Monoclonal Antibodies, Gene Silencing and Gene Editing (CRISPR) Therapies for the Treatment of Hyperlipidemia—The Future Is Here
Abstract
1. Introduction
An Area of Unmet Need
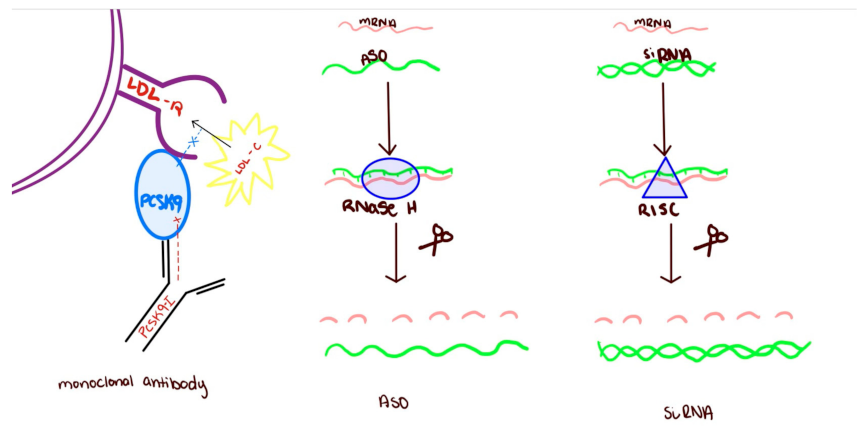
2. Monoclonal Antibodies
2.1. Target: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK-9)
2.2. Target: Angiopoietin-Like Protein 3 (ANGPTL3)
3. Gene Silencing: Antisense Oligonucleotides (ASO)
3.1. Target: PCSK9
3.2. Target: ANGPTL3
3.3. Target: Lp (a)
3.4. Target: APOC3
3.5. Target: ApoB-100
4. Gene Silencing: Short Interfering RNA (SiRNA)
4.1. Target: PCSK9
4.2. Target: ANGPTL3
4.3. Target: Lp (a)
4.4. Target: ApoC3
5. Gene Editing: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
Target: PCSK9
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bravo, I.-G.-F.; Torres-Do-Rego, A.; López-Farré, A.; Galeano-Valle, F.; Demelo-Rodriguez, P.; Alvarez-Sala-Walther, L.A. Undertreatment or Overtreatment with Statins: Where Are We? Front. Cardiovasc. Med. 2022, 9, 808712. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2022.808712 (accessed on 13 July 2022). [CrossRef] [PubMed]
- Griffith, N.; Bigham, G.; Sajja, A.; Gluckman, T.J. Leveraging Healthcare System Data to Identify High-Risk Dyslipidemia Patients. Curr. Cardiol. Rep. 2022, 24, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, C.T.T. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Worldwide burden of LDL cholesterol: Implications in cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 241–244. [Google Scholar] [CrossRef]
- Rodriguez, F.; Knowles, J.W.; Maron, D.J.; Virani, S.S.; Heidenreich, P.A. Frequency of Statin Use in Patients with Low-Density Lipoprotein Cholesterol ≥190 mg/dL from the Veterans Affairs Health System. Am. J. Cardiol. 2018, 122, 756–761. [Google Scholar] [CrossRef]
- Virani, S.S.; Kennedy, K.F.; Akeroyd, J.M.; Morris, P.B.; Bittner, V.A.; Masoudi, F.A.; Stone, N.J.; Petersen, L.A.; Ballantyne, C.M. Variation in Lipid-Lowering Therapy Use in Patients with Low-Density Lipoprotein Cholesterol ≥190 mg/dL: Insights from the National Cardiovascular Data Registry-Practice Innovation and Clinical Excellence Registry. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004652. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Brautbar, A.; Leary, E.; Rasmussen, K.; Wilson, D.P.; Steiner, R.D.; Virani, S. Genetics of familial hypercholesterolemia. Curr. Atheroscler. Rep. 2015, 17, 491. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Stevens, C.A.T.; Lyons, A.R.M.; Dharmayat, K.I.; Freiberger, T.; Hovingh, G.K.; Mata, P.; Raal, F.J.; Santos, R.D.; Soran, H.; et al. Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef]
- Benziger, C.P.; Groth, N. Abstract 15830: Uncontrolled and Undertreated Cholesterol in Patients with Severe Hypercholesterolemia at a Large Healthcare System. Circulation 2020, 142 (Suppl. S3), A15830. [Google Scholar] [CrossRef]
