Abstract
The progress that has been made in computer science positioned in silico studies as an important and well-recognized methodology in the drug discovery and development process. It has numerous advantages in terms of costs and also plays a huge impact on the way the research is conducted since it can limit the use of animal models leading to more sustainable research. Currently, human trials are already being partly replaced by in silico trials. EMA and FDA are both endorsing these studies and have been providing webinars and guidance to support them. For instance, PBPK modeling studies are being used to gather data on drug interactions with other drugs and are also being used to support clinical and regulatory requirements for the pediatric population, pregnant women, and personalized medicine. This trend evokes the need to understand the role of in silico studies in vaccines, considering the importance that these products achieved during the pandemic and their promising hope in oncology. Vaccines are safer than other current oncology treatments. There is a huge variety of strategies for developing a cancer vaccine, and some of the points that should be considered when designing the vaccine technology are the following: delivery platforms (peptides, lipid-based carriers, polymers, dendritic cells, viral vectors, etc.), adjuvants (to boost and promote inflammation at the delivery site, facilitating immune cell recruitment and activation), choice of the targeted antigen, the timing of vaccination, the manipulation of the tumor environment, and the combination with other treatments that might cause additive or even synergistic anti-tumor effects. These and many other points should be put together to outline the best vaccine design. The aim of this article is to perform a review and comprehensive analysis of the role of in silico studies to support the development of and design of vaccines in the field of oncology and infectious diseases. The authors intend to perform a literature review of all the studies that have been conducted so far in preparing in silico models and methods to support the development of vaccines. From this point, it was possible to conclude that there are few in silico studies on vaccines. Despite this, an overview of how the existing work could support the design of vaccines is described.
1. Introduction
Vaccination plays a huge role in the prevention of many infections and has contributed to the eradication of certain diseases, saving millions of people’s lives [1]. More recently, it was possible to assist in the development of COVID-19 vaccines, which, within 1 year, were successfully developed and rapidly approved by health authorities in order to fight the pandemic.
For many diseases, vaccines have been successful. However, there are still many pathogens for which there are no effective vaccines available, such as human immunodeficiency virus (HIV), tuberculosis (TB), respiratory syncytial virus (RSV), cytomegalovirus (CMV), herpes simplex virus (HSV), and Epstein–Barr virus (EBV) [2]. It is known that COVID-19 vaccines have endorsed innovative platforms in terms of technology, such as mRNA [3]. However, even with this technology, there are still pathogens for which successful vaccines have not yet been developed. The same applies to cancer vaccines. These have been investigated over the years, and only a few have been approved by FDA [4,5].
Overall and despite all the progress, there are certain challenges that are always raised during vaccine development: the relationship between the pathogen, the disease, and the population characteristics, and emerging infections, epidemics, or pandemics [6,7].
Strategies related to vaccine design need to be put in place to overcome these challenges. Innovative antigens, adjuvants, and delivery systems need to be outlined in order to achieve a successful vaccine for the diseases mentioned above.
It is undeniable that computational science is nowadays a crucial tool within many fields. Its impact on drug discovery and development enhances many possibilities and has numerous advantages in terms of costs and the way the research is conducted. For instance, it can limit the use of animal models leading to more sustainable research [8,9]. In the future, there is also hope that in silico trials can replace human trials [10]. In fact, this is already a trend for certain populations. EMA and FDA have been endorsing these kinds of studies and providing webinars and guidance to support these trials. Physiologically Based Pharmacokinetic (PBPK) modeling studies, for instance, are being used to gather data on drug interactions with another drugs, pediatric population, pregnancy, and personalized medicine [11]. Overall, in silico studies allow a wide variety of simulations that can be helpful in drug design. From testing drug targets to predicting the drugs pharmacokinetics, pharmacodynamics, and so on. Regarding vaccines, it is also important to test immunologic properties and correlates of protection [12].
This review will highlight what has been performed so far in the field of vaccines for oncology and infectious diseases, using the in silico methodology and what studies have the potential to support the development of the vaccine. Through the exhaustive literature research, an overview of all in silico models created so far to support vaccine design will be described.
