Synergistic Antitumor Potency of a Self-Assembling Cyclodextrin Nanoplex for the Co-Delivery of 5-Fluorouracil and Interleukin-2 in the Treatment of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Studies
2.1.1. Real-Time Cell Viability Analysis (RTCA)
2.1.2. Antitumoral Activity of CD Nanoplexes on 3D Spheroid Tumor Culture
2.1.3. Apoptosis Assays in 3D Spheroid Tumor Culture
2.1.4. Light Microscopy Analysis of 3D Tumor Cells in Plastic Blocks
2.1.5. Immunocytochemical Analysis of 3D Spheroid Tumor Culture
2.1.6. Assessment of Biological Activity of IL-2
2.2. In Vivo Studies
2.2.1. Safety Studies of CD Polymers
2.2.2. Assessment of In vivo Antitumor Activity of CD Nanoplexes
2.2.3. Histomorphometry on Cancer Nodules
2.3. Statistical Analysis
3. Results
3.1. Cell Culture Studies
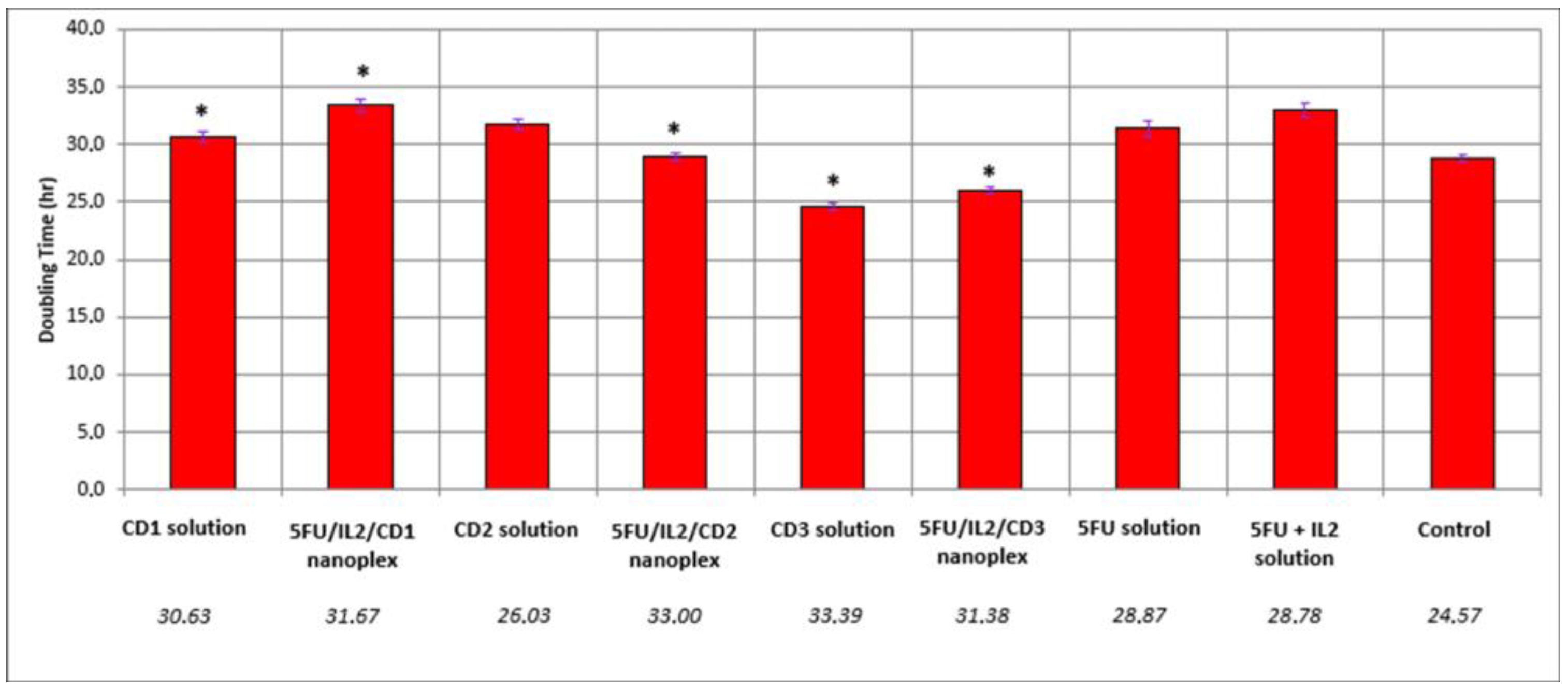
3.1.1. Real-Time Proliferation Analysis
3.1.2. Antitumoral Activity of CD Nanoplexes on 3D Cultured Spheroid Tumor
3.1.3. Apoptosis Assays of 3D Tumor Model Using TUNEL
3.1.4. Light Microscopy Analysis of 3D Tumor Cells on Plastic Blocks
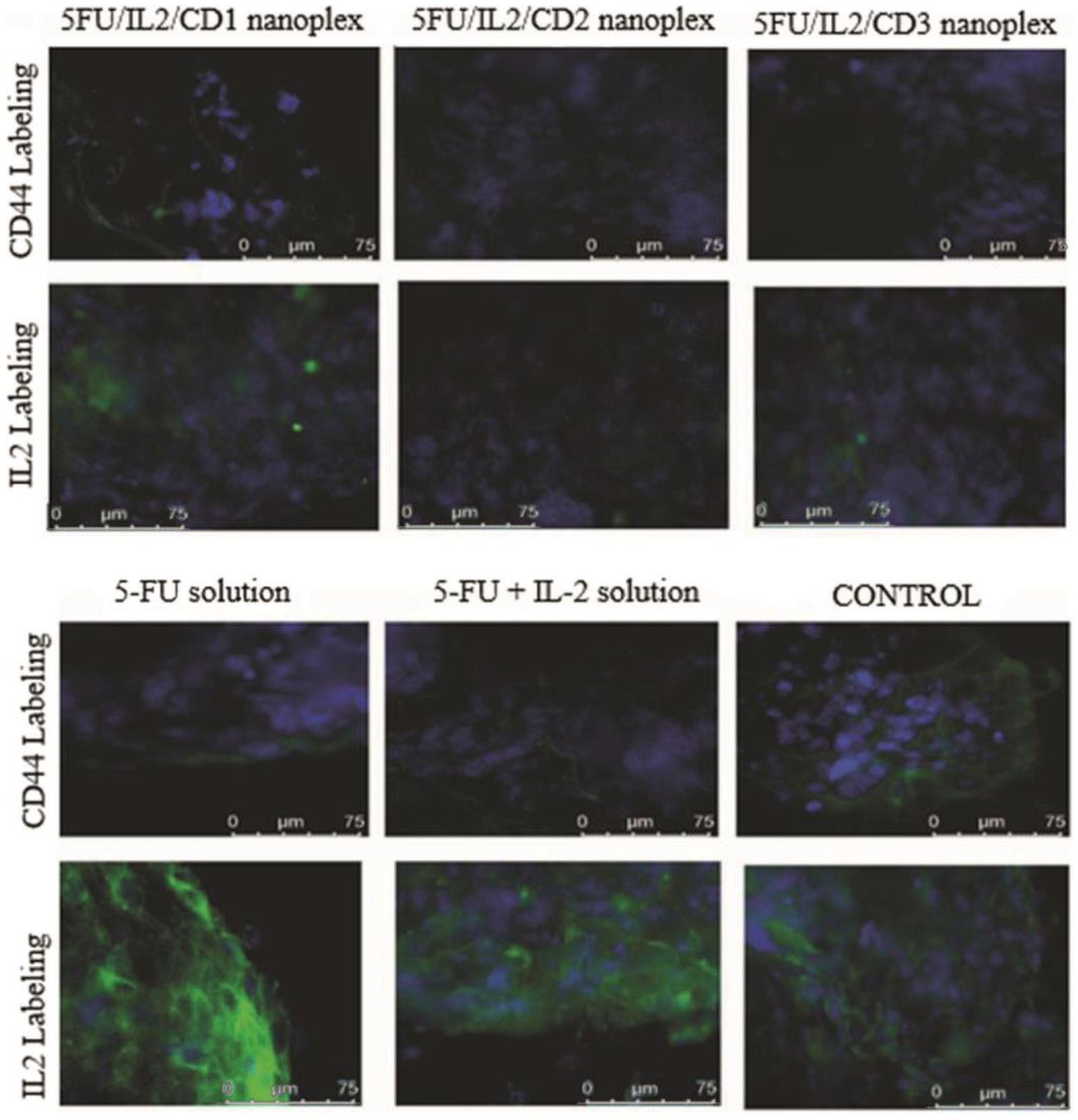
3.1.5. Immunocytochemical Examination in 3D Tumor Cells
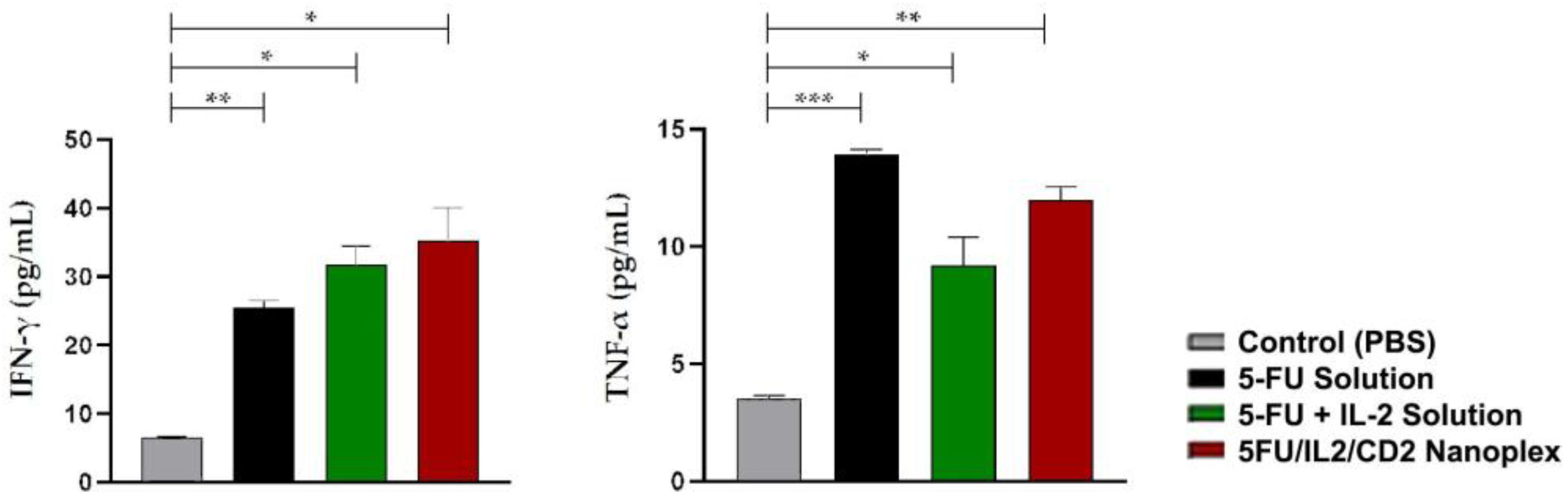
3.1.6. Assessment of Biological Activity of IL-2
3.2. In Vivo Studies
3.2.1. Safety Studies of CD Polymers
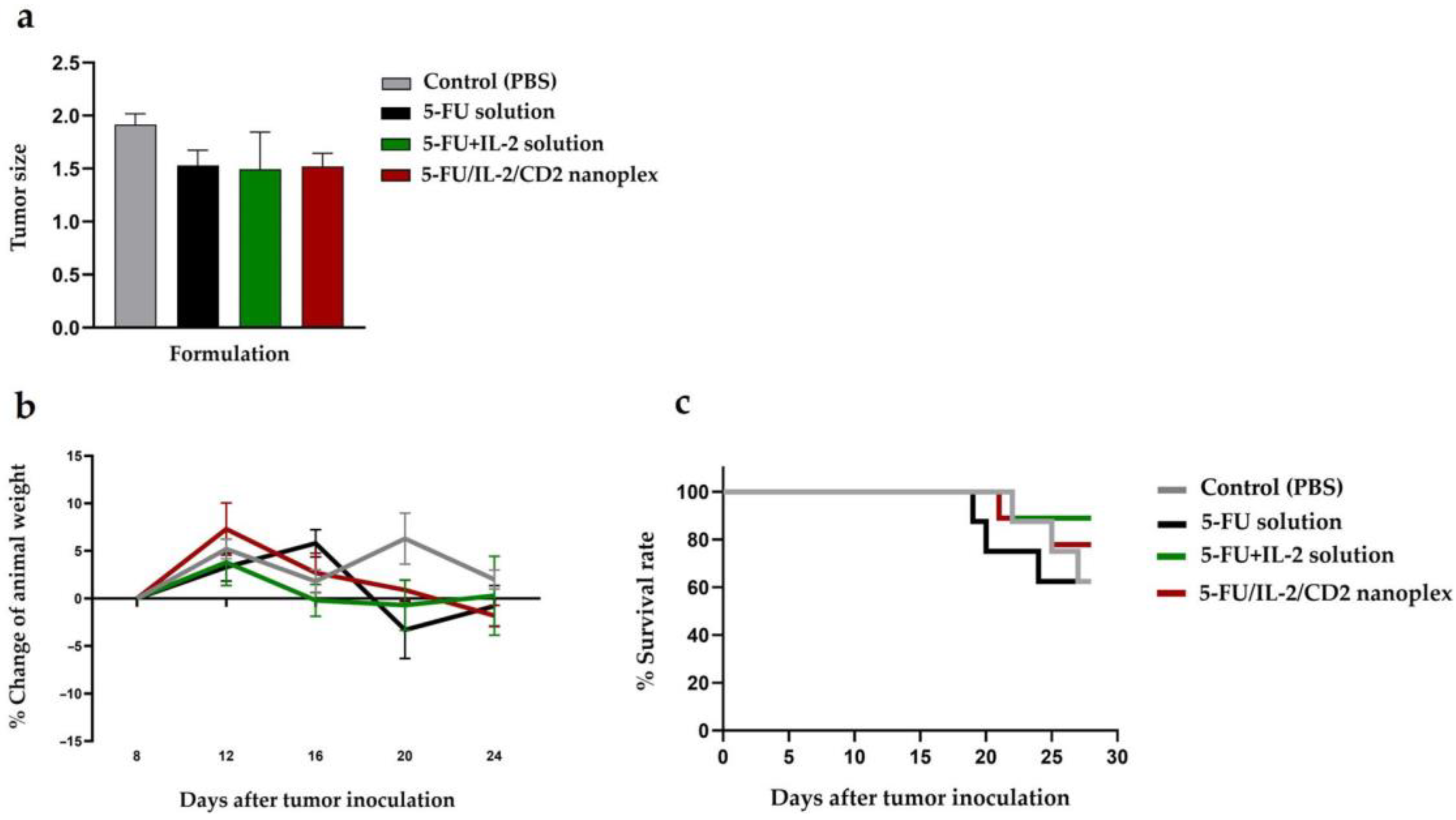
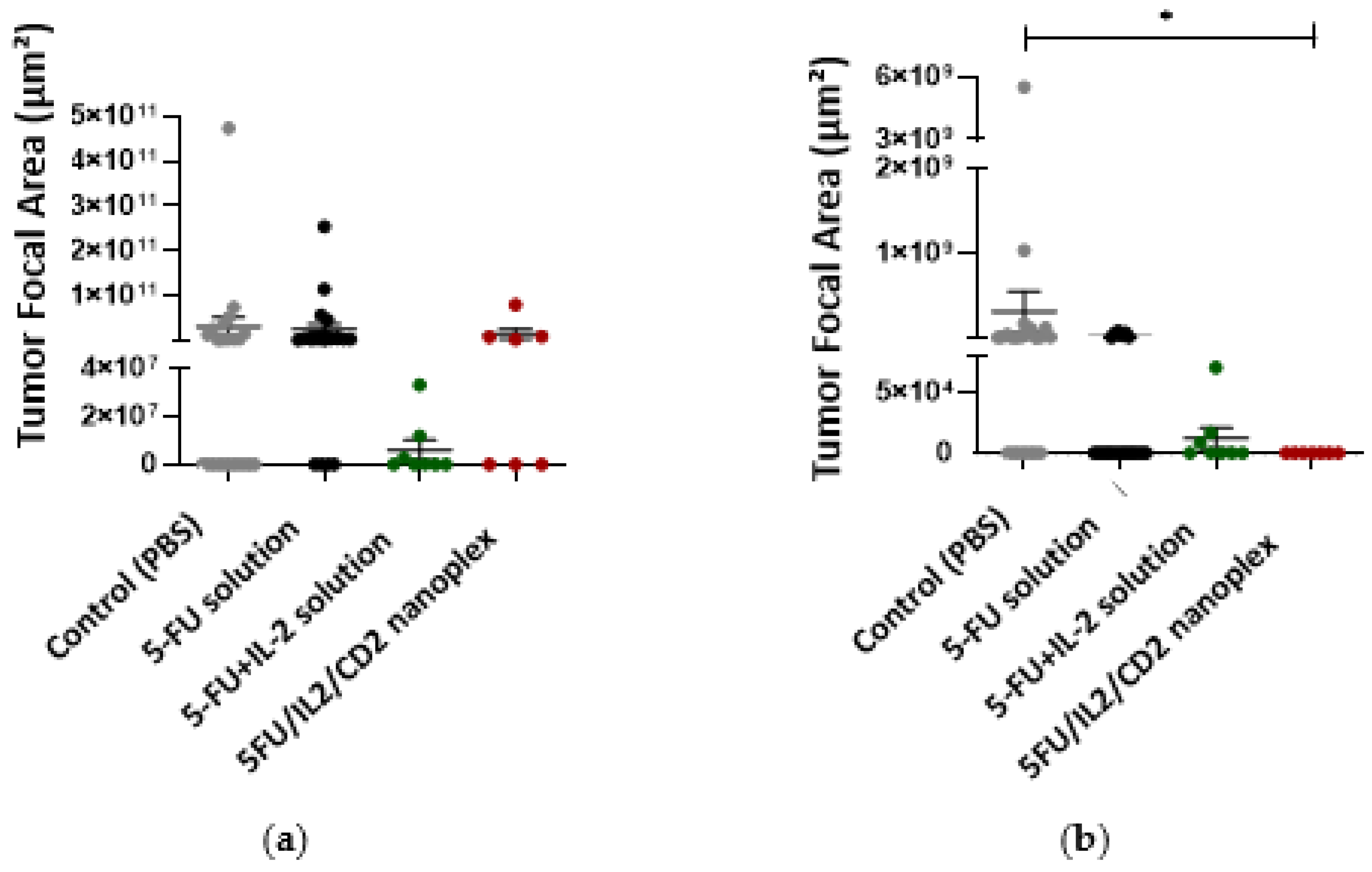
3.2.2. Antitumor Activities of CD Nanoplexes
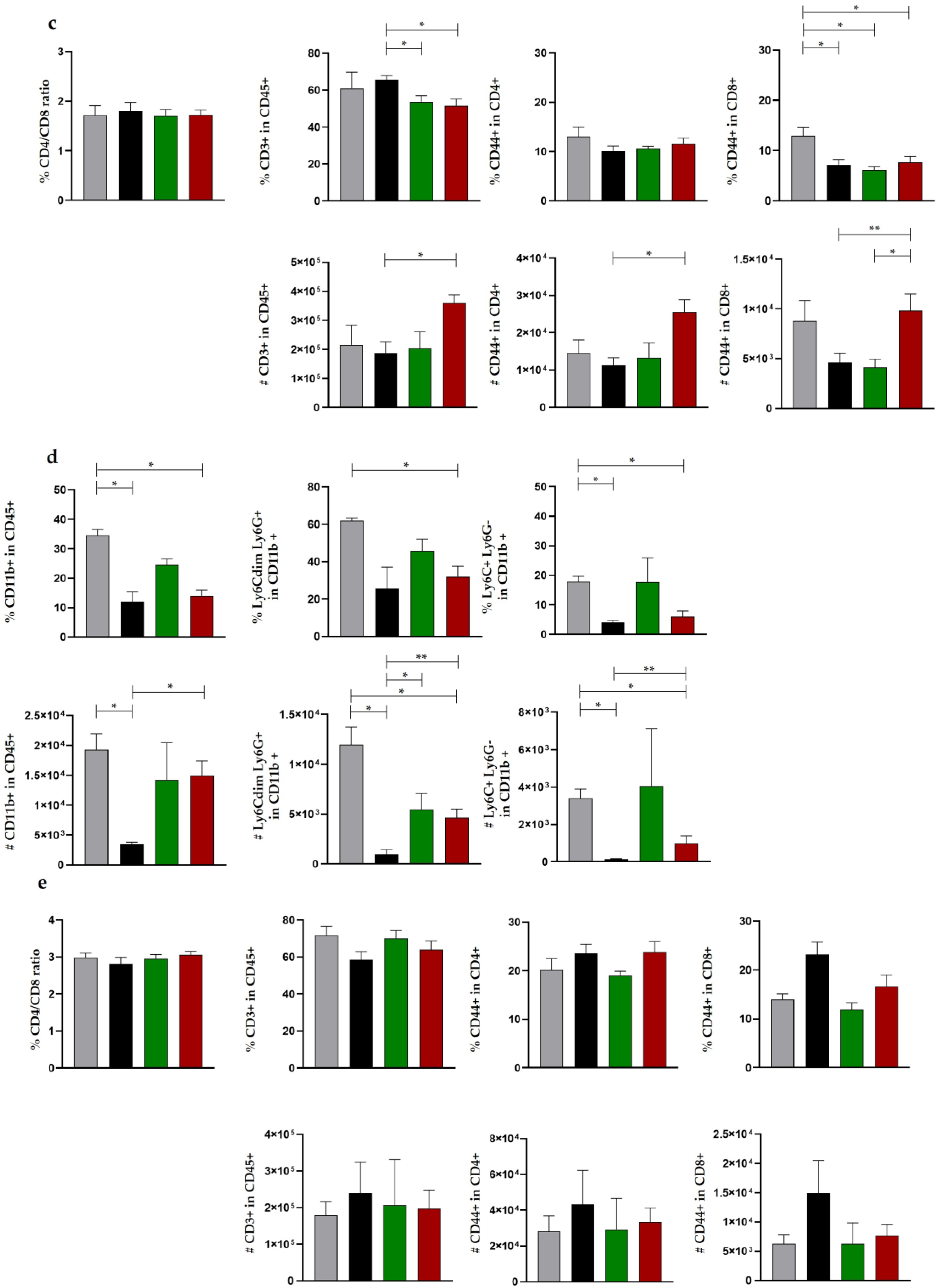
3.2.3. Histomorphometric Evaluation of In Vivo Cancer Nodules
4. Discussion
4.1. Cell Culture Studies
4.1.1. xCELLigence Real-Time Cell Analysis
4.1.2. Antitumoral Activity of CD Nanoplexes on 3D Spheroid Tumor Culture
4.1.3. Apoptosis Assays of 3D Tumor Model Using TUNEL
4.1.4. Light Microscopy Analysis of 3D Tumor Cells at Plastic Blocks
4.1.5. Immunocytochemical Examination in 3D Tumor Cells
4.1.6. Assessment of Biological Activity of IL-2
4.2. In Vivo Studies
4.2.1. Safety Studies of CD Polymers
4.2.2. Antitumor Activities of CD Nanoplexes
4.2.3. Histopathological Evaluation of In Vivo Cancer Nodules
