The Regioselective Conjugation of the 15-nt Thrombin Aptamer with an Optimized Tripeptide Sequence Greatly Increases the Anticoagulant Activity of the Aptamer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Automated Oligonucleotide Synthesis and Conjugation
2.3. MALDI Mass Spectrometry
2.4. Ultraviolet Thermal Denaturation
2.5. Circular Dichroism Spectroscopy
2.6. Fibrinogen Clotting in the Presence of Aptamers
2.7. Molecular Dynamics Simulations of TBA–Peptide Complexes with Thrombin
3. Results and Discussion
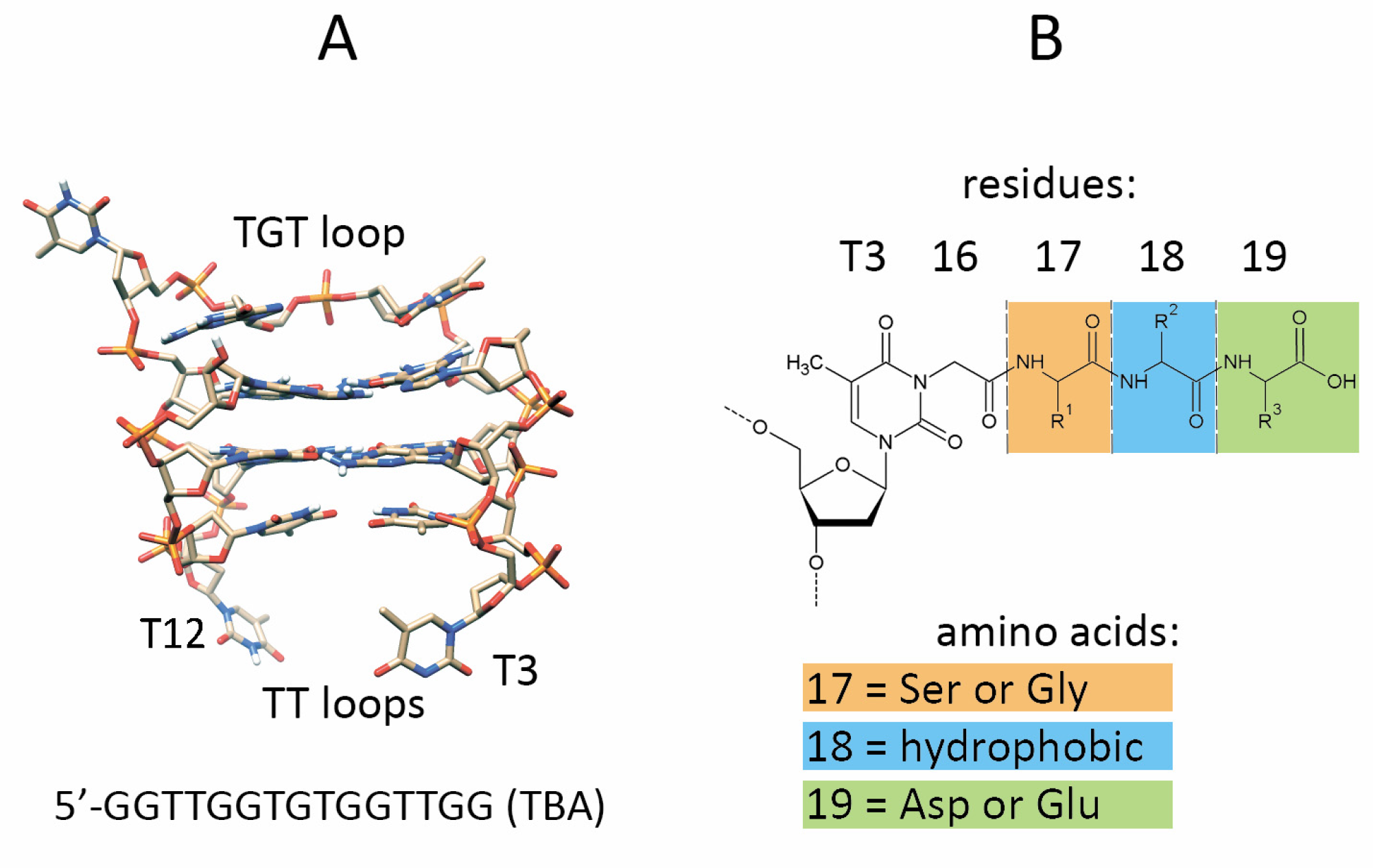
3.1. Design and Synthesis of TBA–Peptide Conjugates
3.2. Biophysical Characteristics of TBA–Peptide Conjugates
3.3. Anticoagulant Properties of TBA–Peptide Conjugates
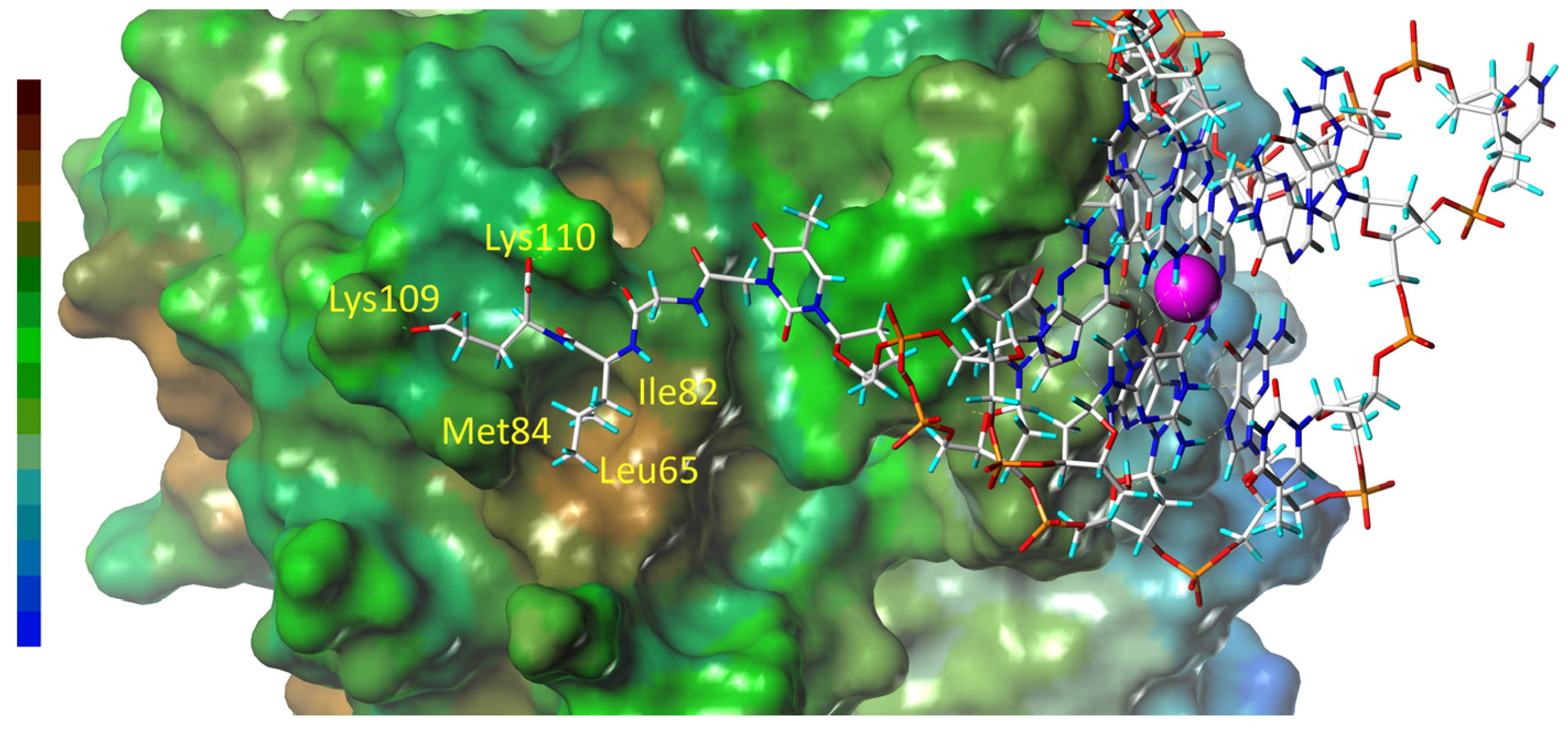
3.4. Molecular Dynamics of the Thrombin Complexes with GLE, SLE, SYE, and SYD Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sinhari, A.; Jain, P.; Jadhav, H.R. A perspective on oligonucleotide therapy: Approaches to patient customization. Front. Pharmacol. 2022, 13, 1006304. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef]
- Liu, M.; Zaman, K.; Fortenberry, Y.M. Overview of the Therapeutic Potential of Aptamers Targeting Coagulation Factors. Int. J. Mol. Sci. 2021, 22, 3897. [Google Scholar] [CrossRef]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug. Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef]
