Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases
Abstract
1. Introduction
2. Cell Membrane Types Currently Being Used to Coat Nanoparticles to Cross the BBB
2.1. Single Species of Cell Membrane
2.1.1. Red-Cell-Membrane-Coated Nanoparticles (RCMCNPs)
2.1.2. White-Cell-Membrane-Coated Nanoparticles (WCMCNPs)
2.1.3. Platelet-Cell-Membrane-Coated Nanoparticles (PCMCNPs)
2.1.4. Cancer-Cell-Membrane-Coated Nanoparticles (CCMCNPs)
2.1.5. Stem-Cell-Membrane-Coated Nanoparticles (SCMCNPs)
2.1.6. Neural-Cell-Membrane-Coated Nanoparticles (NCMCNPs)
2.1.7. Vascular-Endothelial-Cell-Membrane-Coated Nanoparticles (VECMCNPs)
2.2. Hybrid-Cell-Membrane-Coated Nanoparticles (HCMCNPs)
3. Application of Cell Membrane Coated Nanoparticles in NDs
3.1. Ischemic Stroke
3.2. Brain Tumors
3.3. Other NDs
4. Safety and Outcomes after CMCNPs Enter the Circulation
4.1. The Safety of CMCNPs
4.2. The Clearance after CMCNPs Enter the Blood Circulation: Three Scenarios
4.2.1. Normal Condition
4.2.2. Off-Target Effects of CMCNPs before Crossing the BBB
4.2.3. Off-Target Effects of CMCNPs after Crossing the BBB
4.3. Potential Adverse Effects of CMCNPs on the Body
4.3.1. Potential Adverse Effects of Cell Membrane Coatings
4.3.2. Potential Adverse Effects of Long-Term Application of CMCNPs
5. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| neurological diseases | NDs |
| cell membrane-coated nanoparticles | CMCNPs |
| nanoparticles | NPs |
| blood–brain barrier | BBB |
| central nervous system | CNS |
| ischemic stroke | IS |
| traumatic brain injury | TBI |
| multiple sclerosis | MS |
| glioblastoma multiforme | GBM |
| Parkinson’s disease | PD |
| Alzheimer’s disease | AD |
| brain endothelial cells | BECs |
| red blood cells | RBCs |
| white blood cells | WBCs |
| red cell membrane-coated nanoparticles | RCMCNPs |
| N-methyl-D-aspartate receptor | NMDAR |
| reticuloendothelial system | RES |
| signal regulatory proteinα | SIRPα |
| reactive oxygen species | ROS |
| stroke homing peptide | SHP |
| white cell membrane-coated nanoparticles | WCMCNPs |
| monocytes/macrophages | MMs |
| fat extract | FE |
| catalase | CAT |
| resolvin D2 | RvD2 |
| ischemia/reperfusion | I/R |
| genistein | GS |
| curcumin | CUR |
| indinavir | IDV |
| rapamycin | RAPA |
| platelet cell membrane-coated nanoparticles | PCMCNPs |
| cancer cell membrane-coated nanoparticles | CCMCNPs |
| doxorubicin | DOX |
| hydroxychloroquine | HDX |
| 10-hydroxycamptothecin | HCPT |
| paclitaxel | PTX |
| stem cell membrane-coated nanoparticles | SCMCNPs |
| neural stem cells | NSCs |
| oligodendrocyte progenitor cells | OPCs |
| neural cell membrane-coated nanoparticles | NCMCNPs |
| vascular endothelial cells | VECs |
| cerebral malaria | CM |
| hybrid cell membrane-coated nanoparticles | HCMCNPs |
| primary mouse thoracic aorta endothelial cell | PMTAEC |
| blood-brain tumor barrier | BBTB |
| immune tumor microenvironment | iTME |
| β-amyloid protein | Aβ |
| rabies virus glycoprotein | RVG29 |
| triphenylphosphine cation | TPP |
| infected RBCs | iRBCs |
| dihydroartemisinin | DHA |
References
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Zammit, C.; Torzhenskaya, N.; Ozarkar, P.D.; Calleja Agius, J. Neurological disorders vis-a-vis climate change. Early Hum. Dev. 2021, 155, 105217. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Ayala, A. Air Pollution, Ultrafine Particles, and Your Brain: Are Combustion Nanoparticle Emissions and Engineered Nanoparticles Causing Preventable Fatal Neurodegenerative Diseases and Common Neuropsychiatric Outcomes? Environ. Sci. Technol. 2022, 56, 6847–6856. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, M.; Rahimi, S.; Rezaiemanesh, M.R.; Yektay, S. Association between water and sanitation, air and emission pollution and climate change and neurological disease distribution: A study based on GBD data. Chemosphere 2021, 285, 131522. [Google Scholar] [CrossRef]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM(2.5) exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021, 12, 678744. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Fricke, I.B.; Schelhaas, S.; Zinnhardt, B.; Viel, T.; Hermann, S.; Couillard-Després, S.; Jacobs, A.H. In vivo bioluminescence imaging of neurogenesis—The role of the blood brain barrier in an experimental model of Parkinson’s disease. Eur. J. Neurosci. 2017, 45, 975–986. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.; Chen, Q.; Liu, P.; Guo, Q.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhou, W.; Liang, D.; et al. Microthrombus-Targeting Micelles for Neurovascular Remodeling and Enhanced Microcirculatory Perfusion in Acute Ischemic Stroke. Adv. Mater. 2019, 31, e1808361. [Google Scholar] [CrossRef]
- Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545. [Google Scholar] [CrossRef]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The Mystery of Antibodies Against Polyethylene Glycol (PEG)—What do we Know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Haney, M.J.; Zhao, Y.; Manickam, D.S.; Mahajan, V.; Suresh, P.; Hingtgen, S.D.; Mosley, R.L.; Gendelman, H.E.; Kabanov, A.V.; et al. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine 2014, 9, 1403–1422. [Google Scholar] [CrossRef]
- Han, H.; Eyal, S.; Portnoy, E.; Mann, A.; Shmuel, M.; Benifla, M.; Ekstein, D.; Polyak, B. Monocytes as Carriers of Magnetic Nanoparticles for Tracking Inflammation in the Epileptic Rat Brain. Curr. Drug Deliv. 2019, 16, 637–644. [Google Scholar] [CrossRef]
- Brynskikh, A.M.; Zhao, Y.; Mosley, R.L.; Li, S.; Boska, M.D.; Klyachko, N.L.; Kabanov, A.V.; Gendelman, H.E.; Batrakova, E.V. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine 2010, 5, 379–396. [Google Scholar] [CrossRef]
- Correale, J.; Villa, A. The blood-brain-barrier in multiple sclerosis: Functional roles and therapeutic targeting. Autoimmunity 2007, 40, 148–160. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Q.; Long, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N. Research progress on macrophage membrane nano-biomimetic preparations on brain diseases and its inspiration on druggability of active components of traditional Chinese medicine. Chin. Tradit. Herb. Drugs 2020, 51, 4771–4779. [Google Scholar]
- Cui, Y.; Sun, J.; Hao, W.; Chen, M.; Wang, Y.; Xu, F.; Gao, C. Dual-Target Peptide-Modified Erythrocyte Membrane-Enveloped PLGA Nanoparticles for the Treatment of Glioma. Front. Oncol. 2020, 10, 563938. [Google Scholar] [CrossRef]
- Feng, L.; Dou, C.; Xia, Y.; Li, B.; Zhao, M.; Yu, P.; Zheng, Y.; El-Toni, A.M.; Atta, N.F.; Galal, A.; et al. Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery. ACS Nano 2021, 15, 2263–2280. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, C.L.; Pang, L.; Wang, Q.; Liu, J.X.; Wang, B.S.; Liang, J.M.; Guo, Y.Z.; Qin, J.; Wang, J.X. Direct Macromolecular Drug Delivery to Cerebral Ischemia Area using Neutrophil-Mediated Nanoparticles. Theranostics 2017, 7, 3260–3275. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/Chemodynamic Therapy of Orthotopic Glioma. ACS Nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef]
- Yin, T.; Fan, Q.; Hu, F.; Ma, X.; Yin, Y.; Wang, B.; Kuang, L.; Hu, X.; Xu, B.; Wang, Y. Engineered Macrophage-Membrane-Coated Nanoparticles with Enhanced PD-1 Expression Induce Immunomodulation for a Synergistic and Targeted Antiglioblastoma Activity. Nano Lett. 2022, 22, 6606–6614. [Google Scholar] [CrossRef]