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labadie, J.D.; Savas, S.; Harrison, T.A.; Banbury, B.; Huang, Y.; Buchanan, D.D.; Campbell, P.T.; Gallinger, S.J.; Giles, G.G.; Gunter, M.J.; et al. Genome-wide association study identifies tumor anatomical site-specific risk variants for colorectal cancer survival. Sci. Rep. 2022, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Akkin, S.; Varan, G.; Bilensoy, E. A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers. Molecules 2021, 26, 3382. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhao, Z.; Yang, M.; Ji, J.; Zhu, D. T-cell-based immunotherapy in colorectal cancer. Cancer Lett. 2021, 498, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Can Ozturk, S.; Bilgic, E.; Yanik, H.; Korkusuz, P.; Aktas, Y.; Benito, J.M.; Esendagli, G.; Bilensoy, E. Therapeutic efficacy and gastrointestinal biodistribution of polycationic nanoparticles for oral camptothecin delivery in early and late-stage colorectal tumor-bearing animal model. Eur. J. Pharm. Biopharm. 2021, 169, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Colon Cancer: A Clinician’s Perspective in 2019. Gastroenterol. Res. 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharmacol. 2016, 78, 447–464. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Rueda, F.; Lowik, C.W.; Ossendorp, F.; Cruz, L.J. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 2016, 83, 308–320. [Google Scholar] [CrossRef]
- Alessandrino, F.; Qin, L.; Cruz, G.; Sahu, S.; Rosenthal, M.H.; Meyerhardt, J.A.; Shinagare, A.B. 5-Fluorouracil induced liver toxicity in patients with colorectal cancer: Role of computed tomography texture analysis as a potential biomarker. Abdom. Radiol. 2019, 44, 3099–3106. [Google Scholar] [CrossRef]
- McWhirter, D.; Kitteringham, N.; Jones, R.P.; Malik, H.; Park, K.; Palmer, D. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: A review of mechanisms and outcomes. Crit. Rev. Oncol. Hematol. 2013, 88, 404–415. [Google Scholar] [CrossRef]
