Exploring Metabolic Pathways of Anamorelin, a Selective Agonist of the Growth Hormone Secretagogue Receptor, via Molecular Networking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microsomal and cDNA-Expressed Recombinant CYP Isozyme Incubation Assay
2.3. LC/MS/MS Analysis of Anamorelin
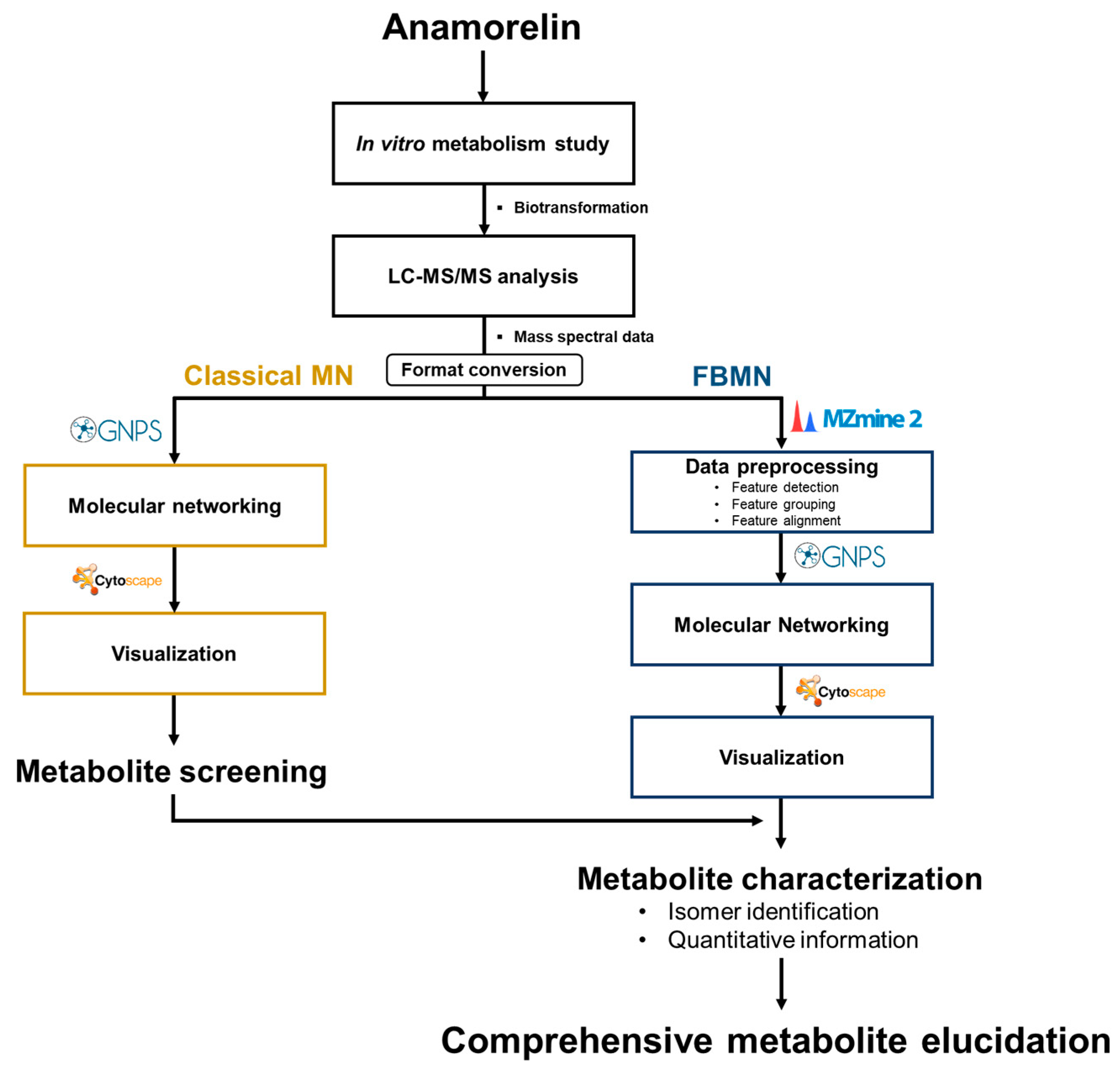
2.4. MN and FBMN Analyses for Metabolite Screening
2.5. FMO Incubation Assay
