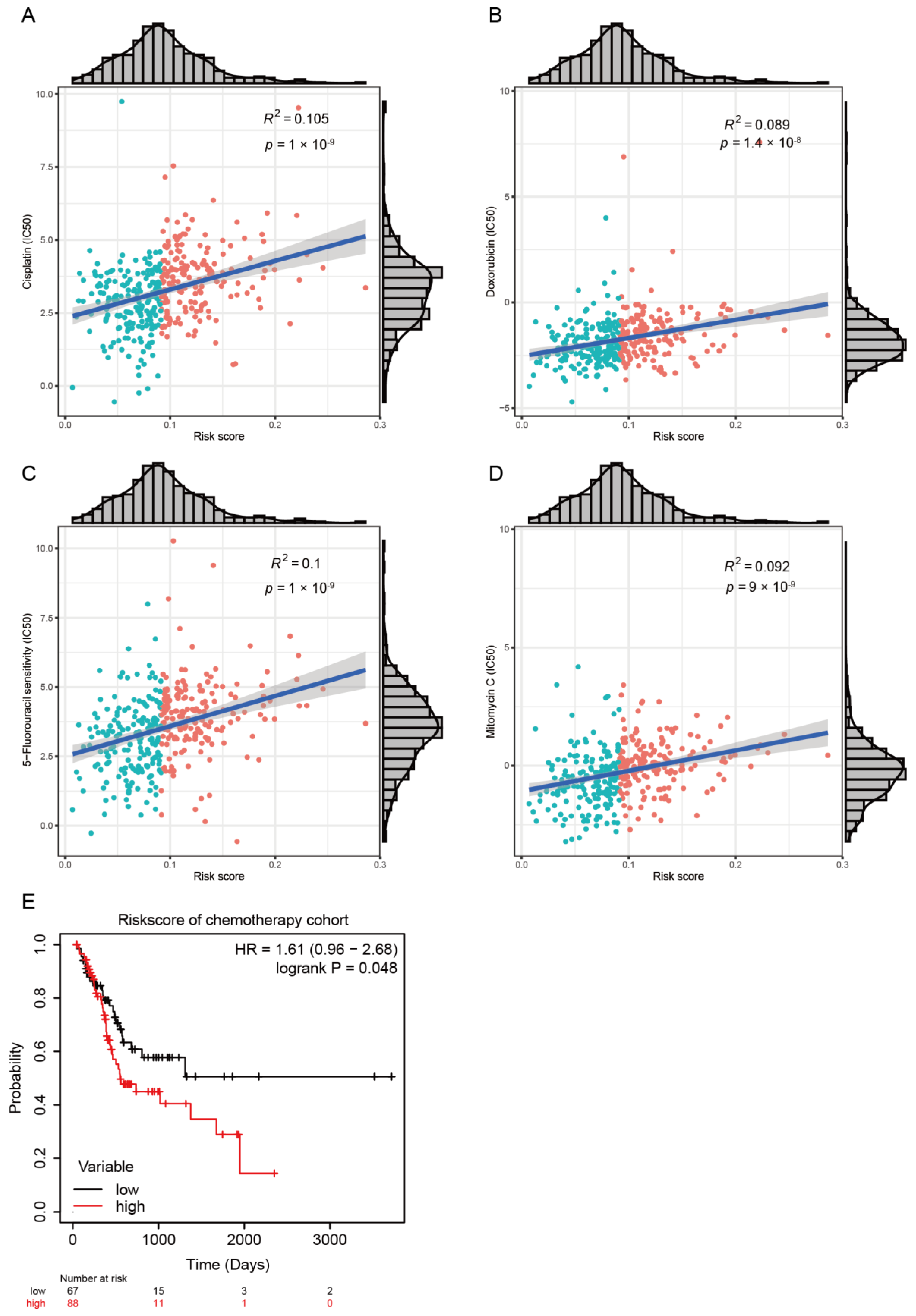
2.4. Chemotherapeutic Response Prediction
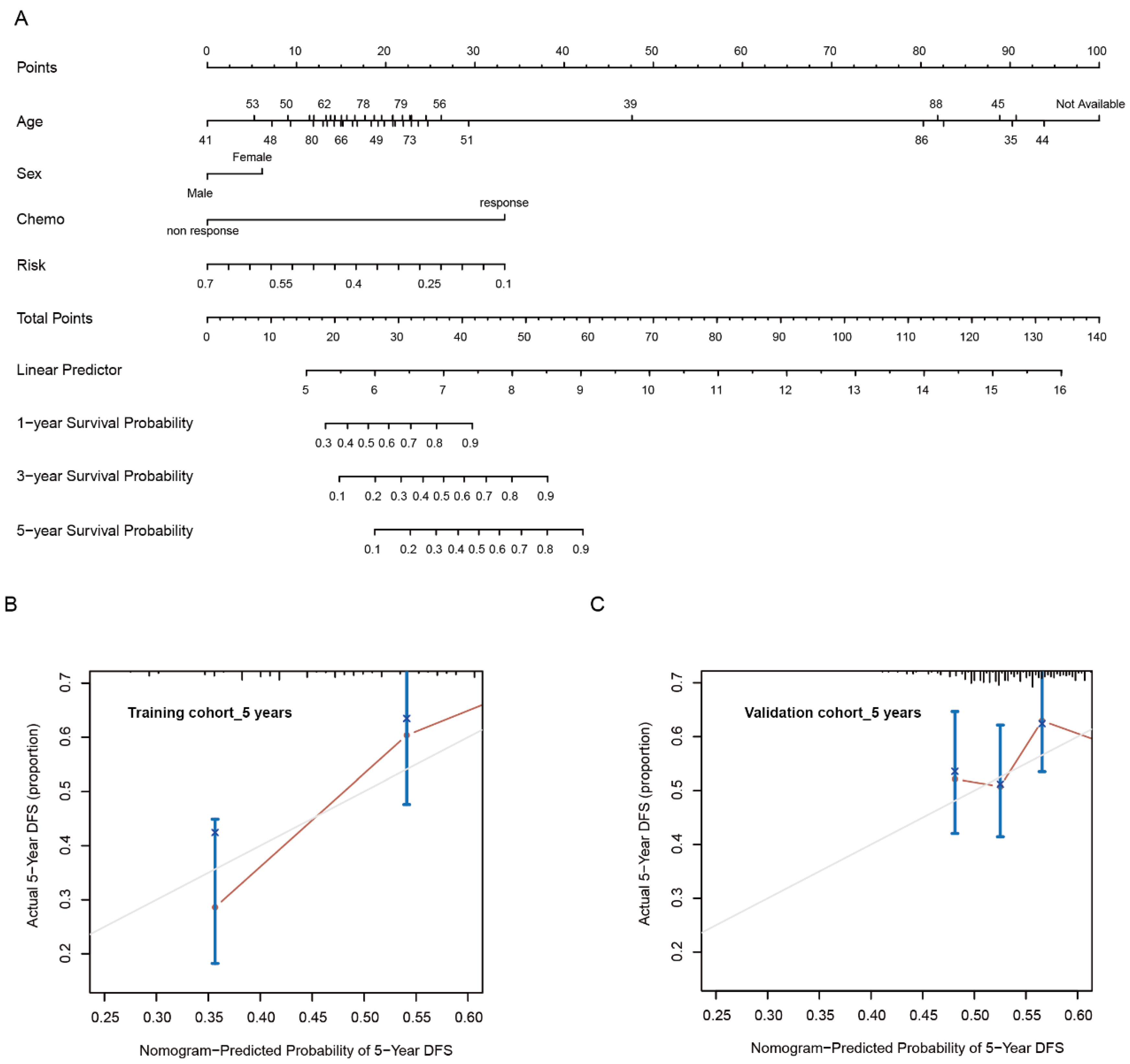
2.5. Establishment and Validation of Risk Model
2.6. RNA Interference and Plasmid Transfection
2.7. Cell Viability Assay
2.8. Western Blot
2.9. Colony Formation Assay
2.10. Cell Migration
2.11. ELISA
2.12. Establishment of GC Subcutaneous Xenograft Models in Nude Mice
2.13. Statistical Analyses
3. Results
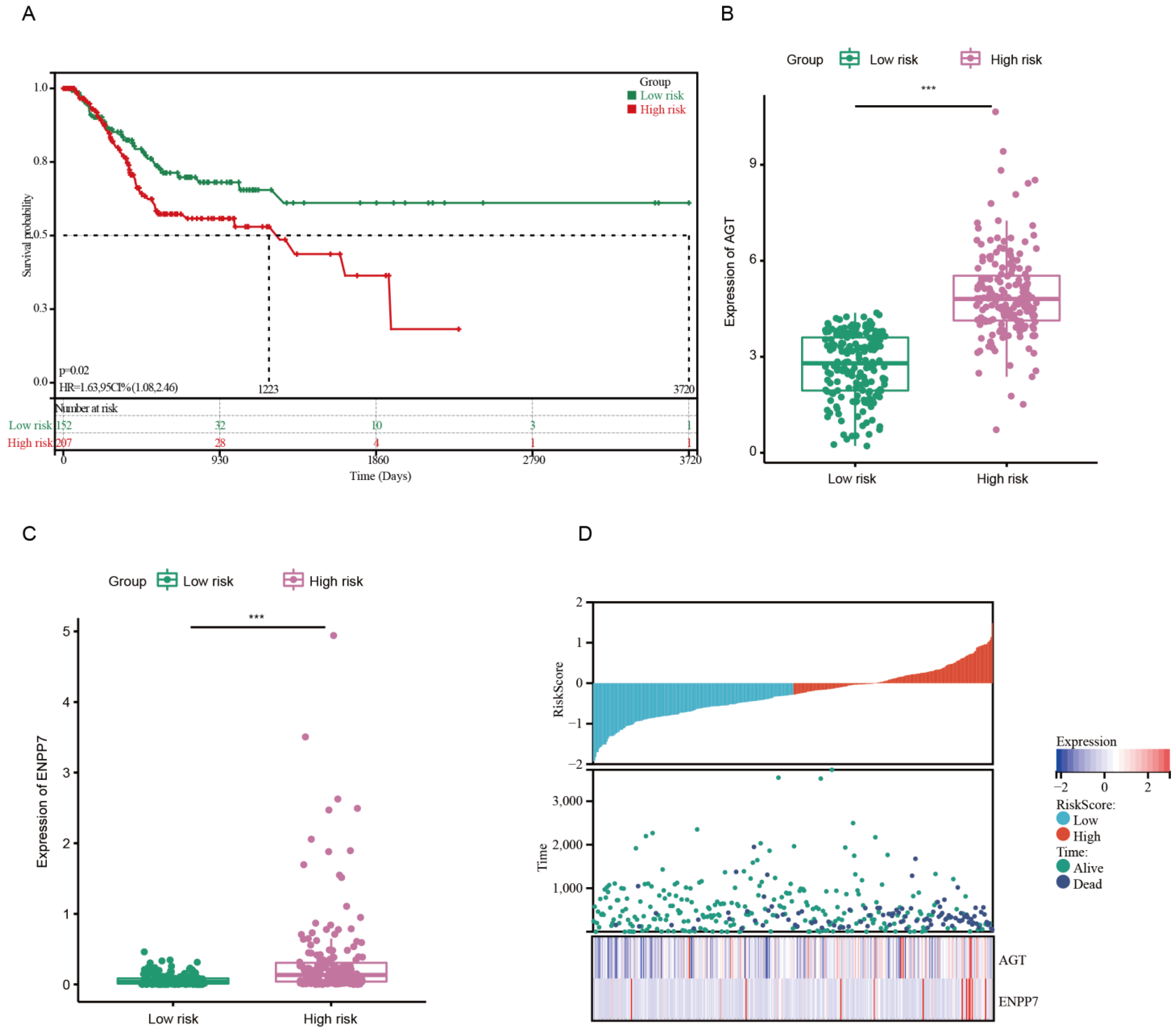
3.1. Development of Risk Model with Prognosis and Response to Chemotherapy Based on LMRGs in Stomach Adenocarcinoma
3.2. Prognostic Value and Validation of the Risk Signature
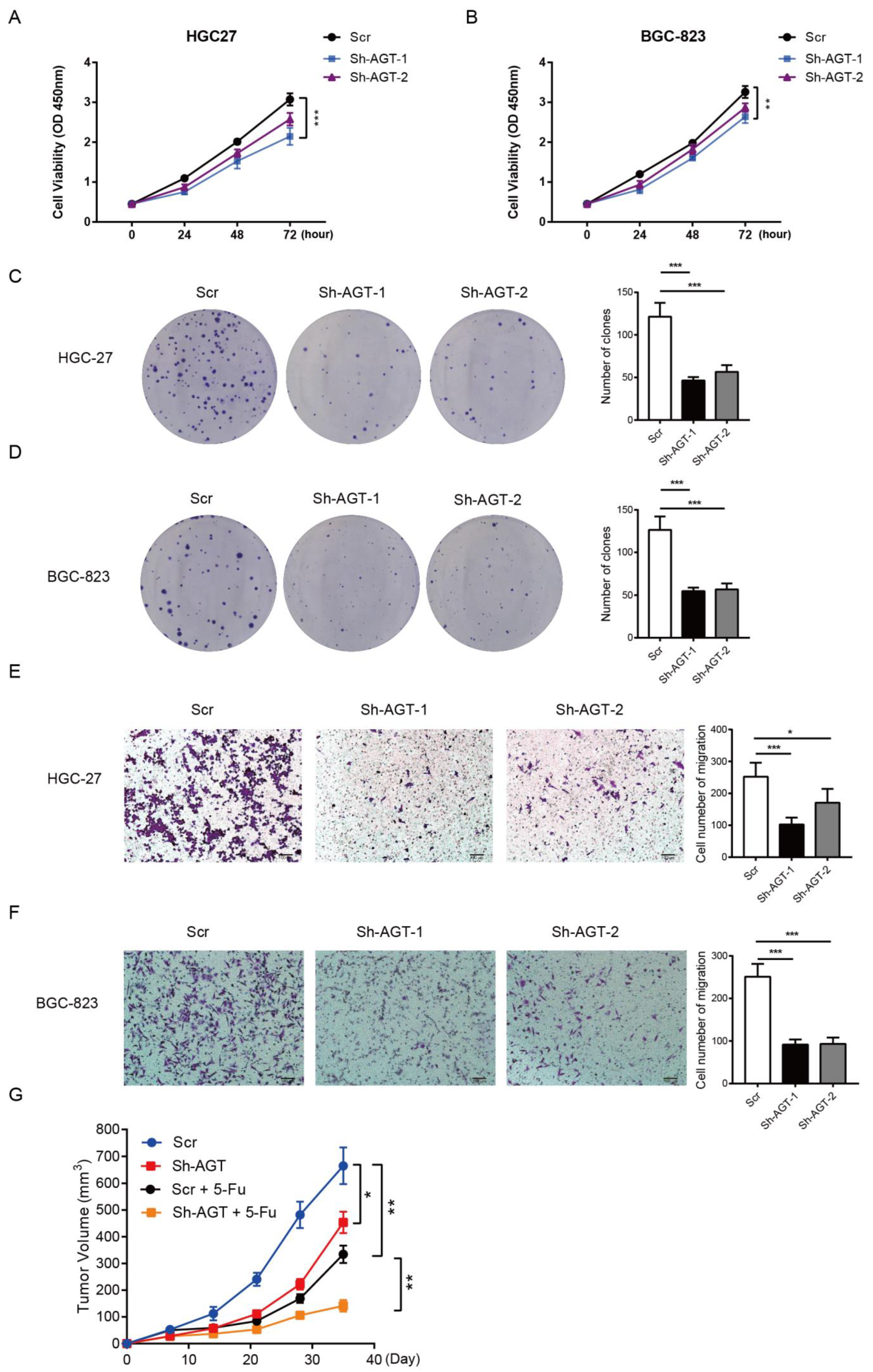
3.3. AGT Promotes GC Proliferation and Migration
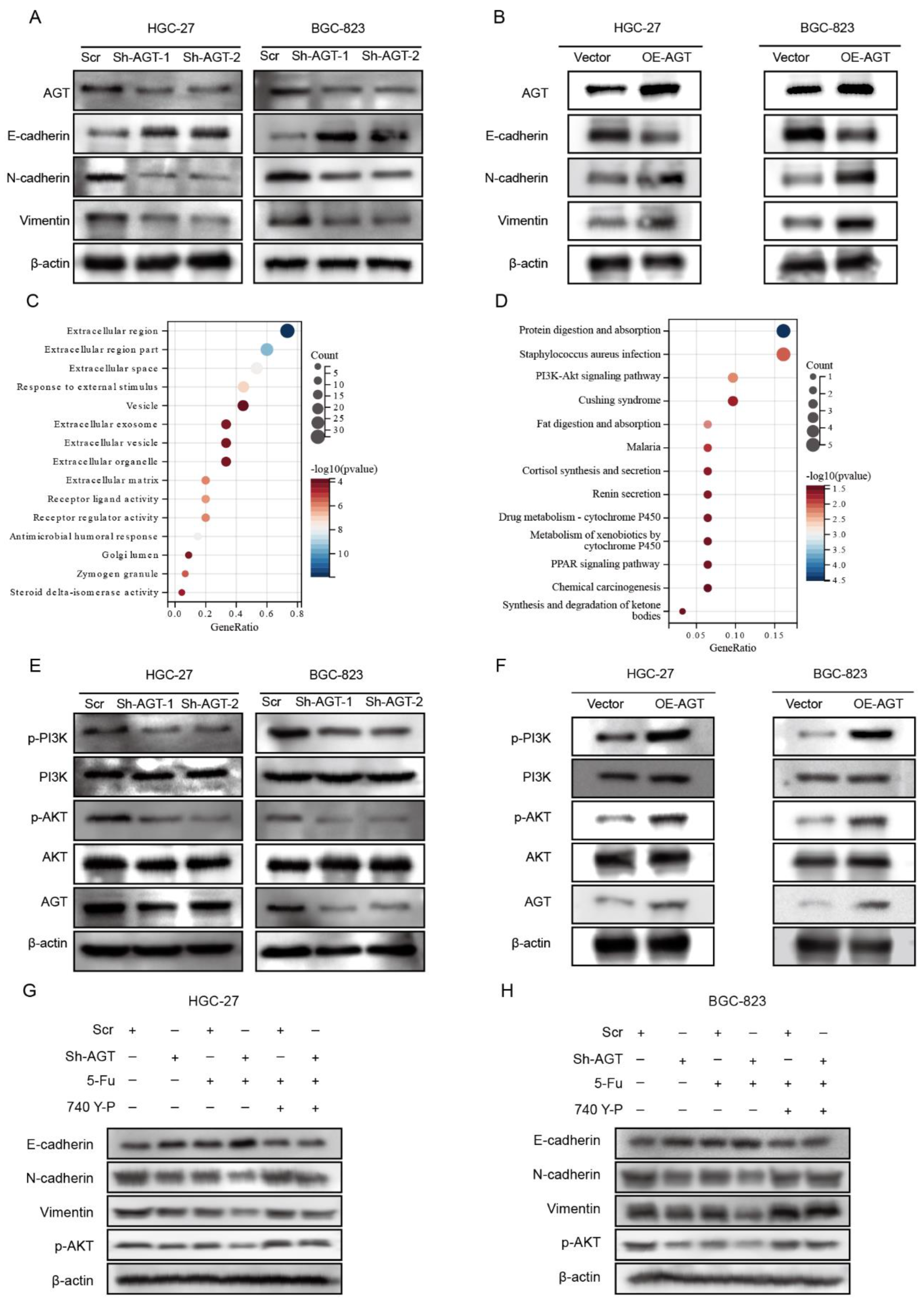
3.4. AGT Activated EMT via PI3K/AKT Pathway in GC Cells
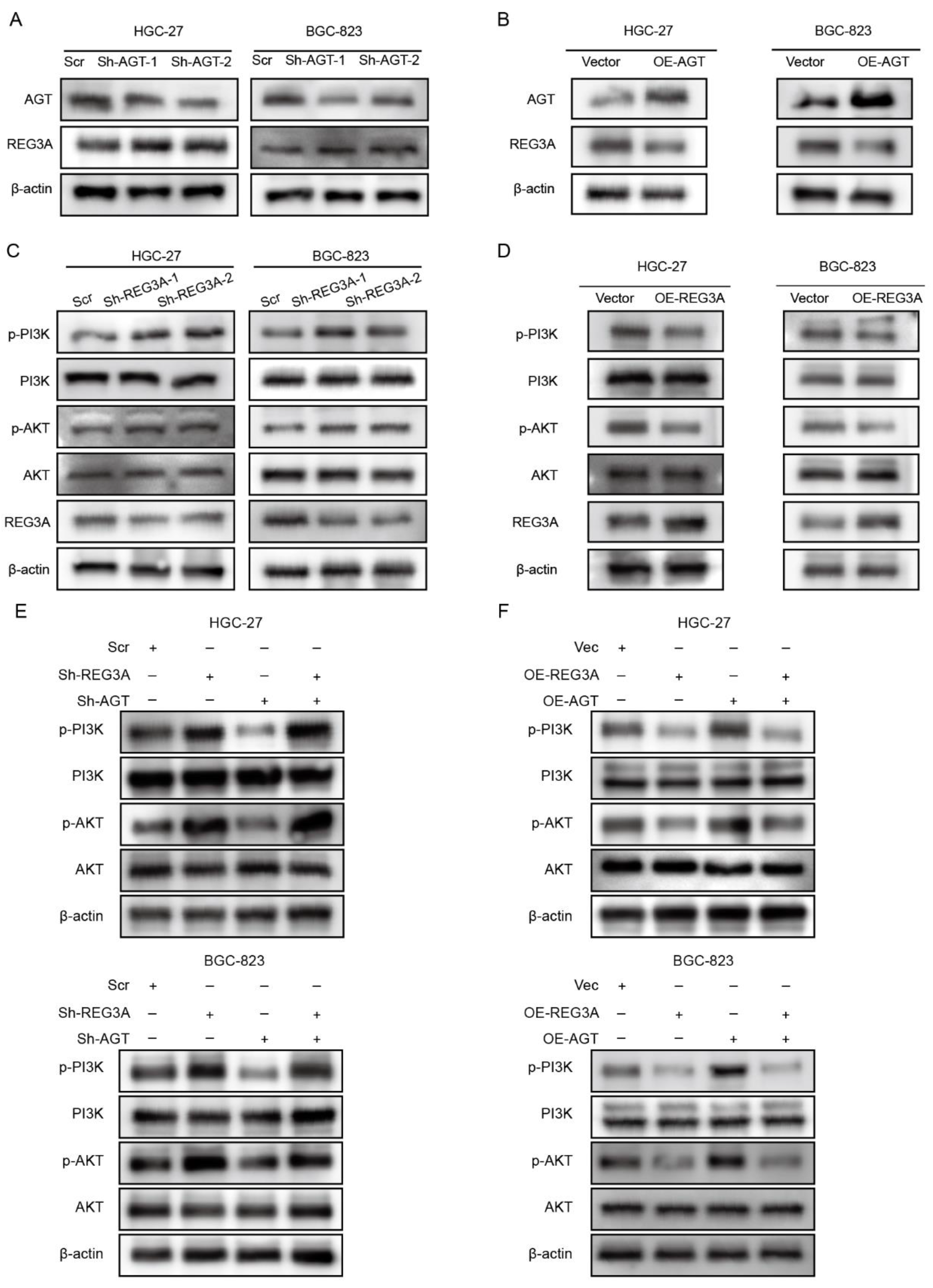
3.5. REG3A Mediated the Activation of the PI3K/AKT Pathway by AGT in GC Cells
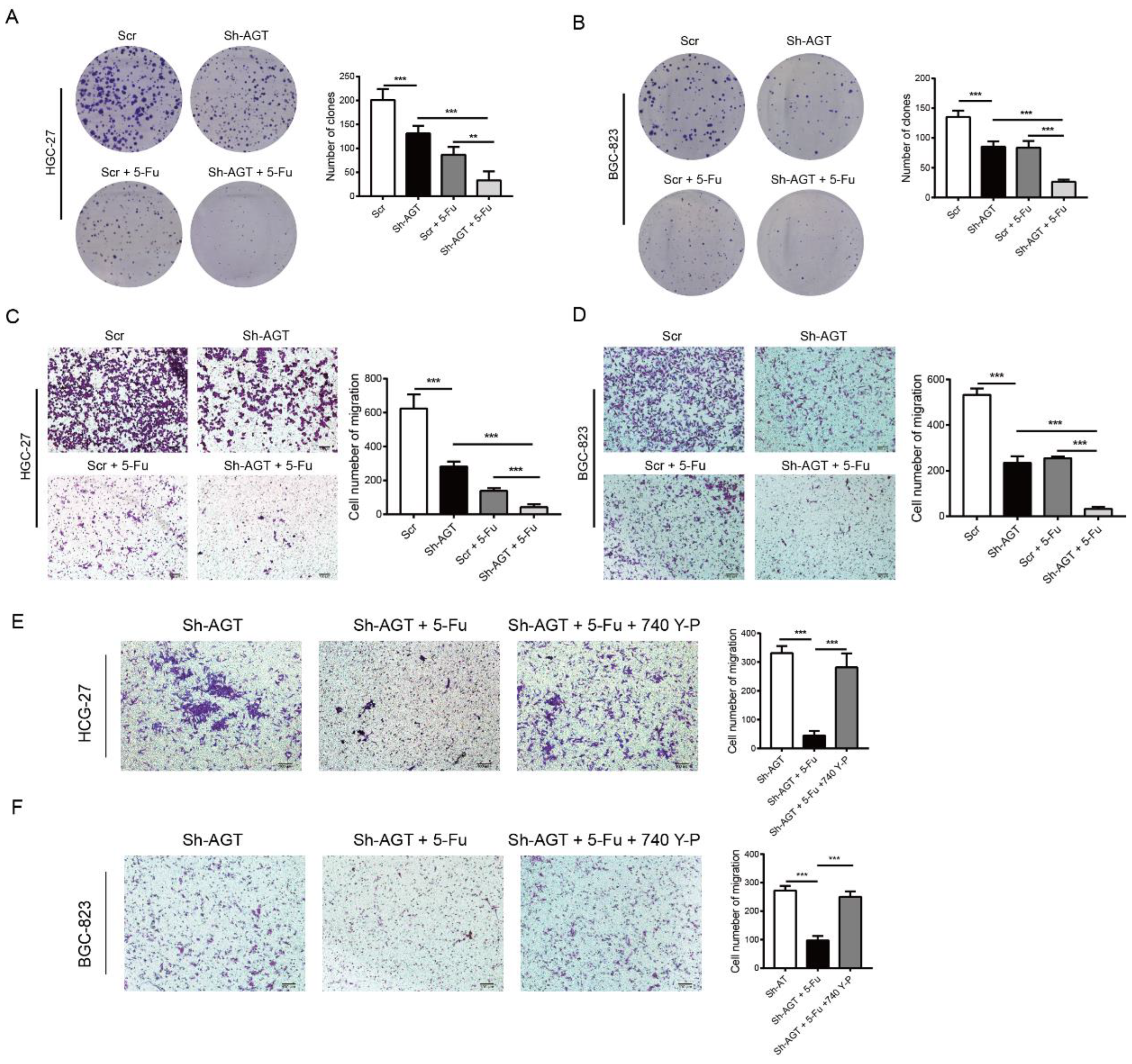
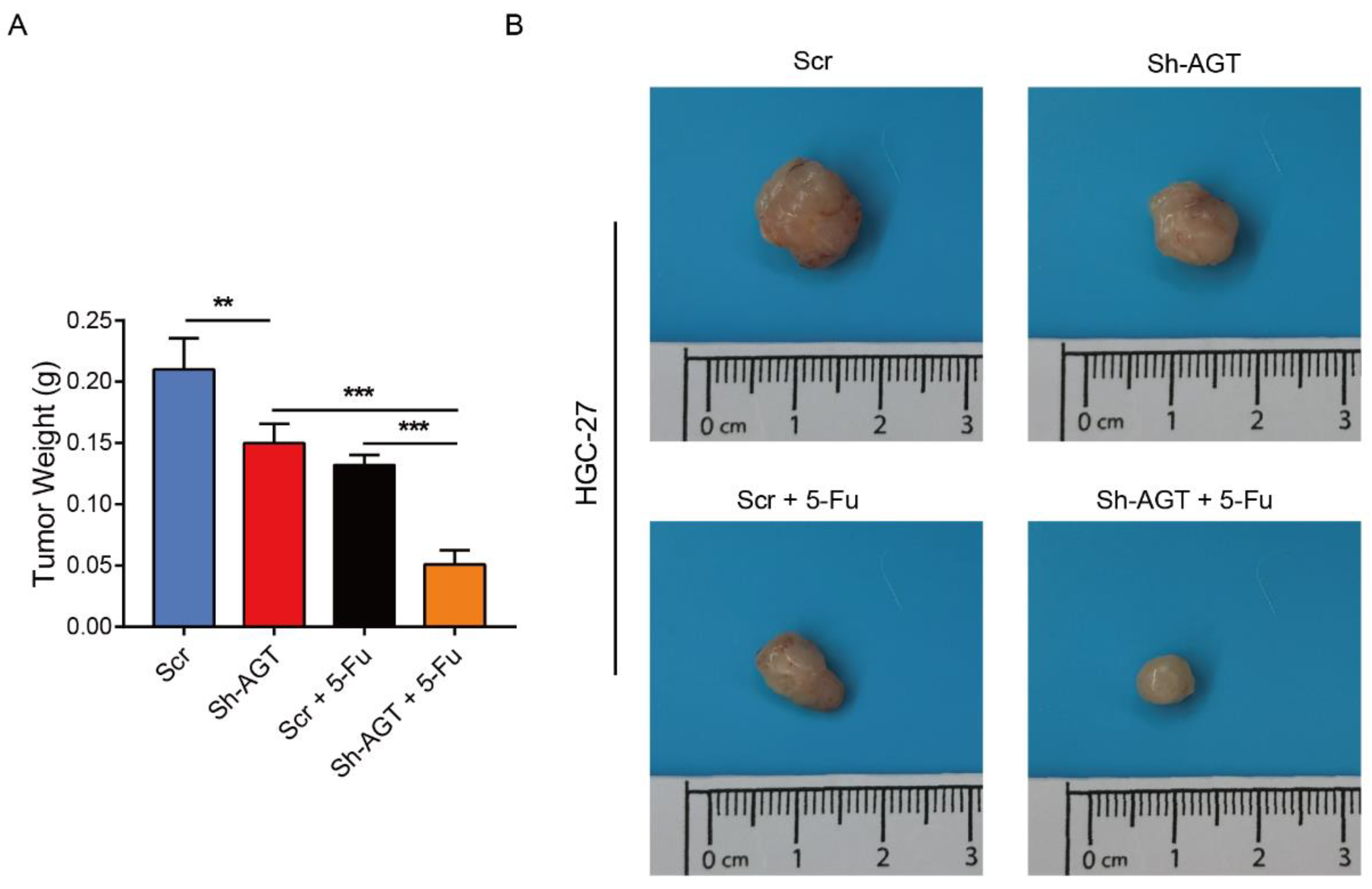
3.6. Targeting AGT Promoted the Efficacy of Chemotherapy of GC through Inhibiting EMT via the PI3K/AKT Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, N.Y.; Tan, P. Molecular classification of gastric cancer. Ann. Oncol. 2016, 27, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Thrumurthy, S.G.; Chaudry, M.A.; Chau, I.; Allum, W. Does surgery have a role in managing incurable gastric cancer? Nat. Rev. Clin. Oncol. 2015, 12, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef] [PubMed]
- Corso, S.; Giordano, S. How Can Gastric Cancer Molecular Profiling Guide Future Therapies? Trends Mol. Med. 2016, 22, 534–544. [Google Scholar] [CrossRef]
- Das, M. Neoadjuvant chemotherapy: Survival benefit in gastric cancer. Lancet Oncol. 2017, 18, e307. [Google Scholar] [CrossRef]