- Goindi, S.; Arora, P.; Kumar, N.; Puri, A. Development of novel ionic liquid-based microemulsion formulation for dermal delivery of 5-Fluorouracil. AAPS PharmSciTech 2014, 15, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifar, T.; Sheybani, S.; Abdouss, M.; Hassani Najafabadi, S.A.; Shafiee Ardestani, M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J. Biomed. Mater. Res. Part A 2018, 106, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Toudeshkchouei, M.; Zahedi, P.; Shavandi, A. Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy. Materials 2020, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.D.; Park, J.; Uhrich, R.; Gao, W.; Horwitz, D.A.; Fahmy, T.M. Paracrine co-delivery of TGF-beta and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials 2015, 59, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.H.; Rosenberg, S.A. The fate of interleukin-2 after in vivo administration. J. Immunol. 1983, 130, 2203–2208. [Google Scholar] [CrossRef]
- Atkins, M.B. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin. Cancer Res. 2006, 12, 2353s–2358s. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Arik, H.; Wei, L.; Zheng, Y.; Suh, H.; Irvine, D.J.; Tang, L. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomater. Sci. 2019, 7, 1345–1357. [Google Scholar] [CrossRef]
- Mizui, M. Natural and modified IL-2 for the treatment of cancer and autoimmune diseases. Clin. Immunol. 2019, 206, 63–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Bai, X.L.; Liang, T.B. Immunometabolism: A novel perspective of liver cancer microenvironment and its influence on tumor progression. World J. Gastroenterol. 2018, 24, 3500–3512. [Google Scholar] [CrossRef]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef]
- Prinz, D.; Klein, K.; List, J.; Knab, V.M.; Menzl, I.; Leidenfrost, N.; Heller, G.; Polic, B.; Putz, E.M.; Witalisz-Siepracka, A.; et al. Loss of NKG2D in murine NK cells leads to increased perforin production upon long-term stimulation with IL-2. Eur. J. Immunol. 2020, 50, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Licona Limon, P.; et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ercan, A.; Celebier, M.; Oncul, S.; Varan, G.; Kocak, E.; Benito, J.M.; Bilensoy, E. Polycationic cyclodextrin nanoparticles induce apoptosis and affect antitumoral activity in HepG2 cell line: An evaluation at the molecular level. Int. J. Pharm. 2021, 598, 120379. [Google Scholar] [CrossRef]