- Föller, M.; Huber, S.M.; Lang, F. Erythrocyte programmed cell death. IUBMB Life 2008, 60, 661–668. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.; Wang, X.; Li, X.; Xu, Q.; Xin, H. Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef]
- Chai, Z.; Ran, D.; Lu, L.; Zhan, C.; Ruan, H.; Hu, X.; Xie, C.; Jiang, K.; Li, J.; Zhou, J.; et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano 2019, 13, 5591–5601. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Hu, X.; Wei, X.; Zhan, C.; Lu, L.; Jiang, K.; Su, B.; Ruan, H.; Ran, D.; Fang, R.H.; et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Control. Release 2017, 264, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, S.; Ding, F.; Liu, X.; Wu, Y.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. A Virus-Mimicking Nucleic Acid Nanogel Reprograms Microglia and Macrophages for Glioblastoma Therapy. Adv. Mater. 2021, 33, e2006116. [Google Scholar] [CrossRef]
- Sun, H.S.; Doucette, T.A.; Liu, Y.; Fang, Y.; Teves, L.; Aarts, M.; Ryan, C.L.; Bernard, P.B.; Lau, A.; Forder, J.P.; et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke 2008, 39, 2544–2553. [Google Scholar] [CrossRef]
- Ward, N.S. Restoring brain function after stroke—Bridging the gap between animals and humans. Nat. Rev. Neurol. 2017, 13, 244–255. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Chen, L.; Zhou, H.; Zhang, M.; Chang, L.; Shen, H.; Zhou, M.; Su, P.; Zhu, D. Targeted delivery of intranasally administered nanoparticles-mediated neuroprotective peptide NR2B9c to brain and neuron for treatment of ischemic stroke. Nanomedicine 2019, 18, 380–390. [Google Scholar] [CrossRef]
- Hong, H.Y.; Choi, J.S.; Kim, Y.J.; Lee, H.Y.; Kwak, W.; Yoo, J.; Lee, J.T.; Kwon, T.H.; Kim, I.S.; Han, H.S.; et al. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J. Control. Release 2008, 131, 167–172. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Lv, W.; Wang, Z.; Lv, L.; Wang, B.; Liu, X.; Liu, Y.; Hu, Q.; Sun, W.; et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Control. Release 2016, 233, 64–71. [Google Scholar] [CrossRef]
- Shen, K.; DeLano, F.A.; Zweifach, B.W.; Schmid-Schönbein, G.W. Circulating leukocyte counts, activation, and degranulation in Dahl hypertensive rats. Circ. Res. 1995, 76, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Garrood, T.; Lee, L.; Pitzalis, C. Molecular mechanisms of cell recruitment to inflammatory sites: General and tissue-specific pathways. Rheumatology 2006, 45, 250–260. [Google Scholar] [CrossRef]
- Zaremba, J.; Ilkowski, J.; Losy, J. Serial measurements of levels of the chemokines CCL2, CCL3 and CCL5 in serum of patients with acute ischaemic stroke. Folia Neuropathol. 2006, 44, 282–289. [Google Scholar]
- Shi, J.; Li, W.; Zhang, F.; Park, J.H.; An, H.; Guo, S.; Duan, Y.; Wu, D.; Hayakawa, K.; Lo, E.H.; et al. CCL2 (C-C Motif Chemokine Ligand 2) Biomarker Responses in Central Versus Peripheral Compartments After Focal Cerebral Ischemia. Stroke 2021, 52, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Afergan, E.; Epstein, H.; Dahan, R.; Koroukhov, N.; Rohekar, K.; Danenberg, H.D.; Golomb, G. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J. Control. Release 2008, 132, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.J.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J. Immunol. 2009, 183, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Haney, M.J.; Klyachko, N.L.; Li, S.; Booth, S.L.; Higginbotham, S.M.; Jones, J.; Zimmerman, M.C.; Mosley, R.L.; Kabanov, A.V.; et al. Polyelectrolyte complex optimization for macrophage delivery of redox enzyme nanoparticles. Nanomedicine 2011, 6, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Bardhan, R.; Stanton-Maxey, K.J.; Badve, S.; Nakshatri, H.; Stantz, K.M.; Cao, N.; Halas, N.J.; Clare, S.E. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012, 3, 47–54. [Google Scholar] [CrossRef]
