Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications
Abstract
1. Introduction
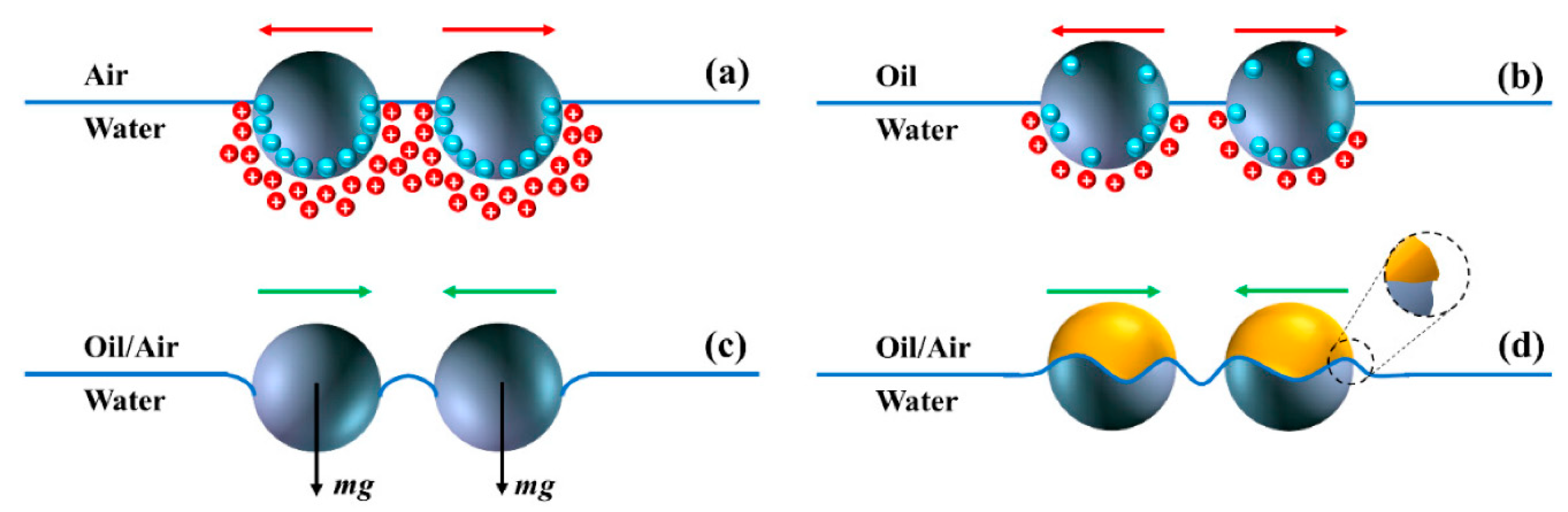
2. Overview of Janus Particles
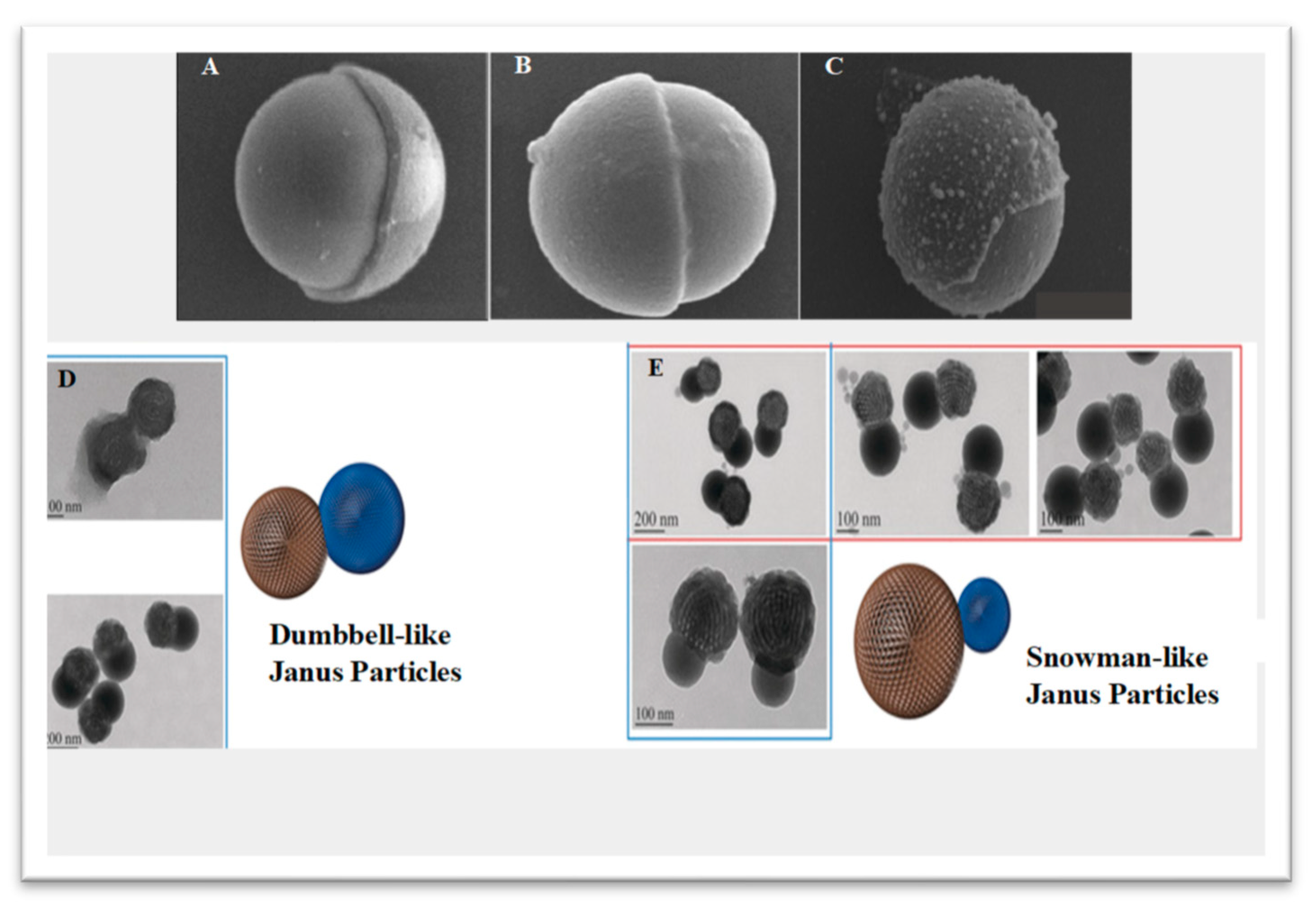
Structural and Functional Characteristics of Janus Particles
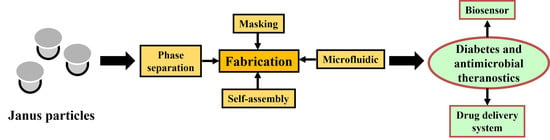
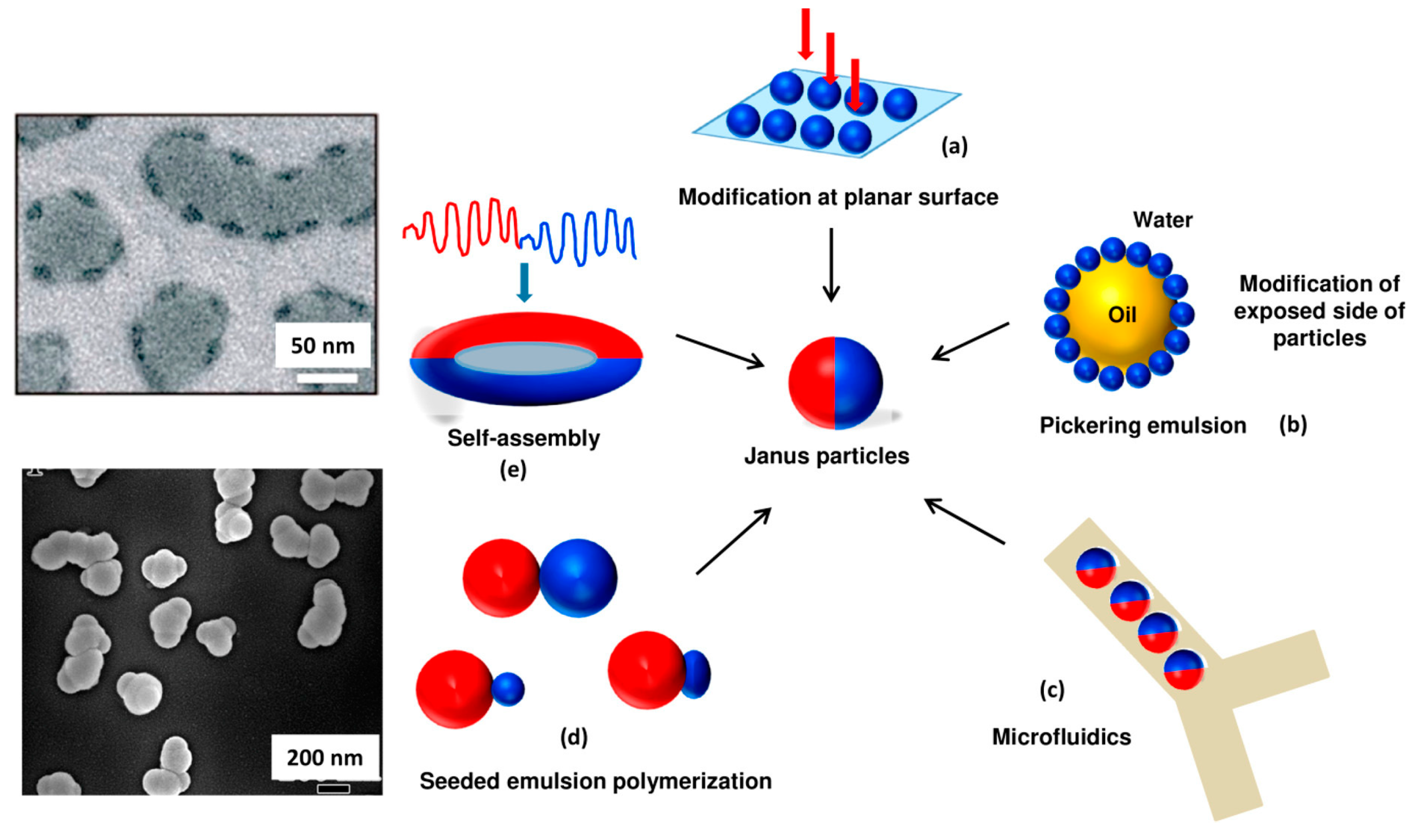
3. Fabrication of Janus Particles
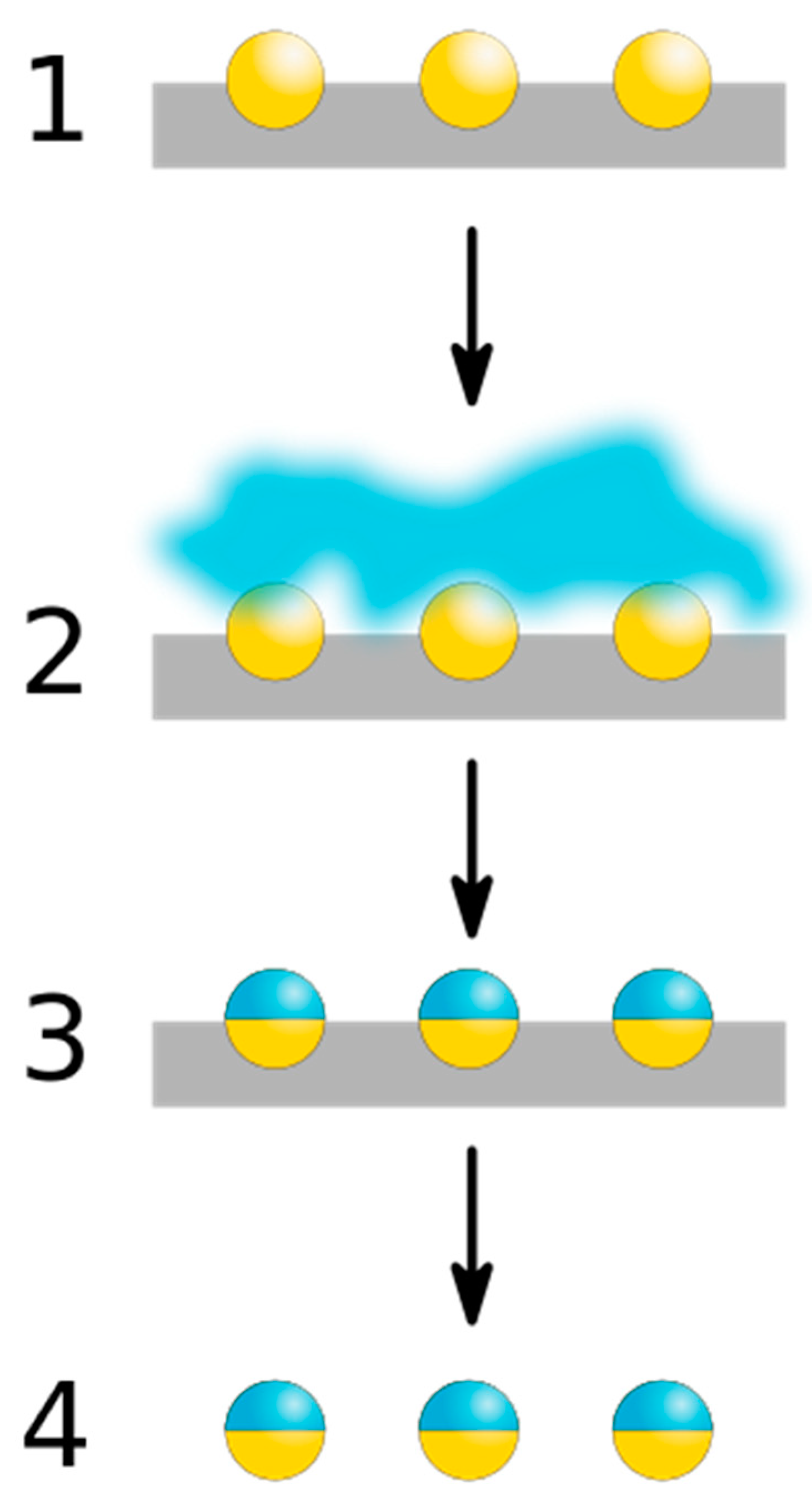
3.1. Masking
3.2. Self-Assembly
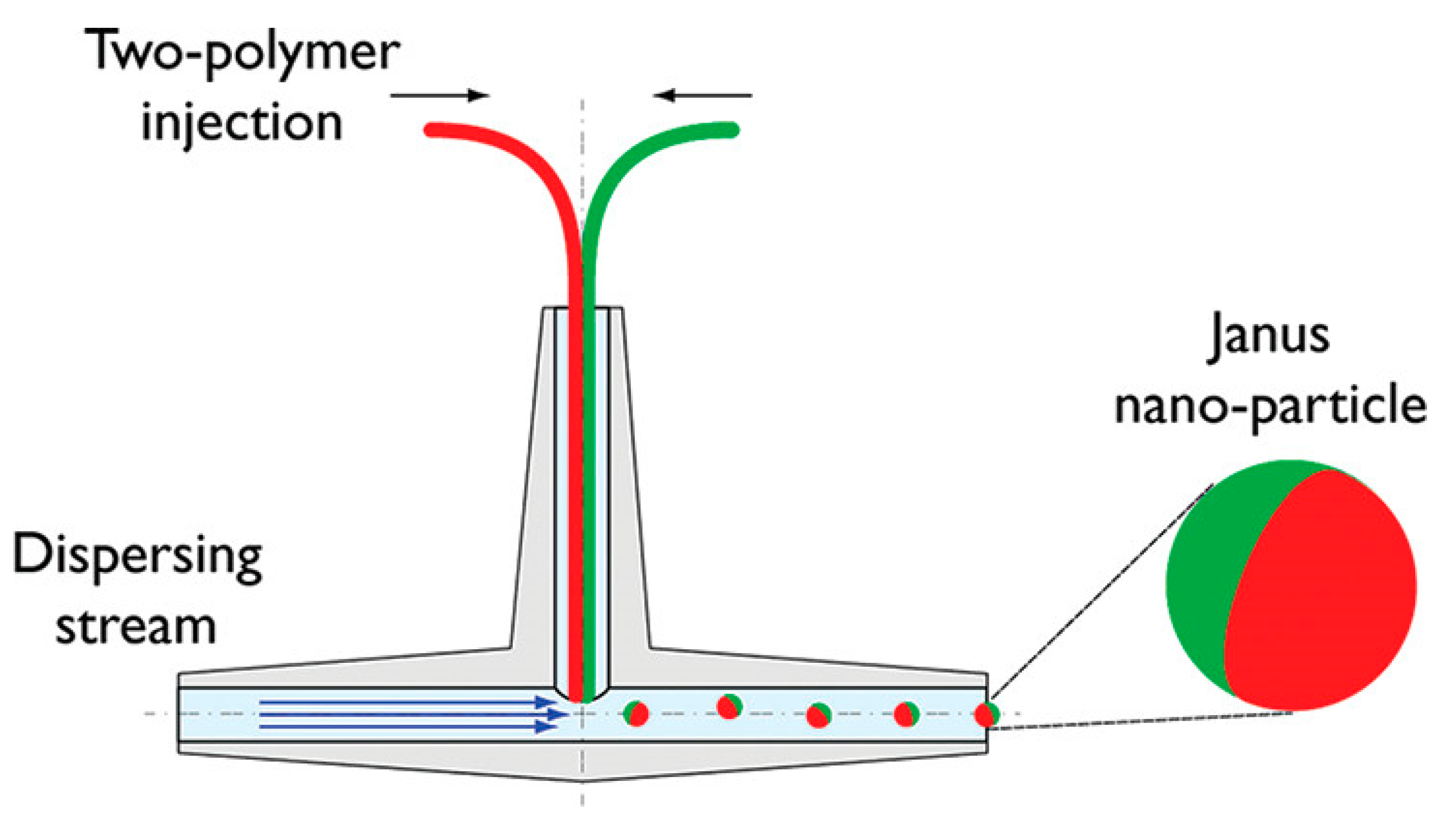
3.3. Microfluidic
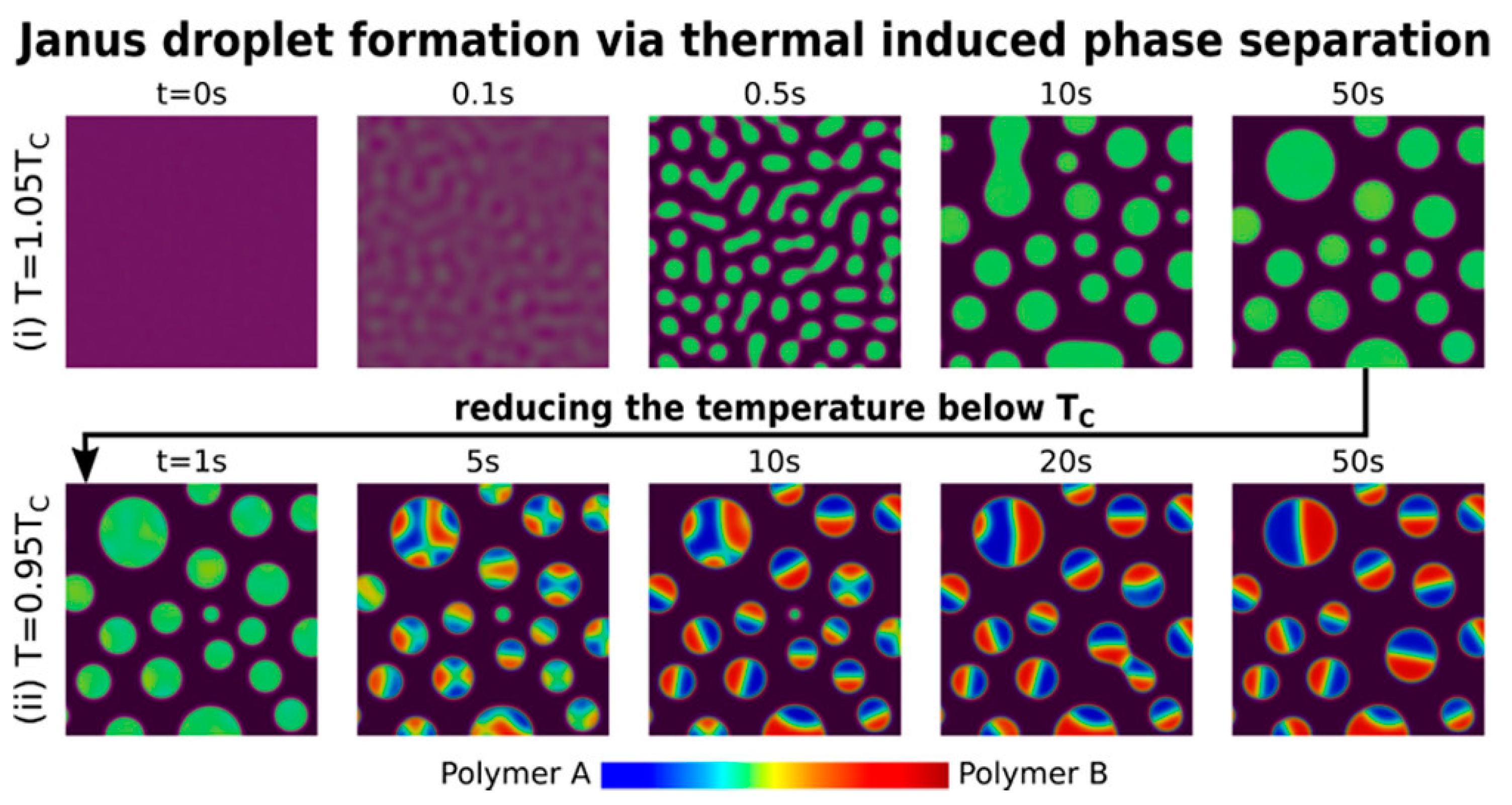
3.4. Phase Separation
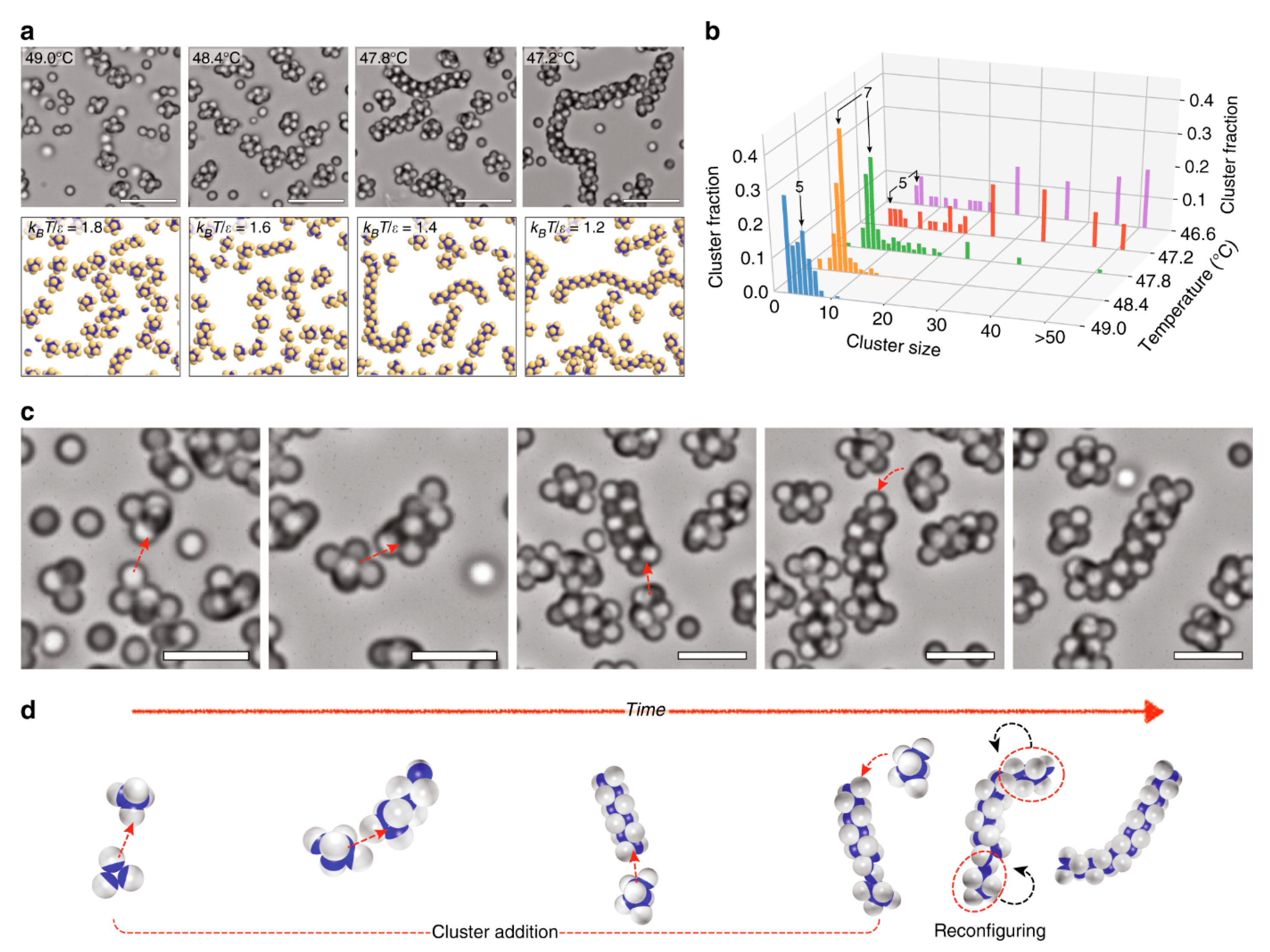
3.5. Factors Affecting the Fabrication of Janus Particles
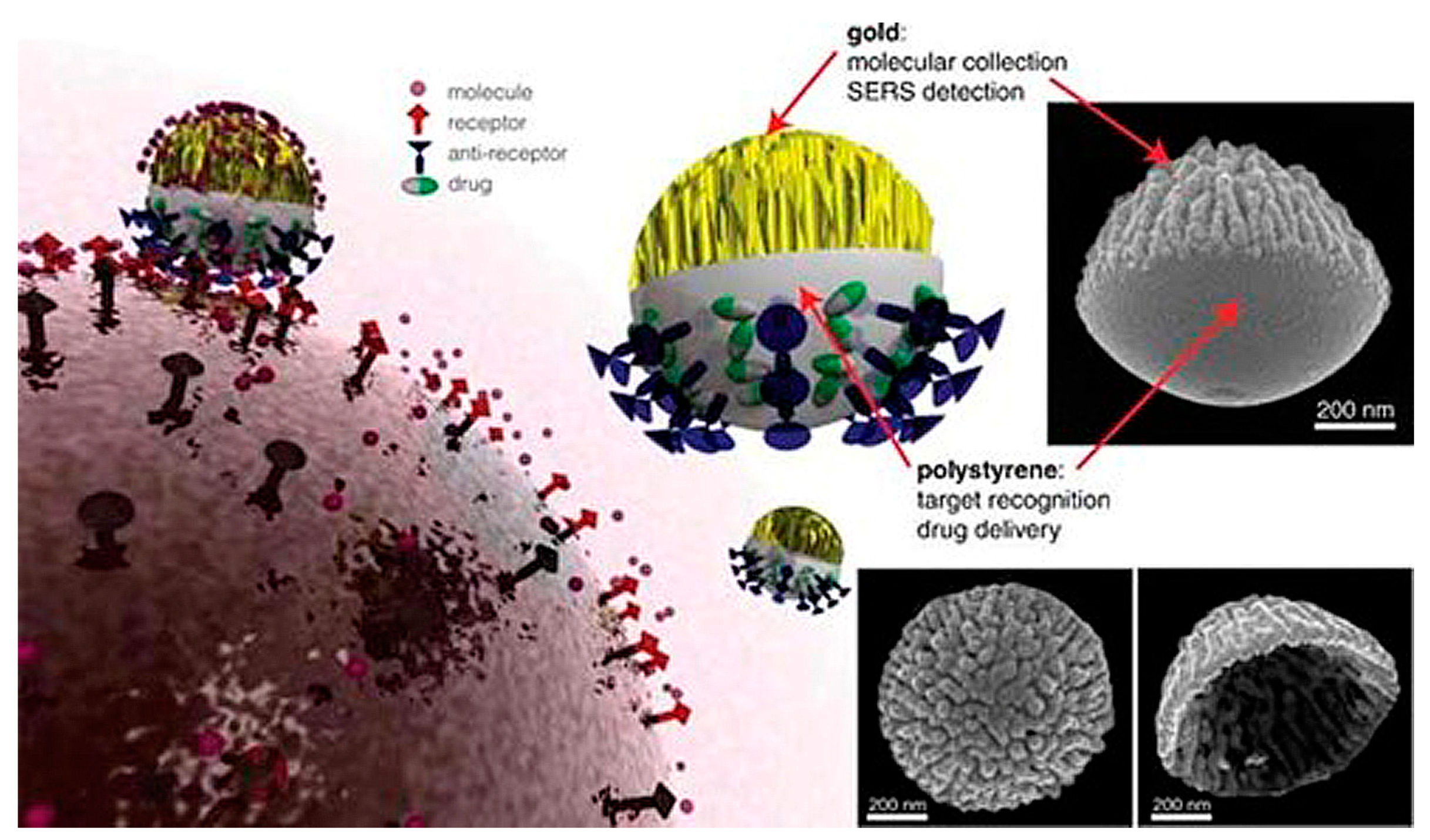
4. Biomedical Applications of Janus Particles
5. Janus Particles as a Potential Delivery System: A Case Study in Diabetes Theranostics
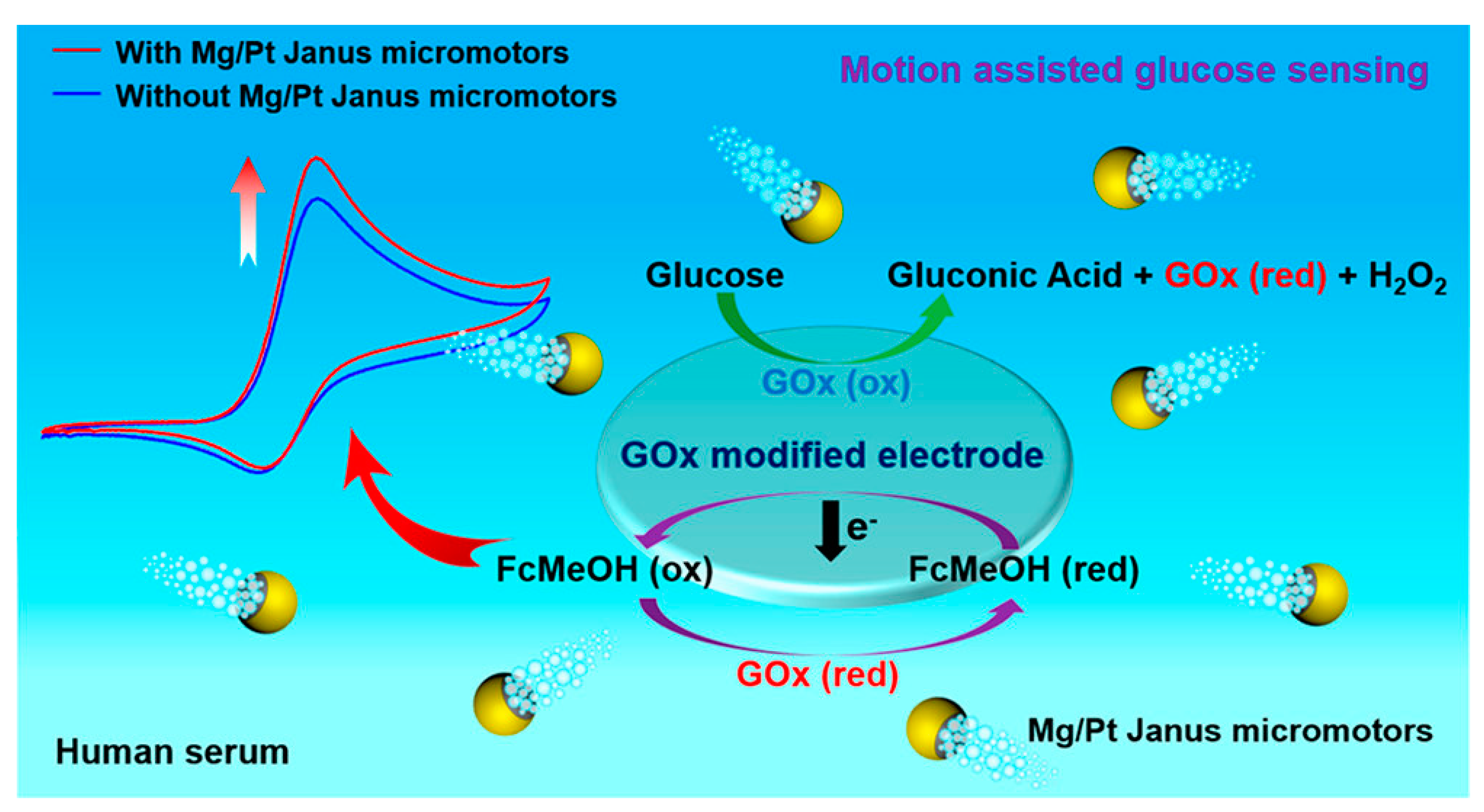
5.1. Biosensor for Diabetes Diagnosis
5.2. Delivery System for Anti-Diabetic Drugs
6. Janus Particles as a Potential Delivery System: A Case Study in Antimicrobial Theranostics
7. Prospects and Challenges of Janus Particles as Drug Delivery Vehicle
7.1. Prospects
7.2. Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. Design and synthesis of Janus micro-and nanoparticles. J. Mater. Chem. 2005, 15, 3745–3760. [Google Scholar] [CrossRef]
- Auschra, S. On the Properties of Self-Thermophoretic Janus Particles. In Von der Fakultät für Physik und Geowissenschaften; der Universität Leipzig: Borna, Germany, 1988. [Google Scholar]
- De Gennes, P.-G. Soft matter. Science 1992, 256, 495–497. [Google Scholar] [CrossRef]
- Rahiminezhad, Z.; Tamaddon, A.M.; Borandeh, S.; Abolmaali, S.S. Janus nanoparticles: New generation of multifunctional nanocarriers in drug delivery, bioimaging and theranostics. Appl. Mater. Today 2020, 18, 100513. [Google Scholar] [CrossRef]
- Tran, L.-T.-C.; Lesieur, S.; Faivre, V. Janus nanoparticles: Materials, preparation and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Grzybowski, B.A.; Granick, S. Janus particle synthesis, assembly, and application. Langmuir 2017, 33, 6964–6977. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, properties and applications of Janus particles. Chem. Soc. Rev. 2012, 41, 4356–4378. [Google Scholar] [CrossRef]
- Su, H.; Hurd Price, C.A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus particles: Design, preparation, and biomedical applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef]
- Lee, K.J.; Yoon, J.; Lahann, J. Recent advances with anisotropic particles. Curr. Opin. Colloid Interface Sci. 2011, 16, 195–202. [Google Scholar] [CrossRef]
- Walther, A.; Muller, A.H.E. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Abbas, Y. Janus Nano-and microparticles as smart drug delivery systems. Curr. Pharm. Biotechnol. 2016, 17, 673–682. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Cui, D.; Jiang, W.; Han, L.; Niu, N. Preparation and application of Janus nanoparticles: Recent development and prospects. Coord. Chem. Rev. 2022, 454, 214318. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Kirillova, A.; Marschelke, C.; Synytska, A. Hybrid Janus particles: Challenges and opportunities for the design of active functional interfaces and surfaces. ACS Appl. Mater. Interfaces 2019, 11, 9643–9671. [Google Scholar] [CrossRef]
- Winkler, J.S.; Barai, M.; Tomassone, M.S. Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds. Exp. Biol. Med. 2019, 244, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Nouri, A. Preparation of Janus nanoparticles and its application in drug delivery. In Design and Development of New Nanocarriers; Elsevier: Amsterdam, Switzerland, 2018; pp. 145–180. [Google Scholar]
- Cho, I.; Lee, K.W. Morphology of latex particles formed by poly(methyl methacrylate)-seeded emulsion polymerization of styrene. J. Appl. Polym. Sci. 1985, 30, 1903–1926. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphael, E.; Veyssié, M. “Janus beads”: Realization and behaviour at water/oil interfaces. EPL (Europhys. Lett.) 1989, 9, 251. [Google Scholar] [CrossRef]