- Giglio, V.; Viale, M.; Bertone, V.; Maric, I.; Vaccarone, R.; Vecchio, G. Cyclodextrin polymers as nanocarriers for sorafenib. Investig. New Drugs 2018, 36, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, H.; Li, J.; Zhong, Y. Drug carrier systems based on water-soluble cationic beta-cyclodextrin polymers. Int. J. Pharm. 2004, 278, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Fancello, S.; Roldo, M.; Malanga, M.; Szente, L.; Migheli, R.; Gavini, E.; Giunchedi, P. Investigation of Cytotoxicity and Cell Uptake of Cationic Beta-Cyclodextrins as Valid Tools in Nasal Delivery. Pharmaceutics 2020, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Koziel, K.; Lagiewka, J.; Girek, B.; Folentarska, A.; Girek, T.; Ciesielski, W. Synthesis of New Amino-beta-Cyclodextrin Polymer, Cross-Linked with Pyromellitic Dianhydride and Their Use for the Synthesis of Polymeric Cyclodextrin Based Nanoparticles. Polymers 2021, 13, 1332. [Google Scholar] [CrossRef]
- Frank, D.; Tyagi, C.; Tomar, L.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Penny, C.; Pillay, V. Overview of the role of nanotechnological innovations in the detection and treatment of solid tumors. Int. J. Nanomed. 2014, 9, 589–613. [Google Scholar] [CrossRef]
- Zhao, N.; Leng, Q.; Woodle, M.C.; Mixson, A.J. Enhanced tumor uptake and activity of nanoplex-loaded doxorubicin. Biochem. Biophys. Res. Commun. 2019, 513, 242–247. [Google Scholar] [CrossRef]
- Sharma, S.; Pukale, S.; Sahel, D.K.; Singh, P.; Mittal, A.; Chitkara, D. Folate targeted hybrid lipo-polymeric nanoplexes containing docetaxel and miRNA-34a for breast cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112305. [Google Scholar] [CrossRef]