- Lordick, F.; Nilsson, M.; Leong, T. Adjuvant radiotherapy for gastric cancer-end of the road? Ann. Oncol. 2021, 32, 287–289. [Google Scholar] [CrossRef]
- de Steur, W.O.; van Amelsfoort, R.M.; Hartgrink, H.H.; Putter, H.; Meershoek-Klein Kranenbarg, E.; van Grieken, N.C.T.; van Sandick, J.W.; Claassen, Y.H.M.; Braak, J.; Jansen, E.P.M.; et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann. Oncol. 2021, 32, 360–367. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Hoy, A.J.; Nagarajan, S.R.; Butler, L.M. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat. Rev. Cancer 2021, 21, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. J. Clin. Investig. 2019, 129, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Z.; Zhang, X.; Yin, K.; Wang, W.; Xu, Z.; Li, B.; Zhang, L.; Xu, J.; Sun, G.; et al. MiR-422a regulates cellular metabolism and malignancy by targeting pyruvate dehydrogenase kinase 2 in gastric cancer. Cell Death Dis. 2018, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Q.; Jiang, Y.; Yu, J.; Hu, Y.; Mou, T.; Chen, G.; Li, G. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2016, 5, e1069936. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Alicea, G.M.; Rebecca, V.W.; Goldman, A.R.; Fane, M.E.; Douglass, S.M.; Behera, R.; Webster, M.R.; Kugel, C.H., 3rd; Ecker, B.L.; Caino, M.C.; et al. Changes in Aged Fibroblast Lipid Metabolism Induce Age-Dependent Melanoma Cell Resistance to Targeted Therapy via the Fatty Acid Transporter FATP2. Cancer Discov. 2020, 10, 1282–1295. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L.; Celerier, J.; Chapuis Bernasconi, C.; Nguyen, G.; Wostl, W.; Maerki, H.P.; Janzer, R.C.; Corvol, P.; Gasc, J.M. Renin and angiotensinogen expression and functions in growth and apoptosis of human glioblastoma. Br. J. Cancer 2004, 90, 1059–1068. [Google Scholar] [CrossRef]
