Rapid Study on Mefloquine Hydrochloride Complexation with Hydroxypropyl-β-Cyclodextrin and Randomly Methylated β-Cyclodextrin: Phase Diagrams, Nuclear Magnetic Resonance Analysis, and Stability Assessment
Abstract
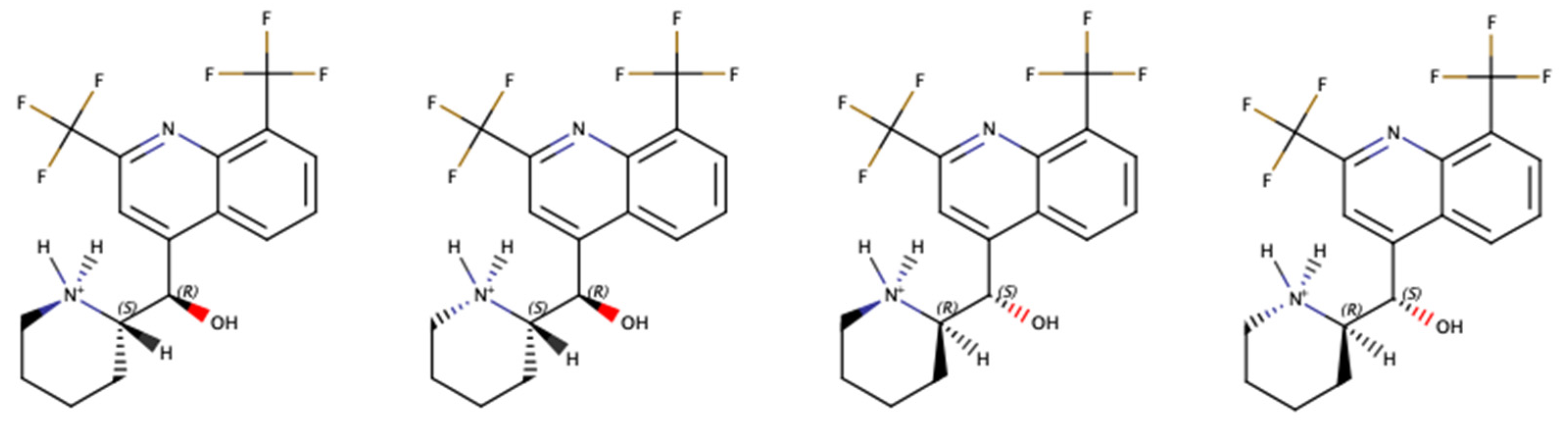
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Studies
2.3. Quantification of Mefloquine
2.4. Complexation Efficiency
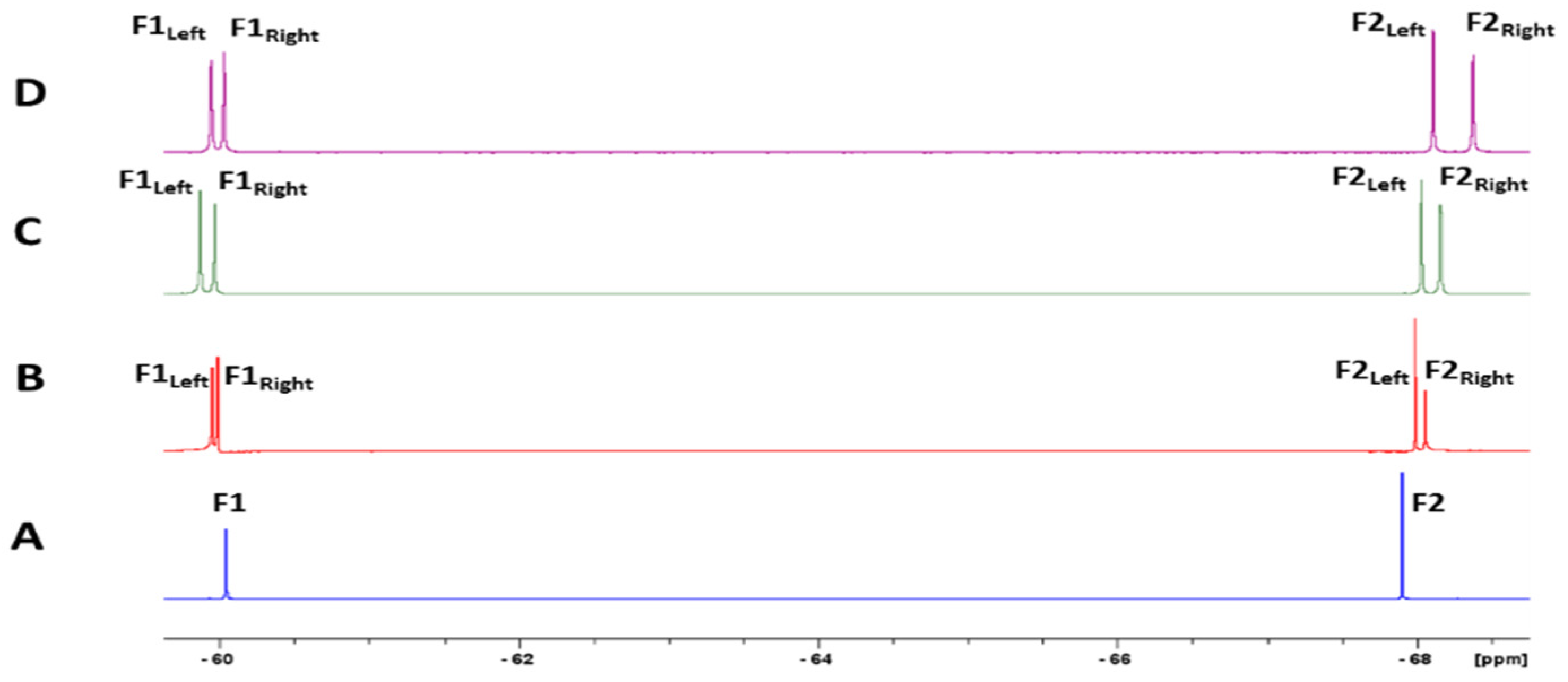
2.5. Nuclear Magnetic Resonance Analysis of Complex Formation
2.6. Photostability Studies
3. Results and Discussion
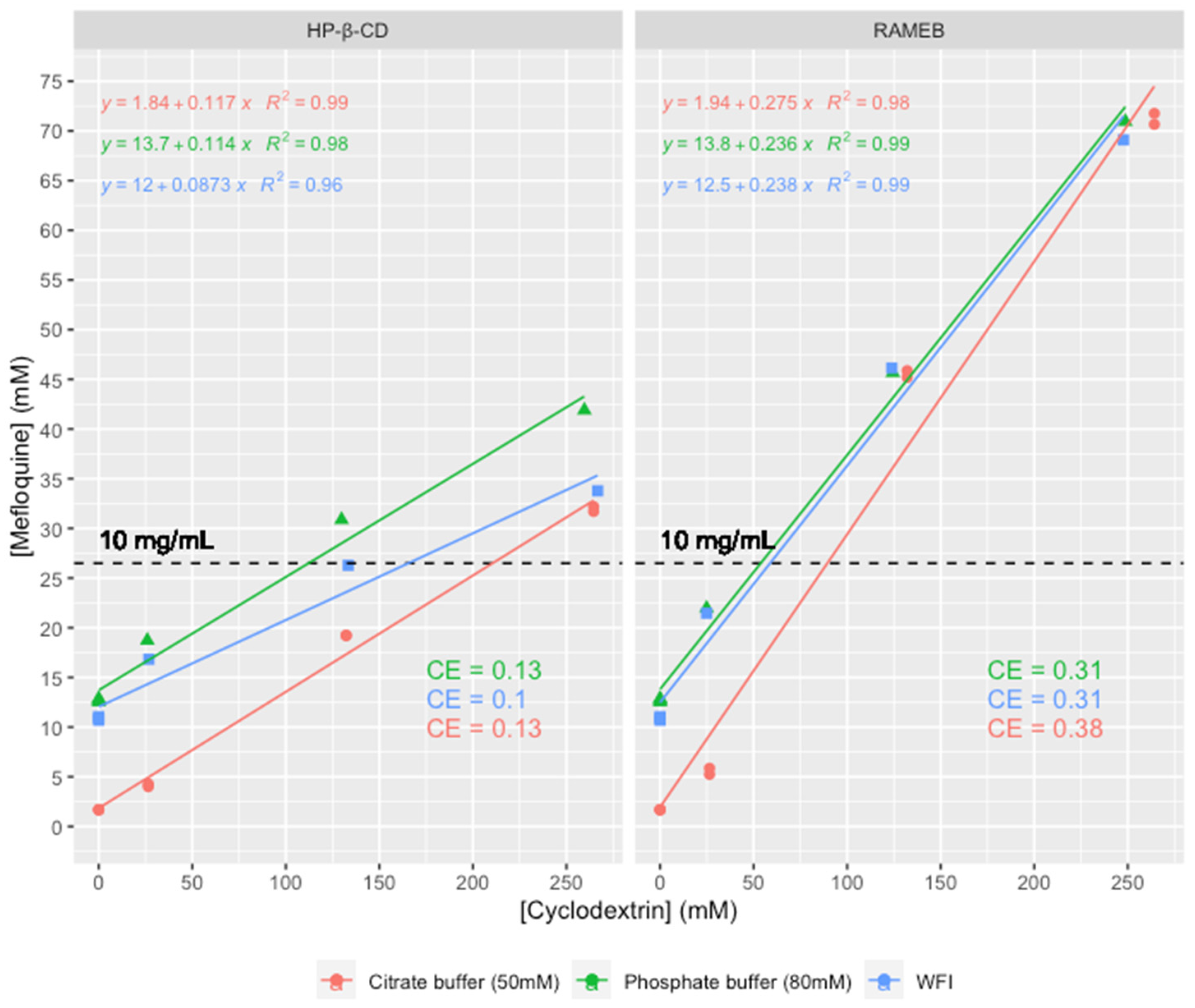
3.1. Analysis of Mefloquine Solubilization with Hydroxypropyl-β-Cyclodextrin (HP-β-CD) and Randomly Methylated β-Cyclodextrin (RAMEB)
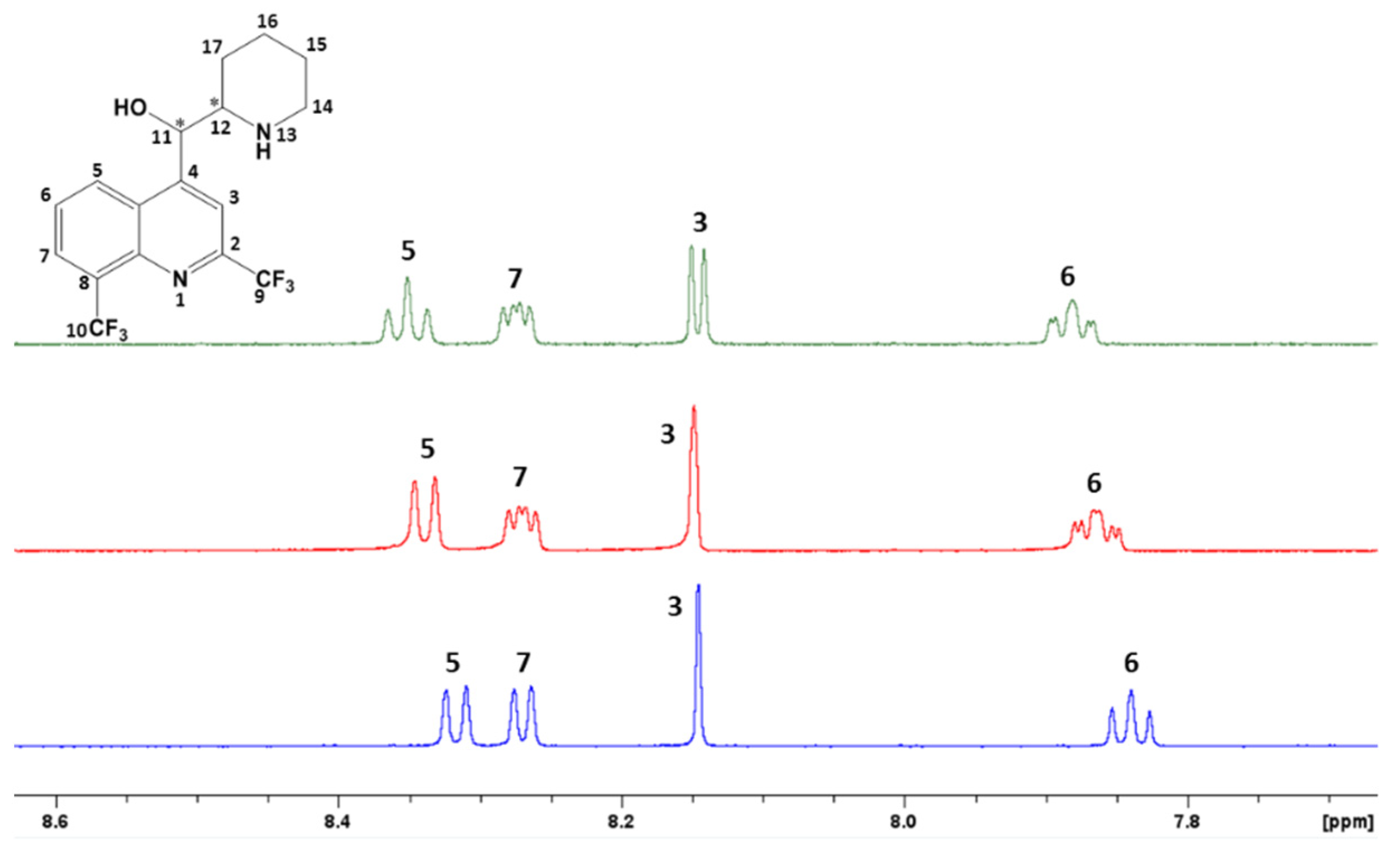
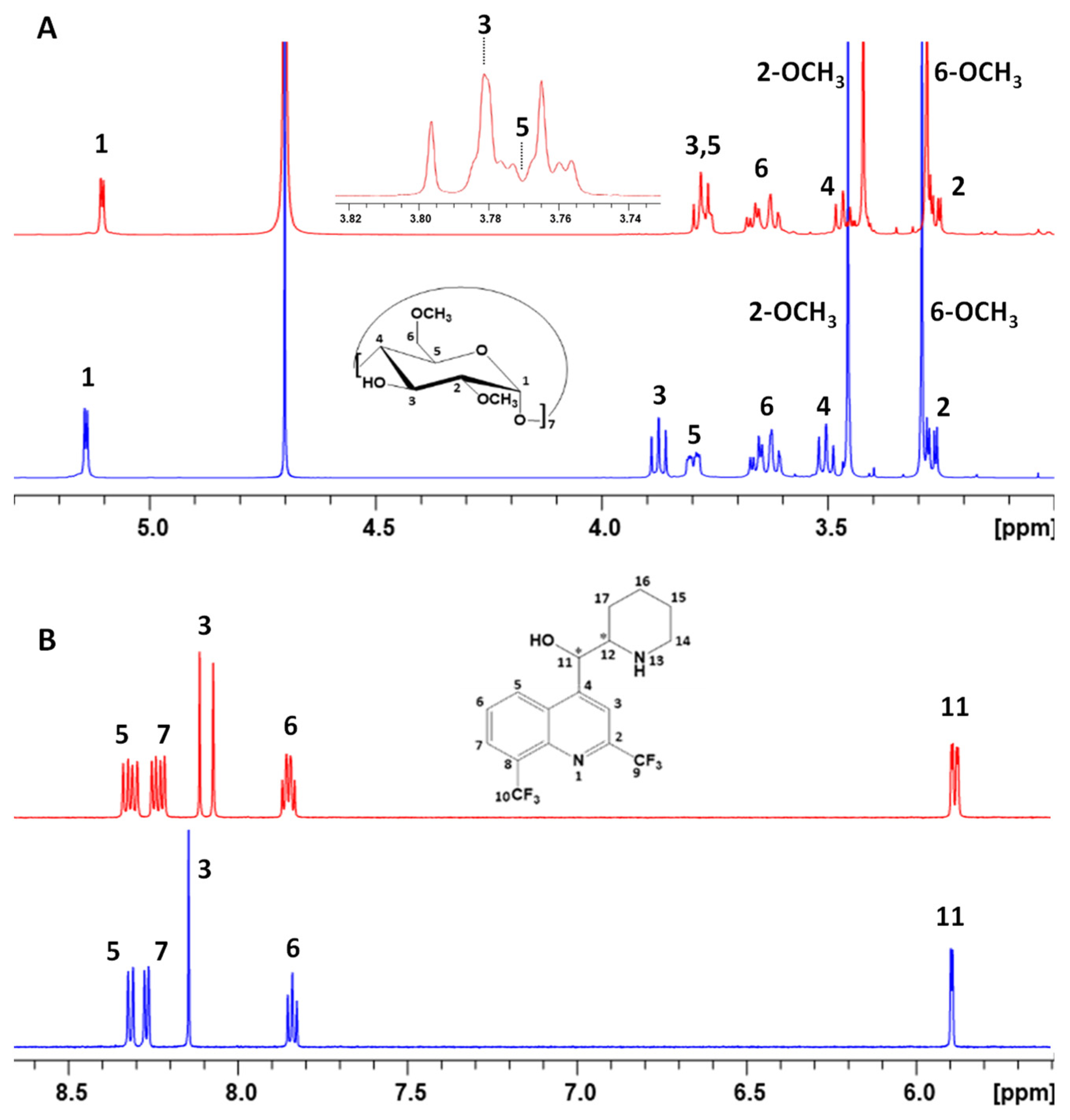
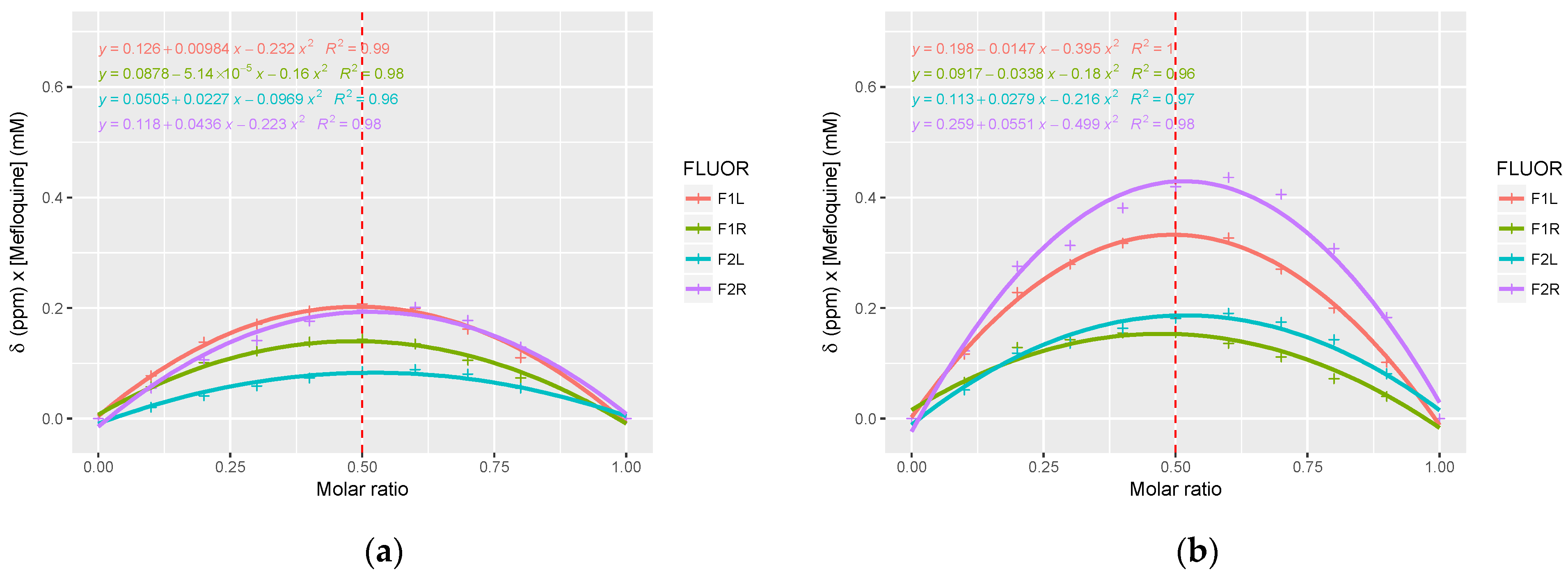
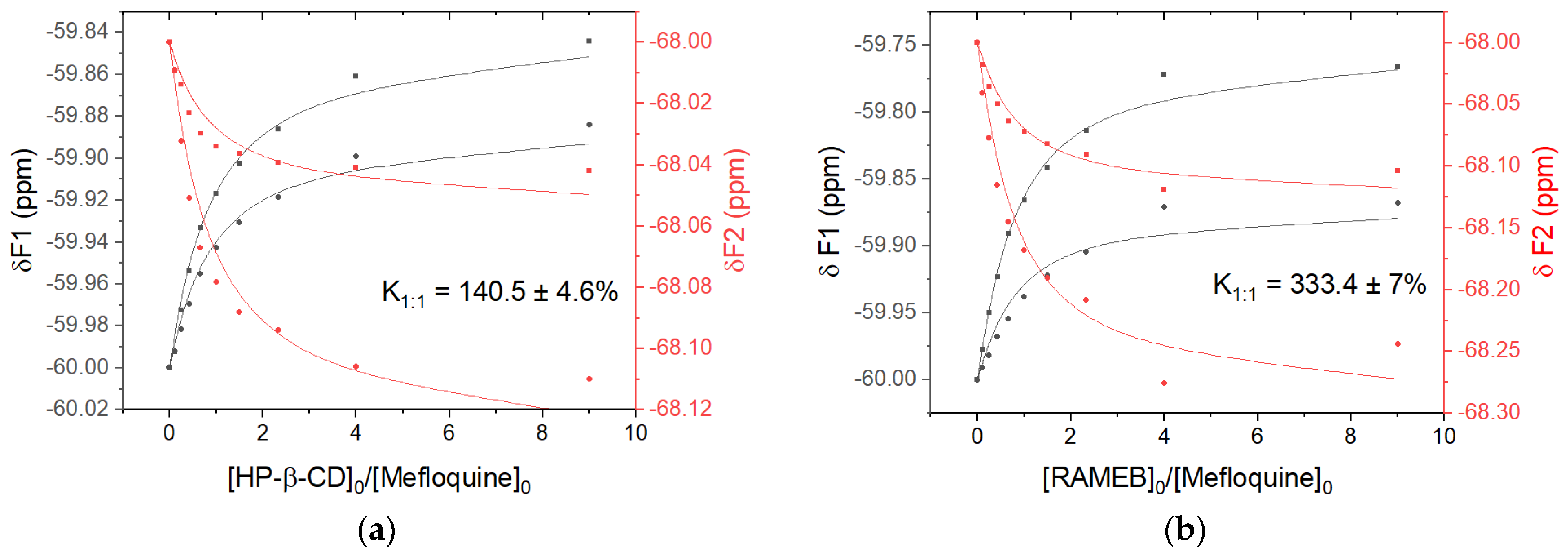
3.2. Nuclear Magnetic Resonance Analysis of Mefloquine–CD Interaction