- Bucholz, E.M.; Rodday, A.M.; Kolor, K.; Khoury, M.J.; de Ferranti, S.D. Prevalence and Predictors of Cholesterol Screening, Awareness, and Statin Treatment Among US Adults with Familial Hypercholesterolemia or Other Forms of Severe Dyslipidemia (1999–2014). Circulation 2018, 137, 2218–2230. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommansino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Gibson, C.A.; Mount, R.R.; Lee, J.; Backes, J.M. Identifying patient perceptions and attitudes regarding statin-associated diabetes mellitus: A mixed-methods study. Future Cardiol. 2022, 18, 817–828. [Google Scholar] [CrossRef]
- Labos, C.; Brophy, J.M.; Smith, G.D.; Sniderman, A.D.; Thanassoulis, G. Evaluation of the pleiotropic effects of statins: A reanalysis of the randomized trial evidence using Egger regression. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 262–265. [Google Scholar] [CrossRef]
- Virani, S.S.; Akeroyd, J.M.; Ahmed, S.T.; Krittanawong, C.; Martin, L.A.; Slagle, J.; Gobbel, G.T.; Matheny, M.E.; Ballantyne, C.M.; Petersen, L.A. The use of structured data elements to identify ASCVD patients with statin-associated side effects: Insights from the Department of Veterans Affairs. J. Clin. Lipidol. 2019, 13, 797–803.e1. [Google Scholar] [CrossRef]
- Jia, X.; Lee, M.T.; Ramsey, D.J.; Mahtta, D.; Akeroyd, J.M.; Turchin, A.; Navar, A.M.; Matheny, M.E.; Gobbel, G.; Stone, N.J.; et al. Association of patient, provider and facility related characteristics with statin associated side effects and statin use: Insight from the Veteran’s Affairs healthcare system. J. Clin. Lipidol. 2021, 15, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lee, M.T.; Ramsey, D.J.; Al Rifai, M.; Mahtta, D.; Krittanawong, C.; Akeroyd, J.M.; Matheny, M.E.; Gobbel, G.; Stone, N.J.; et al. Facility-Level Variation in Reported Statin-Associated Side Effects Among Patients with Atherosclerotic Cardiovascular Disease-Perspective from the Veterans Affair Healthcare System. Cardiovasc. Drugs Ther. 2022, 36, 295–300. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Akeroyd, J.M.; Mahtta, D.; Street, R.; Slagle, J.; Navar, A.M.; Stone, N.J.; Ballantyne, C.M.; Petersen, L.A.; Virani, S.S. Shared Decisions: A Qualitative Study on Clinician and Patient Perspectives on Statin Therapy and Statin-Associated Side Effects. J. Am. Heart Assoc. 2020, 9, e017915. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Tan, H.; Wang, P.; Zha, X.; Chong, W.; Zhou, L.; Fang, F. Efficacy and safety of bempedoic acid for prevention of cardiovascular events and diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2020, 19, 128. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.G.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2020, 27, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Ballantyne, C.M. Bempedoic Acid (ETC-1002): A Current Review. Cardiol. Clin. 2018, 36, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Granowitz, C.; Hull, M.; Liassou, D.; Anderson, A.; Philip, S. High Triglycerides Are Associated with Increased Cardiovascular Events, Medical Costs, and Resource Use: A Real-World Administrative Claims Analysis of Statin-Treated Patients with High Residual Cardiovascular Risk. J. Am. Heart Assoc. 2018, 7, e008740. [Google Scholar] [CrossRef]
- Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients with Versus without Diabetes Mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Available online: https://pubmed.ncbi.nlm.nih.gov/29263150/ (accessed on 13 July 2022).
