Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier
Abstract
1. Introduction
2. Classification and Biogenesis of Extracellular Vesicles
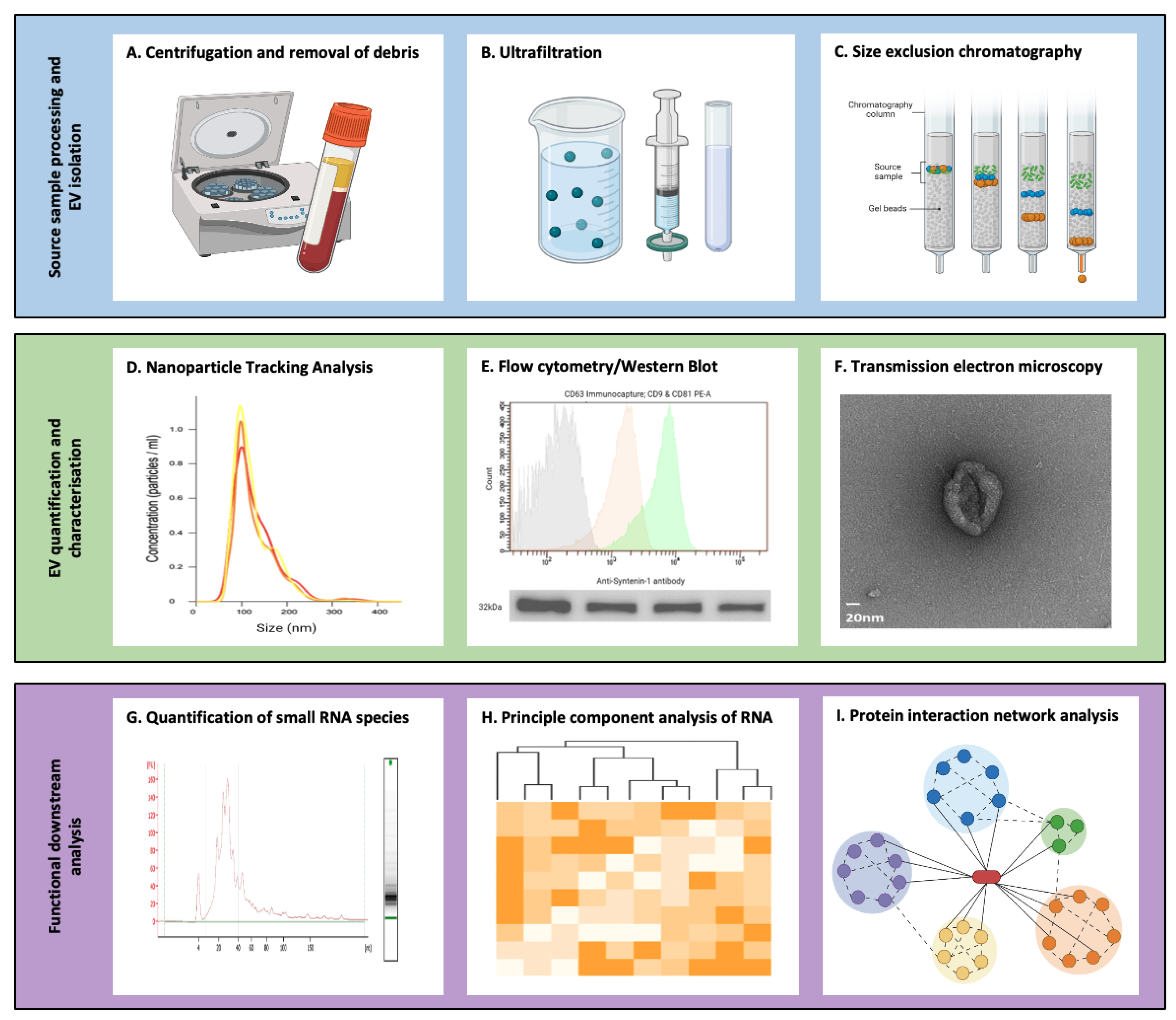
3. Isolation and Characterisation of Extracellular Vesicles
4. Extracellular Vesicles as Therapeutics
4.1. Native EVs as Drug Delivery Systems
4.2. Bioengineered Extracellular Vesicles as Therapeutics
5. Extracellular Vesicles in Organ Transplantation
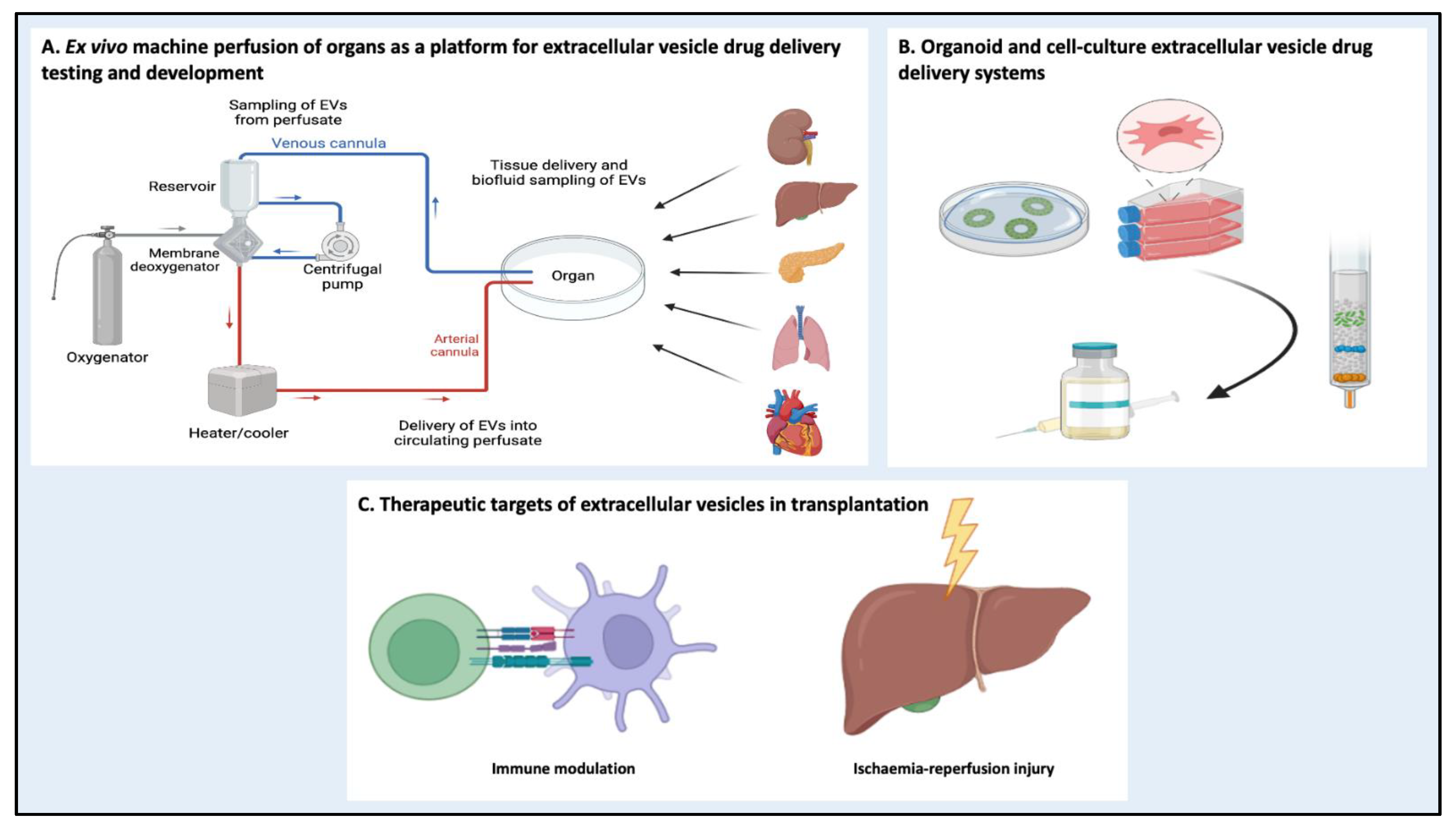
5.1. Alloimmune Response Modulation
5.2. Ischemia-Reperfusion Injury
5.3. Machine Perfusion as a Platform for EV Drug Delivery Systems
5.4. Barriers to Clinical Translation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- PNAS. Human Tumor Virus Utilizes Exosomes for Intercellular Communication. Available online: https://www.pnas.org/doi/full/10.1073/pnas.1014194107 (accessed on 18 November 2022).