- Han, Y.; Gao, C.; Wang, H.; Sun, J.; Liang, M.; Feng, Y.; Liu, Q.; Fu, S.; Cui, L.; Gao, C.; et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact. Mater. 2021, 6, 529–542, Erratum in Bioact. Mater. 2022, 7, 73. [Google Scholar] [CrossRef]
- Shen, L.M.; Li, M.C.; Wei, W.J.; Guan, X.; Liu, J. In Vitro Neuroprotective Effects of Macrophage Membrane-Derived Curcumin-Loaded Carriers against 1-Methyl-4-phenylpyridinium-Induced Neuronal Damage. ACS Omega 2021, 6, 32133–32141. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, S.; Cui, Y.; Liang, X.; Shan, J.; Gu, W.; Qiu, J.; Li, Y.; Wang, G. Functionalized nanoparticles with monocyte membranes and rapamycin achieve synergistic chemoimmunotherapy for reperfusion-induced injury in ischemic stroke. J. Nanobiotechnol. 2021, 19. [Google Scholar] [CrossRef]
- Perez-de-Puig, I.; Miró-Mur, F.; Ferrer-Ferrer, M.; Gelpi, E.; Pedragosa, J.; Justicia, C.; Urra, X.; Chamorro, A.; Planas, A.M. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015, 129, 239–257. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Harker, L.A.; Roskos, L.K.; Marzec, U.M.; Carter, R.A.; Cherry, J.K.; Sundell, B.; Cheung, E.N.; Terry, D.; Sheridan, W. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood 2000, 95, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Burger, P.C. Platelets in inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Chen, J.; Liu, Y.; Cheng, X.; Yang, F.; Gu, N. Platelet Membrane Biomimetic Magnetic Nanocarriers for Targeted Delivery and in Situ Generation of Nitric Oxide in Early Ischemic Stroke. ACS Nano 2020, 14, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Chen, J.; Liu, T.; Gu, Z.; Zhang, J.; Gu, X.; Teng, G.; Yang, F.; Gu, N. Platelet bio-nanobubbles as microvascular recanalization nanoformulation for acute ischemic stroke lesion theranostics. Theranostics 2018, 8, 4870–4883. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yin, H.; Cao, X.; Hu, Q.; Lv, W.; Xu, Q.; Gu, Z.; Xin, H. Sequentially Site-Specific Delivery of Thrombolytics and Neuroprotectant for Enhanced Treatment of Ischemic Stroke. ACS Nano 2019, 13, 8577–8588. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Sintnicolaas, K.; van Marwijk Kooij, M.; van Prooijen, H.C.; van Dijk, B.A.; van Putten, W.L.; Claas, F.H.; Novotny, V.M.; Brand, A. Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: A randomized prospective study. Blood 1995, 85, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Jiang, Y.; Qi, L.; Zhuge, D.; Xu, T.; Guo, Y.; Deng, M.; Zhang, W.; Tian, D.; et al. Targeted delivery of fat extract by platelet membrane-cloaked nanocarriers for the treatment of ischemic stroke. J. Nanobiotechnol. 2022, 20, 249. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, Y.; Deng, M.; Li, D.; Wang, X.; Zheng, H.; Xu, Y.; Li, W.; Zhang, W. Fat extract promotes angiogenesis in a murine model of limb ischemia: A novel cell-free therapeutic strategy. Stem Cell Res. Ther. 2018, 9, 294. [Google Scholar] [CrossRef]
- Becker, R.C.; Sexton, T.; Smyth, S.S. Translational Implications of Platelets as Vascular First Responders. Circ. Res. 2018, 122, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Jeong, J.M. Angiogenesis Imaging Using (68)Ga-RGD PET/CT: Therapeutic Implications. Semin. Nucl. Med. 2016, 46, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Gachet, C.; Hanau, D.; Spehner, D.; Brisson, C.; Garaud, J.C.; Schmitt, D.A.; Ohlmann, P.; Cazenave, J.P. Alpha IIb beta 3 integrin dissociation induced by EDTA results in morphological changes of the platelet surface-connected canalicular system with differential location of the two separate subunits. J. Cell Biol. 1993, 120, 1021–1030. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef]
