A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
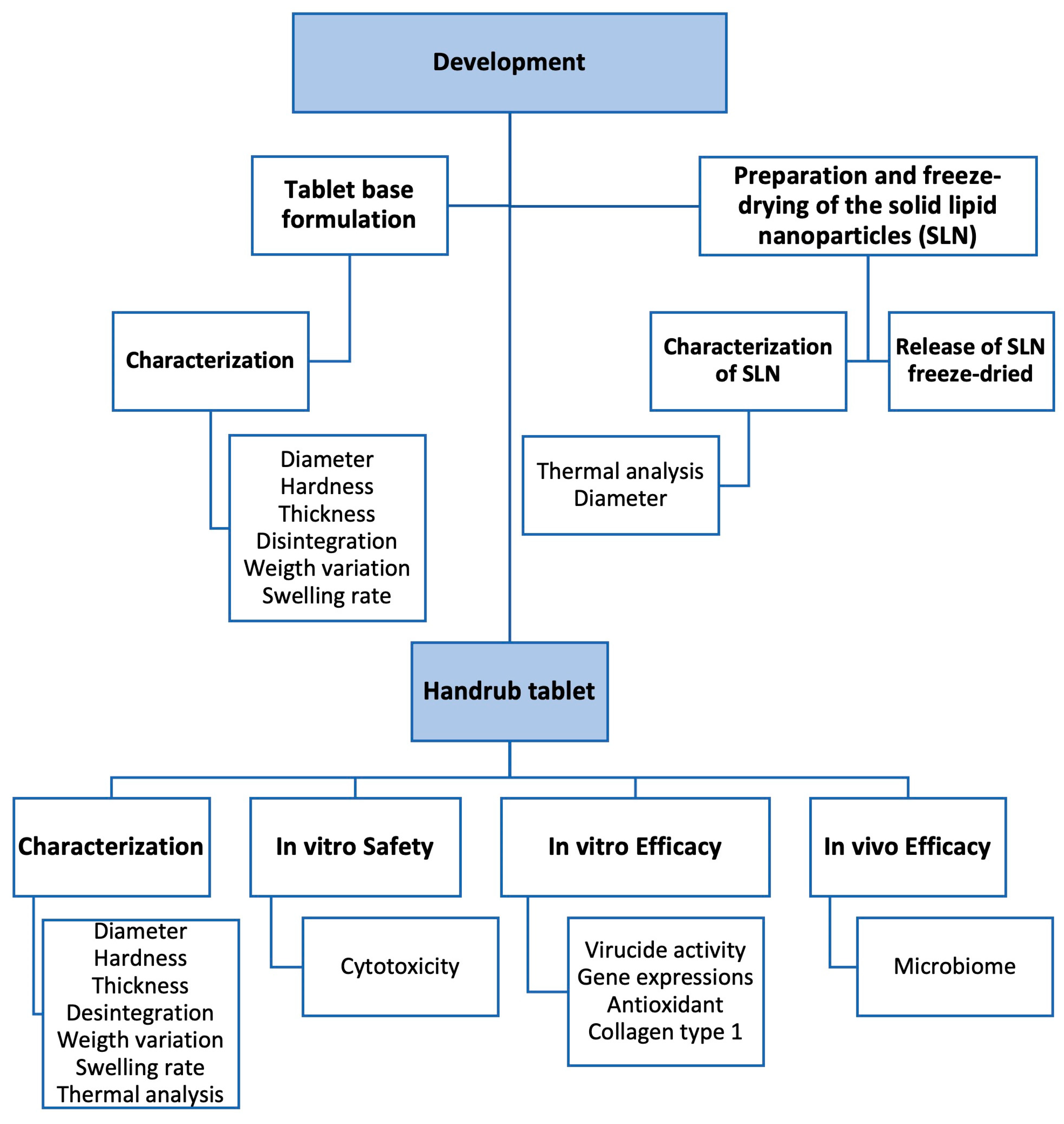
2.2. Development of Tablet Base Formulation
2.2.1. Characterization of Tablet Base
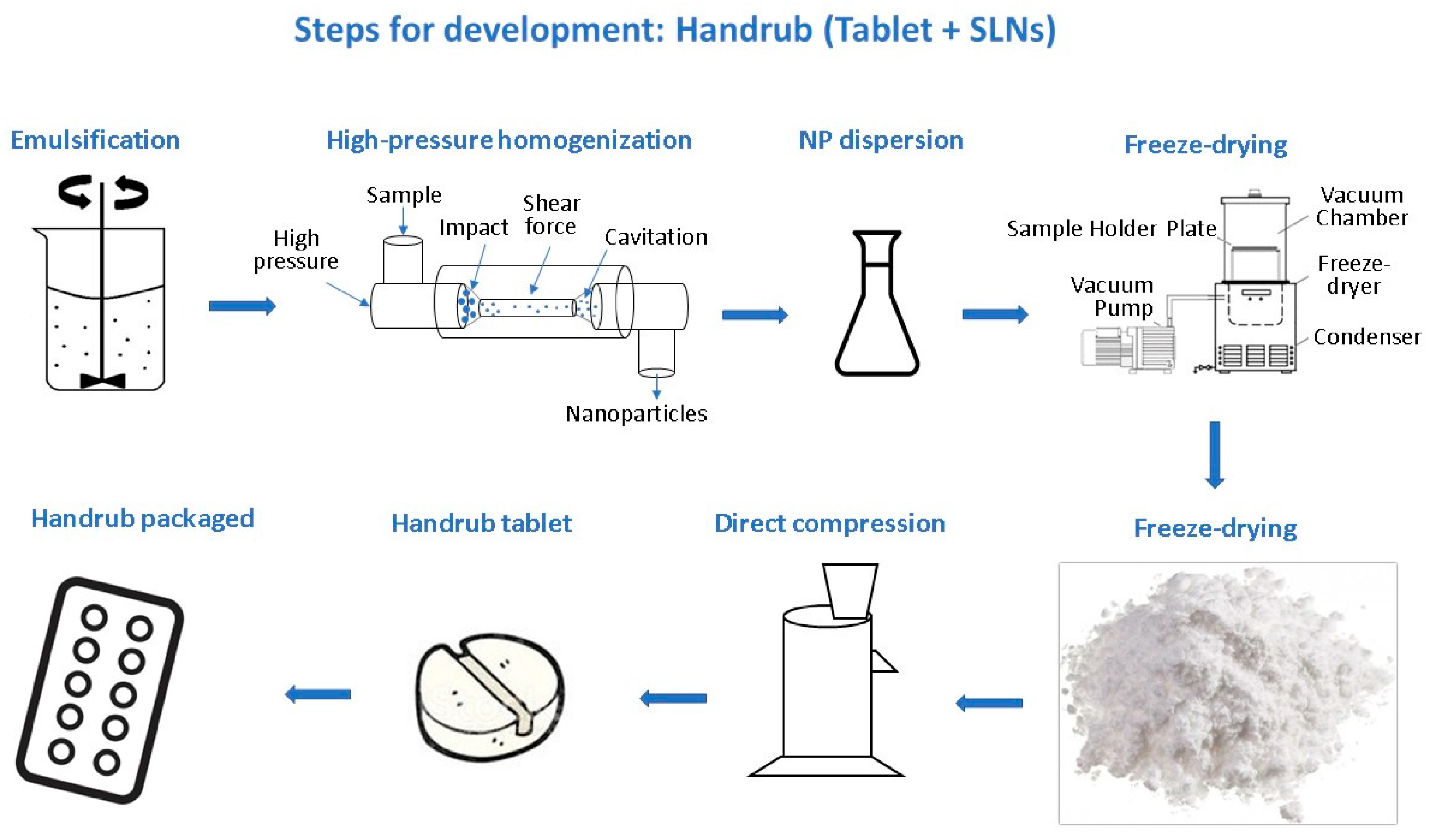
2.3. Preparation of Solid Lipid Nanoparticles (SLNs)
2.3.1. Characterization of Solid Lipid Nanoparticles
2.3.2. Prepare of SLN Dispersion as a Solid Component
2.3.3. Thermal Analysis: Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.3.4. Desorption of SLN from Lyophilized Powder Mixture
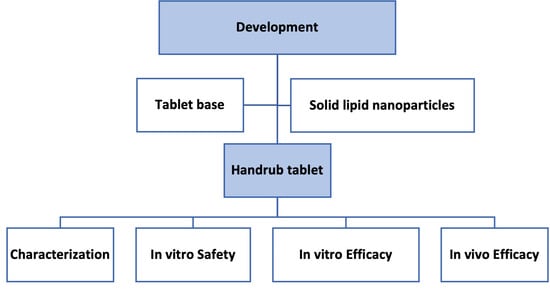
2.4. Development of the Handrub Tablet
Characterization of the Handrub Tablet
2.5. Safety and Efficacy Evaluation of the Handrub Tablet
2.5.1. In Vitro Cell Preparations
2.5.2. In Vitro Cytotoxicity
2.5.3. In Vitro Virucidal Activity
2.5.4. In Vitro Gene Expression
2.5.5. In Vitro Antioxidant Activity
2.5.6. In Vitro Collagen Synthesis
2.5.7. In Vivo Efficacy: Microbiome Analysis
2.6. Data Analysis and Statistics
3. Results
3.1. Characterization of Tablet Base
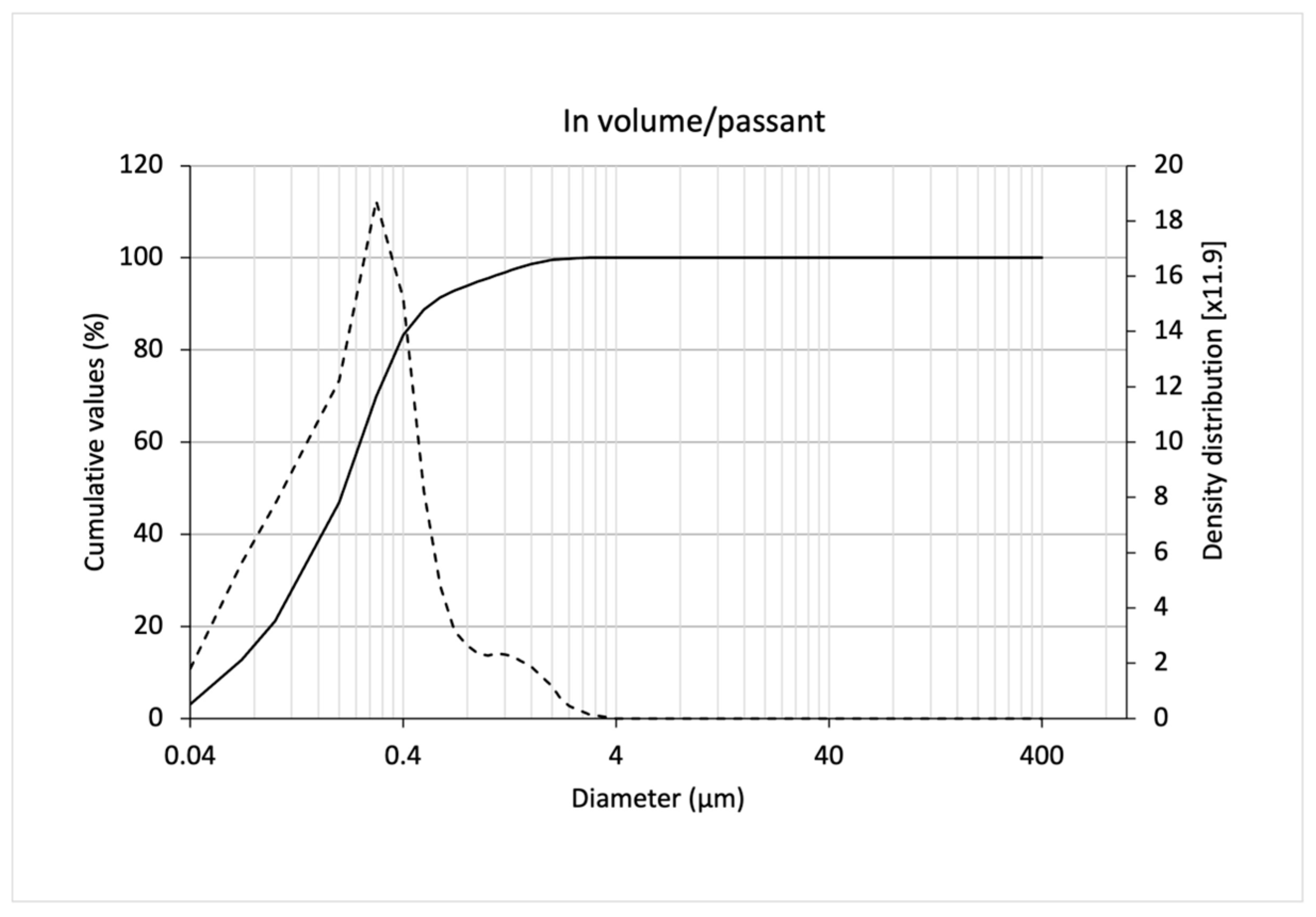
3.2. Characterization of SLNs
Thermal Analysis of SLNs