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. Available online: https://www.annualreviews.org/doi/10.1146/annurev-cellbio-101512-122326?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed on 18 November 2022). [CrossRef]
- Möller, A.; Lobb, R.J. The Evolving Translational Potential of Small Extracellular Vesicles in Cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Baumann, K.; Przybilla, D.; Schmitz, T.; Flender, A.; Paul, K.; Alhusseiny, A.; Nickenig, G.; Werner, N. Endothelial Microparticles Reduce ICAM-1 Expression in a MicroRNA-222-dependent Mechanism. J. Cell. Mol. Med. 2015, 19, 2202–2214. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jcmm.12607 (accessed on 18 November 2022). [CrossRef]
- van Balkom, B.W.M.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.J.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial Cells Require MiR-214 to Secrete Exosomes That Suppress Senescence and Induce Angiogenesis in Human and Mouse Endothelial Cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Huang, Z.; Gurley, E.C.; Wang, X.; Wang, J.; He, H.; Yang, H.; Lai, G.; Zhang, L.; et al. Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Cholestatic Liver Injury in Mouse and Human. Hepatology 2018, 68, 599–615. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Romero-García, N.; Mas-Bargues, C.; Monleón, D.; Gordevicius, J.; Brooke, R.T.; Dromant, M.; Díaz, A.; Derevyanko, A.; Guío-Carrión, A.; et al. Small Extracellular Vesicles from Young Adipose-Derived Stem Cells Prevent Frailty, Improve Health Span, and Decrease Epigenetic Age in Old Mice. Sci. Adv. 2022, 8, eabq2226. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation in vitro and Promote Bone Regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. BES 2018, 31, 87–96. [Google Scholar] [CrossRef]
- Fourcade, O.; Simon, M.-F.; Viodé, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournié, B.; Sarda, L.; Chap, H. Secretory Phospholipase A2 Generates the Novel Lipid Mediator Lysophosphatidic Acid in Membrane Microvesicles Shed from Activated Cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef]
- Ikeda, C.; Haga, H.; Makino, N.; Inuzuka, T.; Kurimoto, A.; Ueda, T.; Matsuda, A.; Kakizaki, Y.; Ishizawa, T.; Kobayashi, T.; et al. Utility of Claudin-3 in Extracellular Vesicles from Human Bile as Biomarkers of Cholangiocarcinoma. Sci. Rep. 2021, 11, 1195. [Google Scholar] [CrossRef]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human Milk Extracellular Vesicle MiRNA Expression and Associations with Maternal Characteristics in a Population-Based Cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef]
- Spitzer, P.; Mulzer, L.-M.; Oberstein, T.J.; Munoz, L.E.; Lewczuk, P.; Kornhuber, J.; Herrmann, M.; Maler, J.M. Microvesicles from Cerebrospinal Fluid of Patients with Alzheimer’s Disease Display Reduced Concentrations of Tau and APP Protein. Sci. Rep. 2019, 9, 7089. [Google Scholar] [CrossRef]
- Sun, I.O.; Lerman, L.O. Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics 2020, 10, 311. [Google Scholar] [CrossRef]
- Liu, J.; Gao, A.; Liu, Y.; Sun, Y.; Zhang, D.; Lin, X.; Hu, C.; Zhu, Y.; Du, Y.; Han, H.; et al. MicroRNA Expression Profiles of Epicardial Adipose Tissue-Derived Exosomes in Patients with Coronary Atherosclerosis. Rev. Cardiovasc. Med. 2022, 23, 206. [Google Scholar] [CrossRef]
- Li, T.; Wang, B.; Ding, H.; Chen, S.; Cheng, W.; Li, Y.; Wu, X.; Wang, L.; Jiang, Y.; Lu, Z.; et al. Effect of Extracellular Vesicles From Multiple Cells on Vascular Smooth Muscle Cells in Atherosclerosis. Front. Pharmacol. 2022, 13, 1430. [Google Scholar] [CrossRef]
- Brown, P.A.; Brown, P.D. Extracellular Vesicles and Atherosclerotic Peripheral Arterial Disease. Cardiovasc. Pathol. 2023, 63, 107510. [Google Scholar] [CrossRef]
- Newman, L.A.; Muller, K.; Rowland, A. Circulating Cell-Specific Extracellular Vesicles as Biomarkers for the Diagnosis and Monitoring of Chronic Liver Diseases. Cell. Mol. Life Sci. 2022, 79, 232. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Shen, D.; Luo, Y.; Che, Y.-Q. Identification of Exosomal MiRNAs Associated with the Anthracycline-Induced Liver Injury in Postoperative Breast Cancer Patients by Small RNA Sequencing. PeerJ 2020, 8, e9021. [Google Scholar] [CrossRef]
- Lu, M.; DiBernardo, E.; Parks, E.; Fox, H.; Zheng, S.-Y.; Wayne, E. The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Autoimmune Disorders. Front. Immunol. 2021, 12, 566299. [Google Scholar] [CrossRef]
- Blommer, J.; Pitcher, T.; Mustapic, M.; Eren, E.; Yao, P.J.; Vreones, M.P.; Pucha, K.A.; Dalrymple-Alford, J.; Shoorangiz, R.; Meissner, W.G.; et al. Extracellular Vesicle Biomarkers for Cognitive Impairment in Parkinson’s Disease. Brain 2023, 146, 195–208. [Google Scholar] [CrossRef]
- Burgelman, M.; Dujardin, P.; Vandendriessche, C.; Vandenbroucke, R.E. Free Complement and Complement Containing Extracellular Vesicles as Potential Biomarkers for Neuroinflammatory and Neurodegenerative Disorders. Front. Immunol. 2022, 13, 1055050. [Google Scholar] [CrossRef]
- Manna, I.; Quattrone, A.; Benedittis, S.D.; Vescio, B.; Iaccino, E.; Quattrone, A. Exosomal MiRNA as Peripheral Biomarkers in Parkinson’s Disease and Progressive Supranuclear Palsy: A Pilot Study. Park. Relat. Disord. 2021, 93, 77–84. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Tran, B.V.; Wang, J.J.; Liang, I.Y.; You, S.; Zhu, Y.; Agopian, V.G.; Tseng, H.-R.; Yang, J.D. The Role of Extracellular Vesicles in Disease Progression and Detection of Hepatocellular Carcinoma. Cancers 2021, 13, 3076. [Google Scholar] [CrossRef]
- Chang, C.-H.; Pauklin, S. Extracellular Vesicles in Pancreatic Cancer Progression and Therapies. Cell Death Dis. 2021, 12, 973. [Google Scholar] [CrossRef]
- Jordan, K.R.; Hall, J.K.; Schedin, T.; Borakove, M.; Xian, J.J.; Dzieciatkowska, M.; Lyons, T.R.; Schedin, P.; Hansen, K.C.; Borges, V.F. Extracellular Vesicles from Young Women’s Breast Cancer Patients Drive Increased Invasion of Non-Malignant Cells via the Focal Adhesion Kinase Pathway: A Proteomic Approach. Breast Cancer Res. 2020, 22, 128. [Google Scholar] [CrossRef]