- Kahlon, T.; Carlisle, S.; Otero Mostacero, D.; Williams, N.; Trainor, P.; DeFilippis, A.P. Angiotensinogen: More Than its Downstream Products: Evidence From Population Studies and Novel Therapeutics. JACC Heart Fail. 2022, 10, 699–713. [Google Scholar] [CrossRef]
- Carlos-Escalante, J.A.; Gómez-Flores-Ramos, L.; Bian, X.; Perdomo-Pantoja, A.; de Andrade, K.C.; Mejía-Pérez, S.I.; Cacho-Díaz, B.; González-Barrios, R.; Reynoso-Noverón, N.; Soto-Reyes, E.; et al. Landscape of Germline Genetic Variants in AGT, MGMT, and TP53 in Mexican Adult Patients with Astrocytoma. Cell. Mol. Neurobiol. 2021, 41, 1285–1297. [Google Scholar] [CrossRef]
- Vincent, F.; Bonnin, P.; Clemessy, M.; Contrerès, J.O.; Lamandé, N.; Gasc, J.M.; Vilar, J.; Hainaud, P.; Tobelem, G.; Corvol, P.; et al. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009, 69, 2853–2860. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Zhang, K.; Yang, W.; Li, X.; Zhao, J.; Liu, K.; Dong, Z.; Lu, J. AGT serves as a potential biomarker and drives tumor progression in colorectal carcinoma. Int. Immunopharmacol. 2021, 101, 108225. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yang, M. Identification by Bioinformatics Analysis of Potential Key Genes Related to the Progression and Prognosis of Gastric Cancer. Front. Oncol. 2022, 12, 881015. [Google Scholar] [CrossRef]
- Geeleher, P.; Cox, N.; Huang, R.S. pRRophetic: An R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE 2014, 9, e107468. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 3: Comparing Groups: Numerical Variables. Indian J. Dermatol. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Chen, W.; Shi, K.; Liu, J.; Yang, P.; Han, R.; Pan, M.; Yuan, L.; Fang, C.; Yu, Y.; Qian, Z. Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer. Bioact. Mater. 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Liu, L.; Pang, H.; He, Q.; Pan, B.; Sun, X.; Shan, J.; Wu, L.; Wu, K.; Yao, X.; Guo, Y. A novel strategy to identify candidate diagnostic and prognostic biomarkers for gastric cancer. Cancer Cell Int. 2021, 21, 335. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Yoon, C.; Till, J.; Cho, S.J.; Chang, K.K.; Lin, J.X.; Huang, C.M.; Ryeom, S.; Yoon, S.S. KRAS Activation in Gastric Adenocarcinoma Stimulates Epithelial-to-Mesenchymal Transition to Cancer Stem-Like Cells and Promotes Metastasis. Mol. Cancer Res. MCR 2019, 17, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, J.; Zou, Y.; Tao, C.; Chen, J. Xanthotoxol suppresses non-small cell lung cancer progression and might improve patients’ prognosis. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 105, 154364. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Taheriazam, A.; Daneii, P.; Hassanpour, A.; Kakavand, A.; Rezaei, S.; Hejazi, E.S.; Aboutalebi, M.; Gholamrezaie, H.; Saebfar, H.; et al. Targeting PI3K/Akt signaling in prostate cancer therapy. J. Cell Commun. Signal. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Arakawa-Hoyt, J.; Aguilar, O.A.; Damm, I.; Towfighi, P.; Sigdel, T.; Tamaki, S.; Babdor, J.; Spitzer, M.H.; Reed, E.F.; et al. Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2116588119. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lu, X.; Hong, C.; Cai, R.; Wang, P.; Xiong, L.; Wang, X.; Chen, Q.; Lin, J. Downregulation of TUSC3 promotes EMT and hepatocellular carcinoma progression through LIPC/AKT axis. J. Transl. Med. 2022, 20, 485. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhang, S.; Li, X.; Yu, K.; Zhao, X.; Chen, X.; Jin, B.; Bai, X. Valsartan ameliorates ageing-induced aorta degeneration via angiotensin II type 1 receptor-mediated ERK activity. J. Cell. Mol. Med. 2014, 18, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef] [PubMed]