2. Materials and Methods
The methodology used in this article can be divided into two phases. Phase 1 involved an exhaustive review of the literature in two major articles databases, PubMed and Web of Science. The research strategy for this review of the literature is outlined in Table 1 below. All the studies found were manually screened to verify their scope. The criteria established below in Table 2 were used to exclude articles during their assessment.

Table 1.
Constructs, Strings, and identified articles from WoS and PubMed.

Table 2.
Reasons to exclude identified articles.
Phase 2 of the methodology was to compile all the in silico approaches prepared so far to aid vaccine development. Anything related to computer sciences that were endorsed in its scope for the support of vaccine development was considered (Table 3).

Table 3.
Results from Phase 2 (Compilation of all in silico models to support vaccine development).
3. Results
Results from Phase 1 (Literature Review and Screening)
While conducting Phase 1 of the methodology, the below-adapted PRISMA flow-chart approach illustrates how the screening was screened. The process can be checked in Figure 1.
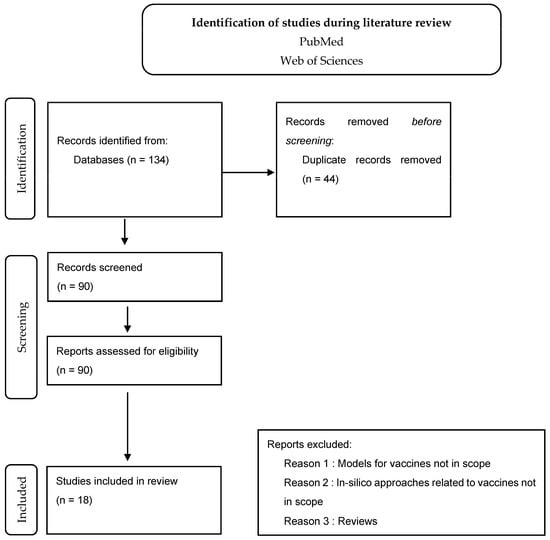
Figure 1.
PRISMA flow-chart approach illustrates how the screening was performed.
4. Discussion
It is important to highlight that results reflect what is available in the public domain and that it is possible that some studies are being performed and sponsored by pharmaceutical companies and not yet being available. This is the main limitation of this review. Additionally, the term “in silico” is general. In this study, the authors considered “in silico” as any study involving computer science models to support drug and vaccine development and design.
According to the results from our study, there are seven studies using PBPK models to support vaccine development; three studies using PopPK; six studies using ABMs models; four ODEs models; 1 MP; and one study using different Computational Vaccine Design techniques. Some of these models combined two or more in silico approaches. For instance, for the Recombinant multi-epitope vaccine against influenza A virus model, a complex Computational Vaccine Design approach to predicting epitopes and selecting adjuvants, to evaluate physicochemical properties and solubility, to predict secondary structure properties, and finally, to immunogenicity and related immune. For these, many software and modeling techniques were combined.
It is possible to verify that there are more models for infectious disease vaccines than for cancer vaccines by looking at the overall results. Additionally, PBPK modeling was the most used in silico approach to aid vaccine development, followed by ABM models, ODEs, and PopPK.
The reason behind PBPK models being the most common in terms of in silico approach might be due to the fact that this kind of modeling and simulation can be used to predict the drugs’ pharmacokinetics in humans using preclinical or clinical data. In parallel, population characteristics can be explored as well (i.e., age, ethnicity, or disease status). Furthermore, these models also play an important role in supporting the dose and dose regiment selection and also support predicting drug interactions. EMA and FDA are already currently accepting these kinds of studies to support regulatory decisions and have provided guidance to conduct PBPK modeling and simulations [11,31,32].
To date, multiple PBPK software has been created and used by various to support pharmaceutical drug development. Some of these platforms were discontinued, such as IDEATM (LION Bioscience, Inc.). However, others have remained in mainstream use and are currently being used by pharmaceutical companies and health authorities, such as EMA and FDA. Examples of the most commonly used software are GastroPlus (Simulation Plus, Inc.), Simcyp Simulator (Certara UK), and free tools such as PK-Sim [33].