- Akkin, S.; Varan, G.; Aksut, D.; Malanga, M.; Ercan, A.; Sen, M.; Bilensoy, E. A different approach to immunochemotherapy for colon Cancer: Development of nanoplexes of cyclodextrins and Interleukin-2 loaded with 5-FU. Int. J. Pharm. 2022, 623, 121940. [Google Scholar] [CrossRef] [PubMed]
- Sevmis, M.; Yoyen-Ermis, D.; Aydin, C.; Bilgic, E.; Korkusuz, P.; Uner, A.; Hamaloglu, E.; Esendagli, G.; Karakoc, D. Splenectomy-Induced Leukocytosis Promotes Intratumoral Accumulation of Myeloid-Derived Suppressor Cells, Angiogenesis and Metastasis. Immunol. Investig. 2017, 46, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, C.; Yin, C. Co-delivery of doxorubicin and interleukin-2 via chitosan based nanoparticles for enhanced antitumor efficacy. Acta Biomater. 2017, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.M.; Mistry, P.; Freathy, C.; Brown, J.L.; Charlton, P.A. Antitumour activity of XR5944 in vitro and in vivo in combination with 5-fluorouracil and irinotecan in colon cancer cell lines. Br. J. Cancer 2005, 92, 722–728. [Google Scholar] [CrossRef]
- Nadalin, S.; Settmacher, U.; Rauchfuss, F.; Balci, D.; Konigsrainer, A.; Line, P.D. RAPID procedure for colorectal cancer liver metastasis. Int. J. Surg. 2020, 82S, 93–96. [Google Scholar] [CrossRef]
- Boyacioglu, O.; Bilgic, E.; Varan, C.; Bilensoy, E.; Nemutlu, E.; Sevim, D.; Kocaefe, C.; Korkusuz, P. ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro. Cell Death Dis. 2021, 12, 56. [Google Scholar] [CrossRef]
- Yersal, N.; Kose, S.; Horzum, U.; Ozkavukcu, S.; Orwig, K.E.; Korkusuz, P. Leptin promotes proliferation of neonatal mouse stem/progenitor spermatogonia. J. Assist. Reprod. Genet. 2020, 37, 2825–2838. [Google Scholar] [CrossRef]
- Castro, F.; Leite Pereira, C.; Helena Macedo, M.; Almeida, A.; Jose Silveira, M.; Dias, S.; Patricia Cardoso, A.; Jose Oliveira, M.; Sarmento, B. Advances on colorectal cancer 3D models: The needed translational technology for nanomedicine screening. Adv. Drug Deliv. Rev. 2021, 175, 113824. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Bulumko, E.; Agyare, E. Evaluation of in-vitro cytotoxic effect of 5-FU loaded-chitosan nanoparticles against spheroid models. J. Nat. Sci. 2018, 4, e535. [Google Scholar]