- Fan, Y.; Hao, W.; Cui, Y.; Chen, M.; Chu, X.; Yang, Y.; Wang, Y.; Gao, C. Cancer Cell Membrane-Coated Nanosuspensions for Enhanced Chemotherapeutic Treatment of Glioma. Molecules 2021, 26, 5103. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Hao, W.; Fan, Y.; Zhang, J.; Liu, Q.; Jiang, M.; Yang, Y.; Wang, Y.; Gao, C. Ligand-modified homologous targeted cancer cell membrane biomimetic nanostructured lipid carriers for glioma therapy. Drug Deliv. 2021, 28, 2241–2255. [Google Scholar] [CrossRef]
- Kumar, P.; Treuren, T.V.; Ranjan, A.P.; Chaudhary, P.; Vishwanatha, J.K. In vivo imaging and biodistribution of near infrared dye loaded brain-metastatic-breast-cancer-cell-membrane coated polymeric nanoparticles. Nanotechnology 2019, 30, 265101. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, H.; Xu, J.; Shen, C. CD47: The next checkpoint target for cancer immunotherapy. Crit. Rev. Oncol. Hematol. 2020, 152, 103014. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Glinsky, G.V.; Glinskii, O.V.; Huxley, V.H.; Turk, J.R.; Mossine, V.V.; Deutscher, S.L.; Pienta, K.J.; Quinn, T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003, 63, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, J.; Liu, X.; Wu, Q.; Shen, W.; Gao, Y.; Liu, Y.; Wu, C. Preparation of C6 cell membrane-coated doxorubicin conjugated manganese dioxide nanoparticles and its targeted therapy application in glioma. Eur. J. Pharm. Sci. 2022, 180, 106338. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Chen, X.; Li, M.; Liu, W.; Zhao, W.; Lim, L.Y.; Tilley, R.D.; Gooding, J.J.; Li, Q. Upconversion Nanoparticle-Based Cell Membrane-Coated cRGD Peptide Bioorthogonally Labeled Nanoplatform for Glioblastoma Treatment. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef]
- Tapeinos, C.; Tomatis, F.; Battaglini, M.; Larrañaga, A.; Marino, A.; Telleria, I.A.; Angelakeris, M.; Debellis, D.; Drago, F.; Brero, F.; et al. Cell Membrane-Coated Magnetic Nanocubes with a Homotypic Targeting Ability Increase Intracellular Temperature due to ROS Scavenging and Act as a Versatile Theranostic System for Glioblastoma Multiforme. Adv. Healthc. Mater. 2019, 8, e1900612. [Google Scholar] [CrossRef]
- De Pasquale, D.; Marino, A.; Tapeinos, C.; Pucci, C.; Rocchiccioli, S.; Michelucci, E.; Finamore, F.; McDonnell, L.; Scarpellini, A.; Lauciello, S.; et al. Homotypic targeting and drug delivery in glioblastoma cells through cell membrane-coated boron nitride nanotubes. Mater. Des. 2020, 192, 108742. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Chi, S.; Zhu, M.; Wang, C.; Liu, Z. Brain Tumor Cell Membrane-Coated Lanthanide-Doped Nanoparticles for NIR-IIb Luminescence Imaging and Surgical Navigation of Glioma. Adv. Healthc. Mater. 2022, 11, e2200521. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, e1902011. [Google Scholar] [CrossRef]
- Caffes, N.; Kurland, D.B.; Gerzanich, V.; Simard, J.M. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int. J. Mol. Sci. 2015, 16, 4973–4984. [Google Scholar] [CrossRef]
- Deng, G.; Ma, C.; Zhao, H.; Zhang, S.; Liu, J.; Liu, F.; Chen, Z.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [CrossRef]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, J.; Wang, W.; Yang, Z.; Hu, Z.; Hu, M.; Ding, P. The effect of stromal cell-derived factor 1 in the migration of neural stem cells. Cell Biochem. Biophys. 2014, 70, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lin, J.; Chew, S.Y. Neural Cell Membrane-Coated Nanoparticles for Targeted and Enhanced Uptake by Central Nervous System Cells. ACS Appl. Mater. Interfaces 2021, 13, 55840–55850. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Wang, K.; Yang, C.; Luo, Y.; Zhang, Y.; Cao, B.; Kang, Y.; Wang, M. Highly fluorescent and bioresorbable polymeric nanoparticles with enhanced photostability for cell imaging. Nanoscale 2015, 7, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Lee-Liu, D.; Edwards-Faret, G.; Tapia, V.S.; Larrain, J. Spinal cord regeneration: Lessons for mammals from non-mammalian vertebrates. Genesis 2013, 51, 529–544. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.B.; Subileau, E.A.; Perriere, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Gong, J.; Wang, J.; Fan, Y.; Cai, J.; Wang, Y.; Qiu, Y.; Wei, Y.; Xiong, C.; et al. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat. Commun. 2020, 11, 5196. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zang, G.; Liu, B.; Qin, X.; Zhang, Y.; Chen, Y.; Zhang, H.; Wu, W.; Wang, G. Bioengineering CXCR4-overexpressing cell membrane functionalized ROS-responsive nanotherapeutics for targeting cerebral ischemia-reperfusion injury. Theranostics 2021, 11, 8043–8056. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, P.; Liao, F.; Yu, L.; Gan, B. Cell membrane-coated biomimetic magnetic nanoparticles for the bio-specific extraction of components from Gualou Guizhi decoction exhibiting activities against oxygen-glucose deprivation/reperfusion injury. J. Pharm. Biomed. Anal. 2022, 209, 114528. [Google Scholar] [CrossRef]
- Wei, W.; Cheng, W.; Dai, W.; Lu, F.; Cheng, Y.; Jiang, T.; Ren, Z.; Xie, Y.; Xu, J.; Zhao, Q.; et al. A Nanodrug Coated with Membrane from Brain Microvascular Endothelial Cells Protects against Experimental Cerebral Malaria. Nano Lett. 2022, 22, 211–219. [Google Scholar] [CrossRef]
- Lennartz, F.; Adams, Y.; Bengtsson, A.; Olsen, R.W.; Turner, L.; Ndam, N.T.; Ecklu-Mensah, G.; Moussiliou, A.; Ofori, M.F.; Gamain, B.; et al. Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria. Cell Host. Microbe 2017, 21, 403–414. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef]
- Hao, W.; Cui, Y.; Fan, Y.; Chen, M.; Yang, G.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Yang, Y.; et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J. Nanobiotechnol. 2021, 19, 378. [Google Scholar] [CrossRef]
- Wu, L.; Li, Q.; Deng, J.; Shen, J.; Xu, W.; Yang, W.; Chen, B.; Du, Y.; Zhang, W.; Ge, F.; et al. Platelet-Tumor Cell Hybrid Membrane-Camouflaged Nanoparticles for Enhancing Therapy Efficacy in Glioma. Int. J. Nanomed. 2021, 16, 8433–8446. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, W.; Ma, X.; Tang, L.; Zhang, Y.; Yang, M.; Hu, F.; Li, G.; Wang, Y. Biomimetic neutrophil and macrophage dual membrane-coated nanoplatform with orchestrated tumor-microenvironment responsive capability promotes therapeutic efficacy against glioma. Chem. Eng. J. 2022, 433. [Google Scholar] [CrossRef]
- Shi, W.; Cao, X.; Liu, Q.; Zhu, Q.; Liu, K.; Deng, T.; Yu, Q.; Deng, W.; Yu, J.; Wang, Q.; et al. Hybrid Membrane-Derived Nanoparticles for Isoliquiritin Enhanced Glioma Therapy. Pharmaceuticals 2022, 15, 1059. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Yu, X.; Gong, C.; Zhu, H.; Zhang, B.; Wang, R.; Yuan, Y. Erythrocyte-cancer Hybrid Membrane-camouflaged Mesoporous Silica Nanoparticles Loaded with Gboxin for Glioma-targeting Therapy. Curr. Pharm. Biotechnol. 2022, 23, 835–846. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Loza, M.I.; Mirelman, D.; Brea, J.; Blanco, M.; Sobrino, T.; Campos, F. A novel mechanism of neuroprotection: Blood glutamate grabber. J. Cereb. Blood Flow Metab. 2016, 36, 292–301. [Google Scholar] [CrossRef]
- Lewén, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. J. Neurotrauma 2000, 17, 871–890. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef]