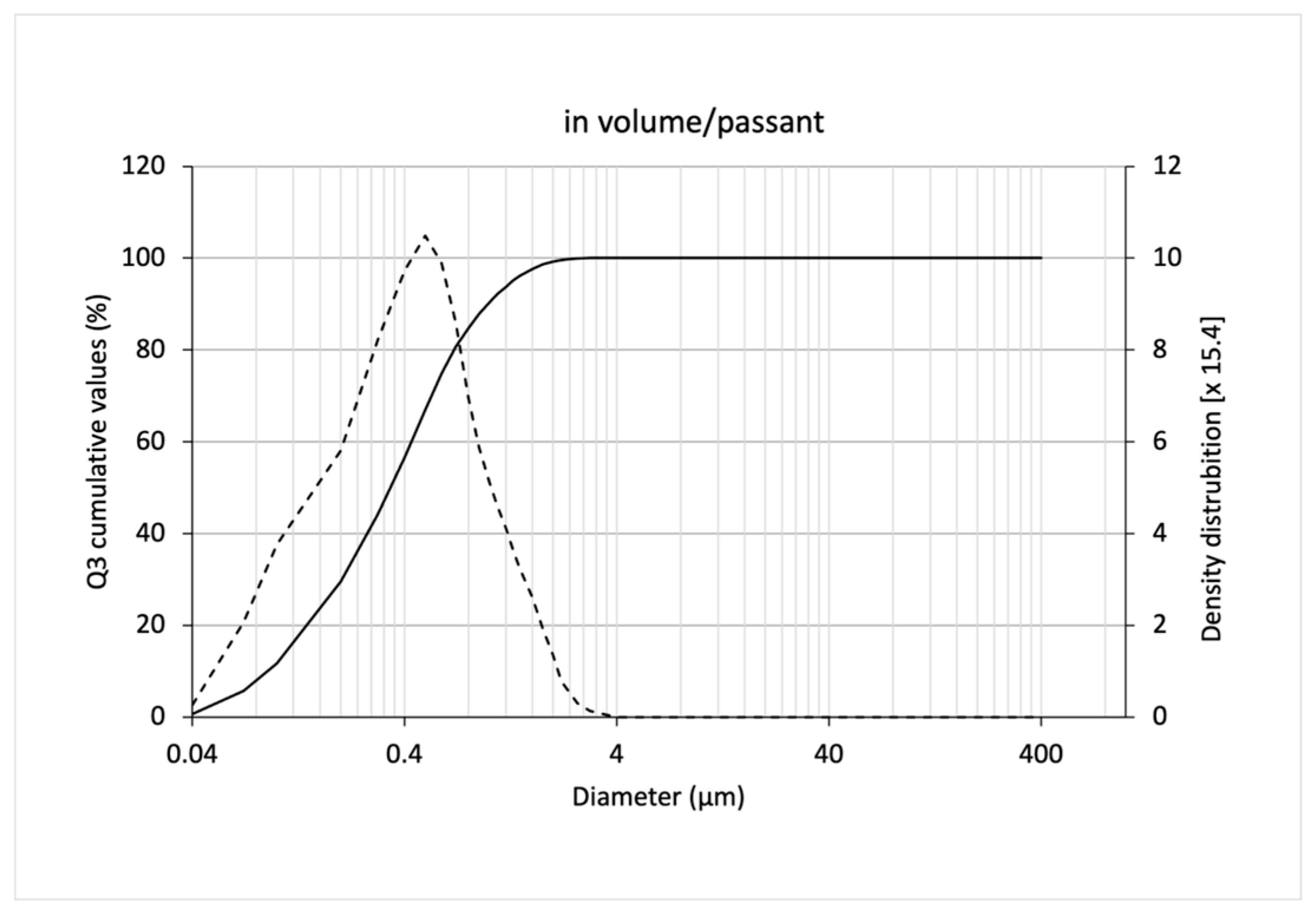
3.3. Release of Freeze-Dried SLNs
3.4. Characterization of the Handrub Tablet
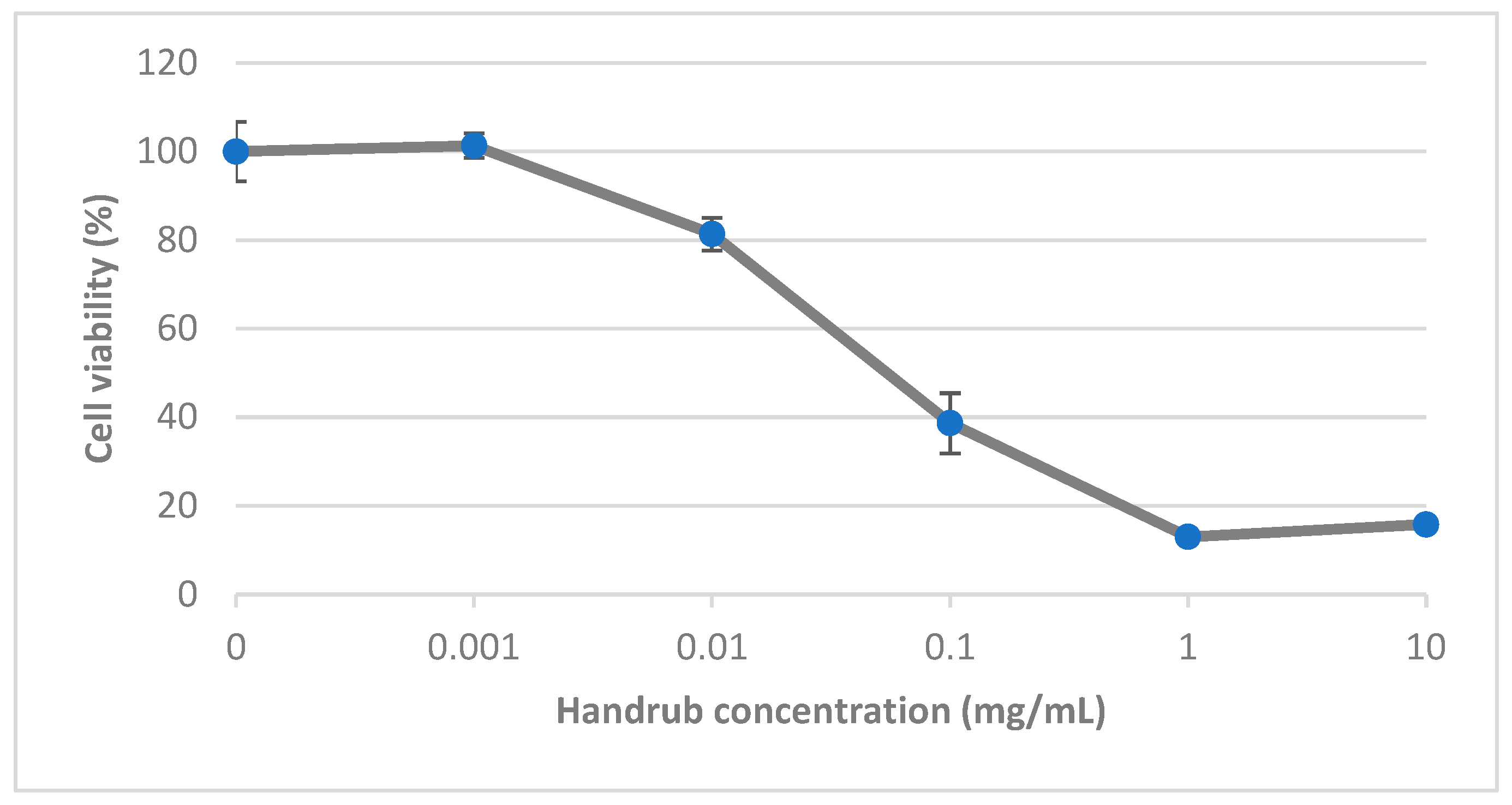
3.5. In Vitro Cytotoxicity
3.6. In Vitro Virucidal Activity
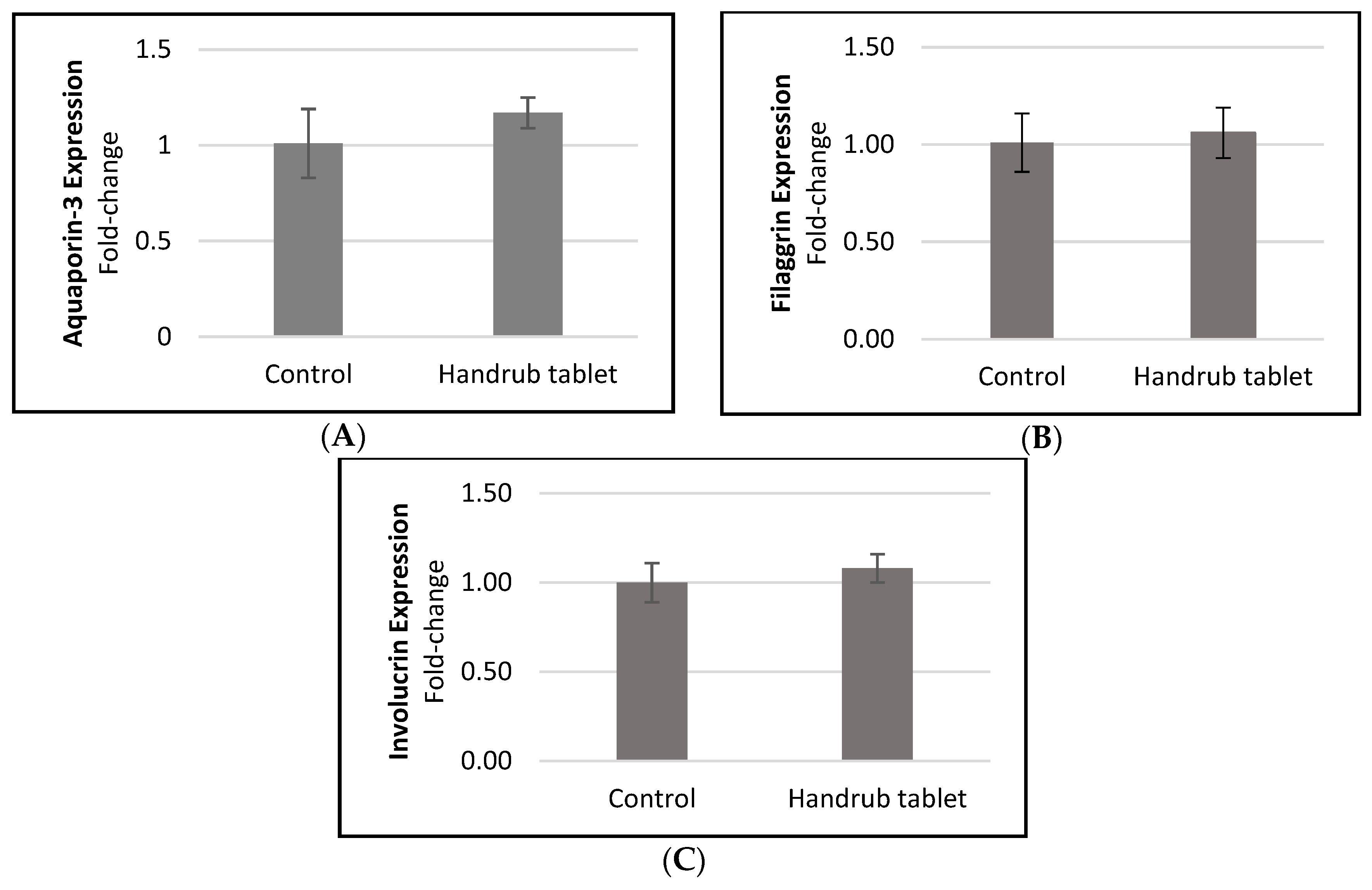
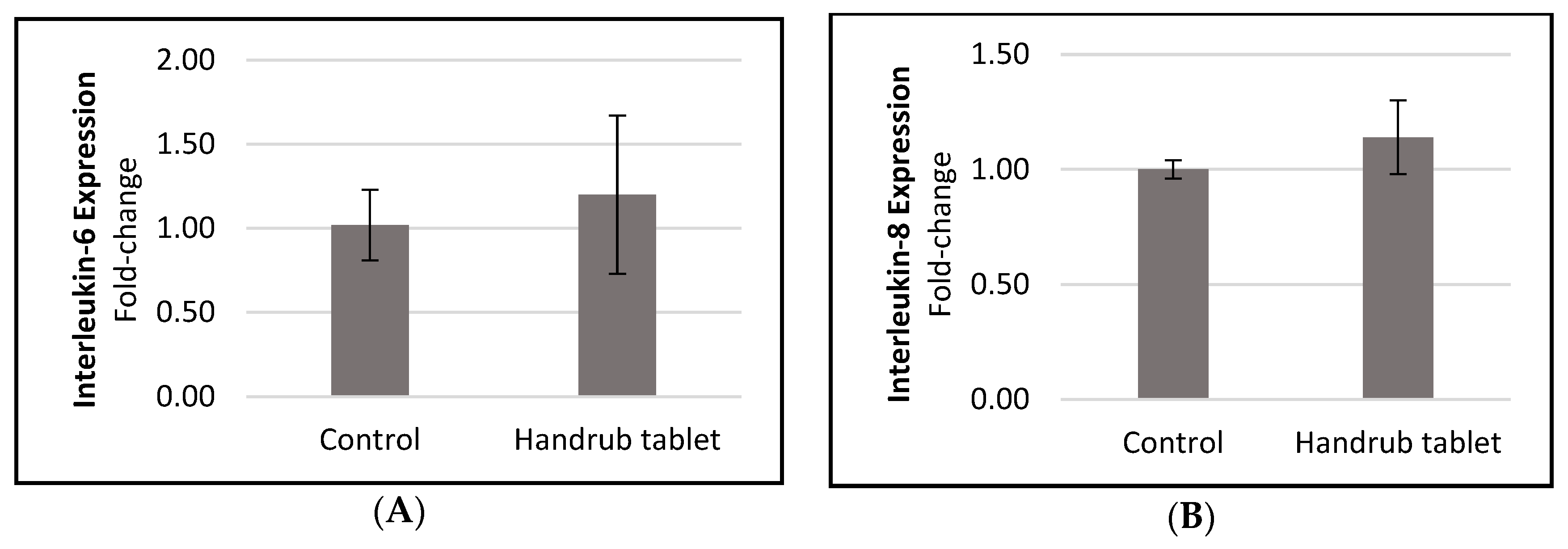
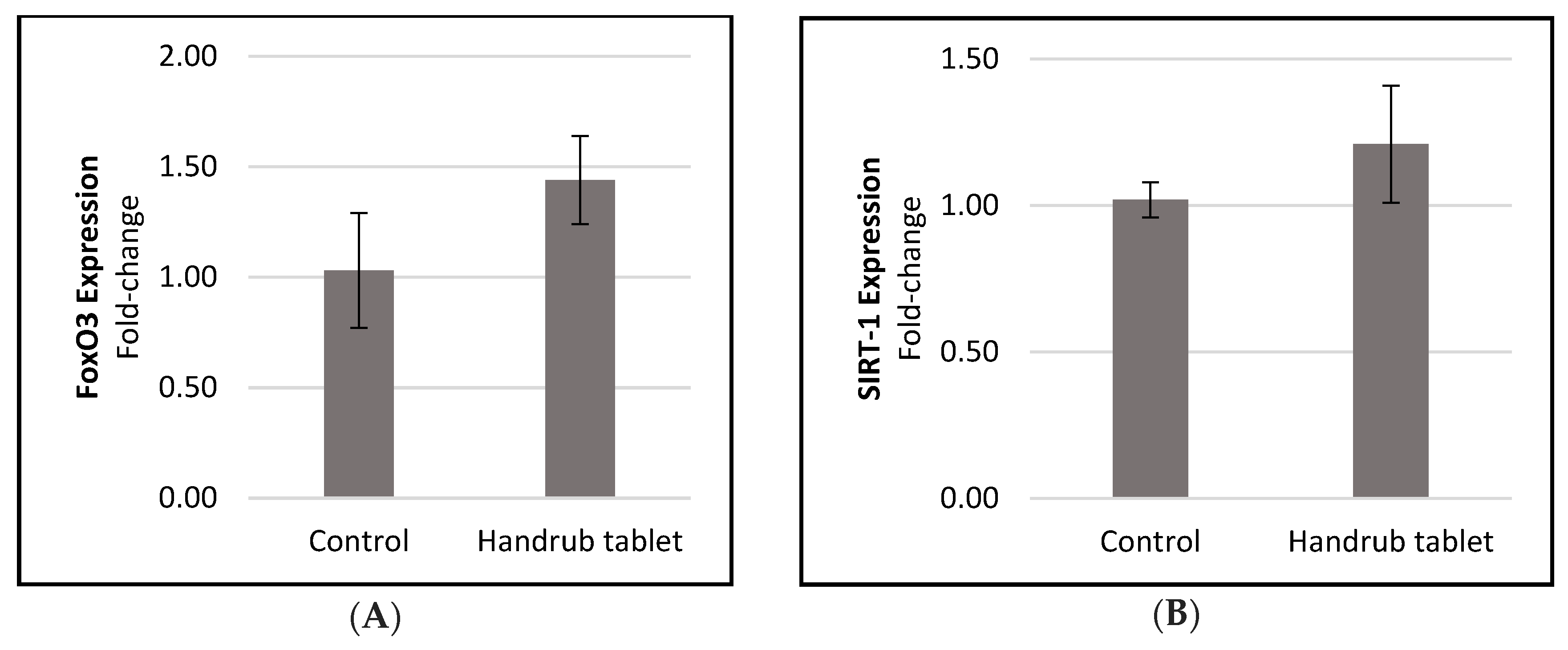
3.7. In Vitro Gene Expression
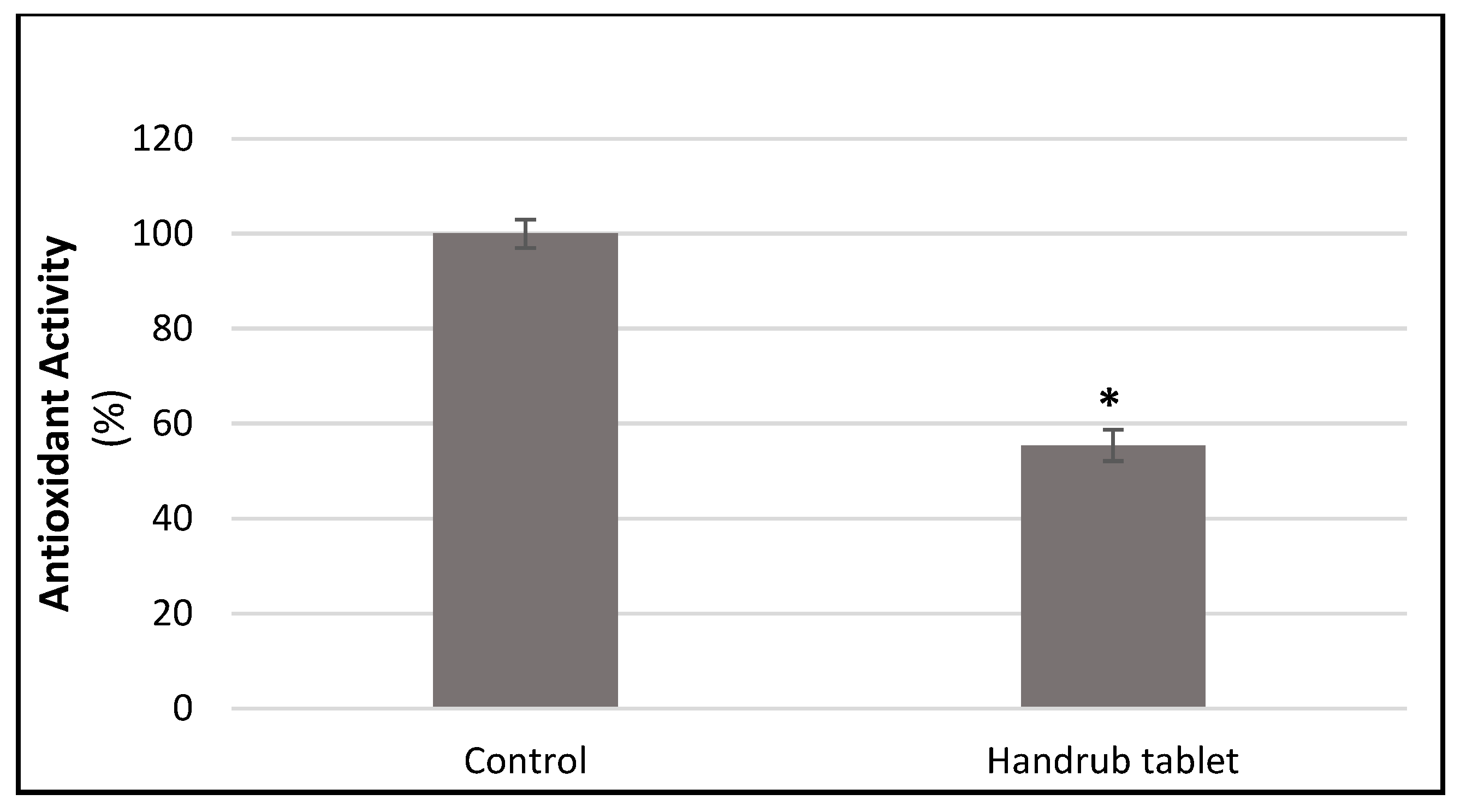
3.8. In Vitro Antioxidant Activity
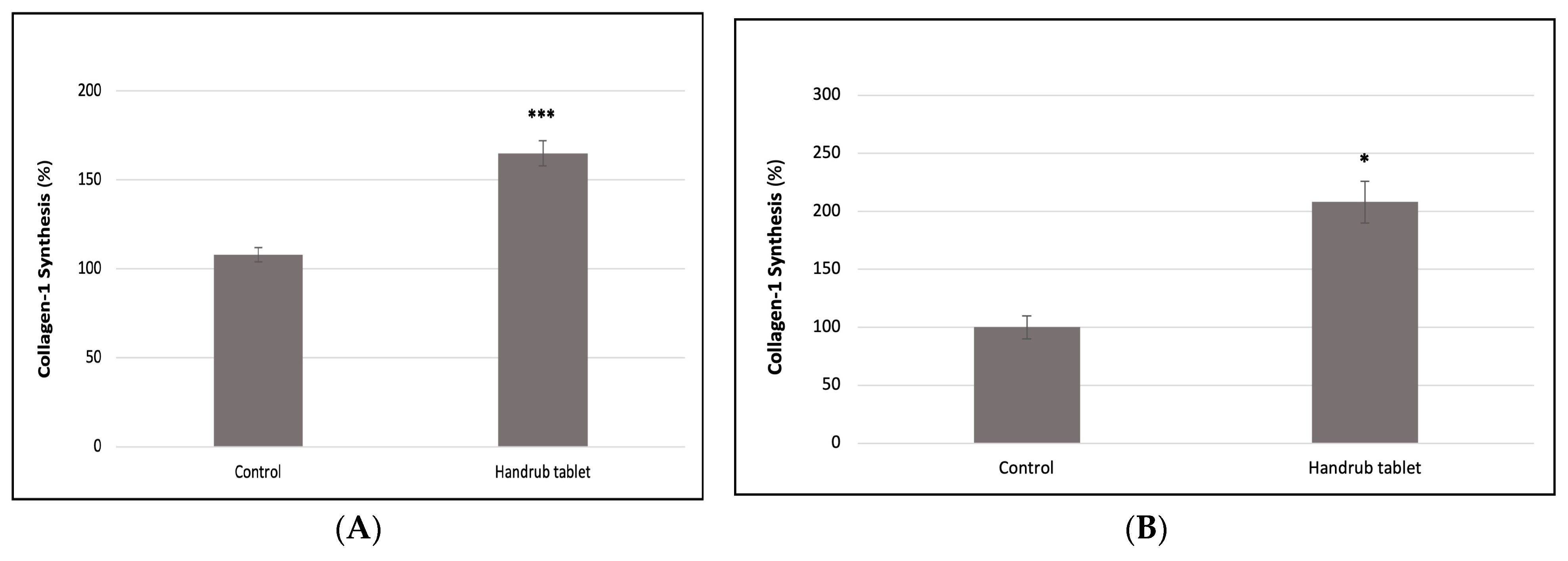
3.9. In Vitro Collagen Synthesis
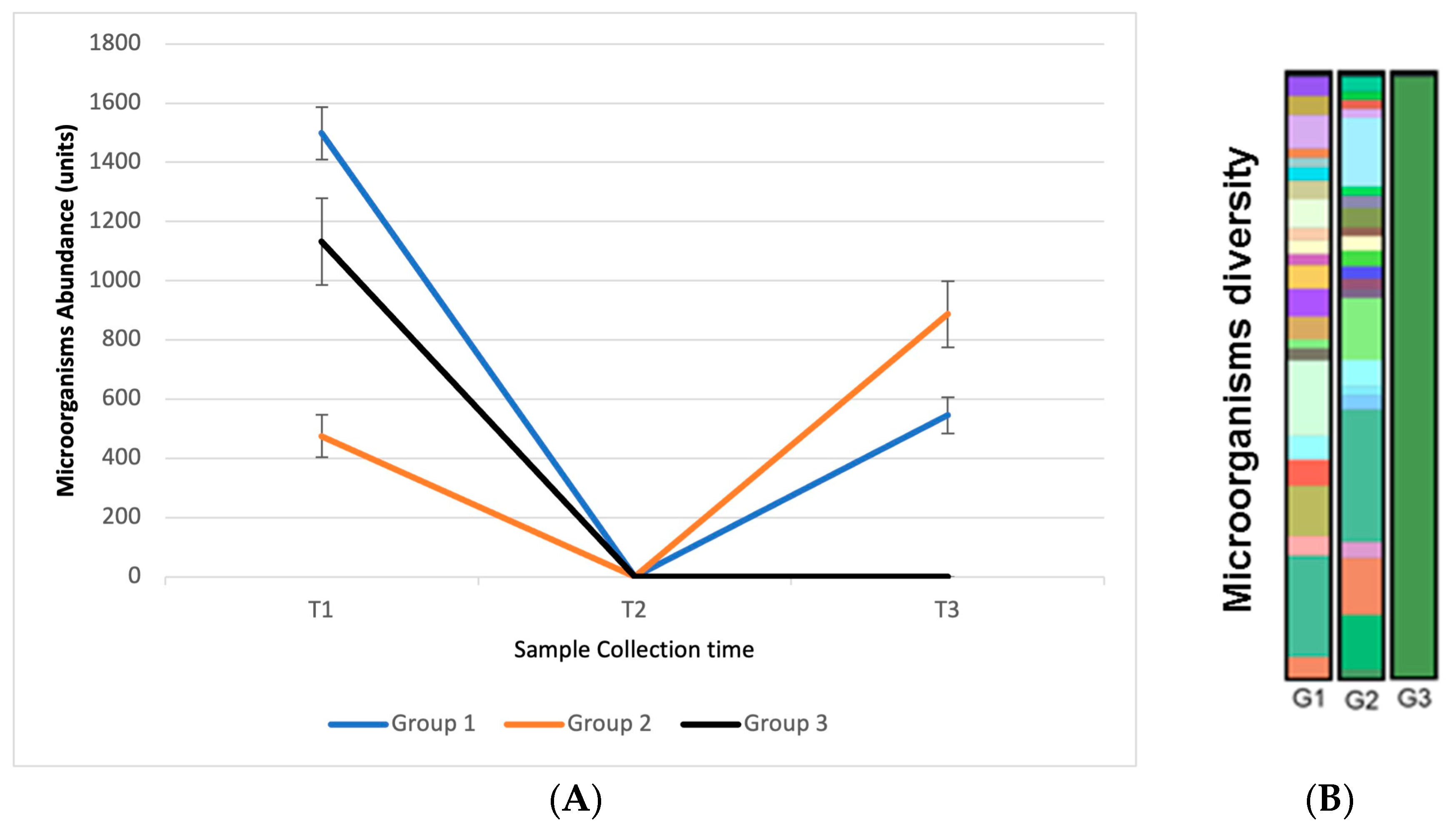
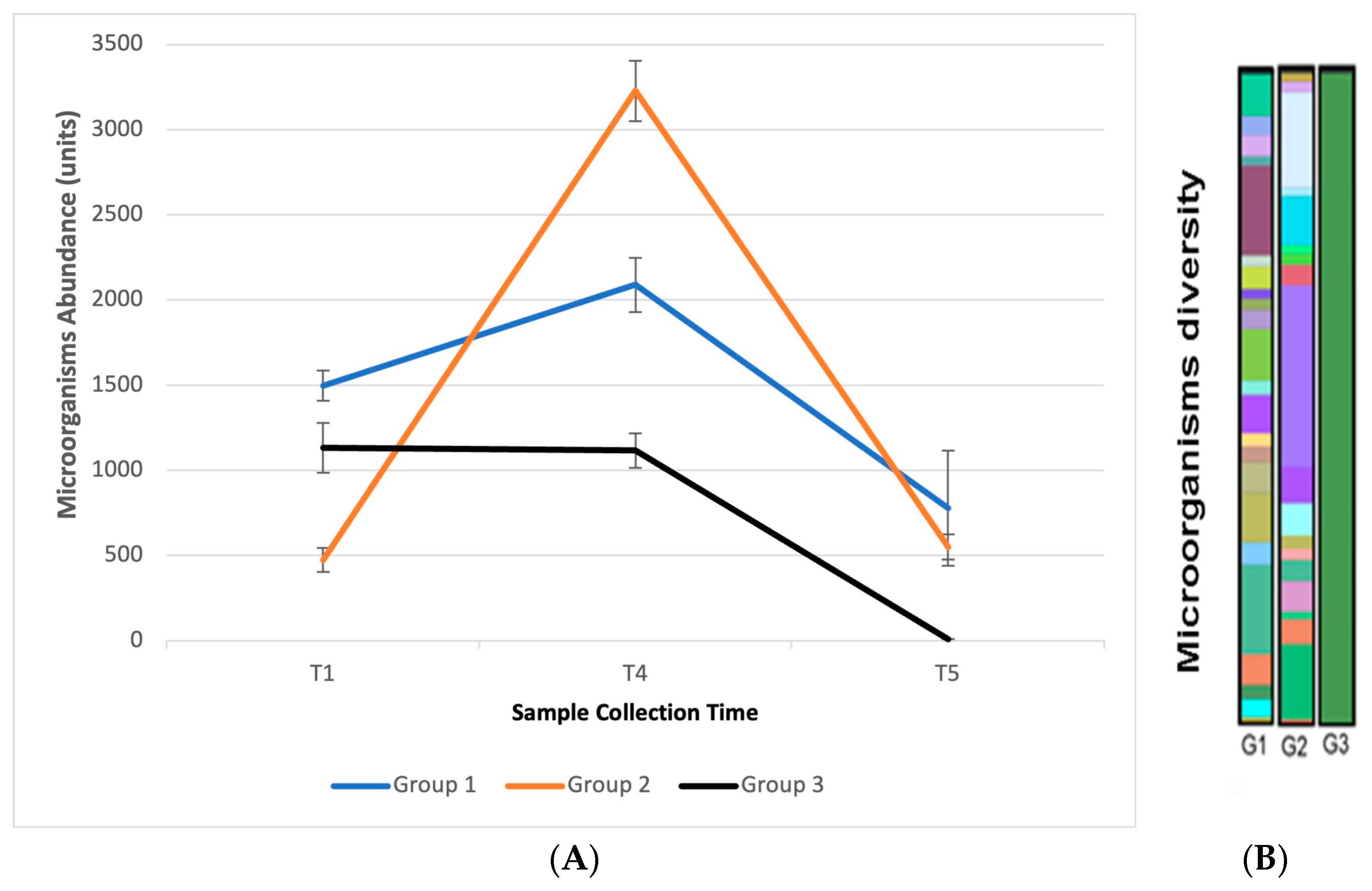
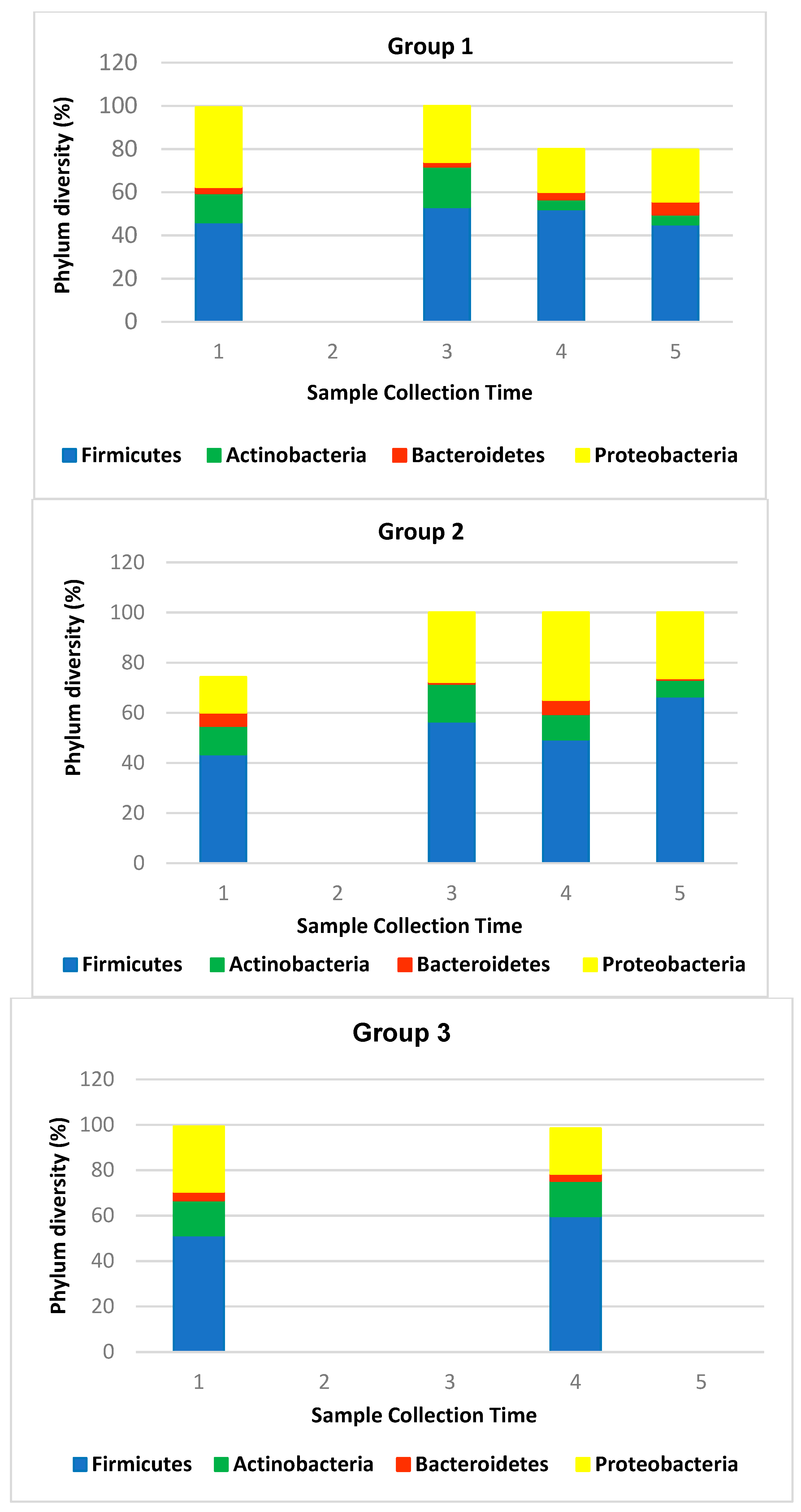
3.10. In Vivo Efficacy: Microbiome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filipe, H.A.L.; Fiuza, S.M.; Henriques, C.A.; Antunes, F.E. Antiviral and antibacterial activity of hand sanitizer and surface disinfectant formulations. Int. J. Pharm. 2021, 609, 121139. [Google Scholar] [CrossRef] [PubMed]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Beiu, C.; Mihai, M.; Popa, L.; Cima, L.; Popescu, M.N. Frequent Hand Washing for COVID-19 Prevention Can Cause Hand Dermatitis: Management Tips. Cureus 2020, 12, e7506. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.L.J.; Yi, T.P.; Bose, R.J.C.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand sanitizers: A review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Nixon, R. Irritant Contact Dermatitis—A Review. Curr. Dermatol. Rep. 2022, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; NISC Comparative Sequencing Program; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Edmonds-Wilson, S.L.; Nurinova, N.I.; Zapka, C.A.; Fierer, N.; Wilson, M. Review of human hand microbiome research. J. Dermatol. Sci. 2015, 80, 3–12. [Google Scholar] [CrossRef]
- Zárate, S.; Taboada, B.; Yocupicio-Monroy, M.; Arias, C.F. Human Virome. Arch. Med. Res. 2017, 48, 701–716. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The Human Skin Double-Stranded DNA Virome: Topographical and Temporal Diversity, Genetic Enrichment, and Dynamic Associations with the Host Microbiome. mBio 2015, 6, e01578-15. [Google Scholar] [CrossRef]
- Mills, J.G.; Brookes, J.D.; Gellie, N.J.C.; Liddicoat, C.; Lowe, A.J.; Sydnor, H.R.; Thomas, T.; Weinstein, P.; Weyrich, L.S.; Breed, M.F. Relating urban biodiversity to human health with the “Holobiont” concept. Front. Microbiol. 2019, 10, 550. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Hirao, R.; Shigetoh, K.; Inagaki, S.; Ishida, N. Virus Inactivation Based on Optimal Surfactant Reservoir of Mesoporous Silica. ACS Appl. Bio Mater. 2023, 6, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.; Steelandt, J.; Gilbert, E.; Almouazen, E.; Haftek, M.; Pirot, F.; Roussel, L.; Renaud, F.N. Antimicrobial nanocapsules: From new solvent-free process to in vitro efficiency. Int. J. Nanomed. 2014, 9, 4467–4474. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Andréo-Filho, N.; Bim, A.V.K.; Kaneko, T.M.; Kitice, N.A.; Haridass, I.N.; Abd, E.; Lopes, P.S.; Thakur, S.S.; Parekh, H.S.; Roberts, M.S.; et al. Development and Evaluation of Lipid Nanoparticles Containing Natural Botanical Oil for Sun Protection: Characterization and in vitro and in vivo Human Skin Permeation and Toxicity. Skin Pharmacol. Physiol. 2018, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Lee, B.-J.; Tran, T.T. Strategies and formulations of freeze-dried tablets for controlled drug delivery. Int. J. Pharm. 2021, 597, 120373. [Google Scholar] [CrossRef]
