Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of tSD/CsA
2.3. Physicochemical Characterization
2.4. Storage Stability Studies
2.5. Mucoadhesive Property
2.6. Determination of CsA Amount
2.7. Pharmacokinetic Study in Rats
2.8. Statistical Analysis
3. Results and Discussion
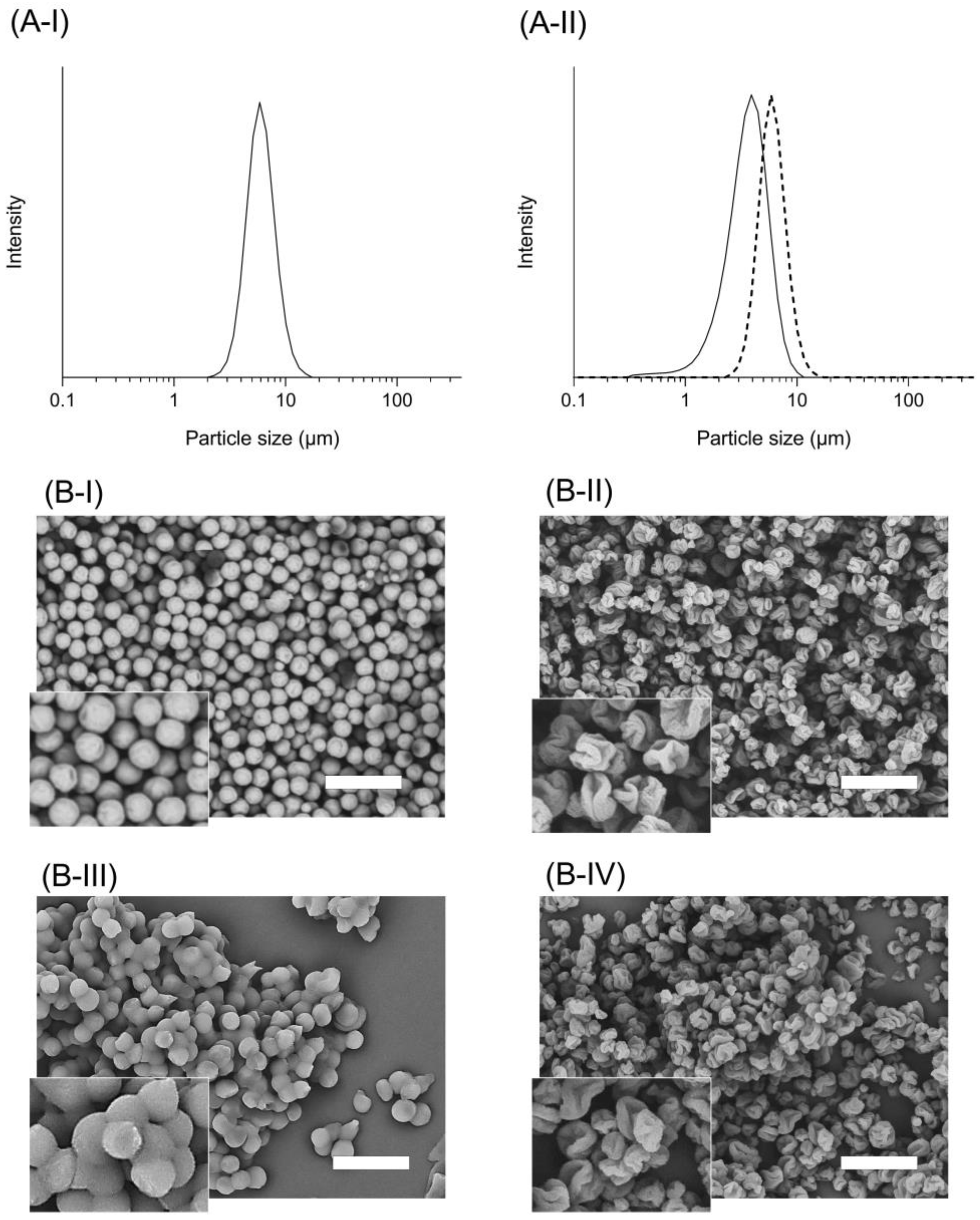
3.1. Appearance and Particle Size Distribution of tSD/CsA
3.2. Storage Stability of tSD/CsA
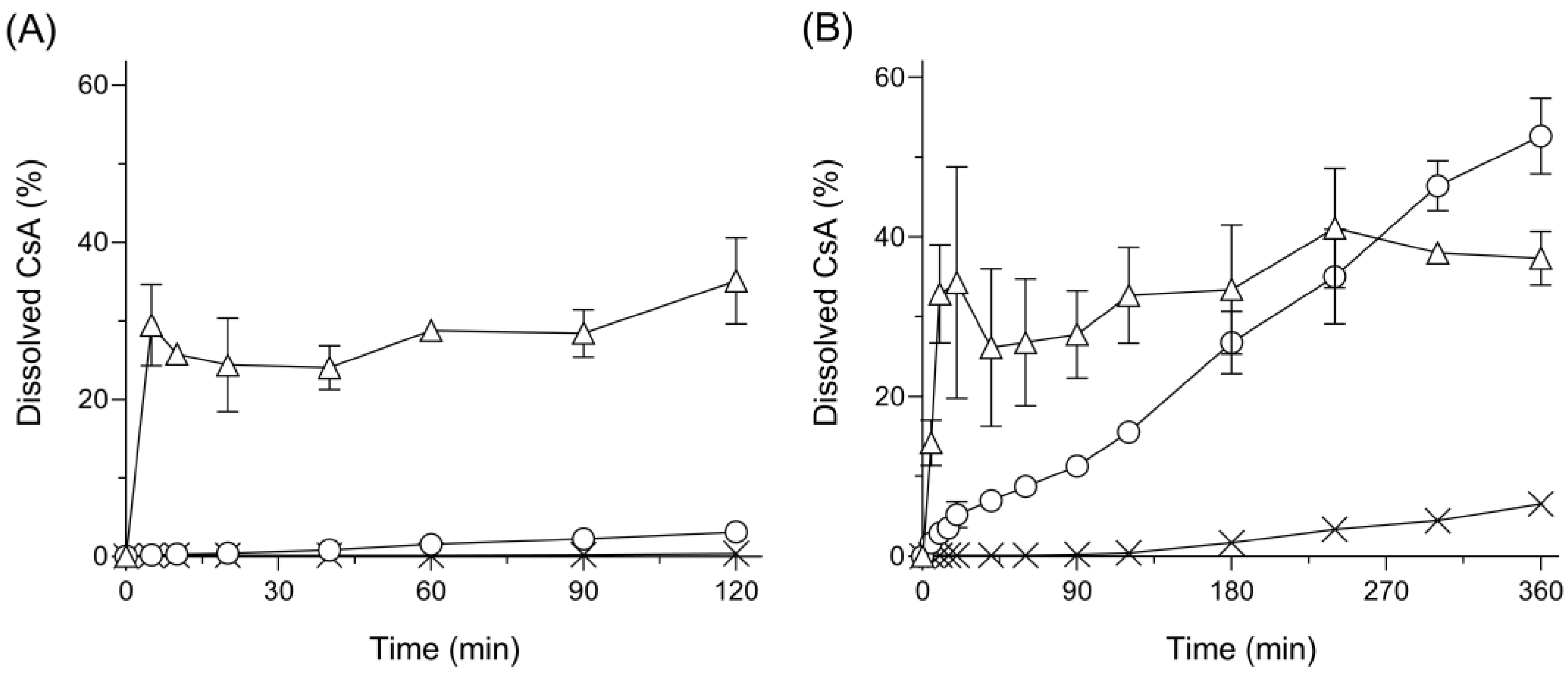
3.3. Dissolution Behavior
3.4. Mucoadhesive Properties
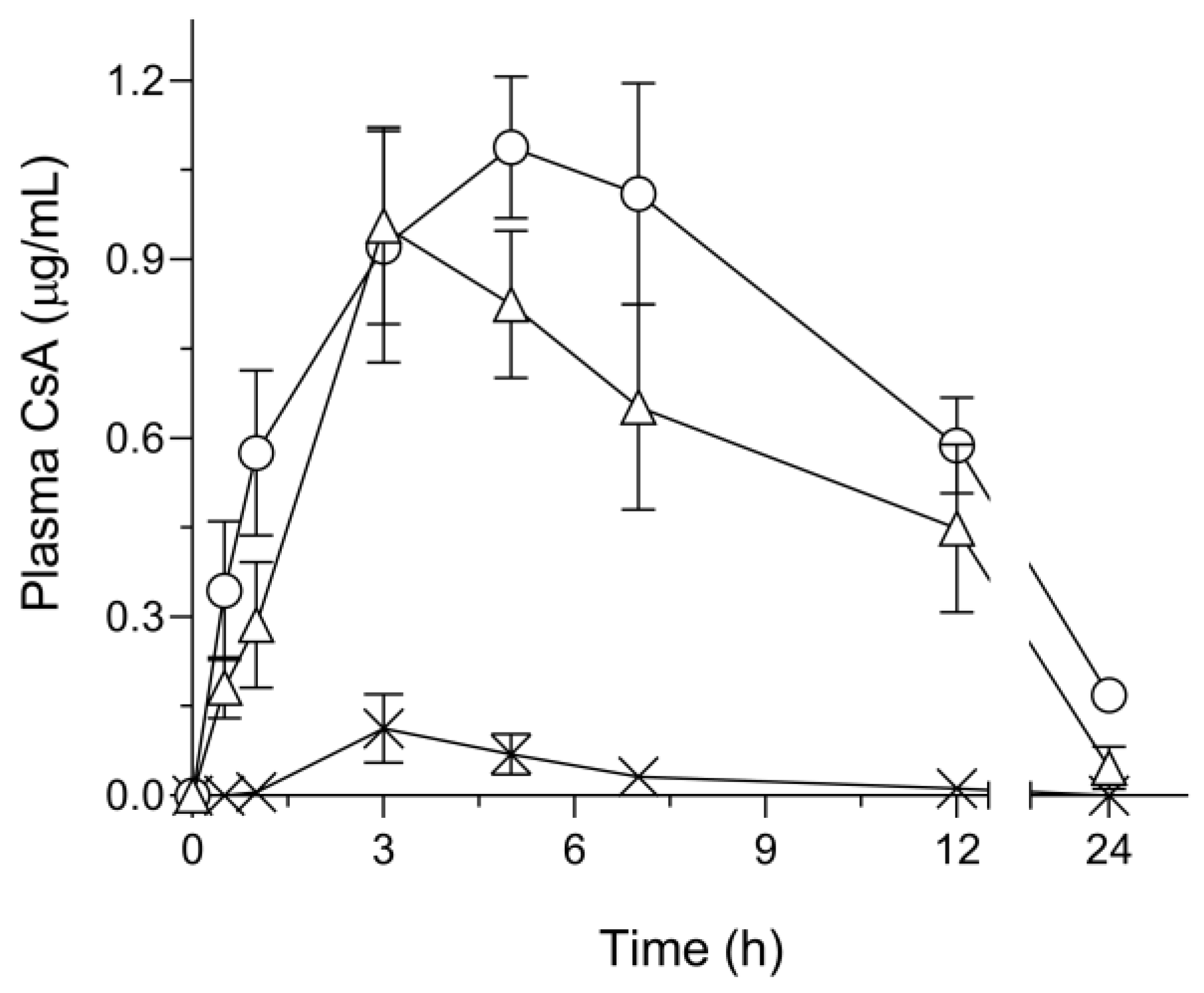
3.5. Pharmacokinetic Profiles after Oral Administration in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anwar-Fadzil, A.F.B.; Yuan, Y.; Wang, L.; Kochhar, J.S.; Kachouie, N.N.; Kang, L. Recent progress in three-dimensionally-printed dosage forms from a pharmacist perspective. J. Pharm. Pharmacol. 2022, 74, 1367–1390. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Moritani, T.; Morinaga, T.; Seto, Y.; Sato, H.; Onoue, S. Amorphous solid dispersion of cyclosporine A prepared with fine droplet drying process: Physicochemical and pharmacokinetic characterization. Int. J. Pharm. 2017, 519, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tabata, A.; Moritani, T.; Morinaga, T.; Mizumoto, T.; Seto, Y.; Onoue, S. Design and Characterizations of Inhalable Poly(lactic-co-glycolic acid) Microspheres Prepared by the Fine Droplet Drying Process for a Sustained Effect of Salmon Calcitonin. Molecules 2020, 25, 1311. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- He, Y.; Ho, C. Amorphous Solid Dispersions: Utilization and Challenges in Drug Discovery and Development. J. Pharm. Sci. 2015, 104, 3237–3258. [Google Scholar] [CrossRef]
- Qiang, W.; Lobmann, K.; McCoy, C.P.; Andrews, G.P.; Zhao, M. Microwave-Induced In Situ Amorphization: A New Strategy for Tackling the Stability Issue of Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 655. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Cao, Y.; Kowalski, J.; Serajuddin, A.T. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol. Pharm. 2008, 5, 994–1002. [Google Scholar] [CrossRef]
- Paudel, A.; Van den Mooter, G. Influence of solvent composition on the miscibility and physical stability of naproxen/PVP K 25 solid dispersions prepared by cosolvent spray-drying. Pharm. Res. 2012, 29, 251–270. [Google Scholar] [CrossRef]
- Borde, S.; Paul, S.K.; Chauhan, H. Ternary solid dispersions: Classification and formulation considerations. Drug Dev. Ind. Pharm. 2021, 47, 1011–1028. [Google Scholar] [CrossRef]
- Kumar, V.; Mintoo, M.J.; Mondhe, D.M.; Bharate, S.B.; Vishwakarma, R.A.; Bharate, S.S. Binary and ternary solid dispersions of an anticancer preclinical lead, IIIM-290: In vitro and in vivo studies. Int. J. Pharm. 2019, 570, 118683. [Google Scholar] [CrossRef]
- Davis, M.T.; Potter, C.B.; Mohammadpour, M.; Albadarin, A.B.; Walker, G.M. Design of spray dried ternary solid dispersions comprising itraconazole, soluplus and HPMCP: Effect of constituent compositions. Int. J. Pharm. 2017, 519, 365–372. [Google Scholar] [CrossRef]