- Yang, T.; Wei, L.; Jing, L.; Liang, J.; Zhang, X.; Tabg, M.; Monteiro, M.J.; Chen, Y.; Wang, Y.; Gu, S.; et al. Dumbbell-Shaped Bi-component Mesoporous Janus Solid Nanoparticles for Biphasic Interface Catalysis. Angew. Chem. Int. Ed. 2017, 56, 8459–8463. [Google Scholar] [CrossRef] [PubMed]
- Shchepelina, O.; Kozlovskaya, V.; Singamaneni, S.; Kharlampieva, E.; Tsukruk, V.V. Replication of anisotropic dispersed particulates and complex continuous templates. J. Mater. Chem. 2010, 20, 6587–6603. [Google Scholar] [CrossRef]
- Zhao, K.; Mason, T.G. Assembly of colloidal particles in solution. Rep. Prog. Phys. 2018, 81, 126601. [Google Scholar] [CrossRef]
- Correia, E.L.; Brown, N.; Razavi, S. Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials 2021, 11, 374. [Google Scholar] [CrossRef]
- Pradhan, S.; Xu, L.; Chen, S. Janus Nanoparticles by Interfacial Engineering. Adv. Funct. Mater. 2007, 17, 2385–2392. [Google Scholar] [CrossRef]
- Tanaka, T.; Okayama, M.; Minami, H.; Okubo, M. Dual stimuli-responsive "mushroom-like" Janus polymer particles as particulate surfactants. Langmuir 2010, 26, 11732–11736. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Okayama, M.; Kitayama, Y.; Kagawa, Y.; Okubo, M. Preparation of "mushroom-like" Janus particles by site-selective surface-initiated atom transfer radical polymerization in aqueous dispersed systems. Langmuir 2010, 26, 7843–7847. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-H.; Gao, X. Nanocomposites with Spatially Separated Functionalities for Combined Imaging and Magnetolytic Therapy. J. Am. Chem. Soc. 2010, 132, 7234–7237. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Walther, A.; Müller, A.H.E. Janus Triad: Three Types of Nonspherical, Nanoscale Janus Particles from One Single Triblock Terpolymer. Macromolecules 2011, 44, 9221–9229. [Google Scholar] [CrossRef]
- Jeong, J.; Um, E.; Park, J.-K.; Kim, M.W. One-step preparation of magnetic Janus particles using controlled phase separation of polymer blends and nanoparticles. RSC Adv. 2013, 3, 11801–11806. [Google Scholar] [CrossRef]
- Lan, Y.; Wu, J.; Han, S.H.; Yadavali, S.; Issadore, D.; Stebe, K.J.; Lee, D. Scalable Synthesis of Janus particles with high naturality. ACS Sustain. Chem. Eng. 2020, 8, 17680–17686. [Google Scholar] [CrossRef]
- Anitas, E.M. Structural characterization of Janus nanoparticles with tunable geometric and chemical asymmetries by small-angle scattering. Phys. Chem. Chem. Phys. 2020, 22, 536–548. [Google Scholar] [CrossRef]
- Lone, S.; Cheong, I.W. Fabrication of polymeric Janus particles by droplet microfluidics. Rsc Adv. 2014, 4, 13322–13333. [Google Scholar] [CrossRef]
- Honciuc, A. Amphiphilic Janus particles at interfaces. In Flowing Matter; Elsevier: Amsterdam, Switzerland, 2019; p. 95. [Google Scholar]
- Poggi, E.; Gohy, J.-F. Janus particles: From synthesis to application. Colloid Polym. Sci. 2017, 295, 2083–2108. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Ding, Y. Gold nanoparticle-conjugated nanomedicine: Design, construction, and structure–efficacy relationship studies. J. Mater. Chem. B 2020, 8, 4813–4830. [Google Scholar] [CrossRef]
- Rucinskaite, G.; Thompson, S.A.; Paterson, S.; de la Rica, R. Enzyme-coated Janus nanoparticles that selectively bind cell receptors as a function of the concentration of glucose. Nanoscale 2017, 9, 5404–5407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; McClements, D.J.; Shi, A.; Zhi, L.; Tian, Y.; Jiao, B.; Liu, H.; Wang, Q. Janus particles: A review of their applications in food and medicine. Crit. Rev. Food Sci. Nutr. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Sun, S.; Wang, Y.; Wei, Y.; Jiang, Y. Biodegradable T2-phage-like Janus nanoparticles for actively-targeted and chemo-photothermal synergistic therapy. Chem. Eng. J. 2022, 428, 131284. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, Y.; Qi, K.; Song, Y.; Li, L.; Zeng, J.; Wang, C.; Zhao, Z. Precise engineering of acorn-like Janus nanoparticles for cancer theranostics. Acta Biomater. 2021, 130, 423–434. [Google Scholar] [CrossRef]