- Le, N.D.; Tran, P.H.; Lee, B.-J.; Tran, T.T. Solid lipid particle-based tablets for buccal delivery: The role of solid lipid particles in drug release. J. Drug Deliv. Sci. Technol. 2019, 52, 96–102. [Google Scholar] [CrossRef]
- Knox, S.; O’boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L.; Bs, M.Y.; Bs, M.Z.; Jia, Y.; He, C. A review of fatty acids influencing skin condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Cuevas-González, P.; Liceaga, A.; Aguilar-Toalá, J. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.B.; Martins, A.M.; Almeida, C.; Ribeiro, H.M.; Marto, J. Water sustainability: A waterless life cycle for cosmetic products. Sustain. Prod. Consum. 2022, 32, 35–51. [Google Scholar] [CrossRef]
- Ciardiello, T.; Pinto, D.; Marotta, L.; Giuliani, G.; Rinaldi, F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics 2020, 7, 34. [Google Scholar] [CrossRef]
- Leite, C.d.S.; Bonafé, G.A.; Pires, O.C.; dos Santos, T.W.; Pereira, G.P.; Pereira, J.A.; Rocha, T.; Martinez, C.A.R.; Ortega, M.M.; Ribeiro, M.L. Dipotassium Glycyrrhizininate Improves Skin Wound Healing by Modulating Inflammatory Process. Int. J. Mol. Sci. 2023, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection? Front. Immunol. 2020, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Aswathy, K.; Asdaq, S.M.B.; Saritha, C.; Thomas, L.; Haridas, N.; Viswanad, V.; Sahu, R.K.; Fattepur, S.; Alamri, A.S.; Alsanie, W.F.; et al. Formulation and in-vitro characterization of fast-disintegrating herbal extract sublingual immunotherapy tablet for peanut-induced allergic asthma. Saudi J. Biol. Sci. 2021, 29, 1283–1297. [Google Scholar] [CrossRef]
- Lopes, P.S.; Leite e Silva, V.R.; Andreo Filho, N.; Machado, A.C.H.R. Formulação Cosmética Antiviral e Seus Usos. Brasil: Instituto Nacional da Propriedade Industrial; BR 10 2021 020202 5 A2. 2021. Available online: https://busca.inpi.gov.br/pePI/servlet/PatenteServletController?Action=detail&CodPedido=1630042&SearchParameter=BR%2010%202021%20020202%205%20%20%20%20%20%20&Resumo=&Titulo= (accessed on 5 August 2023).