ABM models were created to predict the immunogenicity of biological compounds and vaccines. This is because the immune system and its multiple agents and components are linked to complex interactions, and the ABM methodology allows these complex behaviors to emerge during simulation. This makes ABM perfect for performing biological simulations (i.e., for studying the complex and dynamic interactions within the biological environment) [34]. The Universal Immune System Simulator (UISS) platform is a type of ABM model and has been successfully applied to a large number of disease-modeling scenarios, including COVID-19, and can simulate, for instance, infection dynamics and its interactions with the host immune system, making it possible as well to predict the immunogenicity response of compounds [20,23].
ODEs represent models that are considered homogeneous, well-mixed systems and suited for traditional pharmacometrics analyses with sufficient data (population PK and PD models and PBPK models) or for simplistic theoretical PKPD models. They can also be used for quantitative clinical pharmacology models in order to study complex biological systems. Its limitation is related to extensive model assumptions, including parameter distributions. ABMs can provide more detailed insights into complex biological systems and are often complemented with ODEs in hybrid multi-scale models [35].
Population PK analyses are used to aid drug development and inform recommendations on therapeutic individualization (e.g., through tailored dosing). FDA states that adequate population PK data collection and analyses submitted in marketing applications, in some cases, have alleviated the need for postmarketing requirements and/or commitments [36]. PopPK models allow the study of variability in drug concentrations between individuals (healthy volunteers or patients). With this model, it is possible to assess the variability within the population and to account for the variability in terms of patient characteristics such as age, renal function, or disease state [37].
It is important to highlight that all methodologies have their strengths and weaknesses. It all depends on the purpose and context [35].
The most important information to retain with this review is that there are multiple in silico approaches that may complement each other and support pharmaceutical drug development. However, when we searched for in silico methodologies in vaccines, we only found 18 studies where models were prepared to support vaccine development. This means that research must continue in this field.
Within the most common in silico approaches that were found for vaccine development, which is PBPK modeling, it can be verified that the most usual software, the GastroPlus and Simcyp Simulator, were not used.
An interesting approach to future research would be to try to implement one of those existing PBPK models into one of the most common software and use their capacity to study different parameters to verify their applicability to vaccines. Performing simulations to test new adjuvants, improving formulation, targeting new antigens, and finding the best dose for different populations (considering age, ethnicity, or disease status on human pharmacokinetics) could be completed [14,31,38,39]. Sequentially, immunogenicity could be explored using UISS as an ABM through the simulation of the dynamics within the immune system. Currently, there are studies in the literature where UISS is applied to a broad range of diseases and not only to infections. An example of that is the application of UISS to multiple sclerosis pathogenesis, supporting the prediction of the disease and the treatment efficacy [40]. This reflects the flexibility of in silico software and highlights their capabilities to support the development of treatments for complex diseases with complex dynamics, such as cancer.
However, the results from this review show that there are not many models developed for vaccines in general and especially for cancer vaccines. This might be related to the complexity and challenges of vaccine development and the diseases themselves.
Firstly, vaccine development itself is complex. It is known that most vaccine candidates fall in preclinical and early clinical development, and less than 1 in 15 candidates that enter Phase II will be approved. This is due to the lack of understanding of correlates of protection, not using appropriate animal models to predict responses in humans, complex dynamics and responses of the human immune system to antigens, and the synergies and impacts across the various components that can be combined in a vaccine [41].
Furthermore, in terms of vaccine efficacy, it is important to consider not only immediate protection but also long-term protection. Therefore, it is important to understand how to stimulate long-term memory, and this point is still not resolved. As an example, hepatitis B antigen vaccines produce lifelong protection, whereas for other vaccines, the protection is very short in terms of time. For this reason, it would be very important across the scientific community to develop in silico modeling to understand immune responses in humans [42].