- Eynali, S.; Khoei, S.; Khoee, S.; Esmaelbeygi, E. Evaluation of the cytotoxic effects of hyperthermia and 5-fluorouracil-loaded magnetic nanoparticles on human colon cancer cell line HT-29. Int. J. Hyperth. 2017, 33, 327–335. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-delta. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, M.; Bayram, D.; Yavuz Turel, G.; Togay, V.A.; Sahin Calapoglu, N. Secoisolariciresinol diglucoside induces caspase-3-mediated apoptosis in monolayer and spheroid cultures of human colon carcinoma cells. J. Food Biochem. 2021, 45, e13719. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Monch, D.; Maass, A.; Gromoll, C.; Hehr, T.; Leibold, T.; Schlitt, H.J.; Dahlke, M.H.; Renner, P. Three dimensional cultivation increases chemo- and radioresistance of colorectal cancer cell lines. PLoS ONE 2021, 16, e0244513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; He, B.; Wan, T.; Zhu, W.; Han, J.; Zha, B.; Chen, H.; Yang, F.; Li, Q.; Wang, W.; et al. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE 2012, 7, e47115. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Ogura, M.; Sakaguchi, Y.; Hasegawa, T.; Atobe, S.; Terai, K. Mechanism of jet injector-induced plasmid DNA uptake: Contribution of shear stress and endocytosis. Int. J. Pharm. 2021, 609, 121200. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; Osman, M.A.; El-Gizawy, S.A.; Goda, A.E.; Shamloula, M.M.; Faheem, A.M.; McCarron, P.A. Polymeric nano-encapsulation of 5-fluorouracil enhances anti-cancer activity and ameliorates side effects in solid Ehrlich Carcinoma-bearing mice. Biomed. Pharmacother. 2018, 105, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, Q.; Li, J.; Peng, L.; Ding, L. Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. JBUON 2020, 25, 1413–1422. [Google Scholar]
- Letourneau, S.; van Leeuwen, E.M.; Krieg, C.; Martin, C.; Pantaleo, G.; Sprent, J.; Surh, C.D.; Boyman, O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc. Natl. Acad. Sci. USA 2010, 107, 2171–2176. [Google Scholar] [CrossRef]
