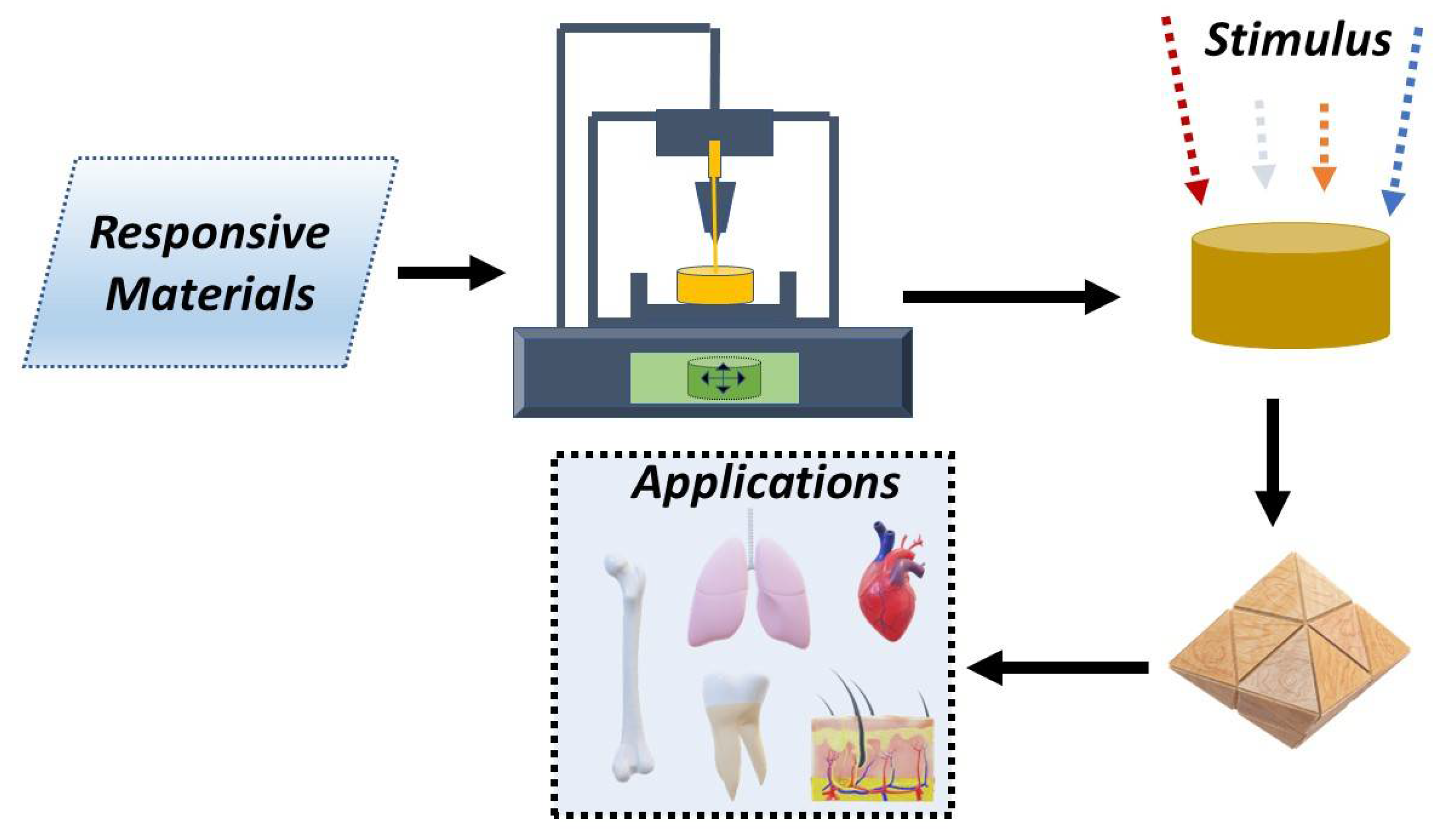
4D Printing: The Development of Responsive Materials Using 3D-Printing Technology
Abstract
1. Introduction
2. Advantages and Disadvantages of Different 3D-Printing Techniques
2.1. Selective Laser Sintering (SLS)
2.2. Directed Energy Deposition (DED)
2.3. Material Extrusion
2.4. Material Jetting
2.5. Stereolithography (SLA)
2.6. Sheet Lamination
2.7. Electrospinning
2.8. Extrusion Bioprinting
2.9. Inkjet Bioprinting
2.10. Laser-Assisted Bioprinting
2.11. Multiphoton Fabrication
3. Materials
3.1. Shape Memory Materials (SMM)
3.1.1. Shape Memory Polymers (SMPs)
3.1.2. Shape Memory Alloys (SMAs)
3.1.3. Shape Memory Ceramics (SMCrs)
3.1.4. Other Materials
3.2. Gels
3.2.1. Hydrogels
3.2.2. Organogels
3.3. Liquid Crystalline Elastomers (LCE)
3.4. Other Functional Materials
3.4.1. Magneto-Responsive Materials
3.4.2. Electro-Responsive Materials
4. Stimulus and Effect on Responsive Materials
4.1. Physical Stimuli
4.1.1. Light
4.1.2. Temperature
4.1.3. Electric Field
4.1.4. Magnetic Field
4.2. Chemical Stimuli
4.2.1. pH
4.2.2. Humidity
4.3. Biological Stimuli
4.3.1. Enzymes
4.3.2. Biomolecules
5. 4D-Bioprinting Applications
5.1. Advantages and Disadvantages of 4D Printing for Biomedical Applications
5.2. Light-Responsive Materials

5.3. Thermo-Responsive Scaffolds
5.4. Electric-Field-Responsive Scaffolds
5.5. Magnetic-Field-Responsive Scaffolds
5.6. Humidity-Responsive Applications
5.7. Enzymes Responsive Materials
| Stimuli | Material | Applications | Ref. |
|---|---|---|---|
| Light | GelMA-filled silicate nanoplatelets containing hMSCs | Promotion of the formation, stability, and maturation of vascular vessels in vitro | [174] |
| PEG tetrabicyclononyne (Mn ~20,000 Da), diazide-functionalized synthetic peptide | Generation of endothelialized 3D vascular networks | [206] | |
| Hyaluronic acid functionalized with photoisomerized tetra-ortho-methoxy-substituted Azo crosslinked to cyclodextrins | Regeneration of functional multi-tissue complex under an external control | [208] | |
| PCL, lauric acid, and melanin | Controlled release of insulin | [211] | |
| Dihydrazide-modified HA hydrogel | Effective, localized delivery of anti-cancer agents | [212] | |
| PCL impregnated with gold nanoparticles-decorated carbon nanofibers | Controlled drug release | [215] | |
| Chitosan microswimmers | Controlled drug release | [218] | |
| Wool keratin modified with TTFAP and AzA | Wound healing and tissue-engineering applications.Antimicrobial activity | [221] | |
| Alginate-gelatin hydrogels coated with PCL and PDA | Controlled release of doxorubicin for cancer therapy | [223] | |
| CNTs-doped N-isopropylacrylamide (NIPAM) composite hydrogel | Biomimetic aortic valve microstructure | [224] | |
| Temperature | PCL triol, poly(hexamethylene diisocyanate), and castor oil | Enhancement of the attachment, proliferation, and differentiation of human bone-marrow-derived mesenchymal stem cells | [225] |
| PNIPAM, hydroxyethyl-chitosan, and dithiol-modified graphene oxide nanosheets | Angiogenic activity | [226] | |
| PEG and partially methacrylated poly[N-(2-hydroxypropyl) methacrylamide mono/dilactate] incorporating polysaccharides | Cartilage repair | [227] | |
| PNIPAM grafted hyaluronan and methacrylated hyaluronan | Chondrogenesis | [228] | |
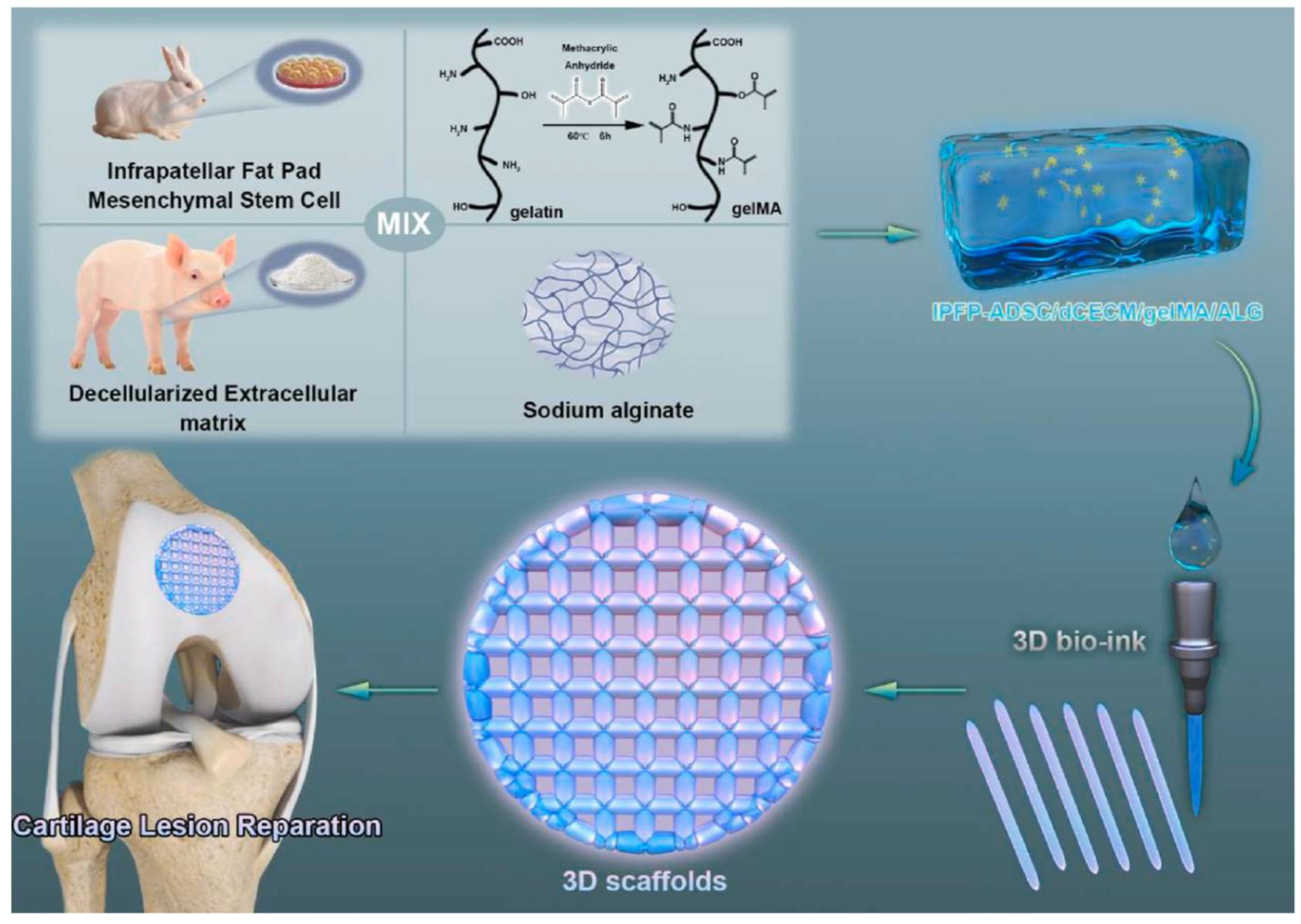
| Decellularized cartilage extracellular matrix, methacrylated gelatin, and sodium alginate | Chondrogenesis | [229] | |
| Poly(lactic-co-glycolic acid)-PEG | Bone repair | [230] | |
| Poly(organophosphazene), BMP-2, and TGF-β1 | Bone regeneration | [231] | |
| PCL-PEG-PCL triblock polymers | Skin regeneration and wound repair | [232] | |
| PU nanoparticles with the inclusion of oligodiols [PCL diol, poly(L-lactide) diol and poly(D,L-lactide) diol] | Promotion of neural stem cells’ proliferation and differentiation | [233] | |
| PU | Promotion of neural stem cells’ proliferation and differentiation | [234] | |
| Sodium alginate grafted with PNIPAM-co-N-tert-butylacrylamide | Promotion of cell spheroid formation | [235] | |
| Electric field | Alginate–gelatin containing PPy:PSS | Promotion of cell adhesion and proliferation | [239] |
| PEDOT coated with collagen | Electrical controlled drug release | [240] | |
| Agarose/alginate–aniline tetramer | Controlled dexamethasone release for neurodegenerative diseases’ treatment | [243] | |
| Magnetorheological elastomer composites with conductive carbon black polylactic acid | Supportive treatments, including tracheal splints and shattered bones | [244] | |
| Magnetic field | PLA/TPU/Fe3O4 | Development of biomimetic structures | [168] |
| Gelatin methacryloyl with iron oxide nanoparticles | Generation of bioinspired soft robotic systems | [213] | |
| Nanoclay-incorporated double-network hydrogel with magnetite nanoparticles | Magnetic guided movement | [214] | |
| Humidity | PEG bilayers | 3D biological studies and tissue engineering | [248] |
| Multisomes | Domain of synthetic biology and medicine | [249] | |
| Zein gel in a supporting bath of Carbopol | Development of controlled self-assembly biomedical materials | [250] | |
| Enzymes | PEGDA hydrogel with alkaline phosphatase and thrombin | Formation of calcified structures and fiber patterns | [252] |
| 2-carboxyethyl acrylate added to photocurable resins and derivatized with urease and glucose oxidase | On-site determination of urea and glucose | [193] |
6. Current Challenges and Future Prospects
7. Conclusions
Funding
Conflicts of Interest
References
- Azlin, M.N.M.; Ilyas, R.A.; Zuhri, M.Y.M.; Sapuan, S.M.; Harussani, M.M.; Sharma, S.; Nordin, A.H.; Nurazzi, N.M.; Afiqah, A.N. 3D Printing and Shaping Polymers, Composites, and Nanocomposites: A Review. Polymers 2022, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Nouri, A.; Rohani Shirvan, A.; Li, Y.; Wen, C. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: A review. J. Mater. Sci. Technol. 2021, 94, 196–215. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Svetlizky, D.; Das, M.; Zheng, B.; Vyatskikh, A.L.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition (DED) additive manufacturing: Physical characteristics, defects, challenges and applications. Mater. Today 2021, 49, 271–295. [Google Scholar] [CrossRef]