Additionally, there are now more complex platforms for outlining the vaccine development strategies to overcome some issues of the standard vaccines, which are composed of inactivated pathogens. Advancements were made, and there are now new platforms related to DNA/RNA technologies, recombinant proteins, and the use of nanoparticles, for instance [43]. The reason for the development of such novel platforms is to aim for a more targeted immune response, to improve efficacy, and to provide long-term protection. There is also the hope that the new technologies will overcome the challenges of unmet needs for certain diseases, such as cancer and other complex diseases. However, as mentioned above, the limitation related to the lack of available data on these technologies may impact their development. This is why in silico studies might be a challenge in the field, but once they become more familiar with the area, they might support developers in important steps across the development of a vaccine, deciding which platform to use, adjuvants, formulations, and which dose and for which populations [43]. The vaccine design and formulation are extremely important in its overall efficacy. The adjuvants, for instance, support in improving the efficacy and the long-term immune response. However, it may also impact in the way the response is conducted [44,45].
Despite all the challenges, it is important to highlight the boost in vaccine development in terms of timelines. It took around 25 years to develop a vaccine for varicella, 5 years for Ebola, and 1 year for COVID-19 [46,47,48,49].
Therefore, in silico approaches, which can be used during all stages of development and discovery, can play an important role and contribute to the “boost” in vaccine development. PBPK studies, for instance, could help to predict the absorption, distribution, metabolism, and excretion (ADME) parameters of the candidates to improve efficacy. With this, it is also possible to save costs since this will reduce animal models and can also replace some trials [50,51].
To sum up, the complexity of vaccine development might be the reason why there are not many in silico models developed so far. The variability of the pathogen and tumors, the immunological responses, antigen selection, and memory of the responses are still the biggest challenges in the field. Due to genetic factors, age, disease status, and other factors, different responses may be expected [52].
Furthermore, when it comes to cancer vaccines, everything is even more complex. It is important to acknowledge that the pathological and immunological setting is different between cancer and infectious diseases. Acute inflammation is representative of infected tissues by pathogens, and this will trigger a potential development of protection in terms of immunity because the inflammation is obvious. Chronic inflammation environment is present with tumors, and these will repress anti-tumor immune responses, while tumor growth will be promoted in order to avoid the immune system. This means that lesions linked to tumors promote a not-so-obvious and, therefore, low-inflammatory environment. As a consequence, it is when the tissue is already very fragile due to tumor growth that the inflammation will become obvious [53]. Despite the complex environment in tumors, it is well known that the clinical translation of vaccines has been an issue. Most cancer vaccine clinical trials failed due to the selection of target antigens and the vaccines’ designs themselves, inducing very low-immunogenicity properties to have proper efficacy. The fact that there are only two therapeutic cancer vaccines approved by FDA and EMA, sipuleucel-T and talimogene laherparepvec [T-VEC], reflects all the complexity within the development of these platforms to treat cancer [54].
However, because so many studies have failed in the past, there is now more knowledge about these strategies, which are related to past failures. This means that lessons learned, together with new technological advancements, might be able to trigger a new era in cancer vaccine development, and in silico approaches will surely be part of it, as they already are for other complex diseases [28].
Considering the above, it means that further advancements are needed in the field of in silico studies for vaccines. Different types of models could be useful to overcome these issues: models to simulate host/pathogen/tumor interactions and models to simulate immune response [55,56,57].
Since regulatory authorities have clearly endorsed in silico models, such as PBPK, and even provided guides and frameworks to developers on how to integrate and achieve valuable data from them in drug development and discovery, considering that vaccines play an important role in the prevention and possibly in the treatment of certain diseases today, it is expected to see more models in the future [58,59,60].
In silico modeling, then, has the ability to save millions in terms of costs and could promote the selection of the best platforms, adjuvants (i.e., liposomes, nanoparticles), antigens (i.e., peptides) and dosages and dosage regimens in order to support the vaccines’ development and design [60,61,62,63,64].