- Le, T.C.; Zhai, J.; Chiu, W.-H.; Tran, P.A.; Tran, N. Janus particles: Recent advances in the biomedical applications. Int. J. Nanomed. 2019, 14, 6749. [Google Scholar] [CrossRef]
- Schick, I.; Lorenz, S.; Gehrig, D.; Tenzer, S.; Storck, W.; Fischer, K.; Strand, D.; Laquai, F.; Tremel, W. Inorganic Janus particles for biomedical applications. Beilstein J. Nanotechnol. 2014, 5, 2346–2362. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Ghosh, D.; Pallavi, P.; Gowtham, P.; Girigoswami, A. Architectural fabrication of multifunctional janus nanostructures for biomedical applications. Nanomed. J. 2022, 9, 180–191. [Google Scholar]
- Percebom, A.M.; Costa, L.H.M. Formation and assembly of amphiphilic Janus nanoparticles promoted by polymer interactions. Adv. Colloid Interface Sci. 2019, 269, 256–269. [Google Scholar] [CrossRef]
- Wu, L.Y.; Ross, B.M.; Hong, S.; Lee, L.P. Bioinspired Nanocorals with Decoupled Cellular Targeting and Sensing Functionality. Small 2010, 6, 503–507. [Google Scholar] [CrossRef]
- Dehghani, E.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Simultaneous two drugs release form Janus particles prepared via polymerization-induced phase separation approach. Colloids Surf. B Biointerfaces 2018, 170, 85–91. [Google Scholar] [CrossRef]
- Aravind, A.; Jeyamohan, P.; Nair, R.; Veeranarayanan, S.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef]
- Alibolandi, M.; Ramezani, M.; Sadeghi, F.; Abnous, K.; Hadizadeh, F. Epithelial cell adhesion molecule aptamer conjugated PEG–PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro. Int. J. Pharm. 2015, 479, 241–251. [Google Scholar] [CrossRef]
- Sayari, E.; Dinarvand, M.; Amini, M.; Azhdarzadeh, M.; Mollarazi, E.; Ghasemi, Z.; Atyabi, F. MUC1 aptamer conjugated to chitosan nanoparticles, an efficient targeted carrier designed for anticancer SN38 delivery. Int. J. Pharm. 2014, 473, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Jo, H.; Song, K.; Cho, M.; Chun, Y.-S.; Jon, S.; Kim, W.J.; Ban, C. Dual-aptamer-based delivery vehicle of doxorubicin to both PSMA (+) and PSMA (−) prostate cancers. Biomaterials 2011, 32, 2124–2132. [Google Scholar] [CrossRef]
- Salem, A.K.; Hung, C.F.; Kim, T.W.; Wu, T.C.; Searson, P.C.; Leong, K.W. Multi-component nanorods for vaccination applications. Nanotechnology 2005, 16, 484. [Google Scholar] [CrossRef]
- Chen, G.; Gibson, K.J.; Liu, D.; Rees, H.C.; Lee, J.-H.; Xia, W.; Lin, R.; Xin, H.L.; Gang, O.; Weizmann, Y. Regioselective surface encoding of nanoparticles for programmable self-assembly. Nat. Mater. 2019, 18, 169–174. [Google Scholar] [CrossRef]
- Shaghaghi, B.; Khoee, S.; Bonakdar, S. Preparation of multifunctional Janus nanoparticles on the basis of SPIONs as targeted drug delivery system. Int. J. Pharm. 2019, 559, 1–12. [Google Scholar] [CrossRef]
- Reguera, J.; de Aberasturi, D.J.; Henriksen-Lacey, M.; Langer, J.; Espinosa, A.; Szczupak, B.; Wilhelm, C.; Liz-Marzán, L.M. Janus plasmonic–magnetic gold–iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale 2017, 9, 9467–9480. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, X.; Liu, W.; Zhang, F.; Zheng, X.; Qiao, P.; Li, J.; Dong, W.-f.; Chen, L. Janus silver-mesoporous silica nanocarriers for SERS traceable and pH-sensitive drug delivery in cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Agrawal, R. Janus Nanoparticles: Recent Advances in Their Interfacial and Biomedical Applications. ACS Appl. Nano Mater. 2019, 2, 1738–1757. [Google Scholar] [CrossRef]
- Lin, C.-C.; Liao, C.-W.; Chao, Y.-C.; Kuo, C. Fabrication and characterization of asymmetric Janus and ternary particles. ACS Appl. Mater. Interfaces 2010, 2, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Safaie, N.; Ferrier Jr, R.C. Janus nanoparticle synthesis: Overview, recent developments, and applications. J. Appl. Phys. 2020, 127, 170902. [Google Scholar] [CrossRef]