- Craig, K.; Abrams, M.; Amiji, M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin. Drug Deliv. 2018, 15, 629–640. [Google Scholar] [CrossRef]
- Fàbrega, C.; Aviñó, A.; Eritja, R. Chemical Modifications in Nucleic Acids for Therapeutic and Diagnostic Applications. Chem. Rec. 2022, 22, e202100270. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Qu, Y.X.; Su, Y.; Peng, Y.; Zhao, Z.; Fu, T.; Wang, X.Q.; Tan, W. Aptamer-Peptide Conjugates as Targeted Chemosensitizers for Breast Cancer Treatment. ACS Appl. Mater. Interfaces 2021, 13, 9436–9444. [Google Scholar] [CrossRef]
- Klabenkova, K.; Fokina, A.; Stetsenko, D. Chemistry of Peptide-Oligonucleotide Conjugates: A Review. Molecules 2021, 26, 5420. [Google Scholar] [CrossRef]
- Aviñó, A.; Jorge, A.F.; Huertas, C.S.; Cova, T.F.G.G.; Pais, A.; Lechuga, L.M.; Eritja, R.; Fabrega, C. Aptamer-peptide conjugates as a new strategy to modulate human α-thrombin binding affinity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1619–1630. [Google Scholar] [CrossRef]
- Tsvetkov, V.B.; Varizhuk, I.V.; Kurochkin, N.N.; Surzhikov, S.A.; Smirnov, I.P.; Stomakhin, A.A.; Kolganova, N.A.; Timofeev, E.N. Anticoagulant Oligonucleotide-Peptide Conjugates: Identification of Thrombin Aptamer Conjugates with Improved Characteristics. Int. J. Mol. Sci. 2022, 23, 3820. [Google Scholar] [CrossRef]