- Bouzaglou, O.; Golan, O.; Lachman, N. Process Design and Parameters Interaction in Material Extrusion 3D Printing: A Review. Polymers 2023, 15, 2280. [Google Scholar] [CrossRef]
- Gülcan, O.; Günaydın, K.; Tamer, A. The State of the Art of Material Jetting—A Critical Review. Polymers 2021, 13, 2829. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Pingale, P.; Dawre, S.; Dhapte-Pawar, V.; Dhas, N.; Rajput, A. Advances in 4D printing: From stimulation to simulation. Drug Deliv. Transl. Res. 2022, 13, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Antezana, P.E.; Municoy, S.; Álvarez-Echazú, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Barui, S. 3D inkjet printing of biomaterials: Principles and applications. Med. Devices Sens. 2021, 4, e10143. [Google Scholar] [CrossRef]
- Baldock, S.J.; Kevin, P.; Harper, G.R.; Griffin, R.; Genedy, H.H.; Fong, M.J.; Zhao, Z.; Zhang, Z.; Shen, Y.; Lin, H.; et al. Creating 3D Objects with Integrated Electronics via Multiphoton Fabrication In Vitro and In Vivo. Adv. Mater. Technol. 2023, 8, 2201274. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D bioprinting of smart polymers for biomedical applications: Recent progress, challenges, and future perspectives. React. Funct. Polym. 2022, 179, 105374. [Google Scholar] [CrossRef]
- Liu, X.; Yuk, H.; Lin, S.; Parada, G.A.; Tang, T.-C.; Tham, E.; de la Fuente-Nunez, C.; Lu, T.K.; Zhao, X. 3D Printing of Living Responsive Materials and Devices. Adv. Mater. 2018, 30, 1704821. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2017, 9, 012001. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Chu, H.; Yang, W.; Sun, L.; Cai, S.; Yang, R.; Liang, W.; Yu, H.; Liu, L. 4D Printing: A Review on Recent Progresses. Micromachines 2020, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Lee, S.J.; Tang, R.; Gasvoda, K.L.; He, F.; Alsberg, E. 4D Cell-Condensate Bioprinting. Small 2022, 18, 2202196. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Jeon, O.; Cleveland, D.; Gasvoda, K.L.; Wells, D.; Lee, S.J.; Alsberg, E. Jammed Micro-Flake Hydrogel for Four-Dimensional Living Cell Bioprinting. Adv. Mater. 2022, 34, 2109394. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, R.D.; Shofner, M.L.; Hague, R.J.M.; McClelland, M.; Schlea, M.R.; Johnson, R.B.; Tuck, C.J. Processing of a Polyamide-12/carbon nanofibre composite by laser sintering. Polym. Test. 2011, 30, 94–100. [Google Scholar] [CrossRef]
- Santos, A.R.C.; Almeida, H.A.; Bártolo, P.J. Additive manufacturing techniques for scaffold-based cartilage tissue engineering. Virtual Phys. Prototyp. 2013, 8, 175–186. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Chiulan, I.; Frone, A.N.; Brandabur, C.; Panaitescu, D.M. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Harun, W.S.W.; Kamariah, M.S.I.N.; Muhamad, N.; Ghani, S.A.C.; Ahmad, F.; Mohamed, Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Paul, C.P.; Jain, A.; Ganesh, P.; Negi, J.; Nath, A.K. Laser rapid manufacturing of Colmonoy-6 components. Opt. Lasers Eng. 2006, 44, 1096–1109. [Google Scholar] [CrossRef]
- Calleja, A.; Tabernero, I.; Fernández, A.; Celaya, A.; Lamikiz, A.; López De Lacalle, L.N. Improvement of strategies and parameters for multi-axis laser cladding operations. Opt. Lasers Eng. 2014, 56, 113–120. [Google Scholar] [CrossRef]
- Zhang, J.; Liou, F. Adaptive Slicing for a Multi-Axis Laser Aided Manufacturing Process. J. Mech. Des. 2004, 126, 254–261. [Google Scholar] [CrossRef]
- Zhang, J.; Di, X.; Li, C.; Zhao, X.; Ba, L.; Jiang, X. Additive manufacturing of Inconel625-HSLA Steel functionally graded material by wire arc additive manufacturing. Metall. Res. Technol. 2021, 118, 502. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Pilipović, A.; Baršić, G.; Katić, M.; Havstad, M.R. Repeatability and Reproducibility Assessment of a PolyJet Technology Using X-ray Computed Tomography. Appl. Sci. 2020, 10, 7040. [Google Scholar] [CrossRef]
- Dilag, J.; Chen, T.; Li, S.; Bateman, S.A. Design and direct additive manufacturing of three-dimensional surface micro-structures using material jetting technologies. Addit. Manuf. 2019, 27, 167–174. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the environmental impacts of 3D printing technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and Proliferative Ability of Various Living Cell Types after Laser-Induced Forward Transfer. Tissue Eng. 2006, 11, 1817–1823. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef] [PubMed]
- Maruo, S.; Fourkas, J.T. Recent progress in multiphoton microfabrication. Laser Photon. Rev. 2008, 2, 100–111. [Google Scholar] [CrossRef]
- Farsari, M. 3D Printing via Multiphoton Polymerization. In Nanomaterials for 2D and 3D Printing; Magdassi, S., Kamyshny, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 83–105. [Google Scholar] [CrossRef]
- Leist, S.K.; Zhou, J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials. Virtual Phys. Prototyp. 2016, 11, 249–262. [Google Scholar] [CrossRef]
- Salimon, A.I.; Senatov, F.S.; Kalyaev, V.A.; Korsunsky, A.M. Shape memory polymer blends and composites for 3D and 4D printing applications. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Sadasivuni, K.K., Deshmukh, K., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–189. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragani, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Li, L. Effects of moisture on the thermomechanical properties of a polyurethane shape memory polymer. Polymer 2006, 47, 1348–1356. [Google Scholar] [CrossRef]
- Cho, J.W.; Kim, J.W.; Jung, Y.C.; Goo, N.S. Electroactive Shape-Memory Polyurethane Composites Incorporating Carbon Nanotubes. Macromol. Rapid Commun. 2005, 26, 412–416. [Google Scholar] [CrossRef]