- Svensson, F.G.; Seisenbaeva, G.A.; Kotov, N.A.; Kessler, V.G. Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells. Materials 2020, 13, 4856. [Google Scholar] [CrossRef]
- Liu, H.; Pang, B.; Tang, Q.; Müller, M.; Zhang, H.; Dervişoğlu, R.; Zhang, K. Multiresponsive Janus-Like Films: Self-Assembly of Surface-Acylated Cellulose Nanowhiskers and Graphene Oxide for Multiresponsive Janus-Like Films with Time-Dependent Dry-State Structures (Small 44/2020). Small 2020, 16, 2070241. [Google Scholar] [CrossRef]
- Liu, F.; Goyal, S.; Forrester, M.; Ma, T.; Miller, K.; Mansoorieh, Y.; Henjum, J.; Zhou, L.; Cochran, E.; Jiang, S. Self-assembly of Janus dumbbell nanocrystals and their enhanced surface plasmon resonance. Nano Lett. 2018, 19, 1587–1594. [Google Scholar] [CrossRef]
- Iida, R.; Kawamura, H.; Niikura, K.; Kimura, T.; Sekiguchi, S.; Joti, Y.; Bessho, Y.; Mitomo, H.; Nishino, Y.; Ijiro, K. Synthesis of Janus-like gold nanoparticles with hydrophilic/hydrophobic faces by surface ligand exchange and their self-assemblies in water. Langmuir 2015, 31, 4054–4062. [Google Scholar] [CrossRef]
- Nandan, B.; Horechyy, A. Hairy core–shell polymer nano-objects from self-assembled block copolymer structures. ACS Appl. Mater. Interfaces 2015, 7, 12539–12558. [Google Scholar] [CrossRef]
- Paulson, J.A.; Mesbah, A.; Zhu, X.; Molaro, M.C.; Braatz, R.D. Control of self-assembly in micro-and nano-scale systems. J. Process Control 2015, 27, 38–49. [Google Scholar] [CrossRef]
- Erhardt, R.; Böker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Müller, A.H.E. Janus micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef]
- Oh, J.S.; Lee, S.; Glotzer, S.C.; Yi, G.-R.; Pine, D.J. Colloidal fibers and rings by cooperative assembly. Nat. Commun. 2019, 10, 3936. [Google Scholar] [CrossRef]
- Xie, H.; She, Z.-G.; Wang, S.; Sharma, G.; Smith, J.W. One-Step Fabrication of Polymeric Janus Nanoparticles for Drug Delivery. Langmuir 2012, 28, 4459–4463. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, M.; Wang, Y.; Xu, Z. Droplet-based microfluidic synthesis of (Au nanorod@ Ag)–polyaniline Janus nanoparticles and their application as a surface-enhanced Raman scattering nanosensor for mercury detection. Anal. Methods 2019, 11, 3966–3973. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Tang, C.; Li, J.; Shen, K.; Liu, J.; Qu, X.; Li, J.; Wang, Q.; Yang, Z. Emulsion interfacial synthesis of asymmetric Janus particles. Macromolecules 2011, 44, 3787–3794. [Google Scholar] [CrossRef]
- Liu, B.; Möhwald, H.; Wang, D. Synthesis of Janus particles via kinetic control of phase separation in emulsion droplets. Chem. Commun. 2013, 49, 9746–9748. [Google Scholar] [CrossRef] [PubMed]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- Rudin, A.; Choi, P. The Elements of Polymer Science and Engineering; Academic Press: Cambridge, MA, USA, 2013; Chapter 12; pp. 495–520. [Google Scholar]
- Zhang, H.; Wang, F.; Nestler, B. Janus Droplet Formation via Thermally Induced Phase Separation: A Numerical Model with Diffusion and Convection. Langmuir 2022, 38, 6882–6895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Schultz, M.J.; Chen, Q.; Moore, J.S.; Granick, S. Solvent-free synthesis of Janus colloidal particles. Langmuir 2008, 24, 10073–10077. [Google Scholar] [CrossRef] [PubMed]
- Kovach, I.; Koetz, J.; Friberg, S.E. Janus emulsions stabilized by phospholipids. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 66–71. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research advances in the synthesis, application, assembly, and calculation of janus materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Bradley, L.C.; Stebe, K.J.; Lee, D. Clickable janus particles. J. Am. Chem. Soc. 2016, 138, 11437–11440. [Google Scholar] [CrossRef]