- Zeskind, B.J.; Jordan, C.D.; Timp, W.; Trapani, L.; Waller, G.; Horodincu, V.; Ehrlich, D.J.; Matsudaira, P. Nucleic acid and protein mass mapping by live-cell deep-ultraviolet microscopy. Nat. Methods 2007, 4, 567–569. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. AMBER 2020; University of California: San Francisco, CA, USA, 2020. [Google Scholar]
- Izadi, S.; Onufriev, A.V. Accuracy limit of rigid 3-point water models. J. Chem. Phys. 2016, 145, 074501. [Google Scholar] [CrossRef]
- Zgarbová, M.; Luque, F.J.; Sponer, J.; Cheatham, T.E., III; Otyepka, M.; Jurecka, P. Toward Improved Description of DNA Backbone: Revisiting Epsilon and Zeta Torsion Force Field Parameters. J. Chem. Theory Comput. 2013, 9, 2339–2354. [Google Scholar] [CrossRef]
- Zgarbová, M.; Sponer, J.; Otyepka, M.; Cheatham, T.E., III; Galindo-Murillo, R.; Jurecka, P. Refinement of the Sugar-Phosphate Backbone Torsion Beta for AMBER Force Fields Improves the Description of Z- and B-DNA. J. Chem. Theory Comput. 2015, 11, 5723–5736. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef]
- De Cristofaro, R.; Di Cera, E. Phenomenological analysis of the clotting curve. J. Protein Chem. 1991, 10, 455–468. [Google Scholar] [CrossRef]
- Waters, E.; Richardson, J.; Schaub, R.; Kurz, J. Effect of NU172 and bivalirudin on ecarin clotting time in human plasma and whole blood. J. Thromb. Haemost 2009, 7, 683. [Google Scholar]