- Mañosa, L.; González-Alonso, D.; Planes, A.; Bonnot, E.; Barrio, M.; Tamarit, J.L.; Aksoy, S.; Acet, M. Giant solid-state barocaloric effect in the Ni–Mn–In magnetic shape-memory alloy. Nat. Mater. 2010, 9, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Gould, O.E.C. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Hardy, J.G.; Palma, M.; Wind, S.J.; Biggs, M.J. Responsive Biomaterials: Advances in Materials Based on Shape-Memory Polymers. Adv. Mater. 2016, 28, 5717–5724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Yi, S.; Lin, Z.; Wang, C.; Chen, Z.; Jiang, L. 4D Printing of Magnetoactive Soft Materials for On-Demand Magnetic Actuation Transformation. ACS Appl. Mater. Interfaces 2021, 13, 4174–4184. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Zengjie, F.; Suthiwanich, K.; Lorestani, F.; Orive, G.; Ostrovidov, S.; Khademhosseini, A. Advances and Future Perspectives in 4D Bioprinting. Biotechnol. J. 2018, 13, 1800148. [Google Scholar] [CrossRef]
- Haskew, M.J.; Hardy, J.G. A Mini-Review of Shape-Memory Polymer-Based Materials: Stimuli-responsive shape-memory polymers. Johns. Matthey Technol. Rev. 2020, 64, 425–442. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Jumahat, A.; Abdullah, N.R.; Frormann, L. Determination of Shape Fixity and Shape Recovery Rate of Carbon Nanotube-filled Shape Memory Polymer Nanocomposites. Procedia Eng. 2012, 41, 1641–1646. [Google Scholar] [CrossRef]
- Monzón, M.D.; Paz, R.; Pei, E.; Ortega, F.; Suárez, L.A.; Ortega, Z.; Alemán, M.E.; Plucinski, T.; Clow, N. 4D printing: Processability and measurement of recovery force in shape memory polymers. Int. J. Adv. Manuf. Technol. 2017, 89, 1827–1836. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [PubMed]
- Hendrikson, W.J.; Rouwkema, J.; Clementi, F.; Van Blitterswijk, C.A.; Farè, S.; Moroni, L. Towards 4D printed scaffolds for tissue engineering: Exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication 2017, 9, 031001. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, G.; Singh, S.; Jha, K.; Prakash, C. 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings. J. Mech. Behav. Biomed. Mater. 2020, 108, 103781. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Xie, H.; Xu, Z.; Li, L.; Jiang, M.N.; Tang, L.; Yang, K.K.; Wang, Y.Z. 4D printing of shape memory aliphatic copolyester via UV-assisted FDM strategy for medical protective devices. Chem. Eng. J. 2020, 396, 125242. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef]
- Hartl, D.J.; Lagoudas, D.C. Aerospace applications of shape memory alloys. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2007, 221, 535–552. [Google Scholar] [CrossRef]
- O’Handley, R.C. Model for strain and magnetization in magnetic shape-memory alloys. J. Appl. Phys. 1998, 83, 3263–3270. [Google Scholar] [CrossRef]
- Mouritz, A.P. Titanium alloys for aerospace structures and engines. In Introduction to Aerospace Materials; Mouritz, A.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 202–223. [Google Scholar] [CrossRef]
- Paul, D.I.; McGehee, W.; O’Handley, R.C.; Richard, M. Ferromagnetic shape memory alloys: A theoretical approach. J. Appl. Phys. 2007, 101, 123917. [Google Scholar] [CrossRef]
- Lu, H.Z.; Yang, C.; Luo, X.; Ma, H.W.; Song, B.; Li, Y.Y.; Zhang, L.C. Ultrahigh-performance TiNi shape memory alloy by 4D printing. Mater. Sci. Eng. A 2019, 763, 138166. [Google Scholar] [CrossRef]
- Khoo, Z.X.; An, J.; Chua, C.K.; Shen, Y.F.; Kuo, C.N.; Liu, Y. Effect of Heat Treatment on Repetitively Scanned SLM NiTi Shape Memory Alloy. Materials 2019, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Z.X.; Liu, Y.; Low, Z.H.; An, J.; Chua, C.K.; Leong, K.F. Fabrication of SLM NiTi Shape Memory Alloy via Repetitive Laser Scanning. Shape Mem. Superelasticity 2018, 4, 112–120. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Speirs, M.; Kruth, J.P.; Schrooten, J.; Luyten, J.; Van Humbeeck, J. Effect of SLM Parameters on Transformation Temperatures of Shape Memory Nickel Titanium Parts. Adv. Eng. Mater. 2014, 16, 1140–1146. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Erkeçoglu, S.; Sezer, A.D.; Bucak, S. Smart Delivery Systems with Shape Memory and Self-Folding Polymers. In Smart Drug Delivery System; Sezer, A.D., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef][Green Version]
- Lai, A.; Du, Z.; Gan, C.L.; Schuh, C.A. Shape memory and superelastic ceramics at small scales. Science 2013, 341, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Speirs, M.; Van Hooreweder, B.; Van Humbeeck, J.; Kruth, J.P. Fatigue behaviour of NiTi shape memory alloy scaffolds produced by SLM, a unit cell design comparison. J. Mech. Behav. Biomed. Mater. 2017, 70, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Liu, J.; Zhao, J.; Zhang, H.; Zhang, Z. Shape memory epoxy composites with high mechanical performance manufactured by multi-material direct ink writing. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105903. [Google Scholar] [CrossRef]
- Ryan, K.R.; Down, M.P.; Banks, C.E. Future of additive manufacturing: Overview of 4D and 3D printed smart and advanced materials and their applications. Chem. Eng. J. 2021, 403, 126162. [Google Scholar] [CrossRef]
- Van Der Linden, H.J.; Herber, S.; Olthuis, W.; Bergveld, P. Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst 2003, 128, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Stimuli-Responsive Hydrogels Based on Polysaccharides Incorporated with Thermo-Responsive Polymers as Novel Biomaterials. Macromol. Biosci. 2006, 6, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Hivare, P.; Gangrade, A.; Swarup, G.; Bhavsar, K.; Singh, A.; Gupta, R.; Thareja, P.; Gupta, S.; Bhatia, D. Peptide functionalized DNA hydrogel enhances neuroblastoma cell growth and differentiation. Nanoscale 2022, 14, 8611–8620. [Google Scholar] [CrossRef]
- Osada, Y.; Matsuda, A. Shape memory in hydrogels. Nature 1995, 376, 219. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, L.M.; Zhao, Q.; Xie, T. 4D Printing: History and Recent Progress. Chin. J. Polym. Sci. 2018, 36, 563–575. [Google Scholar] [CrossRef]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D Printing of Hydrogels for Stretchable Ionotronic Devices. Adv. Funct. Mater. 2021, 31, 2107437. [Google Scholar] [CrossRef]