- Prasad, D.; Chauhan, H.; Atef, E. Role of Molecular Interactions for Synergistic Precipitation Inhibition of Poorly Soluble Drug in Supersaturated Drug-Polymer-Polymer Ternary Solution. Mol. Pharm. 2016, 13, 756–765. [Google Scholar] [CrossRef]
- Meeus, J.; Scurr, D.J.; Chen, X.; Amssoms, K.; Davies, M.C.; Roberts, C.J.; Mooter, G.V.D. Combination of (M)DSC and surface analysis to study the phase behaviour and drug distribution of ternary solid dispersions. Pharm. Res. 2015, 32, 1407–1416. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose-A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release Off. J. Control. Release Soc. 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Sakurai, H.; Ikeuchi-Takahashi, Y.; Kobayashi, A.; Yoshimura, N.; Ishihara, C.; Aomori, T.; Onishi, H. Formulation Development of Mucoadhesive Microparticle-Laden Gels for Oral Mucositis: An In Vitro and In Vivo Study. Pharmaceutics 2020, 12, 603. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Rizi, K.; Green, R.J.; Donaldson, M.; Williams, A.C. Production of pH-responsive microparticles by spray drying: Investigation of experimental parameter effects on morphological and release properties. J. Pharm. Sci. 2011, 100, 566–579. [Google Scholar] [CrossRef]