- Gaba, P.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; Doyle Jr, R.T.; et al. Prevention of Cardiovascular Events and Mortality with Icosapent Ethyl in Patients With Prior Myocardial Infarction. J. Am. Coll. Cardiol. 2022, 79, 1660–1671. [Google Scholar] [CrossRef]
- Klempfner, R.; Erez, A.; S’agit, B.-Z.; Goldenberg, I.; Fisman, E.; Kopel, E.; Shlomo, N.; Israel, A.; Tenenbaum, A. Elevated Triglyceride Level Is Independently Associated With Increased All-Cause Mortality in Patients With Established Coronary Heart Disease: Twenty-Two-Year Follow-Up of the Bezafibrate Infarction Prevention Study and Registry. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased Cardiovascular Risk in Hypertriglyceridemic Patients with Statin-Controlled LDL Cholesterol. J. Clin. Endocrinol. Metab. 2018, 103, 3019–3027. [Google Scholar] [CrossRef]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes. Metab. 2019, 21, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Virani, S.S.; Koschinsky, M.L.; Maher, L.; Maher, L.; Mehta, A.; Orringer, C.E.; Santos, R.D.; Shapiro, M.D.; Saseen, J.J. Global think tank on the clinical considerations and management of lipoprotein(a): The top questions and answers regarding what clinicians need to know. Prog. Cardiovasc. Dis. 2022, 73, 32–40. [Google Scholar] [CrossRef]
- Saeed, A.; Virani, S.S. Lipoprotein(a) and cardiovascular disease: Current state and future directions for an enigmatic lipoprotein. Front. Biosci. Landmark Ed. 2018, 23, 1099–1112. [Google Scholar] [PubMed]
- Malik, B.; Ghatol, A. Understanding How Monoclonal Antibodies Work; StatPearls Publishing: Tampa, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572118/ (accessed on 13 July 2022).
- Abifadel, M.; Varret, M.; Rabès, J.-P.; Allard, D.; Ouguerram, M.D.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickman, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Maligłówka, M.; Kosowski, M.; Hachuła, M.; Cyrnek, M.; Bułdak, Ł.; Basiak, M.; Bołdys, M.; Machnik, G.; Bułdak, R.J.; Okopień, B. Insight into the Evolving Role of PCSK9. Metabolites 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef]
- Cameron, J.; Holla, Ø.L.; Ranheim, T.; Kulseth, M.A.; Berge, K.E.; Leren, T.P. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum. Mol. Genet. 2006, 15, 1551–1558. [Google Scholar] [CrossRef]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, S.; Sever, P.S.; Lira Pineda, A.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Clinical Approach to the Inflammatory Etiology of Cardiovascular Diseases. Available online: https://moh-it.pure.elsevier.com/en/publications/clinical-approach-to-the-inflammatory-etiology-of-cardiovascular- (accessed on 13 July 2022).
- Koren, M.J.; Giugliano, R.P.; Raal, F.J.; Sullivan, D.; Bolognese, M.; Langslet, G.; Civeira, F.; Somaratne, R.; Nelson, P.; Liu, T.; et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 2014, 129, 234–243. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients with Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J. Results of the GLAGOV trial. Cleve. Clin. J. Med. 2017, 84 (Suppl. S4), e1–e5. [Google Scholar]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Hermann, J.P.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Repatha®. High Cholesterol (LDL-C) Treatment (Evolocumab). Available online: https://www.repatha.com (accessed on 13 July 2022).
- DailyMed—PRALUENT—Alirocumab Injection, Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=446f6b5c-0dd4-44ff-9bc2-c2b41f2806b4#S5.1 (accessed on 13 July 2022).
- Ridker, P.M.; Tardif, J.-C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.P.; Kim, A.M.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N. Engl. J. Med. 2017, 376, 1517–1526. [Google Scholar] [CrossRef]
- Ridker, P.M.; Revkin, J.; Amarenco, P.; Brunell, R.; Curto, M.; Civeira, F.; Flather, M.; Glynn, R.J.; Gregoire, J.; Jukema, J.W.; et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N. Engl. J. Med. 2017, 376, 1527–1539. [Google Scholar] [CrossRef]
- Kazi, D.S.; Virani, S.S. Implications of cost-effectiveness analyses of lipid-lowering therapies: From the policy-maker’s desk to the patient’s bedside. Prog. Cardiovasc. Dis. 2019, 62, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Rifai, M.A.; Saeed, A.; Ballantyne, C.M.; Virani, S.S. PCSK9 Inhibitors in the Management of Cardiovascular Risk: A Practical Guidance. Vasc. Health Risk Manag. 2022, 18, 555–566. [Google Scholar] [CrossRef]
- Lee, M.T.; George, J.; Shahab, H.; Hermel, M.; Rana, J.S.; Virani, S.S. Highlights of Cardiovascular Disease Studies Presented at the 2021 American Heart Association Scientific Sessions. Curr. Atheroscler. Rep. 2022, 24, 61–72. [Google Scholar] [CrossRef]
- PCSK9 Forum. AHA Scientific Sessions 2021 (Virtual). Available online: https://www.pcsk9forum.org/articles/meeting-reports/aha-2021/ (accessed on 13 July 2022).