- Pinho, A.C.; Buga, C.S.; Piedade, A.P. The chemistry behind 4D printing. Appl. Mater. Today 2020, 19, 100611. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Tang, M.; Hou, X.; Ke, C. Rapid macroscale shape morphing of 3D-printed polyrotaxane monoliths amplified from pH-controlled nanoscale ring motions. J. Mater. Chem. C 2018, 6, 11956–11960. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Tok, A.I.Y.; Lipik, V. Body temperature-responsive two-way and moisture-responsive one-way shape memory behaviors of poly(ethylene glycol)-based networks. Polym. Chem. 2017, 8, 3833–3840. [Google Scholar] [CrossRef]
- Sumaru, K.; Katsuhide, O.; Toshiyuki, T.; Toshiyuki, K.; Toshio, S. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Gorkin, R.; Panhuis, M.; Spinks, G.M. 4D Printing with Mechanically Robust, Thermally Actuating Hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. [Google Scholar] [CrossRef]
- Halligan, E.; Zhuo, S.; Colbert, D.M.; Alsaadi, M.; Hieng Tie, B.S.; BEzerra, G.; Keane, G.; Geever, L. Modulation of the Lower Critical Solution Temperature of Thermoresponsive Poly(N-vinylcaprolactam) Utilizing Hydrophilic and Hydrophobic Monomers. Polymers 2023, 15, 1595. [Google Scholar] [CrossRef]
- Debons, N.; Dems, D.; Hélary, C.; Le Grill, S.; Picaut, L.; Renaud, F.; Delsuc, N.; Schanne-Klein, M.-C.; Coradin, T.; Aimé, C. Differentiation of neural-type cells on multi-scale ordered collagen-silica bionanocomposites. Biomater. Sci. 2020, 8, 569–576. [Google Scholar] [CrossRef]
- Mebert, A.M.; Alvarez, G.S.; Peroni, R.; Illoul, C.; Hélary, C.; Coradin, T.; Desimone, M.F. Collagen-silica nanocomposites as dermal dressings preventing infection in vivo. Mater. Sci. Eng. C 2018, 93, 170–177. [Google Scholar] [CrossRef]
- Foglia, M.L.; Mitarotonda, R.; De Marzi, M.C.; Desimone, M.F. Silicified collagen materials: Modulation of the in vitro and in vivo response. Mater. Sci. Eng. C 2019, 99, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Alexa, R.L.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.M.; Ianchis, R. 3D-Printed Gelatin Methacryloyl-Based Scaffolds with Potential Application in Tissue Engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef]
- Deng, K.; Liu, Z.; Hu, J.; Liu, W.; Zhang, L.; Xie, R.; Ju, X.; Wang, W.; Chu, L. Composite bilayer films with organic compound-triggered bending properties. Chin. J. Chem. Eng. 2019, 27, 2587–2595. [Google Scholar] [CrossRef]
- Lee, A.Y.; Zhou, A.; An, J.; Chua, C.K.; Zhang, Y. Contactless reversible 4D-printing for 3D-to-3D shape morphing. Virtual Phys. Prototyp. 2020, 15, 481–495. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Mu, X.; Chen, H.; Qi, H.J.; Fang, D. Desolvation Induced Origami of Photocurable Polymers by Digit Light Processing. Macromol. Rapid Commun. 2017, 38, 1600625. [Google Scholar] [CrossRef]
- Su, J.W.; Tao, X.; Deng, H.; Zhang, C.; Jiang, S.; Lin, Y.; Lin, J. 4D printing of a self-morphing polymer driven by a swellable guest medium. Soft Matter 2018, 14, 765–772. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Camacho-Lopez, M.; Finkelmann, H.; Palffy-Muhoray, P.; Shelley, M. Fast liquid-crystal elastomer swims into the dark. Nat. Mater. 2004, 3, 307–310. [Google Scholar] [CrossRef]
- Ma, B.; Xu, C.; Cui, L.; Zhao, C.; Liu, H. Magnetic Printing of Liquid Metal for Perceptive Soft Actuators with Embodied Intelligence. ACS Appl. Mater. Interfaces 2021, 13, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Winkler, M.; Krause, S.; Finkelmann, H.; Schmidt, A.M. Magnetoactive liquid crystal elastomer nanocomposites. J. Mater. Chem. 2009, 19, 538–543. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid Crystalline Elastomers as Actuators and Sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.; Valenzuela, C.; Zhang, X.; Wang, L.; Feng, W. Mechanochromic, Shape-Programmable and Self-Healable Cholesteric Liquid Crystal Elastomers Enabled by Dynamic Covalent Boronic Ester Bonds. Angew. Chem. Int. Ed. 2022, 61, e202116219. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.Z.; Zuo, B.; Wang, M.; Huang, S.; Chen, X.M.; Liu, Z.Y.; Yang, H. Light-driven continuous rotating Möbius strip actuators. Nat. Commun. 2021, 12, 2334. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, L.; Yu, M.; Zhao, D.; Song, P.; Chi, H.; Guo, L.; Yang, H. Liquid Crystal Elastomer Actuators from Anisotropic Porous Polymer Template. Macromol. Rapid Commun. 2017, 38, 1600699. [Google Scholar] [CrossRef]
- Warner, M.; Modes, C.D.; Corbett, D. Curvature in nematic elastica responding to light and heat. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 2975–2989. [Google Scholar] [CrossRef]
- Zhao, Y.; Chi, Y.; Hong, Y.; Li, Y.; Yang, S.; Yin, J. Twisting for soft intelligent autonomous robot in unstructured environments. Proc. Natl. Acad. Sci. USA 2022, 119, e2200265119. [Google Scholar] [CrossRef]
- Lan, R.; Wang, Q.; Shen, C.; Huang, R.; Bao, J.; Zhang, Z.; Zhang, L.; Yang, H. Humidity-Induced Simultaneous Visible and Fluorescence Photonic Patterns Enabled by Integration of Covalent Bonds and Ionic Crosslinks. Adv. Funct. Mater. 2021, 31, 2106419. [Google Scholar] [CrossRef]
- Traugutt, N.A.; Mistry, D.; Luo, C.; Yu, K.; Ge, Q.; Yakacki, C.M. Liquid-Crystal-Elastomer-Based Dissipative Structures by Digital Light Processing 3D Printing. Adv. Mater. 2020, 32, 2000797. [Google Scholar] [CrossRef]
- Gantenbein, S.; Masania, K.; Woigk, W.; Sesseg, J.P.W.; Tervoort, T.A.; Studart, A.R. Three-dimensional printing of hierarchical liquid-crystal-polymer structures. Nature 2018, 561, 226–230. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Fei, G.; Wang, Z.; Xia, H.; Zhao, Y. 4D Printing of a Liquid Crystal Elastomer with a Controllable Orientation Gradient. ACS Appl. Mater. Interfaces 2019, 11, 44774–44782. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bai, H.; Liu, Z.; Zhang, X.; Huang, C.; Wiesner, L.W.; Silberstein, M.; Shepherd, R.F. Digital light processing of liquid crystal elastomers for self-sensing artificial muscles. Sci. Adv. 2021, 7, eabg3677. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wasylczyk, P.; Cerretti, G.; Martella, D.; Parmeggiani, C.; Wiersma, D.S. Alignment engineering in liquid crystalline elastomers: Free-form microstructures with multiple functionalities. Appl. Phys. Lett. 2015, 106, 111902. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Ula, S.W.; Traugutt, N.A.; Volpe, R.H.; Patel, R.R.; Yu, K.; Yakacki, C.M. Liquid crystal elastomers: An introduction and review of emerging technologies. Liq. Cryst. Rev. 2018, 6, 78–107. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. 4D printing applications in medical field: A brief review. Clin. Epidemiol. Glob. Health 2019, 7, 317–321. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, W.; Wang, R.; Gao, S.; Li, B.; Li, Q. 4D Printing of Complex Structures with a Fast Response Time to Magnetic Stimulus. ACS Appl. Mater. Interfaces 2018, 10, 36435–36442. [Google Scholar] [CrossRef]