- Field, C.S.; Baixauli, F.; Kyle, R.L.; Puleston, D.J.; Cameron, A.M.; Sanin, D.E.; Hippen, K.L.; Loschi, M.; Thangavelu, G.; Corrado, M.; et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2020, 31, 422–437.e425. [Google Scholar] [CrossRef]
- Kobayashi, T.; Lam, P.Y.; Jiang, H.; Bednarska, K.; Gloury, R.; Murigneux, V.; Tay, J.; Jacquelot, N.; Li, R.; Tuong, Z.K.; et al. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood 2020, 136, 3004–3017. [Google Scholar] [CrossRef]
- Yu, W.; Lei, Q.; Yang, L.; Qin, G.; Liu, S.; Wang, D.; Ping, Y.; Zhang, Y. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Tang, Y.H.; Lin, G.J.; Ma, Y.B.; Desiderio, J.; Li, P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; et al. Association of Adjuvant Chemotherapy With Overall Survival Among Patients With Locally Advanced Gastric Cancer After Neoadjuvant Chemotherapy. JAMA Netw. Open 2022, 5, e225557. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, H.; Chi, L.; Fu, M.; Wang, G.; Wu, Z.; Xu, S.; Sun, C.; Xu, X.; Lin, L.; et al. Association of Tumor-Associated Collagen Signature With Prognosis and Adjuvant Chemotherapy Benefits in Patients With Gastric Cancer. JAMA Netw. Open 2021, 4, e2136388. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Li, F.; Hua, K.; Deng, J.; Wang, C.H.; Bowers, R.R.; Bartness, T.J.; Kim, H.S.; Harp, J.B. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007, 6, 506–512. [Google Scholar] [PubMed]