Author Contributions
Conceptualization, L.S. and N.V.; methodology L.S.; formal analysis, Ü.L. and N.V.; investigation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, Ü.L. and N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020 Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through the Fundação para a Ciência e a Tecnologia (FCT) in the framework of projects IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation from the Faculty of Medicine of the University of Porto (FMUP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos Trans. R. Soc. Lond B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, P.J.D.M. Cancer Vaccines. Hematol. Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- EMA. EMA Implements New Measures to Minimise Animal Testing during Medicines Development. 2021. Available online: https://www.ema.europa.eu/en/news/ema-implements-new-measures-minimise-animal-testing-during-medicines-development (accessed on 6 January 2022).
- Pappalardo, F.; Russo, G.; Tshinanu, F.M.; Viceconti, M. In silico clinical trials: Concepts and early adoptions. Brief Bioinform. 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- EMA. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. 2018. Available online: https://www.ema.europa.eu/en/reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation-scientific-guideline (accessed on 13 January 2022).
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.S.; Chapon, C.; Tchitchek, N.; Cheraitia, L.; Marcos-Lopez, E.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines 2021, 9, 579. [Google Scholar] [CrossRef]
- Mitkus, R.J.; Hess, M.A.; Schwartz, S.L. Pharmacokinetic modeling as an approach to assessing the safety of residual formaldehyde in infant vaccines. Vaccine 2013, 31, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Mitkus, R.J. A physiologically-based pharmacokinetic (PBPK) model of squalene-containing adjuvant in human vaccines. J. Pharmacokinet. Pharmacodyn. 2013, 40, 545–556. [Google Scholar] [CrossRef]
- Saylor, K.; Zhang, C. A simple physiologically based pharmacokinetic model evaluating the effect of anti-nicotine antibodies on nicotine disposition in the brains of rats and humans. Toxicol. Appl. Pharmacol. 2016, 307, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Mitkus, R.J. A first-generation physiologically based pharmacokinetic (PBPK) model of alpha-tocopherol in human influenza vaccine adjuvant. Regul. Toxicol. Pharmacol. 2015, 71, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Badhan, R.K.S.; Khadke, S.; Perrie, Y. Application of Pharmacokinetics Modelling to Predict Human Exposure of a Cationic Liposomal Subunit Antigen Vaccine System. Pharmaceutics 2017, 9, 57. [Google Scholar] [CrossRef]
- Capuani, S.; Hernandez, N.; Paez-Mayorga, J.; Dogra, P.; Wang, Z.; Cristini, V.; Chua, C.Y.X.; Nichols, J.E.; Grattoni, A. Localization of drug biodistribution in a 3D-bioengineered subcutaneous neovascularized microenvironment. Mater. Today Bio 2022, 16, 100390. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.; Ziemys, A.; Dogra, P.; Ferrari, M. A modeling platform for the lymphatic system. J. Theor. Biol. 2020, 493, 110193. [Google Scholar] [CrossRef]
- Pennisi, M.; Russo, G.; Sgroi, G.; Bonaccorso, A.; Parasiliti Palumbo, G.A.; Fichera, E.; Mitra, D.K.; Walker, K.B.; Cardona, P.J.; Amat, M.; et al. Predicting the artificial immunity induced by RUTI® vaccine against tuberculosis using universal immune system simulator (UISS). BMC Bioinform. 2019, 20, 504. [Google Scholar] [CrossRef]
- Russo, G.; Sgroi, G.; Parasiliti Palumbo, G.A.; Pennisi, M.; Juarez, M.A.; Cardona, P.-J.; Motta, S.; Walker, K.B.; Fichera, E.; Viceconti, M.; et al. Moving forward through the in silico modeling of tuberculosis: A further step with UISS-TB. BMC Bioinform. 2020, 21, 458. [Google Scholar] [CrossRef]