- Abd, E.; Gomes, J.; Sales, C.C.; Yousef, S.; Forouz, F.; Telaprolu, K.C.; Roberts, M.S.; Grice, J.E.; Lopes, P.S.; Leite-Silva, V.R.; et al. Deformable liposomes as enhancer of caffeine penetration through human skin in a Franz diffusion cell test. Int. J. Cosmet. Sci. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). Methods Cell Sci. 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Schlotmann, K.; Kaeten, M.; Black, A.F.; Damour, O.; Waldmann-Laue, M.; Förster, T. Cosmetic efficacy claims in vitro using a three-dimensional human skin model. Int. J. Cosmet. Sci. 2001, 23, 309–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlson, M.W.; Alt-Holland, A.; Egles, C.; Garlick, J.A. Three-Dimensional Tissue Models of Normal and Diseased Skin. Curr. Protoc. Cell Biol. 2008, 41, 19.9.1–19.9.17. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, N.C.; Beier-Sexton, M.; Azad, A.F. Growth and Maintenance of Vero Cell Lines. Curr. Protoc. Microbiol. 2008, 11, A.4E.1–A.4E.7. [Google Scholar] [CrossRef] [PubMed]
- Zapka, C.; Leff, J.; Henley, J.; Tittl, J.; De Nardo, E.; Butler, M.; Griggs, R.; Fierer, N.; Edmonds-Wilson, S. Comparison of Standard Culture-Based Method to Culture-Independent Method for Evaluation of Hygiene Effects on the Hand Microbiome. mBio 2017, 8, e00093-17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, J.; Yin, J.; Li, S. Computational and experimental research on mechanism of cis/trans isomerization of oleic acid. Heliyon 2018, 4, e00768. [Google Scholar] [CrossRef] [PubMed]
- Kirkebæk, B.; Simoni, G.; Lankveld, I.; Poulsen, M.; Christensen, M.; Quist-Jensen, C.A.; Yu, D.; Ali, A. Oleic acid-coated magnetic particles for removal of oil from produced water. J. Pet. Sci. Eng. 2021, 211, 110088. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; International Standard. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Citotoxicity. ISO: Geneva, Switzerland, 2009.
- World Health Organization. Patient Safety. In WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2011; 262p. [Google Scholar]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Goh, C.; Wu, Y.; Welsh, B.; Abad-Casintahan, M.F.; Tseng, C.; Sharad, J.; Jung, S.; Rojanamatin, J.; Sitohang, I.B.S.; Chan, H.N.K. Expert consensus on holistic skin care routine: Focus on acne, rosacea, atopic dermatitis, and sensitive skin syndrome. J. Cosmet. Dermatol. 2022, 22, 45–54. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Krötzsch, E. Soap or alcohol-based products? The effect of hand hygiene on skin characteristics during the COVID-19 pandemic. J. Cosmet. Dermatol. 2022, 22, 347–353. [Google Scholar] [CrossRef]
- Plum, F.; Yüksel, Y.T.; Agner, T.; Nørreslet, L.B. Skin barrier function after repeated short-term application of alcohol-based hand rub following intervention with water immersion or occlusion. Contact Dermat. 2020, 83, 215–219. [Google Scholar] [CrossRef]
- Aoki, Y.; Wehage, S.L.; Talalay, P. Quantification of skin erythema response to topical alcohol in alcohol-intolerant East Asians. Skin Res. Technol. 2017, 23, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.; Streel, E. Is Alcohol in Hand Sanitizers Absorbed Through the Skin or Lungs? Implications for Disulfiram Treatment. Alcohol Alcohol. 2020, 55, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Kieszak, S.; Wang, A.; Law, R.; Schier, J.; Wolkin, A. Reported Adverse Health Effects in Children from Ingestion of Alcohol-Based Hand Sanitizers-United States. 2017. Volume 66. Available online: https://www.npds.us (accessed on 29 April 2023).
- Baker, N.; Williams, A.J.; Tropsha, A.; Ekins, S. Repurposing Quaternary Ammonium Compounds as Potential Treatments for COVID-19. Pharm. Res. 2020, 37, 104. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. Pre- and probiotics for human skin. J. Dermatol. Sci. 2009, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2016, 137, 855–864. [Google Scholar] [CrossRef]
- Coquette, A.; Berna, N.; Vandenbosch, A.; Rosdy, M.; De Wever, B.; Poumay, Y. Analysis of interleukin-1α (IL-1α) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol. In Vitr. 2003, 17, 311–321. [Google Scholar] [CrossRef]
- Baurecht, H.; Rühlemann, M.C.; Rodríguez, E.; Thielking, F.; Harder, I.; Erkens, A.-S.; Stölzl, D.; Ellinghaus, E.; Hotze, M.; Lieb, W.; et al. Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J. Allergy Clin. Immunol. 2018, 141, 1668–1676.e16. [Google Scholar] [CrossRef]
- Hoyer, A.; Rehbinder, E.; Färdig, M.; Asad, S.; Carlsen, K.L.; Endre, K.; Granum, B.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; et al. Filaggrin mutations in relation to skin barrier and atopic dermatitis in early infancy. Br. J. Dermatol. 2021, 186, 544–552. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, K.-M. Skin barrier dysfunction and filaggrin. Arch. Pharmacal Res. 2021, 44, 36–48. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, S.; Zhou, L.; He, K.; Song, L.; Liang, H.; He, C. Effect of cosmetic chemical preservatives on resident flora isolated from healthy facial skin. J. Cosmet. Dermatol. 2018, 18, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z. Aquaporins: An introduction to a key factor in the mechanism of skin hydration. J. Clin. Aesthetic Dermatol. 2012, 5, 53–56. [Google Scholar]
- Tsitsipatis, D.; Klotz, L.-O.; Steinbrenner, H. Multifaceted functions of the forkhead box transcription factors FoxO1 and FoxO3 in skin. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Lee, M.-K.; Hwang, T.; Choi, C.W.; Kim, M.S.; Kim, H.-R.; Lee, B. The multi-functional roles of forkhead box protein O in skin aging and diseases. Redox Biol. 2021, 46, 102101. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Sommerfeldt, J.M.; Andrus, M.B. Resveratrol: Influences on gene expression in human skin. J. Funct. Foods 2014, 10, 377–384. [Google Scholar] [CrossRef]
- Senthil, K.K.; Gokila, V.M.; Mau, J.L.; Lin, C.C.; Chu, F.H.; Wei, C.C.; Liao, V.H.-C.; Wang, S.-Y. A steroid like phytochemical Antcin M is an anti-aging reagent that eliminates hyperglycemia-accelerated premature senescence in dermal fibroblasts by direct activation of Nrf2 and SIRT-1. Oncotarget 2016, 7, 62836. Available online: www.impactjournals.com/oncotarget/ (accessed on 1 September 2023). [CrossRef]
- Qiong, H.; Han, L.; Zhang, N.; Chen, H.; Yan, K.; Zhang, Z.; Ma, Y.; Xu, J. Glycyrrhizin improves the pathogenesis of psoriasis partially through IL-17A and the SIRT1-STAT3 axis. BMC Immunol. 2021, 22, 34. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef]