- Santos, S.H.; Braga, J.F.; Mario, E.G.; Pôrto, L.C.; Rodrigues-Machado Mda, G.; Murari, A.; Botion, L.M.; Alenina, N.; Bader, M.; Santos, R.A. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler. Thromb. Vasc. Biol. 2010, 30, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Quignard-Boulangé, A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Cheng, Y.; Hertervig, E.; Ohlsson, L.; Nilsson, A.; Duan, R.D. Alkaline sphingomyelinase (NPP7) promotes cholesterol absorption by affecting sphingomyelin levels in the gut: A study with NPP7 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G903–G908. [Google Scholar] [CrossRef]
- Duan, R.D.; Cheng, Y.; Hansen, G.; Hertervig, E.; Liu, J.J.; Syk, I.; Sjostrom, H.; Nilsson, A. Purification, localization, and expression of human intestinal alkaline sphingomyelinase. J. Lipid Res. 2003, 44, 1241–1250. [Google Scholar] [CrossRef]
- Xie, G.; Cheng, T.; Lin, J.; Zhang, L.; Zheng, J.; Liu, Y.; Xie, G.; Wang, B.; Yuan, Y. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J. Immunother. Cancer 2018, 6, 88. [Google Scholar] [CrossRef]
- Zhang, F.; Li, K.; Yao, X.; Wang, H.; Li, W.; Wu, J.; Li, M.; Zhou, R.; Xu, L.; Zhao, L. Corrigendum to “A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer” [EBioMedicine 44 (2019) 311- 321]. EBioMedicine 2021, 69, 103469. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, Y.; Li, J.; Zhang, X.; Peng, Z.; Hu, Y.; Zhao, X.; Dong, K.; Zhang, B.; et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022, 20, 133. [Google Scholar] [CrossRef]
- Jang, M.; An, J.; Oh, S.W.; Lim, J.Y.; Kim, J.; Choi, J.K.; Cheong, J.H.; Kim, P. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat. Biomed. Eng. 2021, 5, 114–123. [Google Scholar] [CrossRef]