- Russo, G.; Pappalardo, F.; Juarez, M.A.; Pennisi, M.; Cardona, P.J.; Coler, R.; Fichera, E.; Viceconti, M. Evaluation of the efficacy of RUTI and ID93/GLA-SE vaccines in tuberculosis treatment: In silico trial through UISS-TB simulator. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 2197–2201. [Google Scholar]
- Russo, G.; Pennisi, M.; Fichera, E.; Motta, S.; Raciti, G.; Viceconti, M.; Pappalardo, F. In silico trial to test COVID-19 candidate vaccines: A case study with UISS platform. BMC Bioinform. 2020, 21, 527. [Google Scholar] [CrossRef]
- Silva, L.d.L.e.; Xavier, M.P.; Santos, R.W.d.; Lobosco, M.; Reis, R.F. Uncertain Quantification of Immunological Memory to Yellow Fever Virus. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020; pp. 1281–1288. [Google Scholar]
- Tegenge, M.A.; Von Tungeln, L.S.; Mitkus, R.J.; Anderson, S.A.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Pharmacokinetics and biodistribution of squalene-containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul. Toxicol. Pharmacol. 2016, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Naidoo, L.; Zhang, L.; Carpp, L.N.; Rudnicki, E.; Randhawa, A.; Gonzales, P.; McDermott, A.; Ledgerwood, J.; Lorenzo, M.M.G.; et al. Pharmacokinetics and predicted neutralisation coverage of VRC01 in HIV-uninfected participants of the Antibody Mediated Prevention (AMP) trials. EBioMedicine 2021, 64, 103203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gilbert, P.B.; Capparelli, E.; Huang, Y. Simulation-Based Pharmacokinetics Sampling Design for Evaluating Correlates of Prevention Efficacy of Passive HIV Monoclonal Antibody Prophylaxis. Stat. Biopharm. Res. 2022, 14, 611–625. [Google Scholar] [CrossRef]
- Lőrincz, O.; Tóth, J.; Molnár, L.; Miklós, I.; Pántya, K.; Megyesi, M.; Somogyi, E.; Csiszovszki, Z.; Tőke, E.R. In Silico Model Estimates the Clinical Trial Outcome of Cancer Vaccines. Cells 2021, 10, 3048. [Google Scholar] [CrossRef]
- Linderman, J.J.; Cilfone, N.A.; Pienaar, E.; Gong, C.; Kirschner, D.E. A multi-scale approach to designing therapeutics for tuberculosis. Integr. Biol. 2015, 7, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Russo, G.; Parasiliti Palumbo, G.A.; Pappalardo, F. In silico design of recombinant multi-epitope vaccine against influenza A virus. BMC Bioinform. 2022, 22, 617. [Google Scholar] [CrossRef]
- Zhuang, X.; Lu, C. PBPK Modeling and Simulation in Drug Research and Development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- FDA. How Simulation Can Transform Regulatory Pathways. 2018. Available online: https://www.fda.gov/science-research/about-science-research-fda/how-simulation-can-transform-regulatory-pathways (accessed on 6 January 2022).
- El-Khateeb, E.; Burkhill, S.; Murby, S.; Amirat, H.; Rostami-Hodjegan, A.; Ahmad, A. Physiological-based pharmacokinetic modeling trends in pharmaceutical drug development over the last 20-years; in-depth analysis of applications, organizations, and platforms. Biopharm. Drug Dispos. 2021, 42, 107–117. [Google Scholar] [CrossRef]
- Kabiri Chimeh, M.; Heywood, P.; Pennisi, M.; Pappalardo, F.; Richmond, P. Parallelisation strategies for agent based simulation of immune systems. BMC Bioinform. 2019, 20, 579. [Google Scholar] [CrossRef]
- Truong, V.T.; Baverel, P.G.; Lythe, G.D.; Vicini, P.; Yates, J.W.T.; Dubois, V.F.S. Step-by-step comparison of ordinary differential equation and agent-based approaches to pharmacokinetic-pharmacodynamic models. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 133–148. [Google Scholar] [CrossRef]
- FDA. Population Pharmacokinetics Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics (accessed on 13 January 2022).
- EMA. Guideline on Reporting the Results of Population Pharmacokinetics Analysis. 2007. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-results-population-pharmacokinetic-analyses_en.pdf (accessed on 13 January 2022).