- Lobb, R.J.; Visan, K.S.; Wu, L.-Y.; Norris, E.L.; Hastie, M.L.; Everitt, S.; Yang, I.A.; Bowman, R.V.; Siva, S.; Larsen, J.E.; et al. An Epithelial-to-Mesenchymal Transition Induced Extracellular Vesicle Prognostic Signature in Non-Small Cell Lung Cancer. Commun. Biol. 2023, 6, 68. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wollert, T.; Hurley, J.H. Molecular Mechanism of Multivesicular Body Biogenesis by ESCRT Complexes. Nature 2010, 464, 864–869. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–Syntenin–ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Buschow, S.I.; Nolte-‘t Hoen, E.N.M.; Van Niel, G.; Pols, M.S.; Ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in Dendritic Cells Is Targeted to Lysosomes or T Cell-Induced Exosomes Via Distinct Multivesicular Body Pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Koifman, N.; Biran, I.; Aharon, A.; Brenner, B.; Talmon, Y. A Direct-Imaging Cryo-EM Study of Shedding Extracellular Vesicles from Leukemic Monocytes. J. Struct. Biol. 2017, 198, 177–185. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The Biology of Extracellular Microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Vizio, D.D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large Oncosomes in Human Prostate Cancer Tissues and in the Circulation of Mice with Metastatic Disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-Derived Microvesicles: Shedding Light on Novel Microenvironment Modulators and Prospective Cancer Biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extracellular Communication during Cancer Progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, H.; Wang, L.; Xiao, F.; Guo, Z.; Zhang, H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE 2016, 11, e0147360. [Google Scholar] [CrossRef]
- Budnik, V.; Ruiz-Cañada, C.; Wendler, F. Extracellular Vesicles Round off Communication in the Nervous System. Nat. Rev. Neurosci. 2016, 17, 160–172. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; Clancy, J.W.; Olivia Balmert, M.; D’Souza-Schorey, C. Extracellular Microvesicles and Invadopodia Mediate Non-Overlapping Modes of Tumor Cell Invasion. Sci. Rep. 2015, 5, 14748. [Google Scholar] [CrossRef]
- Moss, D.K.; Betin, V.M.; Malesinski, S.D.; Lane, J.D. A Novel Role for Microtubules in Apoptotic Chromatin Dynamics and Cellular Fragmentation. J. Cell Sci. 2006, 119, 2362–2374. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Chiu, Y.-H.; Armstrong, A.J.; Kinchen, J.M.; Juncadella, I.J.; Bayliss, D.A.; Ravichandran, K.S. Unexpected Link between an Antibiotic, Pannexin Channels and Apoptosis. Nature 2014, 507, 329–334. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K.H. A Novel Mechanism of Generating Extracellular Vesicles during Apoptosis via a Beads-on-a-String Membrane Structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef]
- Lane, J.D.; Allan, V.J.; Woodman, P.G. Active Relocation of Chromatin and Endoplasmic Reticulum into Blebs in Late Apoptotic Cells. J. Cell Sci. 2005, 118, 4059–4071. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal SiRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted Exosome-Mediated Delivery of Opioid Receptor Mu SiRNA for the Treatment of Morphine Relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular Vesicle-Based Therapeutics: Natural versus Engineered Targeting and Trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Hulstaert, E.; Morlion, A.; Cobos, F.A.; Verniers, K.; Nuytens, J.; Eynde, E.V.; Yigit, N.; Anckaert, J.; Geerts, A.; Hindryckx, P.; et al. Charting Extracellular Transcriptomes in The Human Biofluid RNA Atlas. Cell Rep. 2020, 33, 108552. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular Vesicle Measurements with Nanoparticle Tracking Analysis—An Accuracy and Repeatability Comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- Rikkert, L.G.; Nieuwland, R.; Terstappen, L.W.M.M.; Coumans, F.A.W. Quality of Extracellular Vesicle Images by Transmission Electron Microscopy Is Operator and Protocol Dependent. J. Extracell. Vesicles 2019, 8, 1555419. [Google Scholar] [CrossRef]
- Morandi, M.I.; Busko, P.; Ozer-Partuk, E.; Khan, S.; Zarfati, G.; Elbaz-Alon, Y.; Abou Karam, P.; Napso Shogan, T.; Ginini, L.; Gil, Z.; et al. Extracellular Vesicle Fusion Visualized by Cryo-Electron Microscopy. PNAS Nexus 2022, 1, pgac156. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2020, 22, 48. [Google Scholar] [CrossRef]
- Maia, J.; Batista, S.; Couto, N.; Gregório, A.C.; Bodo, C.; Elzanowska, J.; Strano Moraes, M.C.; Costa-Silva, B. Employing Flow Cytometry to Extracellular Vesicles Sample Microvolume Analysis and Quality Control. Front. Cell Dev. Biol. 2020, 8, 593750. [Google Scholar] [CrossRef]
- Mizenko, R.R.; Brostoff, T.; Rojalin, T.; Koster, H.J.; Swindell, H.S.; Leiserowitz, G.S.; Wang, A.; Carney, R.P. Tetraspanins Are Unevenly Distributed across Single Extracellular Vesicles and Bias Sensitivity to Multiplexed Cancer Biomarkers. J. Nanobiotechnol. 2021, 19, 250. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-Based ELISA to Characterize and Quantify Exosomes in Both Cell Culture Supernatants and Body Fluids. Methods Enzymol. 2020, 645, 155–180. [Google Scholar] [CrossRef]
- Martínez-Greene, J.A.; Hernández-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D.A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godínez, M.d.P.; et al. Quantitative Proteomic Analysis of Extracellular Vesicle Subgroups Isolated by an Optimized Method Combining Polymer-based Precipitation and Size Exclusion Chromatography. J. Extracell. Vesicles 2021, 10, e12087. [Google Scholar] [CrossRef]
- Carney, R.P.; Hazari, S.; Colquhoun, M.; Tran, D.; Hwang, B.; Mulligan, M.S.; Bryers, J.D.; Girda, E.; Leiserowitz, G.S.; Smith, Z.J.; et al. Multispectral Optical Tweezers for Biochemical Fingerprinting of CD9-Positive Exosome Subpopulations. Anal. Chem. 2017, 89, 5357–5363. [Google Scholar] [CrossRef]