- Harel, M.; Ortenberg, R.; Varanasi, S.K.; Mangalhara, K.C.; Mardamshina, M.; Markovits, E.; Baruch, E.N.; Tripple, V.; Arama-Chayoth, M.; Greenberg, E.; et al. Proteomics of Melanoma Response to Immunotherapy Reveals Mitochondrial Dependence. Cell 2019, 179, 236–250.e218. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Jinesh, G.G.; Brohl, A.S. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal Transduct. Target. Ther. 2022, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel therapeutic strategies: Targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef]
- Qiu, Y.S.; Liao, G.J.; Jiang, N.N. REG3A overexpression suppresses gastric cancer cell invasion, proliferation and promotes apoptosis through PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 41, 3167–3174. [Google Scholar] [CrossRef]
- Khurana, R.N.; Merrill, P.; Yeh, S.; Suhler, E.; Barakat, M.R.; Uchiyama, E.; Henry, C.R.; Shah, M.; Wang, R.C.; Kapik, B.; et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA). Br. J. Ophthalmol. 2022, 106, 1139–1144. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Li, N.; Kong, M.; Ma, Z.; Zhou, D.; Wang, W.; Wang, H.; Wang, H.; He, K.; et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: Early results of a phase 2 study. J. Immunother. Cancer 2022, 10, e003635. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Shu, Y.; Liu, L.; Kong, L.; Yang, L.; Wang, B.; Sun, G.; Ji, Y.; Cao, G.; et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ 2022, 377, e068714. [Google Scholar] [CrossRef]
- Fang, M.; Tian, J.; Dong, D. Non-invasively predicting response to neoadjuvant chemotherapy in gastric cancer via deep learning radiomics. EClinicalMedicine 2022, 46, 101380. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, L.; Wang, L.; Zhang, D. AGT May Serve as a Prognostic Biomarker and Correlated with Immune Infiltration in Gastric Cancer. Int. J. Gen. Med. 2022, 15, 1865–1878. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All (N = 714) | Detailed Data | |
|---|---|---|---|
| TCGA (N = 359) | GEO batch (N = 355) | ||