- Merck Sharp & Dohme LLC. A Phase 2b, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of MK-0616 in Adults with Hypercholesterolemia. Available online: https://clinicaltrials.gov/ct2/show/NCT05261126 (accessed on 13 July 2022).
- Giugliano, R.P.; Mach, F.; Zavitz, K.; Kurtz, C.; Im, K.; Kanevsky, E.; Scheinder, J.; Wang, H.; Keech, A.; Pedersen, T.R.; et al. Cognitive Function in a Randomized Trial of Evolocumab. N. Engl. J. Med. 2017, 377, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Mach, F.; Guo, J.; Im, K.; Ruzza, R.; Wang, H.; Kurtz, C.E.; Pedersen, T.R.; Keech, A.C.; Ott, B.R.; et al. Cognition After Lowering LDL-Cholesterol with Evolocumab. J. Am. Coll. Cardiol. 2020, 75, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Pirruccello, J.P.; Do, R.; Peloso, G.M.; Guiducci, C.; Sougnez, C.; Garimella, K.V.; Fisher, S.; Abreu, J.; Barry, A.J.; et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010, 363, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Minicocci, I.; Montali, A.; Robciuc, M.R.; Quagliarini, F.; Censi, V.; Labbadia, G.; Gabiati, C.; Pigna, G.; Sepe, M.L.; Pannozzo, F.; et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: A clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 2012, 97, E1266–E1275. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef]
- Adam, R.C.; Mintah, I.J.; Alexa-Braun, C.A.; Shihanian, L.M.; Lee, J.S.; Banerjee, P.; Hamon, S.C.; Kim, H.I.; Cohen, J.C.; Hobbs, H.H.; et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020, 61, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Evinacumab: First Approval. Drugs 2021, 81, 1101–1105. [Google Scholar] [CrossRef]
- Ahmad, Z.; Banerjee, P.; Hamon, S.; Chen, K.C.; Bouzelmat, A.; Sasiela, W.J.; Pordy, B.; Mellis, S.; Dansky, H.; Gipe, D.A.; et al. Inhibition of Angiopoietin-Like Protein 3 With a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia. Circulation 2019, 140, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Gipe, D.A.; Pordy, R.; Ahmad, W.; Cuchel, M.; Shah, P.K.; Chyu, K.Y.; Sasiela, W.J.; Chan, K.C.; Brisson, D.; et al. ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2017, 377, 296–297. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; Hamlin, R.L.; Pordy, R.; Dong, Y.; et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.P.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.C.; Gipe, D.A.; et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Meng, F.; Yang, W.; Liang, L.; Wang, H.; Fu, Z. Efficacy and Safety of Evinacumab for the Treatment of Hypercholesterolemia: A Meta-Analysis. J. Cardiovasc. Pharmacol. 2021, 78, 394–402. [Google Scholar] [CrossRef]
- Gareri, C.; Polimeni, A.; Giordano, S.; Tammè, L.; Curcio, A.; Indolfi, C. Antisense Oligonucleotides and Small Interfering RNA for the Treatment of Dyslipidemias. J. Clin. Med. 2022, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Moumné, L.; Marie, A.-C.; Crouvezier, N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics 2022, 14, 260. [Google Scholar] [CrossRef]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef]
- Gennemark, P.; Walter, K.; Clemmensen, N.; Rekić, D.; Nilsson, C.A.M.; Knöchel, J.; Hölttä, M.; Wernevik, L.; Rosengren, B.; Kakol-Palm, D.; et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci. Transl. Med. 2021, 13, eabe9117. [Google Scholar] [CrossRef]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Marston, N.A.; Bramson, C.R.; Curto, M.; Ramos, V.; Jevne, A.; Kuder, J.F.; Park, J.G.; Murphy, S.A.; Verma, S.; et al. Effect of Vupanorsen on Non–High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients with Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation 2022, 145, 1377–1386. [Google Scholar] [CrossRef]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Mason, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants sith Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Karwatowska-Prokopczuk, E.; Su, F.; Dinh, B.; Xia, S.; Witzum, J.L.; Tsimikas, S. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1035–1046. [Google Scholar] [CrossRef]
- Cleveland Clinic. HORIZON: A multicenter Trial Assessing the Impact of Lipoprotein (a) Lowering with TQJ230 on Major Cardiovascular Events in Patients with Cardiovascular Disease. Available online: https://my.clevelandclinic.org/clinical-trials/1424-horizon-a-multicenter-trial-assessing-the-impact-of-lipoprotein-a-lowering-with-tqj230-on-major-cardiovascular-events-in-patients-with-cardiovascular-disease (accessed on 13 July 2022).