2.6. APCI/MS Analysis
3. Results
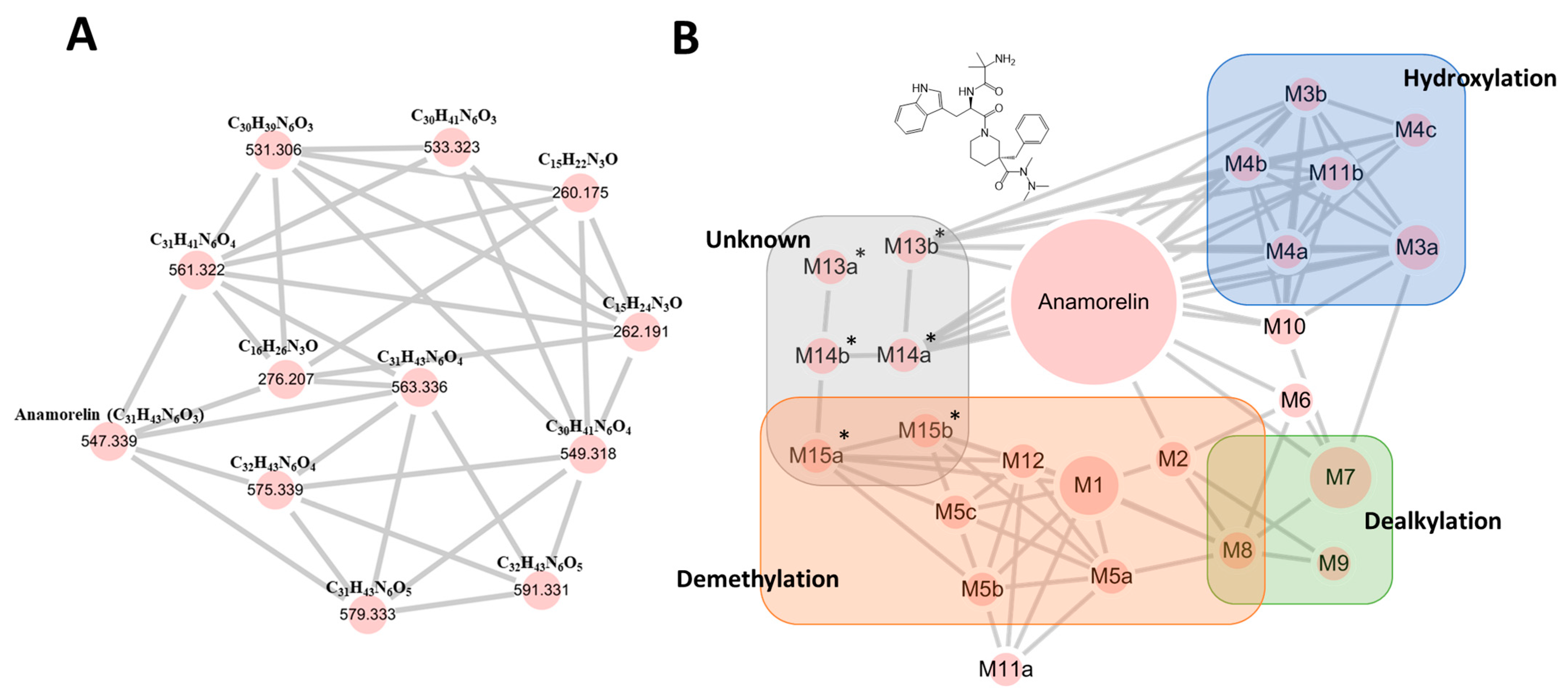
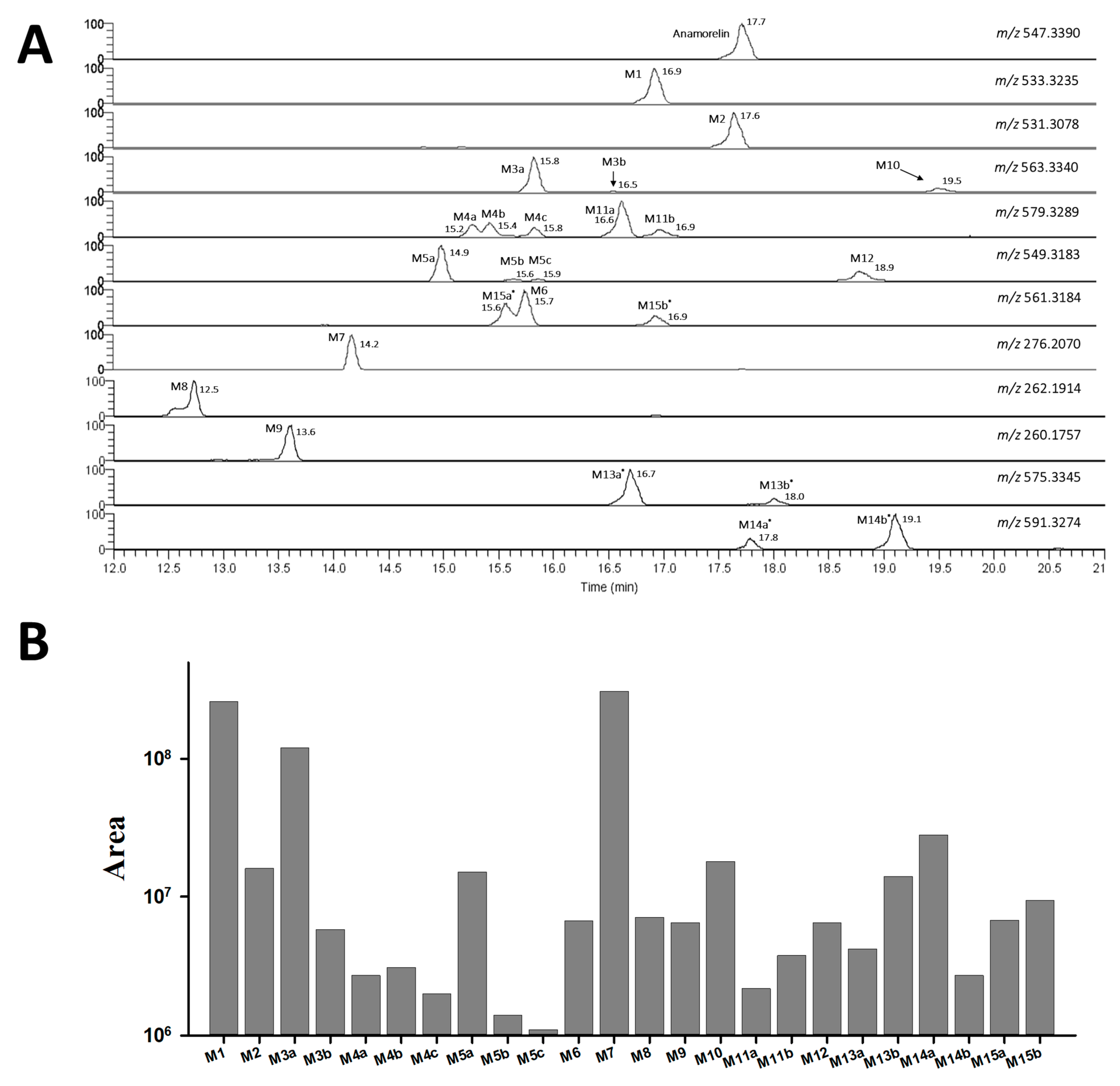