- Amorim, M.G.; Valieris, R.; Drummond, R.D.; Pizzi, M.P.; Freitas, V.M.; Sinigaglia-Coimbra, R.; Calin, G.A.; Pasqualini, R.; Arap, W.; Silva, I.T.; et al. A Total Transcriptome Profiling Method for Plasma-Derived Extracellular Vesicles: Applications for Liquid Biopsies. Sci. Rep. 2017, 7, 14395. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal Stem Cells Modified with Akt Prevent Remodeling and Restore Performance of Infarcted Hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of Myocardial Infarct Size by Human Mesenchymal Stem Cell Conditioned Medium. Stem Cell Res. 2008, 1, 129–137. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma Exosomes Protect the Myocardium From Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (IPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than IPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, Y.; Chen, L.; Wang, X.; Guo, W.; Zhang, X.; Qin, G.; He, S.; Zimmerman, A.; et al. Exosomes/Microvesicles from Induced Pluripotent Stem Cells Deliver Cardioprotective MiRNAs and Prevent Cardiomyocyte Apoptosis in the Ischemic Myocardium. Int. J. Cardiol. 2015, 192, 61–69. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.-Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.M.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human Mesenchymal Stem Cell-Conditioned Medium Improves Cardiac Function Following Myocardial Infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular Vesicles from Human Cardiac Progenitor Cells Inhibit Cardiomyocyte Apoptosis and Improve Cardiac Function after Myocardial Infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Kervadec, A.; Bellamy, V.; El Harane, N.; Arakélian, L.; Vanneaux, V.; Cacciapuoti, I.; Nemetalla, H.; Périer, M.-C.; Toeg, H.D.; Richart, A.; et al. Cardiovascular Progenitor–Derived Extracellular Vesicles Recapitulate the Beneficial Effects of Their Parent Cells in the Treatment of Chronic Heart Failure. J. Heart Lung Transplant. 2016, 35, 795–807. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Yuan, J.; Wu, C.; Yao, K.; Zhang, L.; Ma, L.; Zhu, J.; Zou, Y.; Ge, J. Exosomes Derived from Dendritic Cells Improve Cardiac Function via Activation of CD4+ T Lymphocytes after Myocardial Infarction. J. Mol. Cell. Cardiol. 2016, 91, 123–133. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Laundos, T.L.; Pereira-Carvalho, R.; Batista-Almeida, D.; Pereira, R.; Coelho-Santos, V.; Silva, A.P.; Fernandes, R.; Zuzarte, M.; Enguita, F.J.; et al. Exosomes Secreted by Cardiomyocytes Subjected to Ischaemia Promote Cardiac Angiogenesis. Cardiovasc. Res. 2017, 113, 1338–1350. [Google Scholar] [CrossRef]
- An, M.; Kwon, K.; Park, J.; Ryu, D.-R.; Shin, J.-A.; Lee Kang, J.; Choi, J.H.; Park, E.-M.; Lee, K.E.; Woo, M.; et al. Extracellular Matrix-Derived Extracellular Vesicles Promote Cardiomyocyte Growth and Electrical Activity in Engineered Cardiac Atria. Biomaterials 2017, 146, 49–59. [Google Scholar] [CrossRef]
- Minghua, W.; Zhijian, G.; Chahua, H.; Qiang, L.; Minxuan, X.; Luqiao, W.; Weifang, Z.; Peng, L.; Biming, Z.; Lingling, Y.; et al. Plasma Exosomes Induced by Remote Ischaemic Preconditioning Attenuate Myocardial Ischaemia/Reperfusion Injury by Transferring MiR-24. Cell Death Dis. 2018, 9, 320. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.; Sahoo, S. Angiogenic Mechanisms of Human CD34+ Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer into Macrophages Mediates Cellular Postconditioning de Couto: Exosomal RNA Transfer Modulates Macrophages. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell–Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Barile, L.; Cervio, E.; Lionetti, V.; Milano, G.; Ciullo, A.; Biemmi, V.; Bolis, S.; Altomare, C.; Matteucci, M.; Di Silvestre, D.; et al. Cardioprotection by Cardiac Progenitor Cell-Secreted Exosomes: Role of Pregnancy-Associated Plasma Protein-A. Cardiovasc. Res. 2018, 114, 992–1005. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl. Med. 2017, 6, 51–59. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human Keratinocyte-Derived Microvesicle MiRNA-21 Promotes Skin Wound Healing in Diabetic Rats through Facilitating Fibroblast Function and Angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Narauskaité, D.; Vydmantaite, G.; Rusteikaite, J.; Sampath, R.; Rudaityte, A.; Stašyte, G.; Isabel, M.; Calvente, A.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver MiR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular Vesicles through the Blood–Brain Barrier: A Review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.-K.; Duhan, V.; Radtke, S.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Ameliorate Inflammation-Induced Preterm Brain Injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef]
- Clark, K.; Zhang, S.; Barthe, S.; Kumar, P.; Pivetti, C.; Kreutzberg, N.; Reed, C.; Wang, Y.; Paxton, Z.; Farmer, D.; et al. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells 2019, 8, 1497. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells-Derived Exosomes Are More Immunosuppressive than Microparticles in Inflammatory Arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes Derived from Human Embryonic Mesenchymal Stem Cells Promote Osteochondral Regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-Anchored Anti-EGFR Nanobodies on Extracellular Vesicles Promotes Tumour Cell Targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant Phosphatidylserine-Binding Nanobodies for Targeting of Extracellular Vesicles to Tumor Cells: A Plug-and-Play Approach. Nanoscale 2018, 10, 2413–2426. [Google Scholar] [CrossRef]
- Del Vecchio, K.; Stahelin, R.V. Investigation of the phosphatidylserine binding properties of the lipid biosensor Lactadherin C2 (LactC2), in different membrane environments. J. Bioenerg. Biomembr. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-Rich Cell-Penetrating Peptide-Modified Extracellular Vesicles for Active Macropinocytosis Induction and Efficient Intracellular Delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of Chemotherapeutic Drugs in Tumour Cell-Derived Microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human Peripheral Blood-Derived Exosomes for MicroRNA Delivery. Int. J. Mol. Med. 2019, 43, 2319–2328. [Google Scholar] [CrossRef]
- Youn, S.-W.; Li, Y.; Kim, Y.-M.; Sudhahar, V.; Abdelsaid, K.; Kim, H.W.; Liu, Y.; Fulton, D.J.R.; Ashraf, M.; Tang, Y.; et al. Modification of Cardiac Progenitor Cell-Derived Exosomes by MiR-322 Provides Protection against Myocardial Infarction through Nox2-Dependent Angiogenesis. Antioxidants 2019, 8, 18. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.H.A.M.; Heijnen, H.F.G.; van Bergen en Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and Targeted Extracellular Vesicles Display Enhanced Cell Specificity and Circulation Time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef]
- Annual Activity Report. Available online: https://www.odt.nhs.uk/statistics-and-reports/annual-activity-report/ (accessed on 19 November 2022).