- Sounart, T.L.; Liu, J.; Voigt, J.A.; Hsu, J.W.P.; Spoerke, E.D.; Tian, Z.; Jiang, Y.B. Sequential nucleation and growth of complex nanostructured films. Adv. Funct. Mater. 2006, 16, 335–344. [Google Scholar] [CrossRef]
- Yang, L.; Thérien-Aubin, H. Behavior of colloidal gels made of thermoresponsive anisotropic nanoparticles. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Patnaik, A. Diabetes mellitus: Exploring the challenges in the drug development process. Perspect Clin. Res. 2012, 3, 109. [Google Scholar] [PubMed]
- Morgan, L. Challenges and opportunities in managing type 2 diabetes. Am. Health Drug Benefits 2017, 10, 197. [Google Scholar]
- Wu, A.Y.-H.; Little, V.J.; Low, B. Inbound open innovation for pharmaceutical markets: A case study of an anti-diabetic drug in-licensing decision. J. Bus. Ind. Mark. 2016, 31, 205–218. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.-Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, Silica-Coated, Janus-Like Plasmonic-Magnetic Nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef]
- Raffournier, C.; Saulnier, P.; Boury, F.; Proust, J.E.; Lepault, J.; Erk, I.; Ollivon, M.; Couvreur, P.; Dubernet, C. Oil/water “hand-bag like structures”: How interfacial rheology can help to understand their formation? J. Drug Deliv. Sci. Technol. 2005, 15, 3–9. [Google Scholar] [CrossRef]
- Sun, T.-M.; Du, J.-Z.; Yao, Y.-D.; Mao, C.-Q.; Dou, S.; Huang, S.-Y.; Zhang, P.-Z.; Leong, K.W.; Song, E.-W.; Wang, J. Simultaneous Delivery of siRNA and Paclitaxel via a “Two-in-One” Micelleplex Promotes Synergistic Tumor Suppression. ACS Nano 2011, 5, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, M.; Winkler, J.; Garbuzenko, O.; Minko, T. 337461 Biodegradable Janus Particles for Drug Delivery: Bi-Compartmental Encapsulation of Two API of Disparate Solubility. Presented at the AIChE Annual Meeting, San Francisco, CA, USA, 7 November 2013. [Google Scholar]
- Pan, J.; Wen, M.; Yin, D.; Jiang, B.; He, D.; Guo, L. Design and synthesis of novel amphiphilic Janus dendrimers for bone-targeted drug delivery. Tetrahedron 2012, 68, 2943–2949. [Google Scholar] [CrossRef]
- Hwang, S.; Lahann, J. Differentially degradable janus particles for controlled release applications. Macromol. Rapid Commun. 2012, 33, 1178–1183. [Google Scholar] [CrossRef]
- Misra, A.C.; Bhaskar, S.; Clay, N.; Lahann, J. Multicompartmental Particles for Combined Imaging and siRNA Delivery. Adv. Mater. 2012, 24, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus nanoparticles: From fabrication to (bio) applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zhang, Y.; Xiao, S.; Bi, F.; Zhao, J.; Gai, G.; Ding, J. Glucose oxidase-based glucose-sensitive drug delivery for diabetes treatment. Polymer 2017, 9, 255. [Google Scholar] [CrossRef]
- Rauf, S.; Hayat Nawaz, M.A.; Badea, M.; Marty, J.L.; Hayat, A. Nano-engineered biomimetic optical sensors for glucose monitoring in diabetes. Sensors 2016, 16, 1931. [Google Scholar] [CrossRef]
- Lablanche, S.; Vantyghem, M.-C.; Kessler, L.; Wojtusciszyn, A.; Borot, S.; Thivolet, C.; Girerd, S.; Bosco, D.; Bosson, J.-L.; Colin, C. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 527–537. [Google Scholar] [CrossRef]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.G.; Hoekstra, J.B.; DeVries, J.H. Insulin therapy for type 2 diabetes. Diabetes Care 2009, 32, S253–S259. [Google Scholar] [CrossRef]
- Home, P.; Riddle, M.; Cefalu, W.T.; Bailey, C.J.; Bretzel, R.G.; Del Prato, S.; Leroith, D.; Schernthaner, G.; Van Gaal, L.; Raz, I. Insulin therapy in people with type 2 diabetes: Opportunities and challenges? Diabetes Care 2014, 37, 1499–1508. [Google Scholar] [CrossRef]
- Jabeen, M. Insulin treatment for diabetes. InnovAiT 2020, 13, 739–746. [Google Scholar] [CrossRef]
- Danquah, M.K.; Jeevanandam, J. The current state of diabetes treatment. In Emerging Nanomedicines for Diabetes Mellitus Theranostics; Elsevier: Amsterdam, Switzerland, 2022; pp. 1–31. [Google Scholar]