- Ellison, D.W. Multiple Molecular Data Sets and the Classification of Adult Diffuse Gliomas. N. Engl. J. Med. 2015, 372, 2555–2557. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H.; et al. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, M.; Xi, Z.; Nie, D.; Dai, Z.; Wang, J.; Qian, K.; Weng, H.; Gan, Y.; Xu, L. Cancer Cell Membrane-Camouflaged Nanorods with Endoplasmic Reticulum Targeting for Improved Antitumor Therapy. ACS. Appl Mater. Interfaces 2019, 11, 46614–46625. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Blum, N.T.; Lin, J.; Huang, P. Biomimetic hybrid membrane-based nanoplatforms: Synthesis, properties and biomedical applications. Nanoscale Horiz. 2020, 5, 1293–1302. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 14670–14675. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Selfridge, J.E.; Lezi, E.; Lu, J.; Swerdlow, R.H. Role of mitochondrial homeostasis and dynamics in Alzheimer’s disease. Neurobiol. Dis. 2013, 51, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Keil, U.; Hauptmann, S.; Bonert, A.; Scherping, I.; Eckert, A.; Müller, W.E. Mitochondrial dysfunction induced by disease relevant AbetaPP and tau protein mutations. J. Alzheimers Dis. 2006, 9, 139–146. [Google Scholar] [CrossRef]
- Seydel, K.B.; Kampondeni, S.D.; Valim, C.; Potchen, M.J.; Milner, D.A.; Muwalo, F.W.; Birbeck, G.L.; Bradley, W.G.; Fox, L.L.; Glover, S.J.; et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 2015, 372, 1126–1137. [Google Scholar] [CrossRef]
- Nishanth, G.; Schlüter, D. Blood-Brain Barrier in Cerebral Malaria: Pathogenesis and Therapeutic Intervention. Trends Parasitol. 2019, 35, 516–528. [Google Scholar] [CrossRef]
- Coban, C.; Lee, M.S.J.; Ishii, K.J. Tissue-specific immunopathology during malaria infection. Nat. Rev. Immunol. 2018, 18, 266–278. [Google Scholar] [CrossRef]
- Chen, Y.; Mi, Y.; Zhang, X.; Ma, Q.; Song, Y.; Zhang, L.; Wang, D.; Xing, J.; Hou, B.; Li, H.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef]
- Deng, J.; Wu, Z.; Zhao, Z.; Wu, C.; Yuan, M.; Su, Z.; Wang, Y.; Wang, Z. Berberine-Loaded Nanostructured Lipid Carriers Enhance the Treatment of Ulcerative Colitis. Int. J. Nanomed. 2020, 15, 3937–3951. [Google Scholar] [CrossRef]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Maysinger, D.; Behrendt, M.; Lalancette-Hébert, M.; Kriz, J. Real-time imaging of astrocyte response to quantum dots: In vivo screening model system for biocompatibility of nanoparticles. Nano Lett. 2007, 7, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Barandeh, F.; Nguyen, P.L.; Kumar, R.; Iacobucci, G.J.; Kuznicki, M.L.; Kosterman, A.; Bergey, E.J.; Prasad, P.N.; Gunawardena, S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS ONE 2012, 7, e29424. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Nunes, A.; Bussy, C.; Gherardini, L.; Meneghetti, M.; Herrero, M.A.; Bianco, A.; Prato, M.; Pizzorusso, T.; Al-Jamal, K.T.; Kostarelos, K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine 2012, 7, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.H.; Bae, S.M.; Park, R.W.; Kang, Y.M.; Kim, I.S.; Kwon, I.C.; Choi, K.; Jeong, S.Y.; et al. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
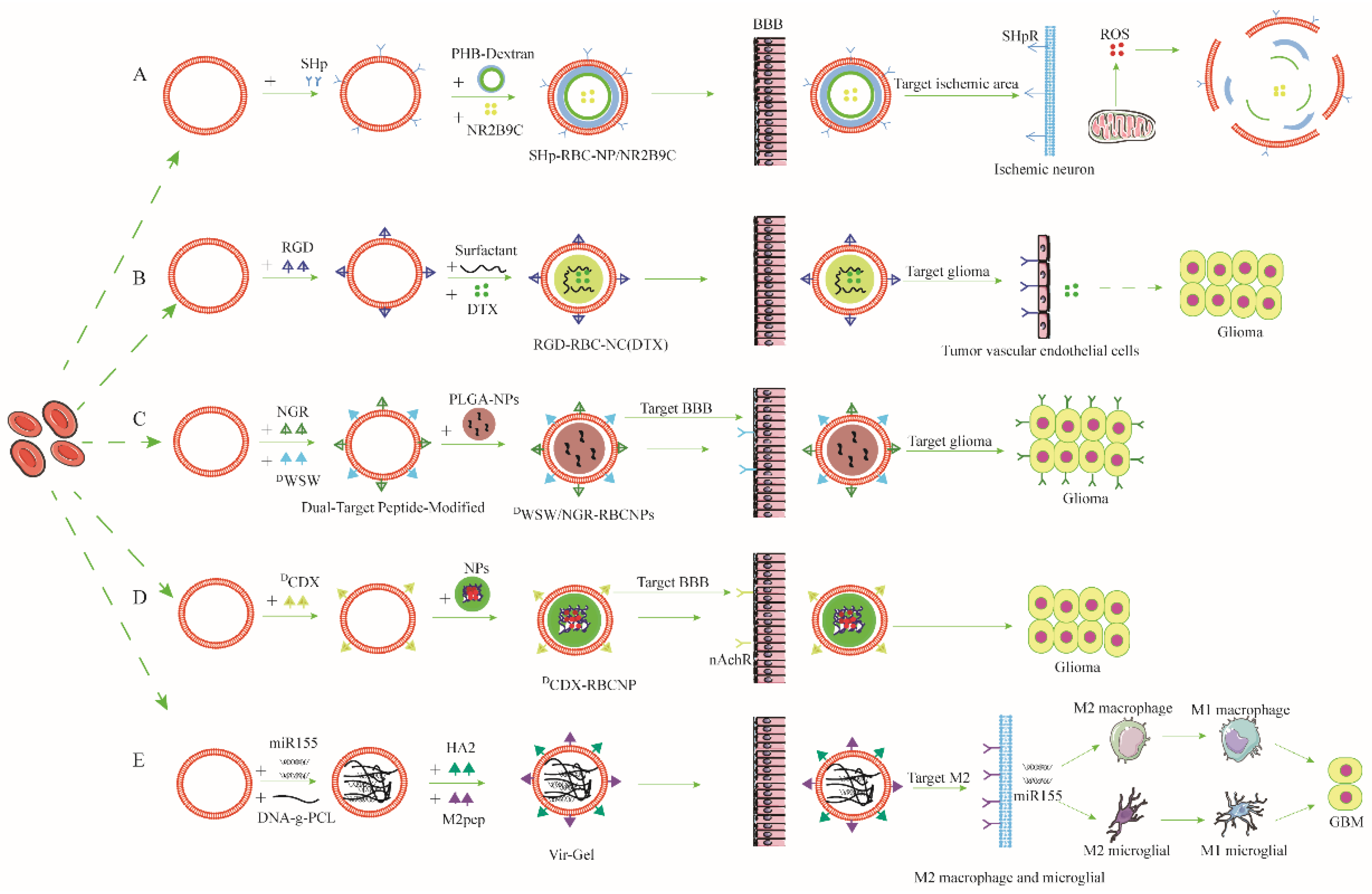
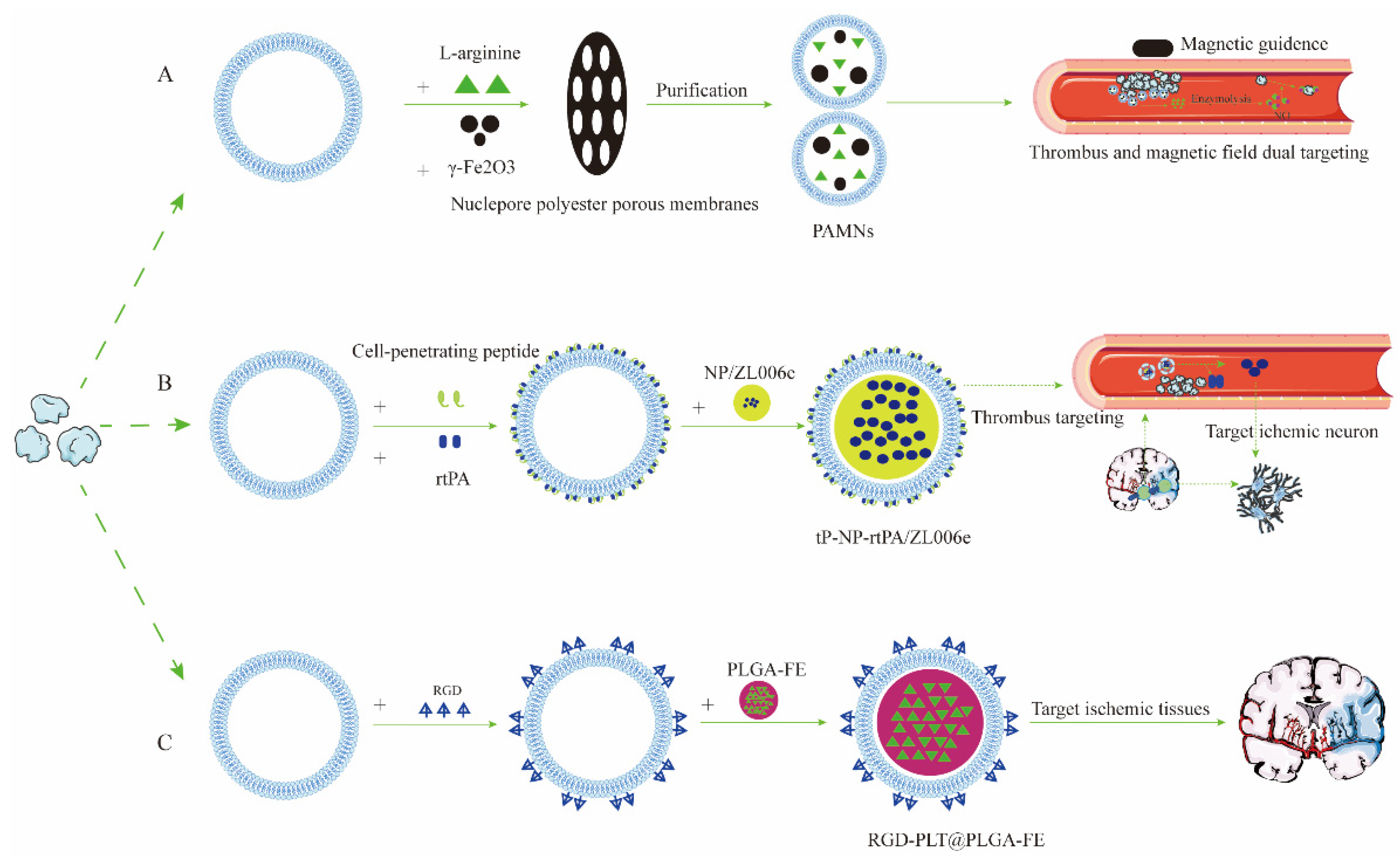

| Drug Targeted Delivery System | Cell Membrane Source | Targeted Modification | Materials of NPs | Drug-Loading | Mechanism of Crossing the BBB | NDs Model | Reference |
|---|---|---|---|---|---|---|---|
| cl PGP-PEG-DGL/CAT-Aco system | Endogenous neutrophils | PGP/CXCR2 | ACO NPs | Catalase (CAT) | Neutrophil-mediated transport through the BBB | MCAO | [34] |
| Neutrophil membrane-derived nanovesicles | Neutrophils | No | - | RvD2 | Integrin β2 and PSGL-1 of neutrophils binding to inflamed endothelium | I/R | [35] |