- Segura, E.; Nicco, C.; Lombard, B.; Véron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Théry, C. ICAM-1 on Exosomes from Mature Dendritic Cells Is Critical for Efficient Naive T-Cell Priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef]
- Li, X.; Li, J.-J.; Yang, J.-Y.; Wang, D.-S.; Zhao, W.; Song, W.-J.; Li, W.-M.; Wang, J.-F.; Han, W.; Zhang, Z.-C.; et al. Tolerance Induction by Exosomes from Immature Dendritic Cells and Rapamycin in a Mouse Cardiac Allograft Model. PLoS ONE 2012, 7, e44045. [Google Scholar] [CrossRef]
- Pang, X.-L.; Wang, Z.-G.; Liu, L.; Feng, Y.-H.; Wang, J.-X.; Xie, H.-C.; Yang, X.-L.; Li, J.-F.; Feng, G.-W. Immature Dendritic Cells Derived Exosomes Promotes Immune Tolerance by Regulating T Cell Differentiation in Renal Transplantation. Aging 2019, 11, 8911–8924. [Google Scholar] [CrossRef]
- Ma, B.; Yang, J.-Y.; Song, W.; Ding, R.; Zhang, Z.; Ji, H.; Zhang, X.; Wang, J.; Yang, X.; Tao, K.; et al. Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci. Rep. 2016, 6, 32971. [Google Scholar] [CrossRef]
- Yang, X.; Meng, S.; Jiang, H.; Zhu, C.; Wu, W. Exosomes Derived from Immature Bone Marrow Dendritic Cells Induce Tolerogenicity of Intestinal Transplantation in Rats. J. Surg. Res. 2011, 171, 826–832. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Schuberth, P.C.; Dunn, A.C.; Miller, L.; Hock, B.D.; MacKay, P.A.; Koch, N.; Jack, R.W.; McLellan, A.D. Induction of Exosome Release in Primary B Cells Stimulated via CD40 and the IL-4 Receptor1. J. Immunol. 2008, 180, 8146–8152. [Google Scholar] [CrossRef]
- Harper, S.J.F.; Ali, J.M.; Wlodek, E.; Negus, M.C.; Harper, I.G.; Chhabra, M.; Qureshi, M.S.; Mallik, M.; Bolton, E.; Bradley, J.A.; et al. CD8 T-Cell Recognition of Acquired Alloantigen Promotes Acute Allograft Rejection. Proc. Natl. Acad. Sci. USA 2015, 112, 12788–12793. [Google Scholar] [CrossRef]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular Vesicles Derived from T Regulatory Cells Suppress T Cell Proliferation and Prolong Allograft Survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef]
- Tung, S.L.; Fanelli, G.; Matthews, R.I.; Bazoer, J.; Letizia, M.; Vizcay-Barrena, G.; Faruqu, F.N.; Philippeos, C.; Hannen, R.; Al-Jamal, K.T.; et al. Regulatory T Cell Extracellular Vesicles Modify T-Effector Cell Cytokine Production and Protect Against Human Skin Allograft Damage. Front. Cell Dev. Biol. 2020, 8, 317. [Google Scholar] [CrossRef]
- Tung, S.L.; Boardman, D.A.; Sen, M.; Letizia, M.; Peng, Q.; Cianci, N.; Dioni, L.; Carlin, L.M.; Lechler, R.; Bollati, V.; et al. Regulatory T Cell-Derived Extracellular Vesicles Modify Dendritic Cell Function. Sci. Rep. 2018, 8, 6065. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine Generation Catalyzed by CD39 and CD73 Expressed on Regulatory T Cells Mediates Immune Suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Lappas, C.M.; Rieger, J.M.; Linden, J. A2A Adenosine Receptor Induction Inhibits IFN-γ Production in Murine CD4+ T Cells1. J. Immunol. 2005, 174, 1073–1080. [Google Scholar] [CrossRef]
- Romio, M.; Reinbeck, B.; Bongardt, S.; Hüls, S.; Burghoff, S.; Schrader, J. Extracellular Purine Metabolism and Signaling of CD73-Derived Adenosine in Murine Treg and Teff Cells. Am. J. Physiol. Cell Physiol. 2011, 301, C530–C539. [Google Scholar] [CrossRef]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.S.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 Expression on Extracellular Vesicles Derived from CD4+CD25+Foxp3+ T Cells Contributes to Their Regulatory Function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Zhang, W.; Ding, F.; Fan, Z.; Zeng, Z. Exosomes Derived From T Regulatory Cells Suppress CD8+ Cytotoxic T Lymphocyte Proliferation and Prolong Liver Allograft Survival. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4877–4884. [Google Scholar] [CrossRef]
- Ponticelli, C. Ischaemia-Reperfusion Injury: A Major Protagonist in Kidney Transplantation. Nephrol. Dial. Transplant. 2014, 29, 1134–1140. [Google Scholar] [CrossRef]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Foutz, J.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am. J. Transplant. 2020, 20, 20–130. [Google Scholar] [CrossRef]
- Taylor, R.; Allen, E.; Richards, J.A.; Goh, M.A.; Neuberger, J.; Collett, D.; Pettigrew, G.J. Survival Advantage for Patients Accepting the Offer of a Circulatory Death Liver Transplant. J. Hepatol. 2019, 70, 855–865. [Google Scholar] [CrossRef]
- Ali, J.M.; Davies, S.E.; Brais, R.J.; Randle, L.V.; Klinck, J.R.; Allison, M.E.D.; Chen, Y.; Pasea, L.; Harper, S.F.J.; Pettigrew, G.J. Analysis of Ischemia/Reperfusion Injury in Time-Zero Biopsies Predicts Liver Allograft Outcomes. Liver Transpl. 2015, 21, 487–499. [Google Scholar] [CrossRef]
- Abraham, S.; Furth, E.E. Quantitative Evaluation of Histological Features in “Time-Zero” Liver Allograft Biopsies as Predictors of Rejection or Graft Failure: Receiver-Operating Characteristic Analysis Application. Hum. Pathol. 1996, 27, 1077–1084. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia–Reperfusion Injury in Liver Transplantation—From Bench to Bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Land, W.G.; Agostinis, P.; Gasser, S.; Garg, A.D.; Linkermann, A. Transplantation and Damage-Associated Molecular Patterns (DAMPs). Am. J. Transplant. 2016, 16, 3338–3361. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β Stimulated Synovial Fibroblasts Induce Osteoarthritic Changes in Articular Chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef]
- Budden, C.F.; Gearing, L.J.; Kaiser, R.; Standke, L.; Hertzog, P.J.; Latz, E. Inflammasome-Induced Extracellular Vesicles Harbour Distinct RNA Signatures and Alter Bystander Macrophage Responses. J. Extracell. Vesicles 2021, 10, e12127. [Google Scholar] [CrossRef]