- Schattling, P.; Thingholm, B.; Stadler, B. Enhanced diffusion of glucose-fueled Janus particles. Chem. Mater. 2015, 27, 7412–7418. [Google Scholar] [CrossRef]
- Fan, Y.L.; Tan, C.H.; Lui, Y.; Zudhistira, D.; Loo, S.C.J. Mechanistic formation of drug-encapsulated Janus particles through emulsion solvent evaporation. RSC Adv. 2018, 8, 16032–16042. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y. Drug Delivery in the Anterior Chamber of the Eye for Islet Transplantation as a Diabetic Treatment. Ph.D. Thesis, Nanyang Technological University, Singapore, 2018. [Google Scholar]
- Jerome Yi Guang, L. Investigation of Polymeric Biodegradable-Biocompatible Janus Particles in Drug Delivery Systems and Further Augmentation via a Facile Synthesis Method. Ph.D. Thesis, Nanyang Technological University, Singapore, 2019. [Google Scholar]
- Lim, Y.G.J.; Poh, K.C.W.; Loo, S.C.J. Hybrid Janus microparticles achieving selective encapsulation for theranostic applications via a facile solvent emulsion method. Macromol. Rapid Commun. 2019, 40, 1800801. [Google Scholar] [CrossRef]
- Díez, P.; Sánchez, A.; Gamella, M.; Martínez-Ruíz, P.; Aznar, E.; De La Torre, C.; Murguía, J.R.; Martínez-Máñez, R.; Villalonga, R.; Pingarrón, J.M. Toward the design of smart delivery systems controlled by integrated enzyme-based biocomputing ensembles. J. Am. Chem. Soc. 2014, 136, 9116–9123. [Google Scholar] [CrossRef]
- Lu, C.; Liu, X.; Li, Y.; Yu, F.; Tang, L.; Hu, Y.; Ying, Y. Multifunctional janus hematite–silica nanoparticles: Mimicking peroxidase-like activity and sensitive colorimetric detection of glucose. ACS Appl. Mater. Interfaces 2015, 7, 15395–15402. [Google Scholar] [CrossRef]
- Ando, M.; Tsuchiya, M.; Itai, S.; Murayama, T.; Kurashina, Y.; Heo, Y.J.; Onoe, H. Janus hydrogel microbeads for glucose sensing with pH calibration. Sensors 2021, 21, 4829. [Google Scholar] [CrossRef]
- Kong, L.; Rohaizad, N.; Nasir, M.Z.M.; Guan, J.; Pumera, M. Micromotor-Assisted Human Serum Glucose Biosensing. Anal. Chem. 2019, 91, 5660–5666. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, X.; Ali, Y.; Berggren, P.-O.; Loo, S.C.J. Local release of rapamycin by microparticles delays islet rejection within the anterior chamber of the eye. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharm. 2021, 894, 173854. [Google Scholar] [CrossRef]
- Janowitz, T.; Kleeman, S.; Vonderheide, R.H. Reconsidering dexamethasone for antiemesis when combining chemotherapy and immunotherapy. Oncologist 2021, 26, 269–273. [Google Scholar] [CrossRef]
- Lim, M.P.A.; Lee, W.L.; Widjaja, E.; Loo, S.C.J. One-step fabrication of core–shell structured alginate–PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater. Sci. 2013, 1, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Wadsworth, S.; Recinos, V.; Mehta, V.; Vellimana, A.; Li, K.; Rosenblatt, J.; Do, H.; Gallia, G.L.; Siu, I.M. Local delivery of rapamycin: A toxicity and efficacy study in an experimental malignant glioma model in rats. Neuro-Oncology 2011, 13, 700–709. [Google Scholar] [CrossRef]
- Loos, J.A.; Churio, M.S.; Cumino, A.C. Anthelminthic activity of glibenclamide on secondary cystic echinococcosis in mice. PLoS Negl. Trop. Dis. 2017, 11, e0006111. [Google Scholar] [CrossRef]
- Korol, S.V.; Jin, Z.; Jin, Y.; Bhandage, A.K.; Tengholm, A.; Gandasi, N.R.; Barg, S.; Espes, D.; Carlsson, P.-O.; Laver, D. Functional characterization of native, high-affinity GABAA receptors in human pancreatic β cells. EBioMedicine 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Sharifi, M.; Soltani, N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur. J. Pharm. 2018, 826, 75–84. [Google Scholar] [CrossRef]
- Dungan, K.; DeSantis, A. Glucagon-like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus. 2016. Available online: https://staffsites.sohag-univ.edu.eg/uploads/1211/1540967016%20-%20Glucagon-like%20peptide-1%20receptor%20agonists%20for%20the%20treatment%20of%20type%202%20diabetes%20mellitus%20-%20UpToDate.pdf (accessed on 4 February 2018).