- Ren, L.; Yang, X.; Guo, W.; Wang, J.; Chen, G. Inclusion Complex of Docetaxel with Sulfobutyl Ether beta-Cyclodextrin: Preparation, In Vitro Cytotoxicity and In Vivo Safety. Polymers 2020, 12, 2336. [Google Scholar] [CrossRef]
- Chai, F.; Maton, M.; Degoutin, S.; Vermet, G.; Simon, N.; Rousseaux, C.; Martel, B.; Blanchemain, N. In vivo evaluation of post-operative pain reduction on rat model after implantation of intraperitoneal PET meshes functionalised with cyclodextrins and loaded with ropivacaine. Biomaterials 2019, 192, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, X.; Law, K.; Chen, Y.; Gu, J.; Zhang, W.; Xin, H.; Sha, X.; Fang, X. Enhanced anti-tumor effect of 9-nitro-camptothecin complexed by hydroxypropyl-beta-cyclodextrin and safety evaluation. Int. J. Pharm. 2011, 415, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Kwon, J.H. Effects of lipid membrane composition on the distribution of biocidal guanidine oligomer with solid supported lipid membranes. RSC Adv. 2020, 10, 22343–22351. [Google Scholar] [CrossRef]
- Garmy, N.; Taieb, N.; Yahi, N.; Fantini, J. Interaction of cholesterol with sphingosine: Physicochemical characterization and impact on intestinal absorption. J. Lipid Res. 2005, 46, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sheng, J.; Chen, G.; Zhu, P.; Shi, C.; Li, B.; Park, C.; Wang, J.; Zhang, B.; Liu, Z.; et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J. Immunother. Cancer 2020, 8, e000973. [Google Scholar] [CrossRef]
- Chang, J.; Bhasin, S.S.; Bielenberg, D.R.; Sukhatme, V.P.; Bhasin, M.; Huang, S.; Kieran, M.W.; Panigrahy, D. Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin. FASEB J. 2019, 33, 114–125. [Google Scholar] [CrossRef]








 ), and metastatic tumor tissue located in the lung parenchyma (**).
), and metastatic tumor tissue located in the lung parenchyma (**).
 ), and metastatic tumor tissue located in the lung parenchyma (**).
), and metastatic tumor tissue located in the lung parenchyma (**).
| Abbreviation | Cyclodextrin Polymers | DS | MW | CLR | CD |
|---|---|---|---|---|---|