- Green, R.A.; Baek, S.; Poole-Warren, L.A.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11, 13. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Wu, W.; Wu, Z.; Yuan, Y.; Wu, J.; Liu, C. Harnessing 4D Printing Bioscaffolds for Advanced Orthopedics. Small 2022, 18, 2106824. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.; Gohy, J.F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Xiong, X.; del Campo, A.; Cui, J. Photoresponsive Polymers. In Smart Polymers and Their Applications; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 87–153. ISBN 9780081024164. [Google Scholar]
- Shin, Y.; Husni, P.; Kang, K.; Lee, D.; Lee, S.; Lee, E.; Youn, Y.; Oh, K. Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics 2021, 13, 725. [Google Scholar] [CrossRef]
- Herath, M.; Epaarachchi, J.; Islam, M.; Fang, L.; Leng, J. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Lee, S.J.; Zhou, X.; Hann, S.Y.; Webster, T.J.; Harris, B.T.; Zhang, L.G. A novel near-infrared light responsive 4D printed nanoarchitecture with dynamically and remotely controllable transformation. Nano Res. 2019, 12, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Sol, J.A.H.P.; Douma, R.F.; Schenning, A.P.H.J.; Debije, M.G. 4D Printed Light-Responsive Patterned Liquid Crystal Elastomer Actuators Using a Single Structural Color Ink. Adv. Mater. Technol. 2022, 8, 2200970. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, X.; Chen, B.; Wei, X. Cell-laden four-dimensional bioprinting using near-infrared-triggered shape-morphing alginate/polydopamine bioinks. Biofabrication 2019, 11, 045019. [Google Scholar] [CrossRef]
- Gayathri, A.; Naveena Sajja, J.; Vidhyashree Daswani, M.; Prabhakaran, V.; Ravindiran, M. An extensive review of shape memory polymers for biomedical applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 993, 012161. [Google Scholar] [CrossRef]
- Ehrmann, G.; Ehrmann, A. 3D printing of shape memory polymers. J. Appl. Polym. Sci. 2021, 138, 50847. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Gonzalez-Gutierrez, J.; Mertz, G.; Ruch, D.; Schmidt, D.F.; Westermann, S. 4D Printing of Multicomponent Shape-Memory Polymer Formulations. Appl. Sci. 2022, 12, 7880. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Yuan, Y.; Shi, R. The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering. Polymers 2023, 15, 556. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Yang, Z.; Ma, R.; Aimaijiang, M.; Xu, J.; Zhang, Y.; Zhou, Y. Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 814. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.S.; Zadorozhnyy, M.Y.; Niaza, K.V.; Medvedev, V.V.; Kaloshkin, S.D.; Anisimova, N.Y.; Kiselevskiy, M.V.; Yang, K.C. Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur. Polym. J. 2017, 93, 222–231. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, D.; Liao, P.; Su, J.-W.; Deng, H.; Vardhanabhuti, B.; Ulery, B.D.; Chen, S.-Y.; Lin, J. 4D Printing of Shape-memory Polymeric Scaffolds for Adaptive Biomedical Implantation. Acta Biomater. 2021, 122, 101–110. [Google Scholar] [CrossRef]
- Teotia, A.K.; Sami, H.; Kumar, A. Thermo-responsive polymers: Structure and design of smart materials. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–43. ISBN 978-0-85709-713-2. [Google Scholar]
- Lee, Y.-W.; Ceylan, H.; Yasa, I.C.; Kilic, U.; Sitti, M. 3D-Printed Multi-Stimuli-Responsive Mobile Micromachines. ACS Appl. Mater. Interfaces 2021, 13, 12759–12766. [Google Scholar] [CrossRef]
- Lin, C.; Huang, Z.; Wang, Q.; Zou, Z.; Wang, W.; Liu, L.; Liu, Y.; Leng, J. Mass-producible near-body temperature-triggered 4D printed shape memory biocomposites and their application in biomimetic intestinal stents. Compos. Part B Eng. 2023, 256, 110623. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Zhang, D.; Zhang, Y.; Chen, J. Structurally Dynamic Gelatin-Based Hydrogels with Self-Healing, Shape Memory, and Cytocompatible Properties for 4D Printing. Biomacromolecules 2023, 24, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Paramshetti, S.; Angolkar, M.; Al Fatease, A.; Alshahrani, S.M.; Hani, U.; Garg, A.; Ravi, G.; Osmani, R.A.M. Revolutionizing Drug Delivery and Therapeutics: The Biomedical Applications of Conductive Polymers and Composites-Based Systems. Pharmaceutics 2023, 15, 1204. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Pegg, E.; Chen, A.; Jin, Z.; Gu, G.X. 4D Printing of Electroactive Materials. Adv. Intell. Syst. 2021, 3, 2100019. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Gonzalez-gutierrez, J.; Farhan, M.; Das, R.; Ruch, D.; Westermann, S.; Schmidt, D.F. 4D Printing of Electroactive Triple-Shape Composites. Polymers 2023, 15, 832. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, F.; Wang, L.; Liu, Y.; Leng, J. 4D printing of electroactive shape-changing composite structures and their programmable behaviors. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106925. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, F.; Liu, Y.; Leng, J. CNT-based electro-responsive shape memory functionalized 3D printed nanocomposites for liquid sensors. Carbon 2019, 155, 77–87. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef]