- Ferdinand, J.R.; Hosgood, S.A.; Moore, T.; Ferro, A.; Ward, C.J.; Castro-Dopico, T.; Nicholson, M.L.; Clatworthy, M.R. Cytokine Absorption during Human Kidney Perfusion Reduces Delayed Graft Function–Associated Inflammatory Gene Signature. Am. J. Transplant. 2021, 21, 2188–2199. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ganster, R.W.; Gambotto, A.; Shao, L.; Kaizu, T.; Wu, T.; Yagnik, G.P.; Nakao, A.; Tsoulfas, G.; Ishikawa, T.; et al. Role of NF-ΚB on Liver Cold Ischemia-Reperfusion Injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1175–G1184. [Google Scholar] [CrossRef]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular Vesicles from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (HiPSC-MSCs) Protect against Renal Ischemia/Reperfusion Injury via Delivering Specificity Protein (SP1) and Transcriptional Activating of Sphingosine Kinase 1 and Inhibiting Necroptosis. Cell Death Dis. 2017, 8, 3200. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.A.; Yoon, H.; Kim, M.Y.; Yoo, J.-K.; Ahn, S.-H.; Park, C.H.; Park, J.; Nam, B.Y.; Park, J.T.; et al. Exosome-Based Delivery of Super-Repressor IκBα Ameliorates Kidney Ischemia-Reperfusion Injury. Kidney Int. 2021, 100, 570–584. [Google Scholar] [CrossRef]
- Viñas, J.L.; Burger, D.; Zimpelmann, J.; Haneef, R.; Knoll, W.; Campbell, P.; Gutsol, A.; Carter, A.; Allan, D.S.; Burns, K.D. Transfer of MicroRNA-486-5p from Human Endothelial Colony Forming Cell–Derived Exosomes Reduces Ischemic Kidney Injury. Kidney Int. 2016, 90, 1238–1250. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Lin, F.; Yin, H.; Li, X.; He, W.; Liu, N.; Gou, X. Exosomes from Human-Bone-Marrow-Derived Mesenchymal Stem Cells Protect against Renal Ischemia/Reperfusion Injury via Transferring MiR-199a-3p. J. Cell. Physiol. 2019, 234, 23736–23749. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, G.; He, W.; Yin, H.; Lin, F.; Gou, X.; Li, X. BMSCs Protect against Renal Ischemia-reperfusion Injury by Secreting Exosomes Loaded with MiR-199a-5p That Target BIP to Inhibit Endoplasmic Reticulum Stress at the Very Early Reperfusion Stages. FASEB J. 2019, 33, 5440–5456. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from Human-Induced Pluripotent Stem Cell–Derived Mesenchymal Stromal Cells (HiPSC-MSCs) Protect Liver against Hepatic Ischemia/Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Z.; Ling, W.; Zhu, D.; Feng, Z.; Kong, L. Exosomes Derived from Dendritic Cells Attenuate Liver Injury by Modulating the Balance of Treg and Th17 Cells After Ischemia Reperfusion. Cell. Physiol. Biochem. 2018, 46, 740–756. [Google Scholar] [CrossRef]
- Block, H.; Rossaint, J.; Zarbock, A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells 2022, 11, 1919. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, J.; Cai, J.; Xiao, J.; Sui, X.; Yuan, X.; Li, R.; Li, Y.; Yao, J.; Lv, G.; et al. Extracellular Vesicles Derived from Mesenchymal Stromal Cells as Nanotherapeutics for Liver Ischaemia–Reperfusion Injury by Transferring Mitochondria to Modulate the Formation of Neutrophil Extracellular Traps. Biomaterials 2022, 284, 121486. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Wakiya, T.; Sadatomo, A.; Ito, H.; Kamata, R.; Watanabe, S.; Komada, T.; Kimura, H.; Sanada, Y.; et al. Iron Overload as a Risk Factor for Hepatic Ischemia-Reperfusion Injury in Liver Transplantation: Potential Role of Ferroptosis. Am. J. Transplant. 2020, 20, 1606–1618. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Tian, X.; Zheng, W.; Yuan, M.; Tian, X.; Zuo, H.; Song, H.; Shen, Z. MiR-29a-3p in Exosomes from Heme Oxygenase-1 Modified Bone Marrow Mesenchymal Stem Cells Alleviates Steatotic Liver Ischemia-Reperfusion Injury in Rats by Suppressing Ferroptosis via Iron Responsive Element Binding Protein 2. Oxidative Med. Cell. Longev. 2022, 2022, e6520789. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Hu, C.-F.; Li, R.; Zhou, Z.; Shen, C.-X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell. Physiol. Biochem. 2017, 43, 52–68. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Gómez-Ferrer, M.; Reinal, I.; Buigues, M.; Villanueva-Bádenas, E.; Ontoria-Oviedo, I.; Hernándiz, A.; González-King, H.; Peiró-Molina, E.; Dorronsoro, A.; et al. MiR-4732-3p in Extracellular Vesicles From Mesenchymal Stromal Cells Is Cardioprotective During Myocardial Ischemia. Front. Cell Dev. Biol. 2021, 9, 734143. [Google Scholar] [CrossRef]
- Del Campo, C.V.; Liaw, N.Y.; Gunadasa-Rohling, M.; Matthaei, M.; Braga, L.; Kennedy, T.; Salinas, G.; Voigt, N.; Giacca, M.; Zimmermann, W.-H.; et al. Regenerative Potential of Epicardium-Derived Extracellular Vesicles Mediated by Conserved MiRNA Transfer. Cardiovasc. Res. 2022, 118, 597–611. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal Stromal Cells-Derived Exosomes Alleviate Ischemia/Reperfusion Injury in Mouse Lung by Transporting Anti-Apoptotic MiR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Lung Ischemia-Reperfusion Injury and Enhance Reconditioning of Donor Lungs after Circulatory Death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef]
- Cai, J.; Gehrau, R.; Tu, Z.; Leroy, V.; Su, G.; Shang, J.; Mas, V.R.; Emtiazjoo, A.; Pelaez, A.; Atkinson, C.; et al. MicroRNA-206 AntagomiR–enriched Extracellular Vesicles Attenuate Lung Ischemia–reperfusion Injury through CXCL1 Regulation in Alveolar Epithelial Cells. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020, 39, 1476–1490. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the Ex Situ Perfusion of Livers for Transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- Gaurav, R.; Butler, A.J.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.; Upponi, S.; Khwaja, S.; Richards, J.; et al. Liver Transplantation Outcomes From Controlled Circulatory Death Donors: SCS vs. in Situ NRP vs. Ex Situ NMP. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef]
- Bogensperger, C.; Hofmann, J.; Messner, F.; Resch, T.; Meszaros, A.; Cardini, B.; Weissenbacher, A.; Oberhuber, R.; Troppmair, J.; Öfner, D.; et al. Ex Vivo Mesenchymal Stem Cell Therapy to Regenerate Machine Perfused Organs. Int. J. Mol. Sci. 2021, 22, 5233. [Google Scholar] [CrossRef]
- Thompson, E.R.; Bates, L.; Ibrahim, I.K.; Sewpaul, A.; Stenberg, B.; McNeill, A.; Figueiredo, R.; Girdlestone, T.; Wilkins, G.C.; Wang, L.; et al. Novel Delivery of Cellular Therapy to Reduce Ischemia Reperfusion Injury in Kidney Transplantation. Am. J. Transplant. 2021, 21, 1402–1414. [Google Scholar] [CrossRef]
- Laing, R.W.; Stubblefield, S.; Wallace, L.; Roobrouck, V.D.; Bhogal, R.H.; Schlegel, A.; Boteon, Y.L.; Reynolds, G.M.; Ting, A.E.; Mirza, D.F.; et al. The Delivery of Multipotent Adult Progenitor Cells to Extended Criteria Human Donor Livers Using Normothermic Machine Perfusion. Front. Immunol. 2020, 11, 1226. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic Human Mesenchymal Stem Cells for Treatment of E. Coli Endotoxin-Induced Acute Lung Injury in the Ex Vivo Perfused Human Lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef]