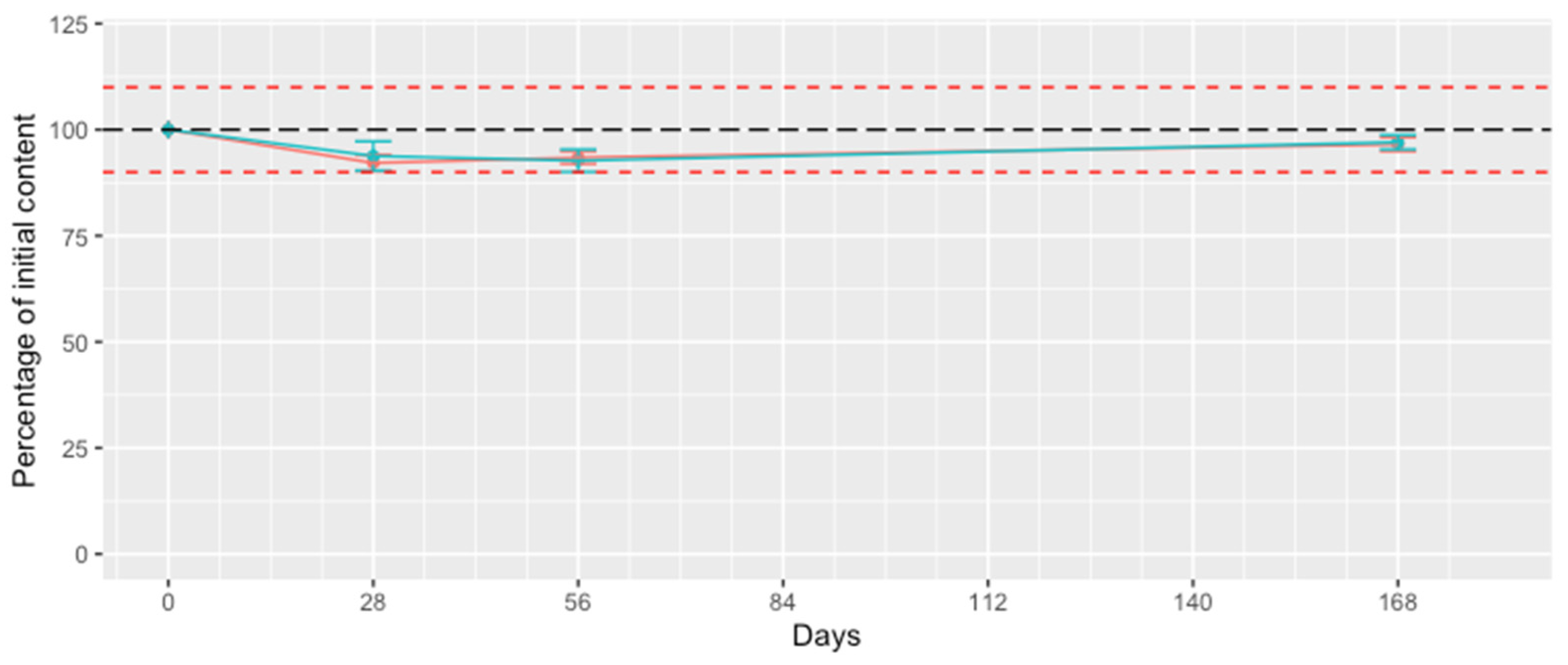
3.3. Analysis of the Photostability of Mefloquine in the Presence of CD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlagenhauf, P.; Adamcova, M.; Regep, L.; Schaerer, M.T.; Bansod, S.; Rhein, H.-G. Use of mefloquine in children—A review of dosage, pharmacokinetics and tolerability data. Malar. J. 2011, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Dassonville-Klimpt, A.; Cézard, C.; Mullié, C.; Agnamey, P.; Jonet, A.; Da Nascimento, S.; Marchivie, M.; Guillon, J.; Sonnet, P. Absolute Configuration and Antimalarial Activity of erythro-Mefloquine Enantiomers. ChemPlusChem 2013, 78, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Strauch, S.; Jantratid, E.; Dressman, J.B.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Mefloquine hydrochloride. J. Pharm. Sci. 2011, 100, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Senarathna, S.M.D.K.G.; Page-Sharp, M.; Crowe, A. The Interactions of P-Glycoprotein with Antimalarial Drugs, Including Substrate Affinity, Inhibition and Regulation. PLoS ONE 2016, 11, e0152677. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Dabke, A.P.; Shete, A.S. Crystal engineering to improve physicochemical properties of mefloquine hydrochloride. Drug Dev. Ind. Pharm. 2010, 36, 1036–1045. [Google Scholar] [CrossRef]
- Mbela, T.K.; Deharo, E.; Haemers, A.; Ludwig, A. Submicron oil-in-water emulsion formulations for mefloquine and halofantrine: Effect of electric-charge inducers on antimalarial activity in mice. J. Pharm. Pharmacol. 1998, 50, 1221–1225. [Google Scholar] [CrossRef]
- Tang, W.-L.; Tang, W.-H.; Chen, W.C.; Diako, C.; Ross, C.F.; Li, S.-D. Development of a Rapidly Dissolvable Oral Pediatric Formulation for Mefloquine Using Liposomes. Mol. Pharm. 2017, 14, 1969–1979. [Google Scholar] [CrossRef]
- Patil, S.S.; Patil, A.L. Preparation, characterization and dissolution enhancement of mefloquine hydrochloride-βCD inclusion complex. Pharmazie 2011, 66, 416–420. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistarà, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Cyclodextrins and the Biopharmaceutics Classification System of Drugs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 63–67. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Grislingaas, A.-L. Photochemical stability of biologically active compounds. II. Photochemical decomposition of mefloquine in water. Int. J. Pharm. 1990, 60, 157–162. [Google Scholar] [CrossRef]
- Marçon, F.; Mathiron, D.; Pilard, S.; Lemaire-Hurtel, A.-S.; Dubaele, J.-M.; Djedaini-Pilard, F. Development and formulation of a 0.2% oral solution of midazolam containing gamma-cyclodextrin. Int. J. Pharm. 2009, 379, 244–250. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Brynn Hibbert, D.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Péan, C.; Créminon, C.; Grassi, J.; Pradelles, P.; Perly, B.; Djedaïni-pilard, F. NMR Investigations of the Inclusion of Thyroxine and Derivatives in Natural Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 1999, 33, 307–319. [Google Scholar] [CrossRef]
- Aubry, A.F.; Tattersall, P.; Ruan, J. Development of Stability Indicating Methods. In Handbook of Stability Testing in Pharmaceutical Development; Huynh-Ba, K., Ed.; Springer: New York, NY, USA, 2009; pp. 139–161. [Google Scholar] [CrossRef]
- Kiss, A.; Répási, J.; Salamon, Z.; Novák, C.; Pokol, G.; Tomor, K. Solid state investigation of mefloquine hydrochloride. J. Pharm. Biomed. Anal. 1994, 12, 889–893. [Google Scholar] [CrossRef]
- Redenti, E.; Szente, L.; Szejtli, J. Drug/Cyclodextrin/Hydroxy Acid Multicomponent Systems. Properties and Pharmaceutical Applications. J. Pharm. Sci. 2000, 89, 1–8. [Google Scholar] [CrossRef]
- Mathiron, D.; Marçon, F.; Dubaele, J.-M.; Cailleu, D.; Pilard, S.; Djedaïni-pilard, F. Benefits of methylated cyclodextrins in the development of midazolam pharmaceutical formulations. J. Pharm. Sci. 2013, 102, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; Mathiron, D.; Bertaut, E.; Landy, D.; Cailleu, D.; Pilard, S.; Clément, C.; Courot, E.; Bonnet, V.; Djedaïni-Pilard, F. Physico-chemical studies of resveratrol, methyl-jasmonate and cyclodextrin interactions: An approach to resveratrol bioproduction optimization. RSC Adv. 2018, 8, 1528–1538. [Google Scholar] [CrossRef]
- Rigaud, S.; Mathiron, D.; Moufawad, T.; Landy, D.; Djedaini-Pilard, F.; Marçon, F. Cyclodextrin Complexation as a Way of Increasing the Aqueous Solubility and Stability of Carvedilol. Pharmaceutics 2021, 13, 1746. [Google Scholar] [CrossRef]