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical review on triglycerides. Eur. Heart J. 2020, 41, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Le, N.A.; Goldberg, I.J.; Gibson, J.C.; Rubinstein, A.; Wang-Iverson, P.; Norum, R.; Brown, W.V. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Investig. 1986, 78, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Alexander, V.J.; Xia, S.; Hurh, E.; Hughes, S.G.; O’Dea, L.; Geary, R.S.; Witzman, J.L.; Tsimikas, S. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur. Heart J. 2019, 40, 2785–2796. [Google Scholar] [CrossRef] [PubMed]
- Pollin, T.I.; Damcott, C.M.; Shen, H.; Ott, S.H.; Shelton, J.; Horenstein, R.B.; Post, W.; McLenithan, J.C.; Bielak, L.F.; Peyser, P.A.; et al. A Null Mutation in Human APOC3 Confers a Favorable Plasma Lipid Profile and Apparent Cardioprotection. Science 2008, 322, 1702–1705. [Google Scholar] [CrossRef]
- TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute; Crosby, J.; Peloso, G.M.; Auer, P.L.; Crosslin, D.R.; Stitzel, N.O.; Lange, L.A.; Lu, Y.; Tang, Z.-Z.; Zhang, H.; et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar]
- Schmitz, J.; Gouni-Berthold, I. APOC-III Antisense Oligonucleotides: A New Option for the Treatment of Hypertriglyceridemia. Curr. Med. Chem. 2018, 25, 1567–1576. [Google Scholar] [CrossRef]
- Calcaterra, I.; Lupoli, R.; Di Minno, A.; Di Minno, M.N.D. Volanesorsen to treat severe hypertriglyceridaemia: A pooled analysis of randomized controlled trials. Eur. J. Clin. Investig. 2022, 52, e13841. [Google Scholar] [CrossRef]
- Akcea and Ionis Receive Complete Response Letter for Waylivra from FDA. Available online: https://www.drugs.com/nda/waylivra_180827.html (accessed on 13 July 2022).
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef]
- Gelrud, A.; Digenio, A.; Alexander, V.; Williams, K.; Hsieh, A.; Gouni-Berthold, I.; Brukert, E.; Stroes, E.; Geary, R.; Hughes, S.; et al. Treatment with Volanesorsen (VLN) Reduced Triglycerides and Pancreatitis in Patients with FCS and sHTG vs. Placebo: Results of the APPROACH and COMPASS †. J. Clin. Lipidol 2018, 12, 537. [Google Scholar] [CrossRef]
- Thomas, G.S.; Cromwell, W.C.; Ali, S.; Chin, W.; Flaim, J.D.; Davidson, M. Mipomersen, an Apolipoprotein B Synthesis Inhibitor, Reduces Atherogenic Lipoproteins in Patients with Severe Hypercholesterolemia at High Cardiovascular Risk. J. Am. Coll. Cardiol. 2013, 62, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadah, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef] [PubMed]
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients with Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef]
- University of Oxford. HPS-4/TIMI 65/ORION-4: A Double-blind Randomized Placebo-controlled Trial Assessing the Effects of Inclisiran on Clinical Outcomes Among People with Atherosclerotic Cardiovascular Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03705234 (accessed on 13 July 2022).
- Nissen, S.E.; Wolski, K.; Balog, C.; Swerdlow, D.I.; Scimgeour, A.C.; Rambaran, C.; Wilson, R.J.; Boyce, M.; Ray, K.K.; Cho, L.; et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals with Elevated Plasma Lipoprotein(a) Levels. JAMA 2022, 327, 1679–1687. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- TIMI STUDY GROUP. OCEAN(a)-Outcomes (TIMI 75). 2022. Available online: https://timi.org/oceana-outcomes-timi-75/ (accessed on 13 July 2022).