- Son, D.O.; Liu, W.; Li, X.; Prud’homme, G.J.; Wang, Q. Combined effect of GABA and glucagon-like peptide-1 receptor agonist on cytokine-induced apoptosis in pancreatic β-cell line and isolated human islets. J. Diabetes 2019, 11, 563–572. [Google Scholar] [CrossRef]
- Bao, F.; Pei, G.; Wu, Z.; Zhuang, H.; Zhang, Z.; Huan, Z.; Wu, C.; Chang, J. Bioactive Self-Pumping Composite Wound Dressings with Micropore Array Modified Janus Membrane for Enhanced Diabetic Wound Healing. Adv. Funct. Mater. 2020, 30, 2005422. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Sun, D.; Jiang, B.; Liang, F.; Yang, Z. Emulsion interfacial synthesis of polymer/inorganic Janus particles. Langmuir 2019, 35, 6032–6038. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Luo, J.-Q.; Li, J.-X.; Huang, Q.-Y.; Shi, X.-X.; Huang, Y.-C.; Leong, K.W.; He, W.-l.; Du, J.-Z. Biofunctional Janus particles promote phagocytosis of tumor cells by macrophages. Chem. Sci. 2020, 11, 5323–5327. [Google Scholar] [CrossRef]
- Fu, J.; An, D.; Song, Y.; Wang, C.; Qiu, M.; Zhang, H. Janus nanoparticles for cellular delivery chemotherapy: Recent advances and challenges. Coord. Chem. Rev. 2020, 422, 213467. [Google Scholar] [CrossRef]
- Marschelke, C.; Fery, A.; Synytska, A. Janus particles: From concepts to environmentally friendly materials and sustainable applications. Colloid Polym. Sci. 2020, 298, 841–865. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Y.; Yu, D.-G.; Chen, Z.; Wang, K.; Liu, Y.; Williams, G.R. Multifunctional fabrics finished using electrosprayed hybrid Janus particles containing nanocatalysts. Chem. Eng. J. 2021, 411, 128474. [Google Scholar] [CrossRef]
- Panwar, K.; Jassal, M.; Agrawal, A.K. Readily dispersible antimicrobial Ag–SiO2 Janus particles and their application on cellulosic fabric. Carbohydr. Polym. 2018, 187, 43–50. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Lerner, M.I.; Pervikov, A.V.; Kazantsev, S.O.; Fomenko, A.N. Development of Fe/Cu and Fe/Ag bimetallic nanoparticles for promising biodegradable materials with antimicrobial effect. Nanotechnologies Russ. 2018, 13, 18–25. [Google Scholar] [CrossRef]
- Jia, R.; Jiang, H.; Jin, M.; Wang, X.; Huang, J. Silver/chitosan-based Janus particles: Synthesis, characterization, and assessment of antimicrobial activity in vivo and vitro. Food Res. Int. 2015, 78, 433–441. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Li, Q.; Dong, G.; Ai, J.; Liu, H.; Chen, Q. Click-Grafting of Cardanol onto Mesoporous Silica/Silver Janus Particles for Enhanced Hemostatic and Antibacterial Performance. ACS Appl. Bio Mater. 2020, 3, 9054–9064. [Google Scholar] [CrossRef]
- Das, D.; Chen, W.-L.; Chuang, H.-S. Rapid and Sensitive Pathogen Detection by DNA Amplification Using Janus Particle-Enabled Rotational Diffusometry. Anal. Chem. 2021, 93, 13945–13951. [Google Scholar] [CrossRef]
- Sinn, I.; Albertson, T.; Kinnunen, P.; Breslauer, D.N.; McNaughton, B.H.; Burns, M.A.; Kopelman, R. Asynchronous Magnetic Bead Rotation Microviscometer for Rapid, Sensitive, and Label-Free Studies of Bacterial Growth and Drug Sensitivity. Anal. Chem. 2012, 84, 5250–5256. [Google Scholar] [CrossRef]
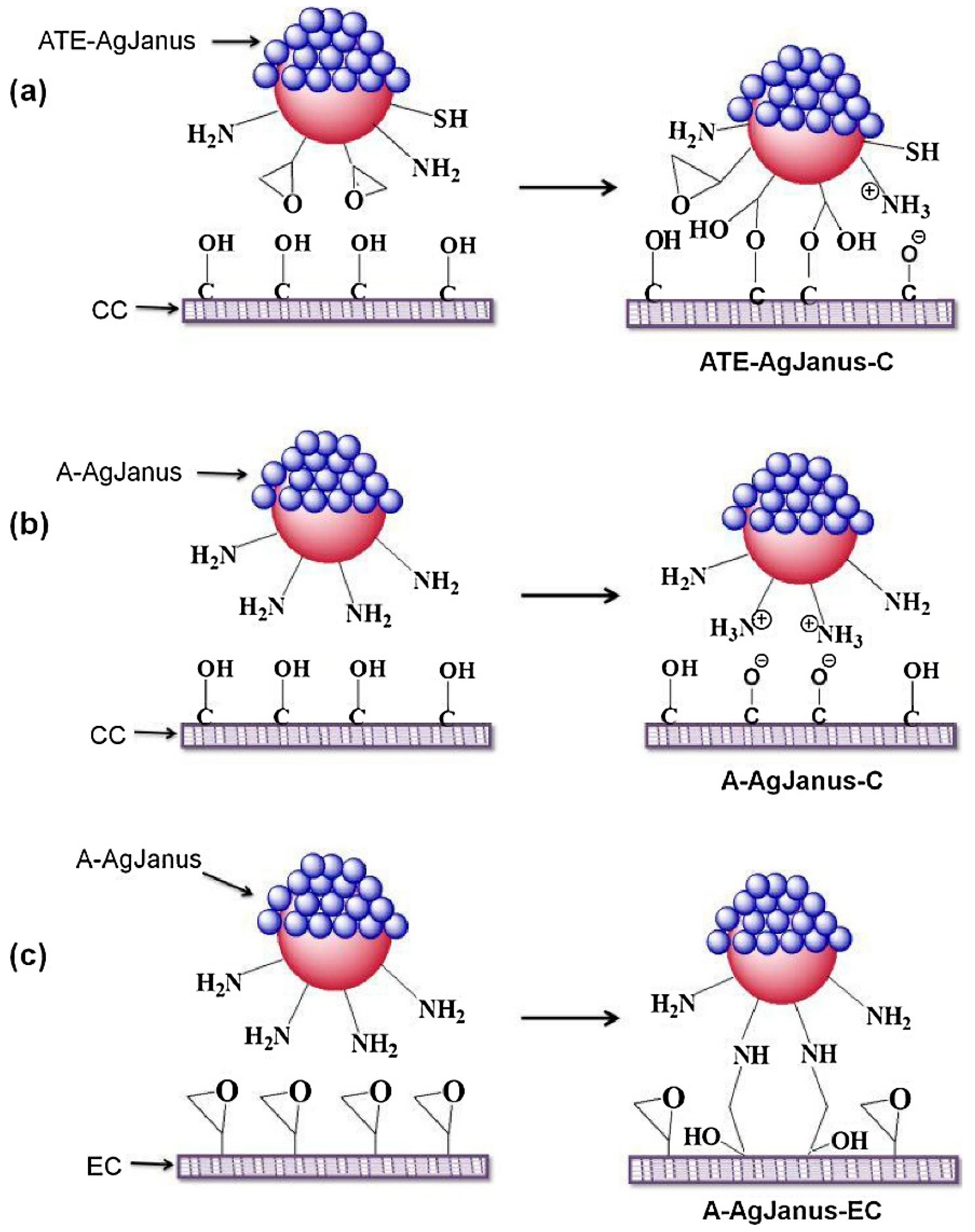
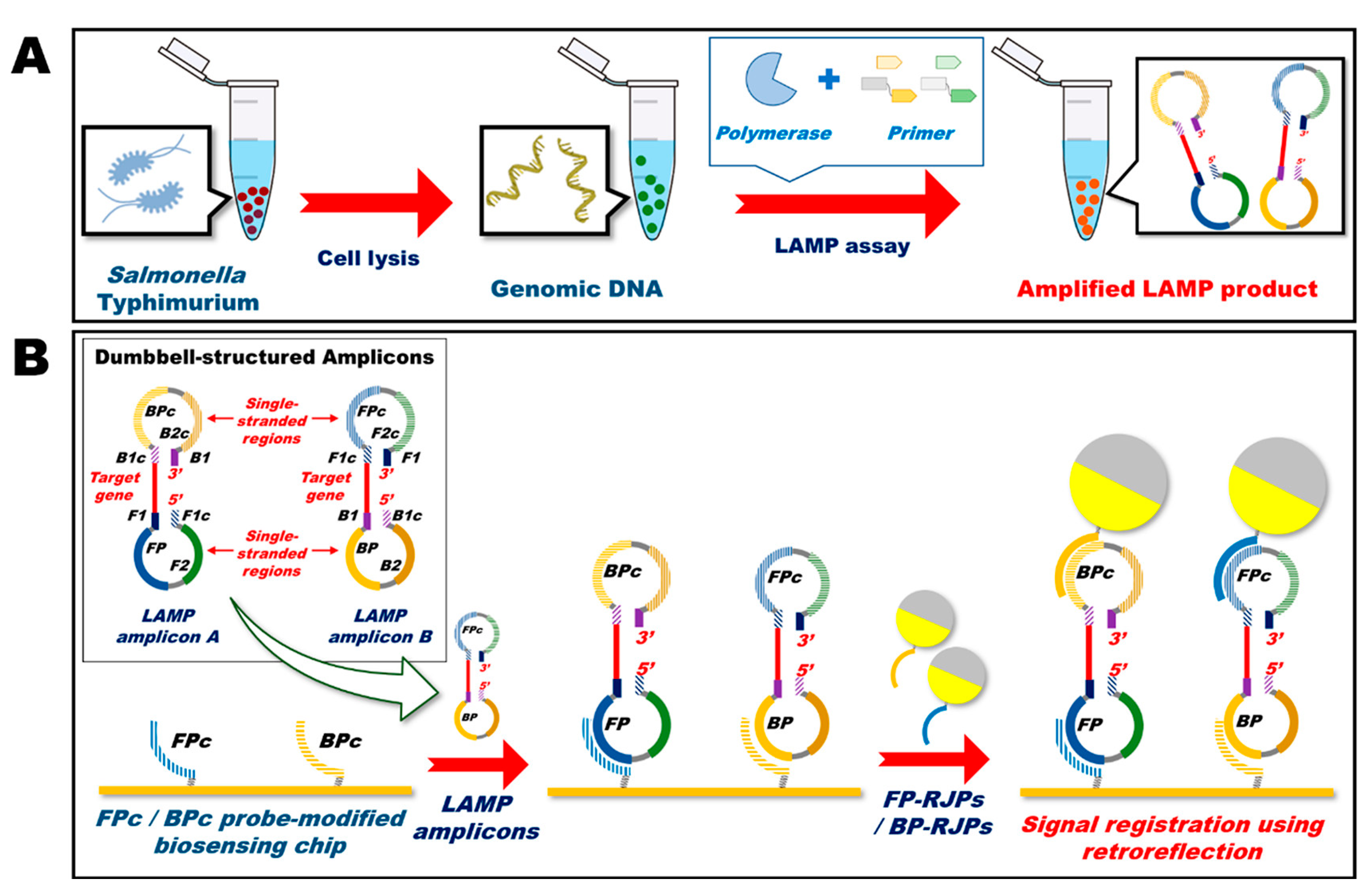
- Chun, H.J.; Kim, S.; Han, Y.D.; Kim, K.R.; Kim, J.-H.; Yoon, H.; Yoon, H.C. Salmonella Typhimurium Sensing Strategy Based on the Loop-Mediated Isothermal Amplification Using Retroreflective Janus Particle as a Nonspectroscopic Signaling Probe. ACS Sens. 2018, 3, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zaman, A.; Sayed, E.; Evans, D.; Morgan, S.; Samwell, C.; Hall, J.; Arshad, M.S.; Singh, N.; Qutachi, O. Electrohydrodynamic atomisation driven design and engineering of opportunistic particulate systems for applications in drug delivery, therapeutics and pharmaceutics. Adv. Drug Deliv. Rev. 2021, 176, 113788. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Alshaer, W.; Ma’mon, M.H.; Lambuk, L.; Ahmed, N.; Mustafa, M.Z.; Low, S.C.; Jaafar, J.; Ferji, K.; Six, J.-L. Applications of Alginate-Based Nanomaterials in Enhancing the Therapeutic Effects of Bee Products. Front. Mol. Biosci. 2022, 9, 865833. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wei, Z.; Xue, C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Naik, R.R.; Maurya, P.; Shakya, A.K. Pulmonary Drug Delivery: Role and Application of Lipid Carriers. Syst. Rev. Pharm. 2020, 11, 1758–1771. [Google Scholar]
- Vandghanooni, S.; Barar, J.; Eskandani, M.; Omidi, Y. Aptamer-conjugated mesoporous silica nanoparticles for simultaneous imaging and therapy of cancer. TrAC Trends Anal. Chem. 2020, 123, 115759. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Doshi, N.; Mitragotri, S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1247–1256. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Djohari, H.; Dormidontova, E.E. Kinetics of nanoparticle targeting by dissipative particle dynamics simulations. Biomacromolecules 2009, 10, 3089–3097. [Google Scholar] [CrossRef]
- Yi, Y.; Sanchez, L.; Gao, Y.; Lee, K.; Yu, Y. Interrogating cellular functions with designer Janus particles. Chem. Mater. 2017, 29, 1448–1460. [Google Scholar] [CrossRef]
- Hassan, N.; Oyarzun-Ampuero, F.; Lara, P.; Guerrero, S.; Cabuil, V.; Abou-Hassan, A.; J Kogan, M. Flow chemistry to control the synthesis of nano and microparticles for biomedical applications. Curr. Top. Med. Chem. 2014, 14, 676–689. [Google Scholar] [CrossRef]