| CD1 | Quaternary-Ammonium-βCD polymer HCl, cross-linked with epichlorohydrin | 2.2 | 40,000 | 11 | 2.2 |
| CD2 | Amino-βCD polymer HCl, cross-linked with epichlorohydrin | 1 | 25,000 | 10 | 1 |
| CD3 | Guanidino-βCD polymer HCl, cross-linked with epichlorohydrin | 1 | 26,000 | 10 | 1 |
| Treatment Groups | ||||
|---|---|---|---|---|
| PBS | CD1 | CD2 | CD3 | |
| AST (55-352 U/L) | 367 ± 2.8 | 748 ± 8.5 | 316.5 ± 4.9 | 586 ± 4.2 |
| ALT (41-131 U/L) | 71 ± 2.8 | 71 ± 1.4 | 53.5 ± 0.7 | 79 ± 2.8 |
| cCRP (mg/dL) | <0.3 | <0.3 | <0.3 | <0.3 |
| AST/ALT | 5.2 ± 0.1 | 10.5 ± 0.4 | 5.9 ± 0 | 7.4 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkın, S.; Varan, G.; Işık, A.; Gökşen, S.; Karakoç, E.; Malanga, M.; Esendağlı, G.; Korkusuz, P.; Bilensoy, E. Synergistic Antitumor Potency of a Self-Assembling Cyclodextrin Nanoplex for the Co-Delivery of 5-Fluorouracil and Interleukin-2 in the Treatment of Colorectal Cancer. Pharmaceutics 2023, 15, 314. https://doi.org/10.3390/pharmaceutics15020314
Akkın S, Varan G, Işık A, Gökşen S, Karakoç E, Malanga M, Esendağlı G, Korkusuz P, Bilensoy E. Synergistic Antitumor Potency of a Self-Assembling Cyclodextrin Nanoplex for the Co-Delivery of 5-Fluorouracil and Interleukin-2 in the Treatment of Colorectal Cancer. Pharmaceutics. 2023; 15(2):314. https://doi.org/10.3390/pharmaceutics15020314
Chicago/Turabian StyleAkkın, Safiye, Gamze Varan, Anıl Işık, Sibel Gökşen, Elif Karakoç, Milo Malanga, Güneş Esendağlı, Petek Korkusuz, and Erem Bilensoy. 2023. "Synergistic Antitumor Potency of a Self-Assembling Cyclodextrin Nanoplex for the Co-Delivery of 5-Fluorouracil and Interleukin-2 in the Treatment of Colorectal Cancer" Pharmaceutics 15, no. 2: 314. https://doi.org/10.3390/pharmaceutics15020314
APA StyleAkkın, S., Varan, G., Işık, A., Gökşen, S., Karakoç, E., Malanga, M., Esendağlı, G., Korkusuz, P., & Bilensoy, E. (2023). Synergistic Antitumor Potency of a Self-Assembling Cyclodextrin Nanoplex for the Co-Delivery of 5-Fluorouracil and Interleukin-2 in the Treatment of Colorectal Cancer. Pharmaceutics, 15(2), 314. https://doi.org/10.3390/pharmaceutics15020314