- De Stefano, N.; Navarro-Tableros, V.; Roggio, D.; Calleri, A.; Rigo, F.; David, E.; Gambella, A.; Bassino, D.; Amoroso, A.; Patrono, D.; et al. Human Liver Stem Cell-derived Extracellular Vesicles Reduce Injury in a Model of Normothermic Machine Perfusion of Rat Livers Previously Exposed to a Prolonged Warm Ischemia. Transpl. Int. 2021, 34, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Rampino, T.; Gregorini, M.; Germinario, G.; Pattonieri, E.F.; Erasmi, F.; Grignano, M.A.; Bruno, S.; Alomari, E.; Bettati, S.; Asti, A.; et al. Extracellular Vesicles Derived from Mesenchymal Stromal Cells Delivered during Hypothermic Oxygenated Machine Perfusion Repair Ischemic/Reperfusion Damage of Kidneys from Extended Criteria Donors. Biology 2022, 11, 350. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell Type-Specific and Common Characteristics of Exosomes Derived from Mouse Cell Lines: Yield, Physicochemical Properties, and Pharmacokinetics. Eur. J. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-Dependent Clearance of Systemically Administered B16BL6-Derived Exosomes from the Blood Circulation in Mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Thompson, E.R.; Bates, L.; Ibrahim, I.K.; Govaere, O.; Shuttleworth, V.; Wang, L.; Figueiredo, R.; Palmer, J.; Bury, Y.; et al. Pharmacological Testing of Therapeutics Using Normothermic Machine Perfusion: A Pilot Study of 2,4-Dinitrophenol Delivery to Steatotic Human Livers. Artif. Organs 2022, 46, 2201–2214. [Google Scholar] [CrossRef]
- Vulto, A.G.; Jaquez, O.A. The Process Defines the Product: What Really Matters in Biosimilar Design and Production? Rheumatology 2017, 56, iv14–iv29. [Google Scholar] [CrossRef]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of Cell Culture Parameters on Production and Vascularization Bioactivity of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Bioeng. Transl. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef]
- Penders, J.; Nagelkerke, A.; Cunnane, E.M.; Pedersen, S.V.; Pence, I.J.; Coombes, R.C.; Stevens, M.M. Single Particle Automated Raman Trapping Analysis of Breast Cancer Cell-Derived Extracellular Vesicles as Cancer Biomarkers. ACS Nano 2021, 15, 18192–18205. [Google Scholar] [CrossRef]
- Maisano, D.; Mimmi, S.; Dattilo, V.; Marino, F.; Gentile, M.; Vecchio, E.; Fiume, G.; Nisticò, N.; Aloisio, A.; Santo, M.P.D.; et al. A Novel Phage Display Based Platform for Exosome Diversity Characterization. Nanoscale 2022, 14, 2998–3003. [Google Scholar] [CrossRef]
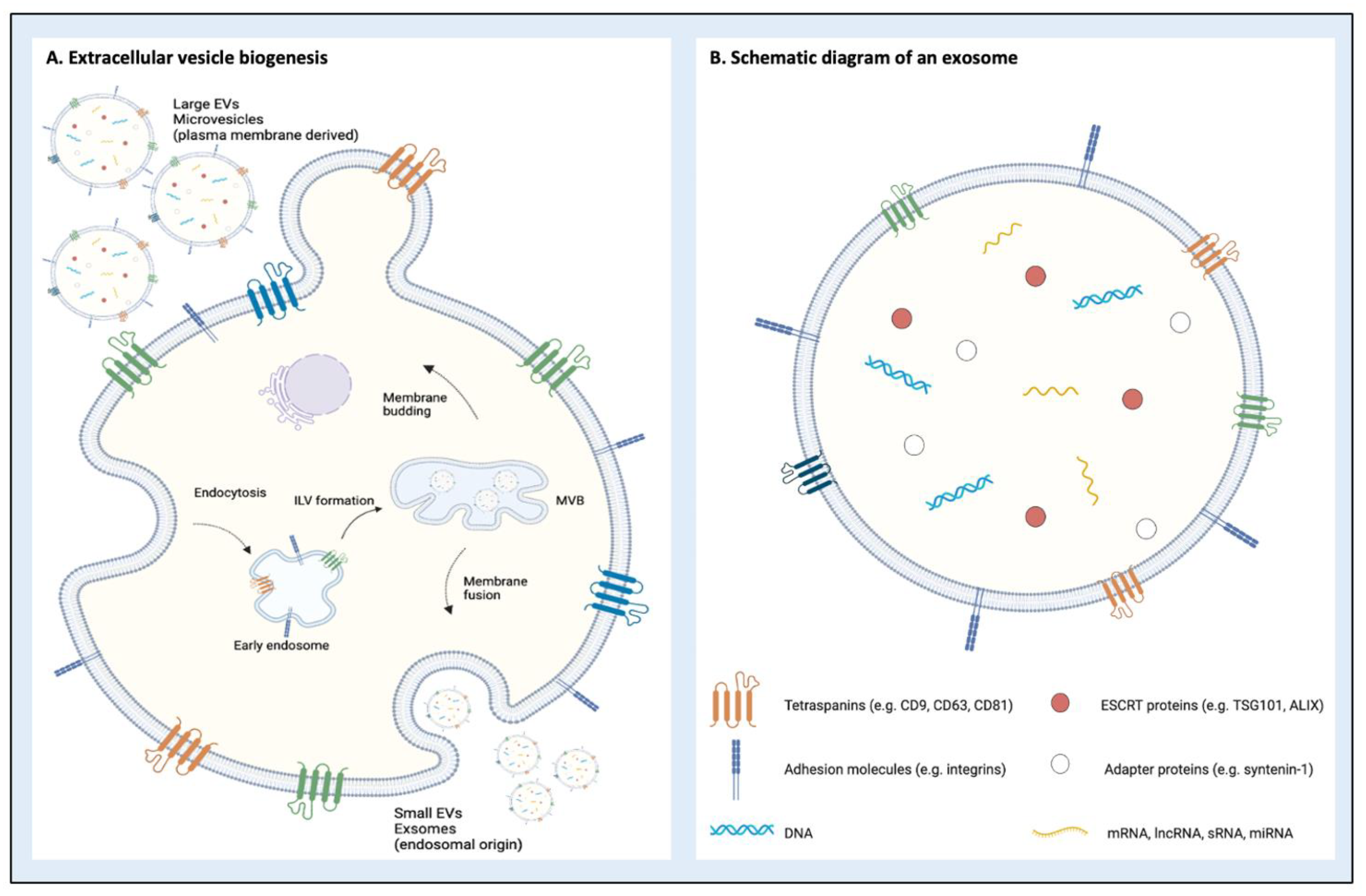
| Subtype | Origin | Size (nm) | Alternative Names | Composition | Biological Cargo |
|---|---|---|---|---|---|
| Exosomes | Endosome | 50–150 | Nanovesicles, proteosomes, exosome-like vesicles | Membrane constituents:
| Enzymes (e.g., peroxidases), nucleic acids (e.g., miRNAs, mRNA, lncRNA) |
| Microvesicles | Plasma membrane | 150–1000 | Microparticles, oncosomes, shedding vesicles, blebbing vesicles | Membrane constituents:
| Nucleic acids (e.g., miRNAs, mRNA, lncRNA, DNA, histones) |
| Apoptotic bodies | Plasma membrane | 500–2000 | Apo-EVs | Membrane constituents:
| Nucleic acids (including histones, large DNA fragments and some miRNAs), organelles |
| Methodology | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Ultrafiltration | Biofluid is passed through a porous membrane to filter particles larger than a predetermined size | Time-effective; high yield | Pores blocked with contaminants; contamination (e.g., proteins and RNA); small particles left on pores; EVs damaged by force used |
| Differential ultracentrifugation | High centrifugal speeds are applied for sufficient time periods to allow individual EVs to travel to the bottom of the tube and accumulate as a pellet; however, the method is less efficient at pelleting smaller/less dense particles | Commonly used; replicable; convenient operation; no sample pre-treatment required | Time-consuming; requires larger volumes of biofluid; unpredictable co-isolation (e.g., lipoproteins); damage to and aggregation and loss of EVs |
| Density gradient ultracentrifugation | EVs are purified based on their buoyant density by using a medium such as iodixanol and centrifugation | Improved separation of EVs from protein complexes; replicable | Time-consuming; low yield; EV damage; co-isolation of non-EV particles of similar densities |
| Polymer precipitation | Volume-expanding polymers reduce the solubility of EVs in solution, with isolation following the subsequent low-speed centrifugation | High yield; time-efficient; commercial kits available | Unclear effects of polymers on downstream applications; coprecipitation of proteins with further protein removal kits needed |
| Size exclusion chromatography | EVs in solution loaded onto a gel bead column, with larger EVs passing around the gel beads and eluting from the column first, whilst smaller particles progress more slowly through the bead matrix and elute later | Vesicle structure and integrity preserved; high purity; reproducible | Time-consuming; post-isolation concentration steps required |
| Immunoaffinity | Immunocapture utilising beads conjugated with antibodies toward EV surface markers | High sensitivity and specificity; EV subtype separation possible | Expensive; low yield; elution techniques can affect EV integrity |
| Number | Name a | Condition | EV Source b | Location | Phase | NCT Number c |
|---|---|---|---|---|---|---|
| Actively recruiting | ||||||
| 1 | Use of Autologous Plasma Rich in Platelets and Extracellular Vesicles in the Surgical Treatment of Chronic Middle Ear Infections | Chronic otitis media | Blood-derived (autologous) | Ljubljana, Slovenia | II/III | NCT04761562 |
| 2 | Safety Evaluation of Intracoronary Infusion of Extracellular Vesicles in Patients With AMI | Myocardial infarction | Blood-derived | Minnesota, USA | I | NCT04327635 |
| 3 | Autologous Serum-derived EV for Venous Trophic Lesions Not Responsive to Conventional Treatments (SER-VES-HEAL) | Venous ulcers | Blood-derived (autologous) | Turin, Italy | NA | NCT04652531 |
| 4 | Bone Marrow Mesenchymal Stem Cell Derived EVs for COVID-19 Moderate-to-Severe Acute Respiratory Distress Syndrome (ARDS): A Phase III Clinical Trial | SARS-CoV-2 | Bone marrow MSC-derived (cargo includes VEGFR, VEGF, ANG1, TIMP-1, TIMP-2, IL-1B, PDGF-AA, TGFb3, bFGF, HGF) | Texas, USA | III | NCT05354141 |