- Álvarez, E.; Estévez, M.; Gallo-Cordova, A.; González, B.; Castillo, R.R.; Morales, M.D.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy. Pharmaceutics 2022, 14, 163. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Ma, Z.; Zhang, C.; Ai, J.; Chen, P.; Yan, C.; Su, B.; Shi, Y. A Material Combination Concept to Realize 4D Printed Products with Newly Emerging Property/Functionality. Adv. Sci. 2020, 7, 1903208. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wu, W.; Dong, X.; Sang, L. 4D printing of mechanically robust PLA/TPU/Fe3O4 magneto-responsive shape memory polymers for smart structures. Compos. Part B Eng. 2023, 248, 110382. [Google Scholar] [CrossRef]
- Wu, H.; Wang, O.; Tian, Y.; Wang, M.; Su, B.; Yan, C.; Zhou, K.; Shi, Y. Selective Laser Sintering-Based 4D Printing of Magnetism-Responsive Grippers. ACS Appl. Mater. Interfaces 2021, 13, 12679–12688. [Google Scholar] [CrossRef] [PubMed]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Dharmayanti, C.; Gillam, T.A.; Klingler-Hoffmann, M.; Albrecht, H.; Blencowe, A. Strategies for the Development of pH-Responsive Synthetic Polypeptides and Polymer-Peptide Hybrids: Recent Advancements. Polymers 2021, 13, 624. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Zhu, X.; Chen, F.; Han, X. Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering 2023, 10, 402. [Google Scholar] [CrossRef]
- Dutta, S.; Cohn, D. Dually responsive biodegradable drug releasing 3D printed structures. J. Appl. Polym. Sci. 2022, 139, e53137. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, H.; Xue, J.; Jiang, J.; Liu, Z.; Liu, Z.; Li, G.; Zhao, Y. Programmable Humidity-Responsive Actuation of Polymer Films Enabled by Combining Shape Memory Property and Surface-Tunable Hygroscopicity. ACS Appl. Mater. Interfaces 2021, 13, 38773–38782. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chatterjee, K. Emerging trends in humidity-responsive 4D bioprinting. Chem. Eng. J. 2023, 455, 140550. [Google Scholar] [CrossRef]
- Tahouni, Y.; Cheng, T.; Lajewski, S.; Benz, J.; Bonten, C.; Wood, D.; Menges, A. Codesign of Biobased Cellulose-Filled Filaments and Mesostructures for 4D Printing Humidity Responsive Smart Structures. 3D Print. Addit. Manuf. 2023, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, L.; Mariani, S.; Ronzan, M.; Mondini, A.; Pugno, N.M.; Mazzolai, B. 4D Printing of Humidity-Driven Seed Inspired Soft Robots. Adv. Sci. 2023, 10, e2205146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. J. Phys. Mater. 2019, 2, 032004. [Google Scholar] [CrossRef]
- Remonatto, D.; Izidoro, B.F.; Mazziero, V.T.; Catarino, B.P.; do Nascimento, J.F.C.; Cerri, M.O.; Andrade, G.S.S.; Paula, A.V. de 3D printing and enzyme immobilization: An overview of current trends. Bioprinting 2023, 33, e00289. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels 2022, 8, 460. [Google Scholar] [CrossRef]
- Shao, Y.; Liao, Z.; Gao, B.; He, B. Emerging 3D Printing Strategies for Enzyme Immobilization: Materials, Methods, and Applications. ACS Omega 2022, 7, 11530–11543. [Google Scholar] [CrossRef]
- Greene, A.F.; Vaidya, A.; Collet, C.; Wade, K.R.; Patel, M.; Gaugler, M.; West, M.; Petcu, M.; Parker, K. 3D-printed enzyme-embedded plastics. Biomacromolecules 2021, 22, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. 3D-4D printed objects: New bioactive material opportunities. Micromachines 2017, 8, 102. [Google Scholar] [CrossRef]
- Pose-Boirazian, T.; Martínez-Costas, J.; Eibes, G. 3D Printing: An Emerging Technology for Biocatalyst Immobilization. Macromol. Biosci. 2022, 22, 2200110. [Google Scholar] [CrossRef]
- Gao, G.; Ahn, M.; Cho, W.-W.; Kim, B.-S.; Cho, D.-W. 3D Printing of Pharmaceutical Application: Drug Screening and Drug Delivery. Pharmaceutics 2021, 13, 1373. [Google Scholar] [CrossRef]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A Versatile Approach for Enzyme Immobilization Using Chemically Modified 3D-Printed Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- Su, C.-K.; Chen, J.-C. One-step three-dimensional printing of enzyme/substrate–incorporated devices for glucose testing. Anal. Chim. Acta 2018, 1036, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, X.; Zheng, Z.; Li, M.; Zhu, X.; Cao, H.; Cui, C. Immobilization of laccase by 3D bioprinting and its application in the biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2020, 164, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Day, G.J.; Zampetakis, I.; Carrabba, M.; Zhang, Z.; Carter, B.M.; Govan, N.; Jackson, C.; Chen, M.; Perriman, A.W. Three-Dimensional Printable Enzymatically Active Plastics. ACS Appl. Polym. Mater. 2021, 3, 6070–6077. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, M.; Singh, G. Recent advancements in urea biosensors for biomedical applications. IET Nanobiotechnol. 2021, 15, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Su, Y.-T.; Su, C.-K. 4D-printed needle panel meters coupled with enzymatic derivatization for reading urea and glucose concentrations in biological samples. Biosens. Bioelectron. 2023, 237, 115500. [Google Scholar] [CrossRef]
- Sahafnejad-Mohammadi, I.; Karamimoghadam, M.; Zolfagharian, A.; Akrami, M.; Bodaghi, M. 4D printing technology in medical engineering: A narrative review. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 1–26. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z. 4D Printing in Biomedical Engineering: Advancements, Challenges, and Future Directions. J. Funct. Biomater. 2023, 14, 347. [Google Scholar] [CrossRef]
- Aldawood, F.K. A Comprehensive Review of 4D Printing: State of the Arts, Opportunities, and Challenges. Actuators 2023, 12, 101. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H. A review on four-dimensional (4D) bioprinting in pursuit of advanced tissue engineering applications. Bioprinting 2022, 27, e00203. [Google Scholar] [CrossRef]
- Gelmi, A.; Schutt, C.E. Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Adv. Healthc. Mater. 2021, 10, 2001125. [Google Scholar] [CrossRef]
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 9, 202000208. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leow, W.R.; Wang, T.; Wang, J.; Yu, J.; He, K.; Qi, D.; Wan, C.; Chen, X. 3D Printed Photoresponsive Devices Based on Shape Memory Composites. Adv. Mater. 2017, 29, 1701627. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef]