- Liu, W.; Wu, W.D.; Selomulya, C.; Chen, X.D. Facile spray-drying assembly of uniform microencapsulates with tunable core-shell structures and controlled release properties. Langmuir ACS J. Surf. Colloids 2011, 27, 12910–12915. [Google Scholar] [CrossRef]
- Shi, Q.; Li, F.; Yeh, S.; Moinuddin, S.M.; Xin, J.; Xu, J.; Chen, H.; Ling, B. Recent Advances in Enhancement of Dissolution and Supersaturation of Poorly Water-Soluble Drug in Amorphous Pharmaceutical Solids: A Review. AAPS PharmSciTech 2021, 23, 16. [Google Scholar] [CrossRef]
- Feng, X.; Vo, A.; Patil, H.; Tiwari, R.V.; Alshetaili, A.S.; Pimparade, M.B.; ARepka, M. The effects of polymer carrier, hot melt extrusion process and downstream processing parameters on the moisture sorption properties of amorphous solid dispersions. J. Pharm. Pharmacol. 2016, 68, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Theil, F.; Lehmkemper, K.; Gessner, D.; Li, Y.; van Lishaut, H. Crystallization Risk Assessment of Amorphous Solid Dispersions by Physical Shelf-Life Modeling: A Practical Approach. Mol. Pharm. 2021, 18, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Friesen, D.T.; Shanker, R.; Crew, M.; Smithey, D.T.; Curatolo, W.J.; Nightingale, J.A. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008, 5, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D. Thermal Stability of Amorphous Solid Dispersions. Molecules 2021, 26, 238. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnurch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. EV 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Elbakry, A.M.; Esmaeil, A.H.; Khaleel, S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J. Pharm. Investig. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: Methods and studies. Fundam. Clin. Pharmacol. 1999, 13, 154–168. [Google Scholar] [CrossRef]

| Cmax (μg/mL) | Tmax (h) | MRT (h) | AUC0–inf. (μg·h/mL) | BA (%) | |
|---|---|---|---|---|---|
| Amorphous CsA | 0.12 ± 0.057 | 3.4 ± 0.40 | 5.9 ± 0.47 | 0.55 ± 0.22 | 0.73 |
| tSD/CsA | 1.3 ± 0.080 | 5.8 ± 0.49 | 8.7 ± 0.31 | 15 ± 1.3 | 19 |
| SD/CsA | 0.97 ± 0.17 | 3.8 ± 0.49 | 7.5 ± 0.84 | 10 ± 2.0 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritani, T.; Usui, H.; Morinaga, T.; Sato, H.; Onoue, S. Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability. Pharmaceutics 2023, 15, 571. https://doi.org/10.3390/pharmaceutics15020571
Moritani T, Usui H, Morinaga T, Sato H, Onoue S. Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability. Pharmaceutics. 2023; 15(2):571. https://doi.org/10.3390/pharmaceutics15020571
Chicago/Turabian StyleMoritani, Tatsuru, Hayato Usui, Tadahiko Morinaga, Hideyuki Sato, and Satomi Onoue. 2023. "Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability" Pharmaceutics 15, no. 2: 571. https://doi.org/10.3390/pharmaceutics15020571
APA StyleMoritani, T., Usui, H., Morinaga, T., Sato, H., & Onoue, S. (2023). Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability. Pharmaceutics, 15(2), 571. https://doi.org/10.3390/pharmaceutics15020571