- Ning, Y.; Wang, C.; Ngai, T.; Tong, Z. Fabrication of tunable Janus microspheres with dual anisotropy of porosity and magnetism. Langmuir 2013, 29, 5138–5144. [Google Scholar] [CrossRef]
- Mills, Z.G.; Mao, W.; Alexeev, A. Mesoscale modeling: Solving complex flows in biology and biotechnology. Trends Biotechnol. 2013, 31, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.J.; Wong, C.H.; Chen, Z. Janus Particle Preparation through UV-Induced Partial Photodegradation of Spin-Coated Particle Films. Langmuir 2021, 37, 8167–8176. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Wang, Y.; Peng, B.; Deng, Z. Bioinspired polydopamine coating as a versatile platform for synthesizing asymmetric Janus particles at an air-water interface. Appl. Surf. Sci. 2020, 509, 145360. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, J.; Xiang, Y.; Zheng, S.; Wu, Y.; Liu, J.; Zhu, X.; Zhang, Y. Shedding Light on Luminescent Janus Nanoparticles: From Synthesis to Photoluminescence and Applications. Small 2022, 18, 2200020. [Google Scholar] [CrossRef] [PubMed]
- Keßler, R.; Bräuer, D.; Dreißigacker, C.; Drescher, J.; Lozano, C.; Bechinger, C.; Born, P.; Voigtmann, T. Direct-imaging of light-driven colloidal Janus particles in weightlessness. Rev. Sci. Instrum. 2020, 91, 013902. [Google Scholar] [CrossRef] [PubMed]
- Jalilvand, Z.; Haider, H.; Cui, J.; Kretzschmar, A.I. Pt-SiO2 Janus particles and the water/oil interface: A competition between motility and thermodynamics. Langmuir 2020, 36, 6880–6887. [Google Scholar] [CrossRef]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial assembly and applications of functional mesoporous materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Lao, J.; Luo, K.; Liu, X.; Sui, K.; Gao, J.; Jiang, L. Reliable and Low Temperature Actuation of Water and Oil Slugs in Janus Photothermal Slippery Tube. ACS Appl. Mater. Interfaces 2022, 14, 17968–17974. [Google Scholar] [CrossRef]


| Janus Particles | Synthesis Technique | Formation and Characteristic Features | Application | References |
|---|---|---|---|---|
| Hexanethiolate-capped Janus gold (AuC6) nanoparticles | Masking |
| Generation of sophisticated and functional nanostructures via careful selection of chemical ligands. | [22] |
| “Mushroom-like” Janus poly(methyl methacrylate) (PMMA)/poly(styrene-2-(2-bromoisobutyryloxy)ethyl methacrylate)-graft-poly(2-(dimethylamino)ethyl methacrylate) (PDM) particles | Phase separation |
| They can be applied as particulate surfactants for o/w emulsion due to their dual stimuli-responsive behavior. | [23,24] |
| Pyrene labelled-magnetic nanoparticles (MNPs) linked-poly(styrene-block-allyl alcohol) (PS-b-PAA) Janus nanoparticles | Phase separation |
|
| [25] |
| Poly (tert-butoxystyrene)-block-polybutadiene-block-poly(tert-butyl methacrylate) (tSBT) polymeric Janus particles | Controlled phase transitions |
| For tuning of bulk morphologies to form soft Janus particles with nano-scaled dimensions and different non-spherical shapes from a single triblock terpolymer. The technique improves water solubility and the stimuli responsiveness of Janus particles, enabling tailored functionalities in aqueous media. | [26] |
| Magnetic Janus particles | Solvent evaporation-induced phase separation and microfluidic technique |
| Facilitates the use of a single emulsification step to create a versatile microfluidic technology that can generate various structural compositions of Janus particles. | [27] |
| Amphiphilic Janus particles | Single-emulsion polymerization method |
| The study showed the synthesis of highly natural amphiphilic Janus particles with plant-derived materials. These Janus particles are identified to possess the ability to stabilize oil-in-water emulsions under flowing conditions. | [28] |
| Janus Particles | Characteristics Features and/or Performance | References |
|---|---|---|
| Hybrid plasmonic-magnetic and biocompatible SiO2-coated Ag/Fe2O3 Janus particles. |
| [80] |
| Biocompatible Janus particles are made up of poly(lactic-co-glycolic acid) (PLGA). |
| [64] |
| ‘Handbags-type’ Janus particles with 2 separate oil and aqueous cores surrounded by a phospholipid. |
| [81] |
| A two-in-one micelle-plex (Janus). |
| [82] |
| Janus nanoparticles with ice-cream coned shape. |
| [83] |
| Amphiphilic Janus dendrimers. |
| [84] |
| Janus particles with differentially degradable compartments. |
| [85] |
| Janus particles for theranostic application. |
| [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, K.X.; Danquah, M.K.; Jeevanandam, J.; Barhoum, A. Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications. Pharmaceutics 2023, 15, 423. https://doi.org/10.3390/pharmaceutics15020423
Tan KX, Danquah MK, Jeevanandam J, Barhoum A. Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications. Pharmaceutics. 2023; 15(2):423. https://doi.org/10.3390/pharmaceutics15020423
Chicago/Turabian StyleTan, Kei Xian, Michael K. Danquah, Jaison Jeevanandam, and Ahmed Barhoum. 2023. "Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications" Pharmaceutics 15, no. 2: 423. https://doi.org/10.3390/pharmaceutics15020423
APA StyleTan, K. X., Danquah, M. K., Jeevanandam, J., & Barhoum, A. (2023). Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications. Pharmaceutics, 15(2), 423. https://doi.org/10.3390/pharmaceutics15020423