- Arakawa, C.K.; Badeau, B.A.; Zheng, Y.; DeForest, C.A. Multicellular Vascularized Engineered Tissues through User-Programmable Biomaterial Photodegradation. Adv. Mater. 2017, 29, 1703156. [Google Scholar] [CrossRef]
- Griffin, D.R.; Kasko, A.M. Photodegradable macromers and hydrogels for live cell encapsulation and release. J. Am. Chem. Soc. 2012, 134, 13103–13107. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wu, X.; Zhang, Y.; Chen, S.; Qiao, Z.; Wei, D.; Sun, J.; Fan, H. Semiconvertible Hyaluronic Hydrogel Enabled Red-Light-Responsive Reversible Mechanics, Adhesion, and Self-Healing. Biomacromolecules 2022, 23, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ji, C.; Zhao, X.; Han, Y.; Müllen, K.; Pan, K.; Yin, M. Green-Light-Triggered Phase Transition of Azobenzene Derivatives toward Reversible Adhesives. J. Am. Chem. Soc. 2019, 141, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Kim, D.; Wu, Y.; Oh, Y.K. On-demand delivery of protein drug from 3D-printed implants. J. Control. Release 2022, 349, 133–142. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Z.; Huang, Z.; Sun, Y.; Huang, Y.; Chen, J.; Ye, J.; Yang, H.; Yang, C.; Zhao, C. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohydr. Polym. 2020, 229, 115394. [Google Scholar] [CrossRef] [PubMed]
- Manemann, S.M.; Gerber, Y.; Bielinski, S.J.; Chamberlain, A.M.; Margolis, K.L.; Weston, S.A.; Killian, J.M.; Roger, V.L. Recent trends in cardiovascular disease deaths: A state specific perspective. BMC Public Health 2021, 21, 1031. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Senapati, S.; Paliwal, P.; Krishnamurthy, S.; Hemalatha, S.; Maiti, P. Nanoparticle-Induced Controlled Drug Delivery Using Chitosan-Based Hydrogel and Scaffold: Application to Bone Regeneration. Mol. Pharm. 2019, 16, 327–338. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Jeong, S.; Kim, S.; Kim, H.J.; Jo, J.; Shanmugasundaram, A.; Kim, H.; Choi, E.; Lee, D.-W. 3D-printed cardiovascular polymer scaffold reinforced by functional nanofiber additives for tunable mechanical strength and controlled drug release. Chem. Eng. J. 2023, 454, 140118. [Google Scholar] [CrossRef]
- Jang, D.; Jeong, J.; Song, H.; Chung, S.K. Targeted drug delivery technology using untethered microrobots: A review. J. Micromech. Microeng. 2019, 29, 053002. [Google Scholar] [CrossRef]
- Liu, D.; Wang, T.; Lu, Y. Untethered Microrobots for Active Drug Delivery: From Rational Design to Clinical Settings. Adv. Healthc. Mater. 2022, 11, 2102253. [Google Scholar] [CrossRef] [PubMed]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, C.; Sotgiu, G.; Sagnella, A.; Varchi, G.; Guerrini, A.; Giuri, D.; Polo, E.; Orlandi, V.T.; Marras, E.; Gariboldi, M.; et al. Wool Keratin 3D Scaffolds with Light-Triggered Antimicrobial Activity. Biomacromolecules 2016, 17, 2882–2890. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Fan, X.; Jiao, B.; Zhang, Z.; Zhang, M.; Chen, F.; Gao, H.; Deng, L.; Xiong, W. Femtosecond Laser 4D Printing of Light-Driven Intelligent Micromachines. Adv. Funct. Mater. 2023, 33, 2211473. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Leng, J.; Zhang, L.G. Four-Dimensional Printing Hierarchy Scaffolds with Highly Biocompatible Smart Polymers for Tissue Engineering Applications. Tissue Eng. Part C Methods 2016, 22, 952–963. [Google Scholar] [CrossRef]
- Nie, L.; Li, J.; Lu, G.; Wei, X.; Deng, Y.; Liu, S.; Zhong, S.; Shi, Q.; Hou, R.; Sun, Y.; et al. Temperature responsive hydrogel for cells encapsulation based on graphene oxide reinforced poly(N- isopropylacrylamide)/hydroxyethyl-chitosan. Mater. Today Commun. 2022, 31, 103697. [Google Scholar] [CrossRef]
- Abbadessa, A.; Mouser, V.H.M.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.A.; Hennink, W.E.; Malda, J.; Vermonden, T. A Synthetic Thermosensitive Hydrogel for Cartilage Bioprinting and Its Biofunctionalization with Polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef]
- Wu, J.; Han, Y.; Fu, Q.; Hong, Y.; Li, L.; Cao, J.; Li, H.; Liu, Y.; Chen, Y.; Zhu, J.; et al. Application of tissue-derived bioink for articular cartilage lesion repair. Chem. Eng. J. 2022, 450, 138292. [Google Scholar] [CrossRef]
- Sawkins, M.J.; Mistry, P.; Brown, B.N.; Shakesheff, K.M.; Bonassar, L.J.; Yang, J. Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication 2015, 7, 035004. [Google Scholar] [CrossRef] [PubMed]
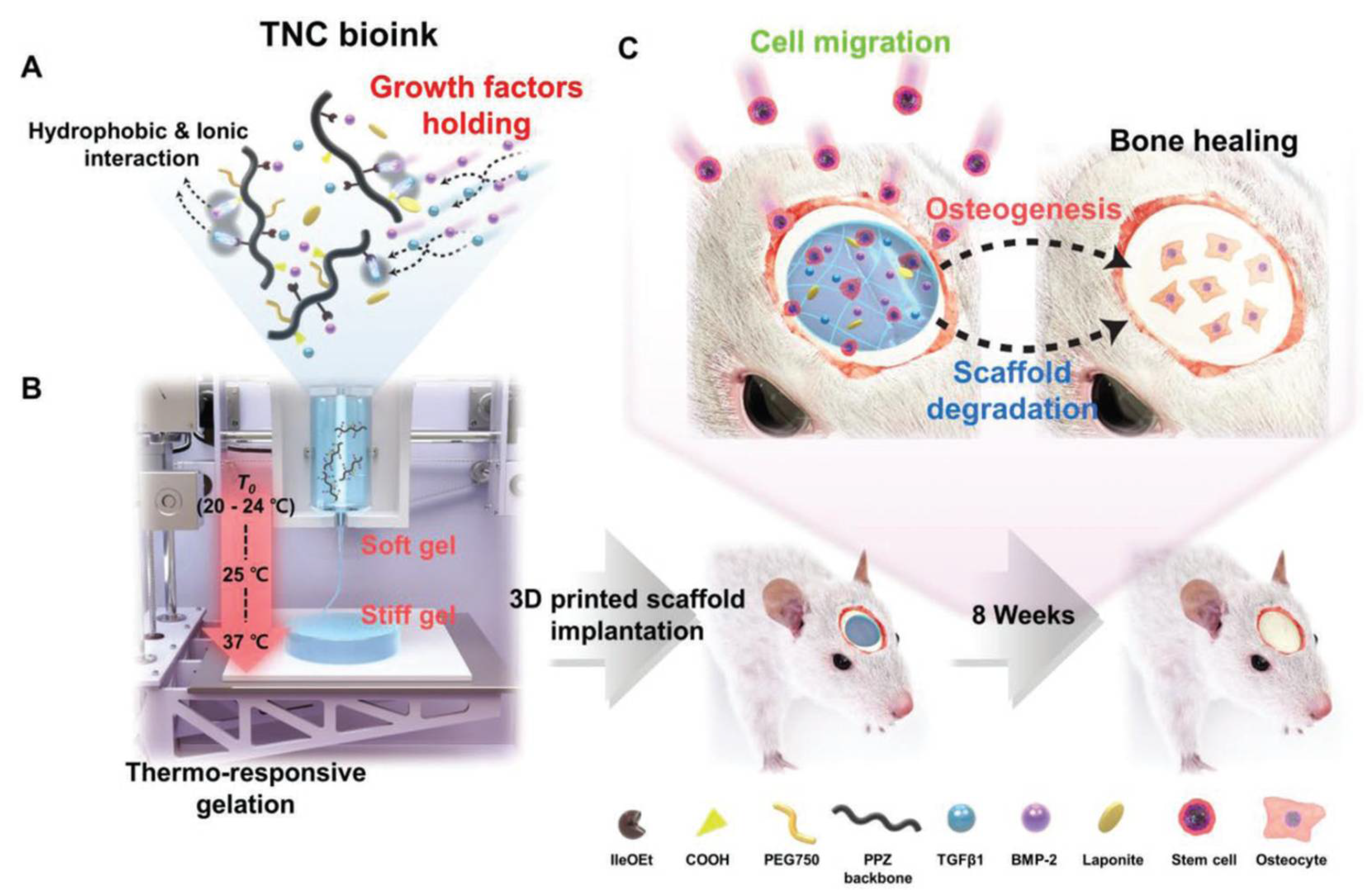
- Kim, J.; Choi, H.S.; Kim, Y.M.; Song, S.C. Thermo-Responsive Nanocomposite Bioink with Growth-Factor Holding and its Application to Bone Regeneration. Small 2023, 19, 2203464. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jin, R.; Zhou, Y.; Yu, M.; Ling, Y.; Wang, L.Q. Crystallization enhanced thermal-sensitive hydrogels of PCL-PEG-PCL triblock copolymer for 3D printing. Biomed. Mater. 2021, 16, 035006. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Hsieh, F.Y.; Tseng, C.S.; Hsu, S.H. Preparation and characterization of a biodegradable polyurethane hydrogel and the hybrid gel with soy protein for 3D cell-laden bioprinting. J. Mater. Chem. B 2016, 4, 6694–6705. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsu, S.H. Synthesis and Characterization of Dual Stimuli-Sensitive Biodegradable Polyurethane Soft Hydrogels for 3D Cell-Laden Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287. [Google Scholar] [CrossRef]
- Saravanou, S.F.; Ioannidis, K.; Dimopoulos, A.; Paxinou, A.; Kounelaki, F.; Varsami, S.M.; Tsitsilianis, C.; Papantoniou, I.; Pasparakis, G. Dually crosslinked injectable alginate-based graft copolymer thermoresponsive hydrogels as 3D printing bioinks for cell spheroid growth and release. Carbohydr. Polym. 2023, 312, 120790. [Google Scholar] [CrossRef]
- Pillay, V.; Tsai, T.-S.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Modi, G.; Naidoo, D.; Tomar, L.K.; Tyagi, C.; Ndesendo, V.M.K. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J. Biomed. Mater. Res. Part A 2014, 102, 2039–2054. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D Printable Electrically Conductive Hydrogel Scaffolds for Biomedical Applications: A Review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole: PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef] [PubMed]