- Mayer, G.; Rohrbach, F.; Pötzsch, B.; Müller, J. Aptamer-based modulation of blood coagulation. Hamostaseologie 2011, 31, 258–263. [Google Scholar] [PubMed]
- Troisi, R.; Napolitano, V.; Spiridonova, V.; Russo Krauss, I.; Sica, F. Several structural motifs cooperate in determining the highly effective anti-thrombin activity of NU172 aptamer. Nucleic Acids Res. 2018, 46, 12177–12185. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, I.; Kolganova, N.; Troisi, R.; Sica, F.; Timofeev, E. Expanding the recognition interface of the thrombin-binding aptamer HD1 through modification of residues T3 and T12. Mol. Ther. Nucleic Acids 2021, 23, 863–871. [Google Scholar] [CrossRef] [PubMed]

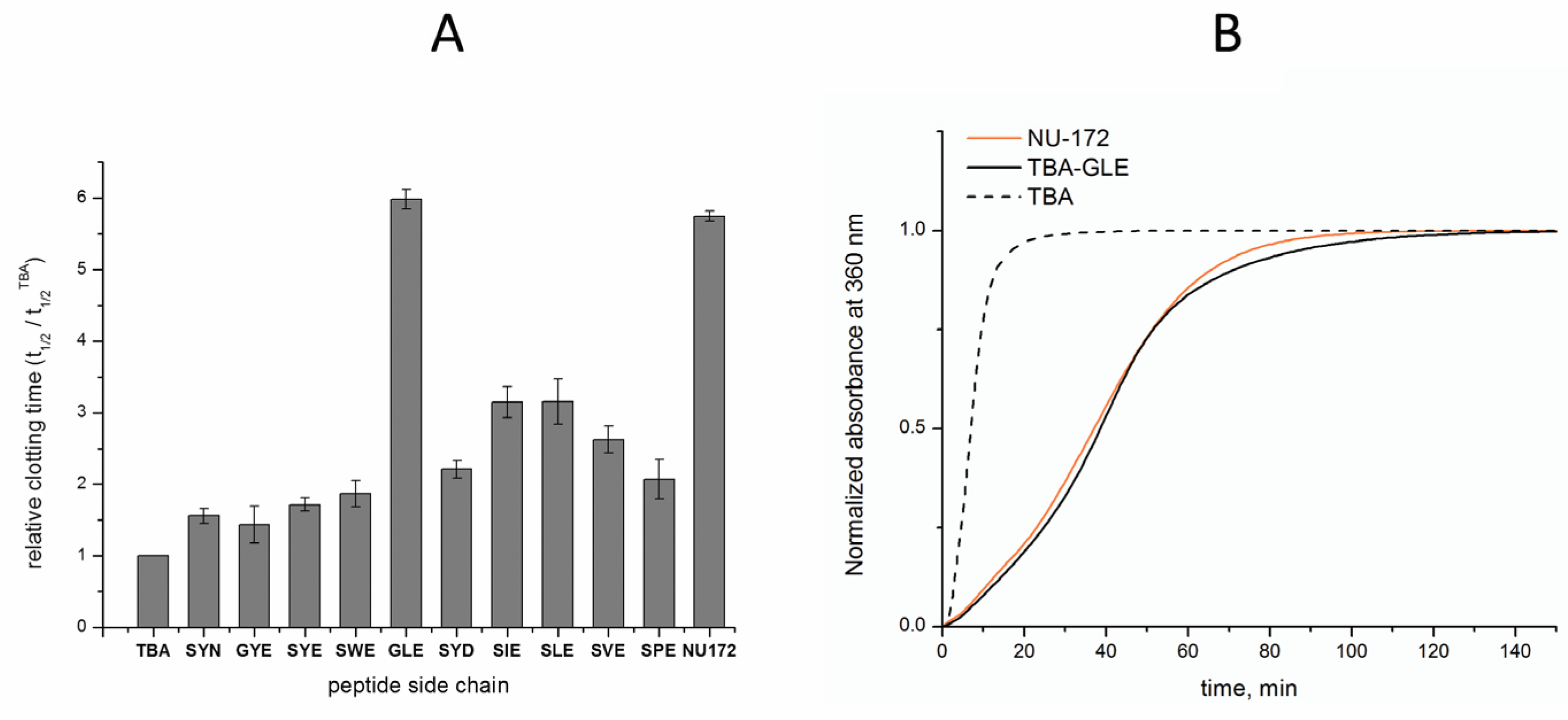

| TBA Conjugate | Mcalc/Mobsvd | Tm, °C 1 | t1/2/t1/2TBA 2 |
|---|---|---|---|
| SYN | 5148.5/5151.5 | 50.9 | 1.56 ± 0.11 |
| GYE | 5133.5/5136.6 | 50.9 | 1.44 ± 0.26 |
| SYE | 5163.5/5171.3 | 50.1 | 1.72 ± 0.09 |
| SWE | 5186.6/5187.4 | 51.3 | 1.87 ± 0.19 |
| GLE | 5083.5/5084.3 | 50.8 | 5.98 ± 0.14 |
| SYD | 5149.5/5138.7 | 49.8 | 2.21 ± 0.12 |
| SIE | 5113.5/5116.5 | 50.7 | 3.15 ± 0.22 |
| SLE | 5113.5/5107.2 | 50.0 | 3.16 ± 0.32 |
| SVE | 5099.5/5098.2 | 50.3 | 2.63 ± 0.19 |