- Wouessidjewe, D.; Crassous, A.; Duchêne, D.; Coleman, A.; Rysanek, N.; Tsoucaris, G.; Perly, B.; Djedaini, F. Inclusion of spironolactone in cyclomaltoheptaose: A guest affected by the hospitality of the host. Carbohydr. Res. 1989, 192, 313–322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durand, A.; Mathiron, D.; Rigaud, S.; Djedaini-Pilard, F.; Marçon, F. Rapid Study on Mefloquine Hydrochloride Complexation with Hydroxypropyl-β-Cyclodextrin and Randomly Methylated β-Cyclodextrin: Phase Diagrams, Nuclear Magnetic Resonance Analysis, and Stability Assessment. Pharmaceutics 2023, 15, 2794. https://doi.org/10.3390/pharmaceutics15122794
Durand A, Mathiron D, Rigaud S, Djedaini-Pilard F, Marçon F. Rapid Study on Mefloquine Hydrochloride Complexation with Hydroxypropyl-β-Cyclodextrin and Randomly Methylated β-Cyclodextrin: Phase Diagrams, Nuclear Magnetic Resonance Analysis, and Stability Assessment. Pharmaceutics. 2023; 15(12):2794. https://doi.org/10.3390/pharmaceutics15122794
Chicago/Turabian StyleDurand, Amaury, David Mathiron, Sébastien Rigaud, Florence Djedaini-Pilard, and Frédéric Marçon. 2023. "Rapid Study on Mefloquine Hydrochloride Complexation with Hydroxypropyl-β-Cyclodextrin and Randomly Methylated β-Cyclodextrin: Phase Diagrams, Nuclear Magnetic Resonance Analysis, and Stability Assessment" Pharmaceutics 15, no. 12: 2794. https://doi.org/10.3390/pharmaceutics15122794
APA StyleDurand, A., Mathiron, D., Rigaud, S., Djedaini-Pilard, F., & Marçon, F. (2023). Rapid Study on Mefloquine Hydrochloride Complexation with Hydroxypropyl-β-Cyclodextrin and Randomly Methylated β-Cyclodextrin: Phase Diagrams, Nuclear Magnetic Resonance Analysis, and Stability Assessment. Pharmaceutics, 15(12), 2794. https://doi.org/10.3390/pharmaceutics15122794