| MPBzyme@NCM | Neutrophils | No | PVP and Fe[(CN6)]3- | MPBzyme | Integrin β2 and Mac-1 of neutrophils targeting to ICAM-1 of inflamed brain microvascular endothelial cells | IS | [33] |
| Monocytes loaded with liposomes | Monocytes | No | Liposomes | Serotonin | Monocyte-mediated transport through the BBB | No | [53] |
| IDV-NP-BMM | Bone marrow macrophages | No | IDV-NP suspensions | Indinavir (IDV) | Macrophage-mediated transport through the BBB | HIV-1 encephalitis | [54] |
| BMM-PEI-PEG/cat | Bone marrow macrophages | No | PEI-PEG | CAT | BMM adhesion to cerebral vascular endothelial cells | PD | [55] |
| BMM loaded with nanozyme | Bone marrow macrophages | No | PEI-PEG | CAT | BMM adhesion to cerebral vascular endothelial cells via integrin α4 | PD | [29] |
| MMs loaded with therapeutic NPs | MMs | No | Gold nano-shells and silica particles | No | MMs-mediated transport through the BBB | Brain metastases of breast cancer | [56] |
| Macrophages loaded with nanozyme | Macrophages | No | PEI-PEG | Catalase | Macrophage-mediated transport through the BBB | Neuroinflammation/PD | [27] |
| Nanoparticles loaded NPs | Monocytes | No | Magnetite- laden NPs | No | Monocyte-mediated transport through the BBB | Epileptic | [28] |
| RVG/TPP-MASLNs | Macrophages | RVG29 and TPP | Solid lipid nanoparticles (SLNs) | Genistein (GS) | RVG29 peptide for crossing the BBB | AD | [57] |
| NVs-CUR | Macrophages | No | No | Curcumin (CUR) | Not mentioned | PD | [58] |
| McM/RNPs | Monocytes | No | PLGA-NPs | Rapamycin (RAPA) | Integrin α4 and integrin β1 of monocytes targeting to inflammatory endothelial cells | MCAO | [59] |
| MPM@P-NGS | Macrophages | No | MnO_2@PVCL NGS | Cisplatin | Cell-carrier-mediated BBB traversing based on endogenous immunocytes | Orthotopic glioma | [36] |
| PD-1-MM@PLGA/RAPA | Macrophages | PD-1 | PLGA-NPs | Rapamycin | Macrophage-mediated transport through the BBB | GBM | [37] |
| Drug Targeted Delivery System | Cancer Cell Membrane | Targeted Modification | Materials of NPs | Drug-Loading | Cancer Model | Reference |
|---|---|---|---|---|---|---|
| CCNP | MDA-MB-831cells | No | mPEG-PLGA-NPs | Doxorubicin (DOX) | Brain and metastatic breast cancer | [82] |
| CM-NCubes | U-251 MG cells | No | Fe3O4/MnO2- NCubes | Sorafenib and AMF | GBM | [88] |
| Dox-CM-BNNTs | U87 MG cells | No | BNNTs | DOX | GBM | [89] |
| DHA-NGR/CCNLC | C6 glioma cells | NGR | NLCs | DHA | Glioma | [81] |
| WSW-CCM-(PTX)NS | C6 glioma cells | DWSW | PVP K30 and SDC nanosuspension | Paclitaxel (PTX) | Glioma | [87] |
| HCPT-NS/CCM | C6 glioma cells | No | Nanosuspension | 10-hydroxycamptothecin (HCPT) | Glioma | [80] |
| MnO2-DOX-C6 | C6 glioma cells | No | MnO2 | Doxorubicin (DOX) | Glioma | [85] |
| HDX@YSN@CCM@cRGD | U87 MG cells | cRGD | YSNs | Hydroxychloroquine (HDX) | Glioblastoma | [86] |
| CC-LnNPs | U87 MG cells | No | LnNPs | No | Glioma | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wei, Y.; Zhang, C.; Bi, R.; Qiu, Y.; Li, Y.; Hu, B. Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics 2023, 15, 621. https://doi.org/10.3390/pharmaceutics15020621
Li J, Wei Y, Zhang C, Bi R, Qiu Y, Li Y, Hu B. Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics. 2023; 15(2):621. https://doi.org/10.3390/pharmaceutics15020621
Chicago/Turabian StyleLi, Jianzhuang, Yanhao Wei, Chunlin Zhang, Rentang Bi, Yanmei Qiu, Yanan Li, and Bo Hu. 2023. "Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases" Pharmaceutics 15, no. 2: 621. https://doi.org/10.3390/pharmaceutics15020621
APA StyleLi, J., Wei, Y., Zhang, C., Bi, R., Qiu, Y., Li, Y., & Hu, B. (2023). Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics, 15(2), 621. https://doi.org/10.3390/pharmaceutics15020621