- Stříteský, S.; Marková, A.; Víteček, J.; Šafaříková, E.; Hrabal, M.; Kubáč, L.; Kubala, L.; Weiter, M.; Vala, M. Printing inks of electroactive polymer PEDOT:PSS: The study of biocompatibility, stability, and electrical properties. J. Biomed. Mater. Res. Part A 2018, 106, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.D.; Cooper, P.A.; Municoy, S.; Desimone, M.F.; Cheneler, D.; Shnyder, S.D.; Hardy, J.G. Controlled Bioactive Delivery Using Degradable Electroactive Polymers. Biomacromolecules 2022, 23, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Au-Yong, S.; Firlak, M.; Draper, E.R.; Municoy, S.; Ashton, M.D.; Akien, G.R.; Halcovitch, N.R.; Baldock, S.J.; Martin-Hirsch, P.; Desimone, M.F.; et al. Electrochemically Enhanced Delivery of Pemetrexed from Electroactive Hydrogels. Polymers 2022, 14, 4953. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Bodaghi, M. Magnetorheological elastomer-based 4D printed electroactive composite actuators. Sens. Actuators A Phys. 2023, 349, 114063. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Nizioł, M.; Szymczyk, P.; Wiśniewski, P.; Guiseppi-Elie, A. 3D printed stimuli-responsive magnetic nanoparticle embedded alginate-methylcellulose hydrogel actuators. Addit. Manuf. 2020, 34, 101275. [Google Scholar] [CrossRef]
- Tognato, R.; Armiento, A.R.; Bonfrate, V.; Levato, R.; Malda, J.; Alini, M.; Eglin, D.; Giancane, G.; Serra, T. A Stimuli-Responsive Nanocomposite for 3D Anisotropic Cell-Guidance and Magnetic Soft Robotics. Adv. Funct. Mater. 2019, 29, 1804647. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, L.; Xia, J.; Mi, S.; Sun, W. 3D Printing Unique Nanoclay-Incorporated Double-Network Hydrogels for Construction of Complex Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2021, 10, 2100036. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Kadam, S.S.; Xiao, R.; Jivan, F.; Onn, T.M.; Fernandes, R.; Nguyen, T.D.; Gracias, D.H. Bio-Origami Hydrogel Scaffolds Composed of Photocrosslinked PEG Bilayers. Adv. Healthc. Mater. 2013, 2, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Villar, G.; Heron, A.J.; Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nanotechnol. 2011, 6, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Raza, A.; Xue, Y.Q.; Yang, G.; Hayat, U.; Yu, J.; Liu, C.; Wang, H.J.; Wang, J.Y. Water-responsive 4D printing based on self-assembly of hydrophobic protein “Zein” for the control of degradation rate and drug release. Bioact. Mater. 2023, 23, 343–352. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. 4D bioprinting of tissues and organs. Bioprinting 2021, 23, e00161. [Google Scholar] [CrossRef]
- Devillard, C.D.; Mandon, C.A.; Lambert, S.A.; Blum, L.J.; Marquette, C.A. Bioinspired Multi-Activities 4D Printing Objects: A New Approach toward Complex Tissue Engineering. Biotechnol. J. 2018, 13, e1800098. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Kim, Y.-H.; Kanczler, J.M.; Lanham, S.A.; Ahlfeld, T.; Lode, A.; Dawson, J.I.; Gelinsky, M.; Oreffo, R.O.C. Nanoclay-based 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication 2020, 12, 035010. [Google Scholar] [CrossRef]
- Gugulothu, S.B.; Chatterjee, K. Visible Light-Based 4D-Bioprinted Tissue Scaffold. ACS Macro Lett. 2023, 12, 494–502. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.; Li, K.; Liu, X.; Wang, J.; Liu, T.; Wu, W. Enhanced multi-material 4D printing hybrid composites based on shape memory polymer/thermoplastic elastomer. Smart Mater. Struct. 2023, 32, 055025. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Singh, M. Multi-material 3D printed PLA/PA6-TiO2 composite matrix: Rheological, thermal, tensile, morphological and 4D capabilities. Adv. Mater. Process. Technol. 2022, 8, 2329–2348. [Google Scholar] [CrossRef]
- Jang, S.; Park, S. 4D printed untethered milli-gripper fabricated using a biodegradable and biocompatible electro- and magneto-active hydrogel. Sens. Actuators B Chem. 2023, 384, 133654. [Google Scholar] [CrossRef]
- Abdullah, T.; Okay, O. 4D Printing of Body Temperature-Responsive Hydrogels Based on Poly (acrylic acid) with Shape-Memory and Self-Healing Abilities. ACS Appl. Bio Mater. 2023, 6, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Regondi, S. Artificial Intelligence-Empowered 3D and 4D Printing Technologies toward Smarter Biomedical Materials and Approaches. Polymers 2022, 14, 2794. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yue, L.; Yu, L.; Shao, H.; Peng, X.; Zhou, K.; Demoly, F.; Zhao, R.; Qi, H.J. Machine Learning-Evolutionary Algorithm Enabled Design for 4D-Printed Active Composite Structures. Adv. Funct. Mater. 2022, 32, 2109805. [Google Scholar] [CrossRef]
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antezana, P.E.; Municoy, S.; Ostapchuk, G.; Catalano, P.N.; Hardy, J.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. 4D Printing: The Development of Responsive Materials Using 3D-Printing Technology. Pharmaceutics 2023, 15, 2743. https://doi.org/10.3390/pharmaceutics15122743
Antezana PE, Municoy S, Ostapchuk G, Catalano PN, Hardy JG, Evelson PA, Orive G, Desimone MF. 4D Printing: The Development of Responsive Materials Using 3D-Printing Technology. Pharmaceutics. 2023; 15(12):2743. https://doi.org/10.3390/pharmaceutics15122743
Chicago/Turabian StyleAntezana, Pablo Edmundo, Sofia Municoy, Gabriel Ostapchuk, Paolo Nicolás Catalano, John G. Hardy, Pablo Andrés Evelson, Gorka Orive, and Martin Federico Desimone. 2023. "4D Printing: The Development of Responsive Materials Using 3D-Printing Technology" Pharmaceutics 15, no. 12: 2743. https://doi.org/10.3390/pharmaceutics15122743
APA StyleAntezana, P. E., Municoy, S., Ostapchuk, G., Catalano, P. N., Hardy, J. G., Evelson, P. A., Orive, G., & Desimone, M. F. (2023). 4D Printing: The Development of Responsive Materials Using 3D-Printing Technology. Pharmaceutics, 15(12), 2743. https://doi.org/10.3390/pharmaceutics15122743