- Blander, G.; Bhimavarapu, A.; Mammone, T.; Maes, D.; Elliston, K.; Reich, C.; Matsui, M.S.; Guarente, L.; Loureiro, J.J. SIRT1 Promotes Differentiation of Normal Human Keratinocytes. J. Investig. Dermatol. 2009, 129, 41–49. [Google Scholar] [CrossRef]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Cheng, J.; Hata, T. Dysbiosis of the Skin Microbiome in Atopic Dermatitis. In Skin Microbiome Handbook; Scrivener Publishing: Beverly, UK, 2020; pp. 185–201. [Google Scholar]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Drake, D. The Gut Microbiome-Skin Axis: Impact on Skin and Systemic Health. In Skin Microbiome Handbook; Scrivener Publishing: Beverly, UK, 2020; pp. 33–44. [Google Scholar]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Whitfill, T.; Dubé, G.R.; Oh, J. Skin Microbiome Alterations in Skin Diseases. In Skin Microbiome Handbook; Scrivener Publishing: Beverly, UK, 2020; pp. 59–78. [Google Scholar]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.-H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]




| Components | Percentual Composition (% w/w) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | |
| Magnesium stearate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Colloidal silicon dioxide | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sodium starch glycolate | 10 | 10 | 20 | 20 | --- | --- | --- | --- | 7.5 | 7.5 | 7.5 |
| Sodium croscarmellose | --- | --- | --- | --- | 10 | 10 | 20 | 20 | 7.5 | 7.5 | 7.5 |
| Microcrystalline cellulose | --- | 84 | --- | 74 | --- | 84 | --- | 74 | 39.5 | 39.5 | 39.5 |
| Pregelatinized Starch | 84 | --- | 74 | --- | 84 | --- | 74 | --- | 39.5 | 39.5 | 39.5 |
| Ingredients | Concentration (% w/w) |
|---|---|
| Cetyltrimethylammonium chloride | 1.0 |
| Polyvinylpyrrolidone K30 | 1.0 |
| Poloxamer 188 | 1.0 |
| Purified water | 90.0 |
| Glyceryl monostearate | 1.5 |
| Stearic acid | 0.9 |
| Palmitic acid | 0.9 |
| Oleic acid | 0.6 |
| Lauric acid | 0.3 |
| Caprylic acid | 1.0 |
| Valeric acid | 0.6 |
| Butyric acid | 0.2 |
| Ingredients | Concentration (% w/w) |
|---|---|
| Blend of Solid lipid nanoparticles and MCC (9% w/w) | 33.0 |
| Prebiotics | 16.51 |
| Postbiotics | 9.0 |
| Glycyrrhizinate dipotassium | 0.15 |
| Menthol | 1.0 |
| Fragrance | 1.0 |
| Sodium croscarmellose | 10.0 |
| Silicon dioxide | 1.0 |
| Magnesium stearate | 5.0 |
| Hydroxypropyl cellulose | 2.0 |
| Microcrystalline cellulose PH101 | 21.34 |
| Parameters (Mean ± SD) * | Formulations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | |
| Weight (mg) | 89.1 | 92.9 | 86.1 | 91.0 | 88.7 | 93.5 | 87.8 | 95.6 | 91.2 | 89.9 | 90.0 |
| ± 2.6 | ± 1.8 | ± 2.9 | ± 3.2 | ± 1.5 | ± 1.7 | ± 1.5 | ± 9.0 | ± 1.2 | ± 3.3 | ± 0.8 | |
| Height (mm) | 2.03 | 2.48 | 2.04 | 2.44 | 2.15 | 2.54 | 2.11 | 2.45 | 2.27 | 2.24 | 2.24 |
| ± 0.07 | ± 0.01 | ± 0.00 | ± 0.03 | ± 0.10 | ± 0.34 | ± 0.04 | ± 0.05 | ± 0.03 | ± 0.12 | ± 0.08 | |
| Diameter (mm) | 7.89 | 7.90 | 7.81 | 7.82 | 7.68 | 7.96 | 7.73 | 7.91 | 7.52 | 7.88 | 7.91 |
| ± 0.08 | ± 0.14 | ± 0.06 | ± 0.03 | ± 0.13 | ± 0.01 | ± 0.01 | ± 0.02 | ± 0.23 | ± 0.01 | ± 0.03 | |
| Disintegration time (s) | 85.67 | 22.00 | 81.00 | 33.00 | 51.33 | 16.00 | 52.00 | 26.00 | 52.67 | 51.67 | 56.00 |
| ± 10.02 | ± 5.57 | ± 16.09 | ± 4.36 | ± 7.77 | ± 2.65 | ± 9.17 | ± 4.36 | ± 8.39 | ± 3.21 | ± 3.00 | |
| Swelling (%) | 466.9 | 677.3 | 610.1 | 931.1 | 369.8 | 421.1 | 493.9 | 499.5 | 656.6 | 684.9 | 688.7 |
| ± 54.06 | ± 19.77 | ± 30.42 | ± 54.63 | ± 3.25 | ± 11.36 | ± 38.60 | ± 24.58 | ± 2.02 | ± 13.37 | ± 7.76 | |
| Hardness (N) | <1 | <1 | <1 | 2.60 ** | 2.30 ** | 6.53 | <1 | 1.63 ** | <1 | <1 | <1 |
| --- | --- | --- | --- | --- | 1.10 | --- | --- | --- | --- | --- | |
| Formulation/Analysis | Size Values (µm) (Mean ± SD) | Dispersity (Spam) | Uniformity Ratio | |||
|---|---|---|---|---|---|---|
| d10 | d50 | d90 | dm | |||
| Emulsion/passing volume * | 0.21 ± 0.03 | 2.91 ± 0.49 | 9.80 ± 0.69 | 3.99 ± 0.40 | 3.33 ± 0.33 | 41.27 ± 2.46 |
| Solid lipid nanoparticles dispersion/passing volume ** | 0.06 ± 0.00 | 0.22 ± 0.01 | 0.59 ± 0.04 | 0.31 ± 0.02 | 2.43 ± 0.13 | 6.27 ± 0.42 |
| Solid lipid nanoparticles dispersion/passing number ** | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.05 ± 0.00 | 1.78 ± 0.19 | --- |
| Samples | Parameters Recorded | |||
|---|---|---|---|---|
| Δw (%) (T Range/°C) | Tonset (°C) | Tpeak DTG (°C) | Tpeak DSC (°C) | |
| Butyric Acid | 99 (36–173) | 151 | 159 | 160 |
| Valeric Acid | 104 (44–188) | 166 | 179 | 179 |
| Caprylic Acid | 91 (52–242) | 215 | 231 | 232 |
| Oleic Acid | 100 (134–490) | 287 | 325 451 | 101 331 492 |
| Oil Phase non-lyophilized | 100 (97–480) | 181 403 | 214 309 425 | 46 97 212 317 437 |
| Oil Phase lyophilized | 95 (94–496) | 247 404 | 309 427 | 49 105 309 438 |
| Lipid Nanoparticles dispersion | 98 (35–474) | 98 221 381 | 104 244 404 | 108 237 448 |
| Lipid Nanoparticles lyophilized | 99 (48–519) | 238 390 | 241 298 409 | 86 467 |
| Formulation/Analysis | Size Values (µm) (Mean ± SD) | Dispersity (Spam) | Residual Humidity (%) | |||
|---|---|---|---|---|---|---|
| d10 | d50 | d90 | dm | |||
| Solid lipid nanoparticles freeze dried (1:1) * | 0.08 ± 0.01 | 0.28 ± 0.05 | 0.96 ± 0.03 | 0.41 ± 0.04 | 3.19 ± 0.47 | 2.51 ± 0.30 |
| Parameters | Handrub Tablet (Mean ± SD) * |
|---|---|
| Mean weight (mg) | 102.6 ± 2.7 |
| Thickness (mm) | 2.78 ± 0.05 |
| Diameter (mm) | 8.02 ± 0.02 |
| Disintegration time (s) | 22.30 ± 2.90 |
| Swelling rate (%) | 332.5 ± 12.2 |
| Hardness (N) | <1 ± 0.00 |
| Group | Cell Culture | Time (min) | Log Quantification | Reduction Percentage |
|---|---|---|---|---|
| Negative control | Monolayer and human equivalent skin | 1, 5 and 120 | Not identified | - |
| Viral control | Monolayer | 1 and 120 | 105.0 | 0 |
| Human equivalent skin | 5 | 104.0 | 0 | |
| Handrub tablet | Human equivalent Skin | 5 | 100.5 | 99.96% |
| Monolayer | 1 | 101.0 | 99.99% | |
| Monolayer | 120 | 102.0 | 99.9% | |
| 70% Alcohol | Human equivalent skin | 5 | 101.0 | 99.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, A.C.H.R.; Marinheiro, L.J.; Benson, H.A.E.; Grice, J.E.; Martins, T.d.S.; Lan, A.; Lopes, P.S.; Andreo-Filho, N.; Leite-Silva, V.R. A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome. Pharmaceutics 2023, 15, 2793. https://doi.org/10.3390/pharmaceutics15122793
Machado ACHR, Marinheiro LJ, Benson HAE, Grice JE, Martins TdS, Lan A, Lopes PS, Andreo-Filho N, Leite-Silva VR. A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome. Pharmaceutics. 2023; 15(12):2793. https://doi.org/10.3390/pharmaceutics15122793
Chicago/Turabian StyleMachado, Ana Carolina Henriques Ribeiro, Laís Júlio Marinheiro, Heather Ann Elizabeth Benson, Jeffrey Ernest Grice, Tereza da Silva Martins, Alexandra Lan, Patricia Santos Lopes, Newton Andreo-Filho, and Vania Rodrigues Leite-Silva. 2023. "A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome" Pharmaceutics 15, no. 12: 2793. https://doi.org/10.3390/pharmaceutics15122793
APA StyleMachado, A. C. H. R., Marinheiro, L. J., Benson, H. A. E., Grice, J. E., Martins, T. d. S., Lan, A., Lopes, P. S., Andreo-Filho, N., & Leite-Silva, V. R. (2023). A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome. Pharmaceutics, 15(12), 2793. https://doi.org/10.3390/pharmaceutics15122793