| 5 | Safety and Efficacy of Injection of Human Placenta Mesenchymal Stem Cells Derived Exosomes for Treatment of Complex Anal Fistula | Fistula-in-ano | Human placenta MSC-derived | Tehran, Iran | I/II | NCT05402748 |
| 6 | Allogenic Mesenchymal Stem Cell Derived Exosome in Patients With Acute Ischemic Stroke | Ischaemic stroke | Allogeneic MSC-derived (cargo enriched for miR-124) | Isfahan, Iran | I/II | NCT03384433 |
| 7 | Efficacy and Safety of EXOSOME-MSC Therapy to Reduce Hyper-inflammation In Moderate COVID-19 Patients (EXOMSC-COV19) | SARS-CoV-2 | MSC-derived | Indonesia | II/III | NCT05216562 |
| 8 | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for the Treatment of Pulmonary Infection | Pulmonary infection | Mesenchymal progenitor MSC-derived | Shanghai, China | I/II | NCT04544215 |
| 9 | Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue | Colon cancer | Plants (cargo of curcumin) | Kentucky, USA | I | NCT01294072 |
| 10 | Evaluation of the Safety of CD24-Exosomes in Patients With COVID-19 Infection | SARS-CoV-2 | CD24-expressing 293-TREx™ cells (EVs enriched for CD24) | Tel Aviv, Israel | I | NCT04747574 |
| 11 | Clinical Efficacy of Exosome in Degenerative Meniscal Injury (KNEEXO) | Degenerative meniscal injury | MSC-derived | Eskisehir, Turkey | II | NCT05261360 |
| 12 | The Effect of Stem Cells and Stem Cell Exosomes on Visual Functions in Patients With Retinitis Pigmentosa | Retinitis pigmentosa | Wharton jelly- derived mesenchymal stem cells | Kayseri, Turkey | II/III | NCT05413148 |
| 14 | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | Dry eye | Umbilical MSC- derived | Guangdong, China | I/II | NCT04213248 |
| 15 | iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation | Pancreatic cancer | MSC-derived (cargo of siRNA against KrasG12D) | Texas, USA | I | NCT03608631 |
| 16 | Safety and Efficacy of Exosomes Overexpressing CD24 in Two Doses for Patients With Moderate or Severe COVID-19 | SARS-CoV-2 | CD24-expressing 293-TREx™ cells (EVs enriched for CD24) | Athens, Greece | II | NCT04902183 |
| 17 | Safety and Effectiveness of Placental Derived Exosomes and Umbilical Cord Mesenchymal Stem Cells in Moderate to Severe Acute Respiratory Distress Syndrome (ARDS) Associated With the Novel Corona Virus Infection (COVID-19) | SARS-CoV-2 | Umbilical cord MSC-derived (cargo of unspecified growth factors) | Missouri, USA | I | NCT05387278 |
| 18 | An Open, Dose-escalation Clinical Study of Chimeric Exosomal Tumor Vaccines for Recurrent or Metastatic Bladder Cancer | Bladder cancer | Chimeric exosomal tumour vaccine | Shanghai, China | I | NCT05559177 |
| 19 | A Study of exoASO-STAT6 (CDK-004) in Patients With Advanced Hepatocellular Carcinoma (HCC) and Patients With Liver Metastases From Primary Gastric Cancer and Colorectal Cancer (CRC) | Hepatocellular carcinoma, metastatic gastric and colorectal cancer | Bioengineered (cargo of STAT6 anti-sense oligonucleotide) | California, USA | I | NCT05375604 |
| Completed | ||||||
| 1 | Efficacy of Platelet- and Extracellular Vesicle-rich Plasma in Chronic Postsurgical Temporal Bone Inflammations (PvRP-ear) | Chronic inflammation of temporal bone post-surgery | Blood-derived (autologous) | Ljubljana, Slovenia | NA | NCT04281901 |
| 2 | Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS (EXIT-COVID19) | SARS-CoV-2 | Bone marrow MSC-derived | Alabama, USA | II | NCT04493242 |
| 3 | Safety and Tolerability Study of MSC Exosome Ointment | Psoriasis | MSC-derived (cargo of VEGFR, VEGF, ANG1, TIMP-1, TIMP-2, IL-1B, PDGF-AA, TGFb3, bFGF, HGF) | Singapore | I | NCT05523011 |
| 4 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | SARS-CoV-2 | MSC-derived | Wuhan, China | I | NCT04276987 (conclusion: inhalation of EVs up to a total amount of 2.0 × 109 was feasible and functioned well, with no evidence of prespecified adverse events, immediate clinical instability or dose-relevant toxicity at any of the doses tested. This safety profile was seemingly followed by CT imaging improvement within 7 days) |
| 5 | Intra-discal Injection of Platelet-rich Plasma (PRP) Enriched With Exosomes in Chronic Low Back Pain | Chronic lower back pain | Blood derived (autologous) | Uttarakhand, India | I | NCT04849429 |
| 6 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia (COVID-19EXO) | SARS-CoV-2 | MSC-derived | Volga, Russia | I/II | NCT04491240 |
| 7 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes In Healthy Volunteers | Nil | Adipose MSC- derived | Shanghai, China | I | NCT04313647 (conclusion: all volunteers tolerated EV nebulization well, with no serious adverse events observed. The authors suggested that nebulised EVs could be a promising therapeutic strategy in lung injury diseases) |
| 8 | Plant Exosomes ± Curcumin to Abrogate Symptoms of Inflammatory Bowel Disease | Inflammatory bowel disease | Plants (cargo of curcumin) | Kentucky, USA | NA | NCT04879810 |
| 9 | Edible Plant Exosome Ability to Prevent Oral Mucositis Associated With Chemoradiation Treatment of Head and Neck Cancer | Oral mucositis | Grapes | Kentucky, USA | I | NCT01668849 |
| 10 | Trial of a Vaccination With Tumor Antigen-loaded Dendritic Cell-derived Exosomes (CSET 1437) | Non-small cell lung cancer | Dendritic cell derived (cargo of melanoma- associated antigen) | Villejuif, France | II | NCT01159288 (conclusion: EVs boost the natural killer cell arm of antitumour immunity in patients with advanced NSCLC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiers, H.V.M.; Stadler, L.K.J.; Smith, H.; Kosmoliaptsis, V. Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier. Pharmaceutics 2023, 15, 891. https://doi.org/10.3390/pharmaceutics15030891
Spiers HVM, Stadler LKJ, Smith H, Kosmoliaptsis V. Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier. Pharmaceutics. 2023; 15(3):891. https://doi.org/10.3390/pharmaceutics15030891
Chicago/Turabian StyleSpiers, Harry V. M., Lukas K. J. Stadler, Hugo Smith, and Vasilis Kosmoliaptsis. 2023. "Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier" Pharmaceutics 15, no. 3: 891. https://doi.org/10.3390/pharmaceutics15030891
APA StyleSpiers, H. V. M., Stadler, L. K. J., Smith, H., & Kosmoliaptsis, V. (2023). Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier. Pharmaceutics, 15(3), 891. https://doi.org/10.3390/pharmaceutics15030891