- Jones, H.M.; Dickins, M.; Youdim, K.; Gosset, J.R.; Attkins, N.J.; Hay, T.L.; Gurrell, I.K.; Logan, Y.R.; Bungay, P.J.; Jones, B.C.; et al. Application of PBPK modelling in drug discovery and development at Pfizer. Xenobiotica 2012, 42, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.A.; Campbell, J.L.; Pithawalla, Y.B.; Pourhashem, H.; Muhammad-Kah, S.R.; Sarkar, M.A.; Liu, J.; McKinney, W.J.; Gentry, R.; Gogova, M. A comprehensive physiologically based pharmacokinetic (PBPK) model for nicotine in humans from using nicotine-containing products with different routes of exposure. Sci. Rep. 2022, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, F.; Russo, G.; Pennisi, M.; Sgroi, G.; Palumbo, G.; Motta, S.; Maimone, D.; Chiacchio, F. Agent based modeling of relapsing multiple sclerosis: A possible approach to predict treatment outcome. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; p. 41. [Google Scholar]
- Douglas, G.; Samant, B. The Vaccine Industry. In Plotkin’s Vaccines, 7th ed.; Plotkin, S., Orenstein, W., Offit, P., Edwards, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4, pp. 41–50. [Google Scholar]
- Abbas, A.; Lichtman, H.; Pillai, S. Immunity to Microbes. In Cellular and Molecular Immunology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 365–388. [Google Scholar]
- Hamley, I.W. Peptides for Vaccine Development. ACS Appl. Bio Mater. 2022, 5, 905–944. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Vaccine Design. In Methods in Molecular Biology; Thomas, S., Ed.; Humana: New York, NY, USA, 2021; Volume 2412, pp. 145–178. [Google Scholar]
- Matić, Z.; Šantak, M. Current view on novel vaccine technologies to combat human infectious diseases. Appl. Microbiol. Biotechnol. 2022, 106, 25–56. [Google Scholar] [CrossRef] [PubMed]
- CDC. Varicella. In Epidemiology and Prevention of Vaccine-Preventable Diseases; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Wolf, J.; Bruno, S.; Eichberg, M.; Jannat, R.; Rudo, S.; VanRheenen, S.; Coller, B.A. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. Npj Vaccines 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2020, 589, 16–18. [Google Scholar] [CrossRef]
- Rowland, M.; Peck, C.; Tucker, G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev. Pharm. Toxicol. 2011, 51, 45–73. [Google Scholar] [CrossRef]
- Jones, H.M.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.A.; Upetri, V.V.; Zheng, M.; Hall, S.D. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Plotkin, S.A. Increasing Complexity of Vaccine Development. J. Infec. Dis. 2015, 212, 12–16. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer Immunity: Lessons From Infectious Diseases. J. Infect. Dis. 2015, 212, S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Gwin, W.R.; Mitchell, D.A. Vaccine Therapies for Cancer: Then and Now. Targ Oncol. 2021, 16, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Six, A.; Bellier, B.; Vaslin, T.V.; Klatzmann, D. Systems biology in vaccine design. Microb. Biotechnol. 2012, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M.; Mata, J. Quantitative modeling of immune responses. Immunol. Rev. 2007, 216, 5–8. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Kenza, A.; Samer, C.F.; Yvonne, G.; Jules, A.D.; Youseff, D. Reviewing Data Integrated for PBPK Model Development to Predict Metabolic Drug-Drug Interactions: Shifting Perspectives and Emerging Trends. Front. Pharmacol. 2021, 12, 708299. [Google Scholar]
- Shekhani, R.; Steinacher, L.; Swen, J.J.; Sundberg, I.M. Evaluation of Current Regulation and Guidelines of Pharmacogenomic Drug Labels: Opportunities for Improvements. Clin. Pharmacol. Ther. 2020, 107, 1240–1255. [Google Scholar] [CrossRef]
- Manolis, E.; Pons, G. Proposals for Model-Based Paediatric Medicinal Development within the Current European Union Regulatory Framework. Br. J. Clin. Pharmacol. 2009, 68, 493–501. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef]
- WHO. How Are Vaccines Developed? Available online: https://www.who.int/news-room/feature-stories/detail/how-are-vaccines-developed (accessed on 22 January 2023).
- Rapin, N.; Lund, O.; Castiglione, F. Immune system simulation online. Bioinformatics 2011, 27, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).