- Hussain, A.; Ballantyne, C.M.; Saeed, A.; Virani, S.S. Triglycerides and ASCVD Risk Reduction: Recent Insights and Future Directions. Curr. Atheroscler. Rep. 2020, 22, 25. [Google Scholar] [CrossRef]
- Quispe, R.; Sweeney, T.; Varma, B.; Agarwala, A.; Michos, E.D. Recent Updates in Hypertriglyceridemia Management for Cardiovascular Disease Prevention. Curr. Atheroscler. Rep. 2022, 24, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Arrowhead Pharmaceuticals. A Phase 1 Single and Multiple Dose-Escalating Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamic Effects of ARO-APOC3 in Adult Healthy Volunteers as Well as in Severely Hypertriglyceridemic Patients and Patients with Familial Chylomicronemia Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT03783377 (accessed on 13 July 2022).
- Arrowhead Pharmaceuticals. A Phase 3 Study to Evaluate the Efficacy and Safety of ARO-APOC3 in Adults With Familial Chylomicronemia Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT05089084 (accessed on 13 July 2022).
- Arrowhead Pharmaceuticals. A Double-Blind, Placebo-Controlled Phase 2b Study to Evaluate the Efficacy and Safety of ARO-APOC3 in Adults with Mixed Dyslipidemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04998201 (accessed on 13 July 2022).
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biol. Targets Ther. 2021, 15, 353–361. [Google Scholar]
- Walker, H.E.; Rizzo, M.; Fras, Z.; Jug, B.; Banach, M.; Penson, P.E. CRISPR Gene Editing in Lipid Disorders and Atherosclerosis: Mechanisms and Opportunities. Metabolites 2021, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.L.; Cupido, A.J.; Laufs, U. Gene Therapy Targeting PCSK9. Metabolites 2022, 12, 70. [Google Scholar] [CrossRef]
- Verve Therapeutics. Verve Therapeutics Announces Clearance of First VERVE-101 Clinical Trial Application and Outlines Global Clinical Development Strategy; Reports First Quarter 2022 Financial Results. Available online: https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-announces-clearance-first-verve-101-clinical (accessed on 13 July 2022).
- Centers for Disease Control and Prevention. Heart Disease Facts. 2022. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 13 July 2022).
- CDC Prevention Programs. Available online: https://www.heart.org/en/get-involved/advocate/federal-priorities/cdc-prevention-programs (accessed on 13 July 2022).
| Target Protein | FDA Approved Therapies | Therapy Under Investigation | Efficacy | Safety |
|---|---|---|---|---|
| PCSK9 | Evolocumab and Alirocumab | Oral PCSK9 inhibitor MK-0616 | FOURIER and FOUIER-OLE trials: Patients on evolocumab in the parent trial and continued its use in the FOURIER-OLE trial had 23% lower risk of CV death as compared to patients who took placebo in the parent trial (HR 0.77, 95% CI 0.60–0.99, p = 0.04). ODYSSEY OUTCOMES trial: there was a significant reduction in MACE for patients on alirocumab vs. placebo (9.5% vs. 11.1%, HR 0.85, 95% CI 0.78–0.93, p < 0.001. | Mild injection site reactions. |
| ANGPTL3 | Evinacumab | Meta-analysis of 5 RCTs: evinacumab reduced LDL-C significantly compared with placebo [MD −33.12%, 95% CI, −48.63% to −17.60%, p < 0.0001], triglycerides (MD −50.95%, 95% CI, −56.55% to −45.36%, p < 0.0001), and HDL-C (MD −12.77%, 95% CI, −16.35% to −9.18%, p < 0.0001). CV outcomes data unknown | Adverse events did not significantly differ in treatment vs. placebo groups. |
| Target Protein | FDA Approved Therapies | Therapy Under Investigation | Efficacy | Safety |
|---|---|---|---|---|
| PCSK9 | None | AZD8233 | ETESIAN phase 2b study: LDL-C and PCSK9 levels were reduced in a dose dependent manner at week 12 for patients on study drug vs. placebo. LDL-C decreased −72% (95% CI −78 to −65) for the 50 mg dose and −79% (95% CI −83 to −74) for the 90 mg dose. | No significant adverse events. |
| CV outcomes unknown. | No significant adverse events. | |||