| Age | |||
| <65 | 374 (52.38%) | 147 (40.95%) | 227 (63.94%) |
| ≥65 | 340 (47.62%) | 212 (59.05%) | 128 (36.06%) |
| Gender | |||
| Male | 471 (65.97%) | 229 (63.79%) | 242 (68.17%) |
| Female | 243 (34.03%) | 130 (36.21%) | 113 (31.83%) |
| Status | |||
| Alive | 408 (57.14%) | 225 (62.67%) | 183 (51.55%) |
| Dead | 306 (42.86%) | 134 (37.33%) | 172 (48.45%) |
| TNM stage | |||
| T1 | 19 (2.66%) | 8 (2.23%) | 11 (3.10%) |
| T2 | 128 (17.93%) | 93 (25.91%) | 35 (9.86%) |
| T3 | 220 (30.81%) | 155 (43.17%) | 65 (18.31%) |
| T4 | 339 (47.48) | 95 (26.46%) | 244 (68.73%) |
| TX | 8 (1.12%) | 8 (2.23%) | 0 (0.00%) |
| Chemotherapy | |||
| Response | — | 73 (47.10%) | — |
| Non-response | — | 50 (32.25%) | — |
| Unavailable | — | 32 (20.65%) | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Ma, M.; Zhang, L.; Wang, S.; Guo, Y.; Ling, X.; Lin, H.; Lai, N.; Lin, S.; Du, L.; et al. System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy. Pharmaceutics 2023, 15, 810. https://doi.org/10.3390/pharmaceutics15030810
Zhu L, Ma M, Zhang L, Wang S, Guo Y, Ling X, Lin H, Lai N, Lin S, Du L, et al. System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy. Pharmaceutics. 2023; 15(3):810. https://doi.org/10.3390/pharmaceutics15030810
Chicago/Turabian StyleZhu, Le, Ming Ma, Lumin Zhang, Shun Wang, Yu Guo, Xinxin Ling, Hanchao Lin, Nannan Lai, Shengli Lin, Ling Du, and et al. 2023. "System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy" Pharmaceutics 15, no. 3: 810. https://doi.org/10.3390/pharmaceutics15030810
APA StyleZhu, L., Ma, M., Zhang, L., Wang, S., Guo, Y., Ling, X., Lin, H., Lai, N., Lin, S., Du, L., & Dong, Q. (2023). System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy. Pharmaceutics, 15(3), 810. https://doi.org/10.3390/pharmaceutics15030810