| SPE | 5097.5/5090.4 | 49.9 | 2.08 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varizhuk, I.V.; Tsvetkov, V.B.; Toropygin, I.Y.; Stomakhin, A.A.; Kolganova, N.A.; Surzhikov, S.A.; Timofeev, E.N. The Regioselective Conjugation of the 15-nt Thrombin Aptamer with an Optimized Tripeptide Sequence Greatly Increases the Anticoagulant Activity of the Aptamer. Pharmaceutics 2023, 15, 604. https://doi.org/10.3390/pharmaceutics15020604
Varizhuk IV, Tsvetkov VB, Toropygin IY, Stomakhin AA, Kolganova NA, Surzhikov SA, Timofeev EN. The Regioselective Conjugation of the 15-nt Thrombin Aptamer with an Optimized Tripeptide Sequence Greatly Increases the Anticoagulant Activity of the Aptamer. Pharmaceutics. 2023; 15(2):604. https://doi.org/10.3390/pharmaceutics15020604
Chicago/Turabian StyleVarizhuk, Irina V., Vladimir B. Tsvetkov, Ilya Yu. Toropygin, Andrey A. Stomakhin, Natalia A. Kolganova, Sergei A. Surzhikov, and Edward N. Timofeev. 2023. "The Regioselective Conjugation of the 15-nt Thrombin Aptamer with an Optimized Tripeptide Sequence Greatly Increases the Anticoagulant Activity of the Aptamer" Pharmaceutics 15, no. 2: 604. https://doi.org/10.3390/pharmaceutics15020604
APA StyleVarizhuk, I. V., Tsvetkov, V. B., Toropygin, I. Y., Stomakhin, A. A., Kolganova, N. A., Surzhikov, S. A., & Timofeev, E. N. (2023). The Regioselective Conjugation of the 15-nt Thrombin Aptamer with an Optimized Tripeptide Sequence Greatly Increases the Anticoagulant Activity of the Aptamer. Pharmaceutics, 15(2), 604. https://doi.org/10.3390/pharmaceutics15020604