| ANGPTL3 | None | Vupanorsen | TRANSLATE-TIMI trial: Vupanorsen achieved a decrease in non-HDL-C up to 27.7% in the 80 mg every 2 weeks arm (p < 0.001). Triglycerides were reduced up to 56.8% (p < 0.001) in a dose dependent manner and LDL-C was reduced up to 16% without a dose dependent association. | |
| Lp (a) | None | Pelacarsen | Phase IIb trial: Significant reductions in direct Lp (a) cholesterol in a dose-dependent manner were observed with pelacarsen compared with pooled placebo, by a mean of 29–67% versus 2% respectively, p = 0.001. Pelacarsen was also associated with a modest decrease in laboratory-reported LDL-C. | No significant adverse events. |
| ApoC3 | None | Volanesorsen (approved in EU) | Pooled analysis of four studies showed significant reduction in TG after 3 months of treatment with volanesorsen compared with placebo (MD: −73.9%; 95%CI: −93.5%, −54.2; p < 0.001 I2 = 89.05%; p < 0.001) with significant decrease in LDL-C and increase in HDL-C as well. | Safety concerns related to thrombocytopenia and bleeding. |
| apoB-100 | ||||
| Mipomersen | ||||
| None | ||||
| During phase I, II and III trials, mipomersen significantly lowered LDL-C, apoB-100 and Lp(a) from baseline. In a phase 3 multicenter blinded randomized placebo-controlled study, mipomersen reduced LDL-C by −36.9% as compared to the placebo group at −4.5% (p < 0.001). | Most common side effects include injection site reactions, flu-like symptoms and hepatotoxicity. |
| Target Protein | FDA Approved Therapies | Therapy Under Investigation | Efficacy | Safety |
|---|---|---|---|---|
| PCSK9 | Inclisiran | A pooled analysis of ORION-9, 10, and 11 trials demonstrated that the placebo-corrected change in LDL-C with inclisiran at day 510 was −50.7% (95% CI: −52.9% to −48.4%; p < 0.0001). CV outcomes trial currently ongoing. | Treatment-emergent adverse events at the injection site were more frequent with inclisiran than placebo (5.0 vs. 0.7%), but were predominantly mild | |
| ANGPTL3 | ARO-ANG3 | Phase I/II clinical Subcutaneous injection of ARO-ANG3 for 16 weeks at doses of 100, 200, and 300 mg reduced circulating levels of ANGPTL3 by 96%, TG by 72%, and LDL-C by 50%. In the FH group, LDL-C levels were reduced by 23%–37%, and TG levels were reduced by 25%–43% at doses of 100, 200, and 300 mg injected subcutaneously. | Most adverse events were mild. | |
| Lp(a) | Olpasiran | OCEAN [a]-DOSE trial: At 36 weeks, olpasiran reduced the Lp(a) concentration in a dose-dependent manner. The placebo-adjusted mean percent change in the LDL-C concentration at 36 weeks ranged from −22.6% to −24.8% across the olpasiran dose levels. A CV outcomes trial is currently underway. | The overall incidence of adverse events was similar across the trial groups. | |
| APOC3 | ARO-APOC3 | Multiple dose double-blind randomized placebo control trials in patients with severe hypertriglyceridemia or FCS are ongoing. | No significant adverse events noted. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermel, M.; Lieberman, M.; Slipczuk, L.; Rana, J.S.; Virani, S.S. Monoclonal Antibodies, Gene Silencing and Gene Editing (CRISPR) Therapies for the Treatment of Hyperlipidemia—The Future Is Here. Pharmaceutics 2023, 15, 459. https://doi.org/10.3390/pharmaceutics15020459
Hermel M, Lieberman M, Slipczuk L, Rana JS, Virani SS. Monoclonal Antibodies, Gene Silencing and Gene Editing (CRISPR) Therapies for the Treatment of Hyperlipidemia—The Future Is Here. Pharmaceutics. 2023; 15(2):459. https://doi.org/10.3390/pharmaceutics15020459
Chicago/Turabian StyleHermel, Melody, Madison Lieberman, Leandro Slipczuk, Jamal S. Rana, and Salim S. Virani. 2023. "Monoclonal Antibodies, Gene Silencing and Gene Editing (CRISPR) Therapies for the Treatment of Hyperlipidemia—The Future Is Here" Pharmaceutics 15, no. 2: 459. https://doi.org/10.3390/pharmaceutics15020459
APA StyleHermel, M., Lieberman, M., Slipczuk, L., Rana, J. S., & Virani, S. S. (2023). Monoclonal Antibodies, Gene Silencing and Gene Editing (CRISPR) Therapies for the Treatment of Hyperlipidemia—The Future Is Here. Pharmaceutics, 15(2), 459. https://doi.org/10.3390/pharmaceutics15020459