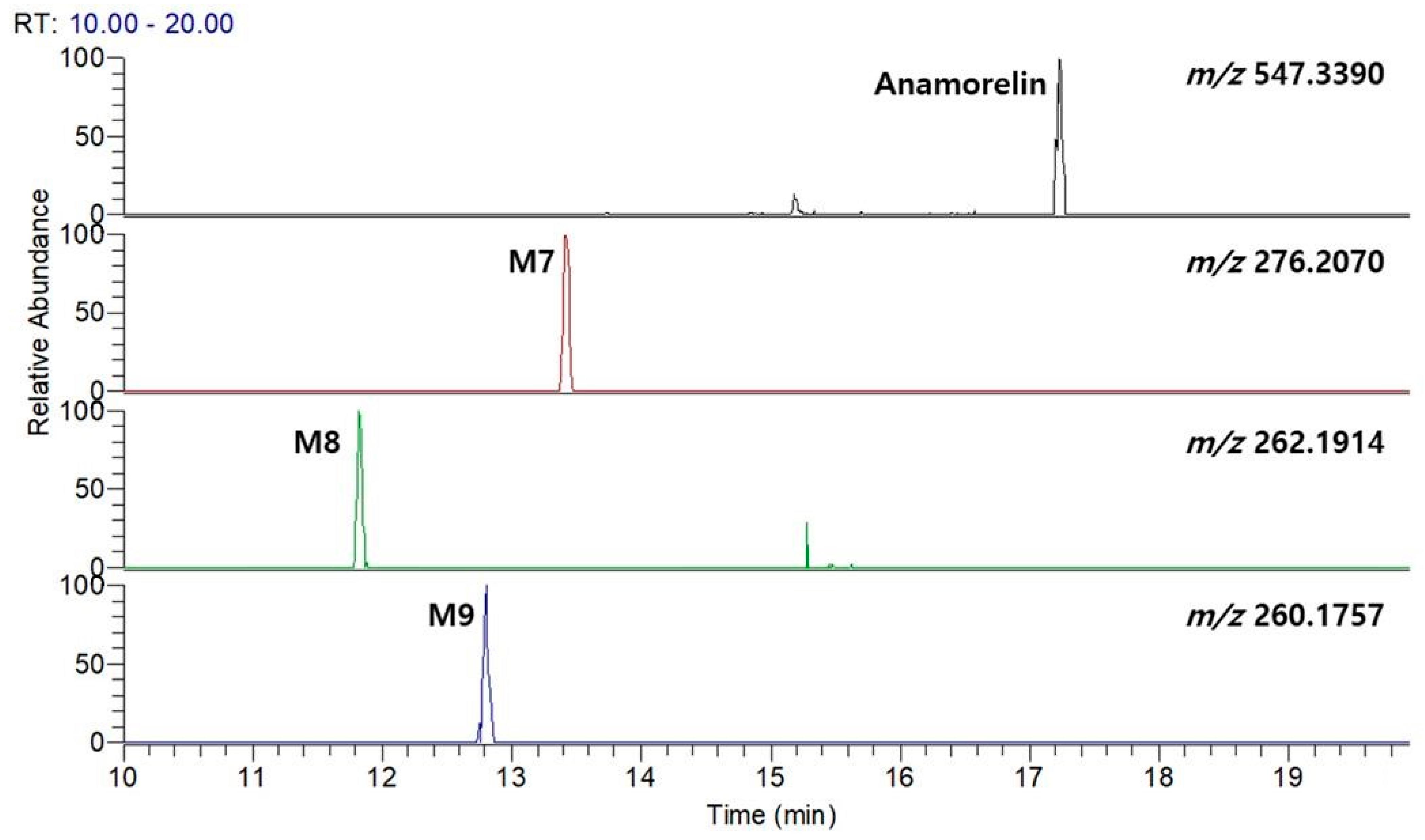
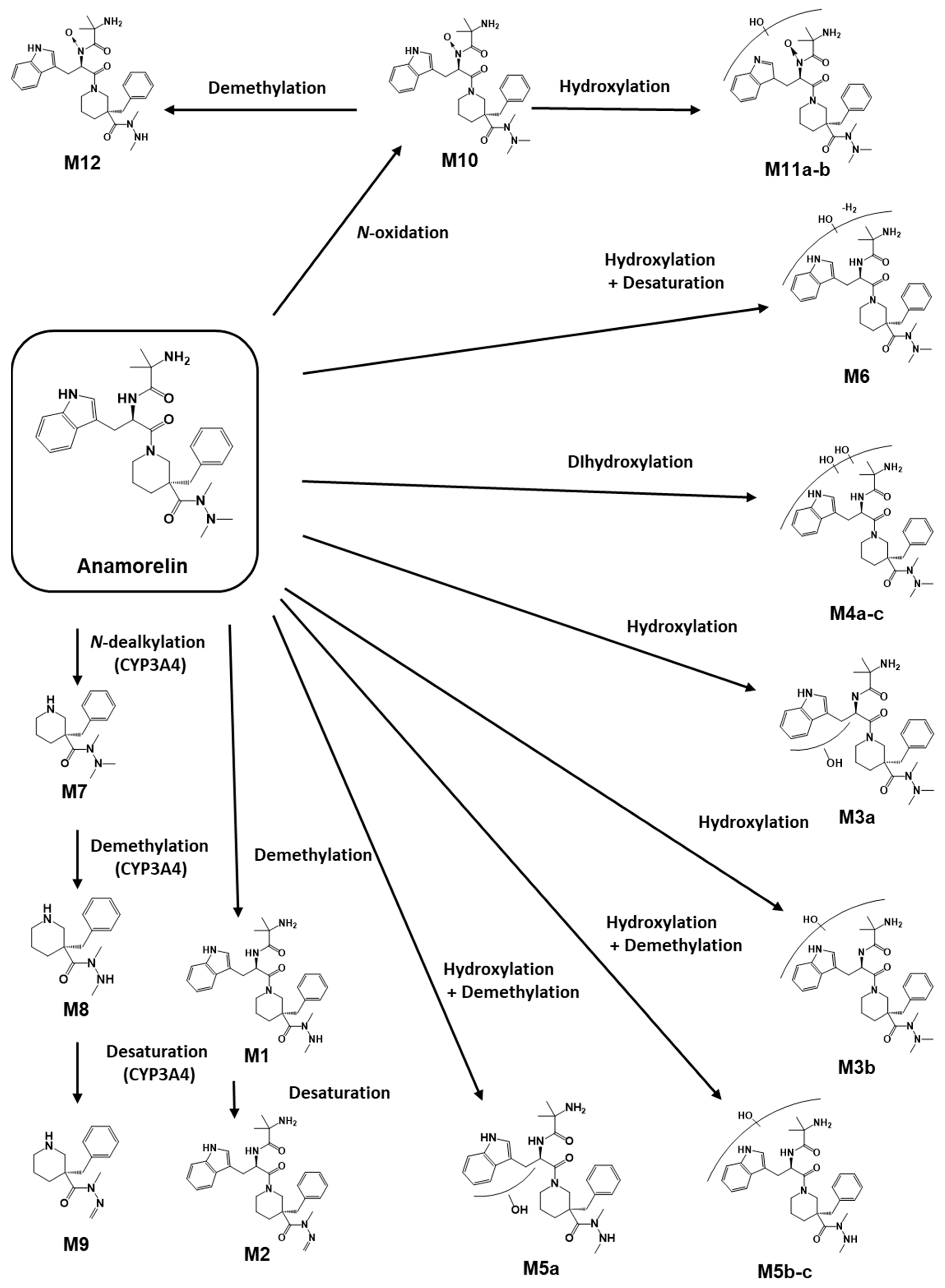
3.1. Anamorelin Metabolite Screening with Molecular Networking
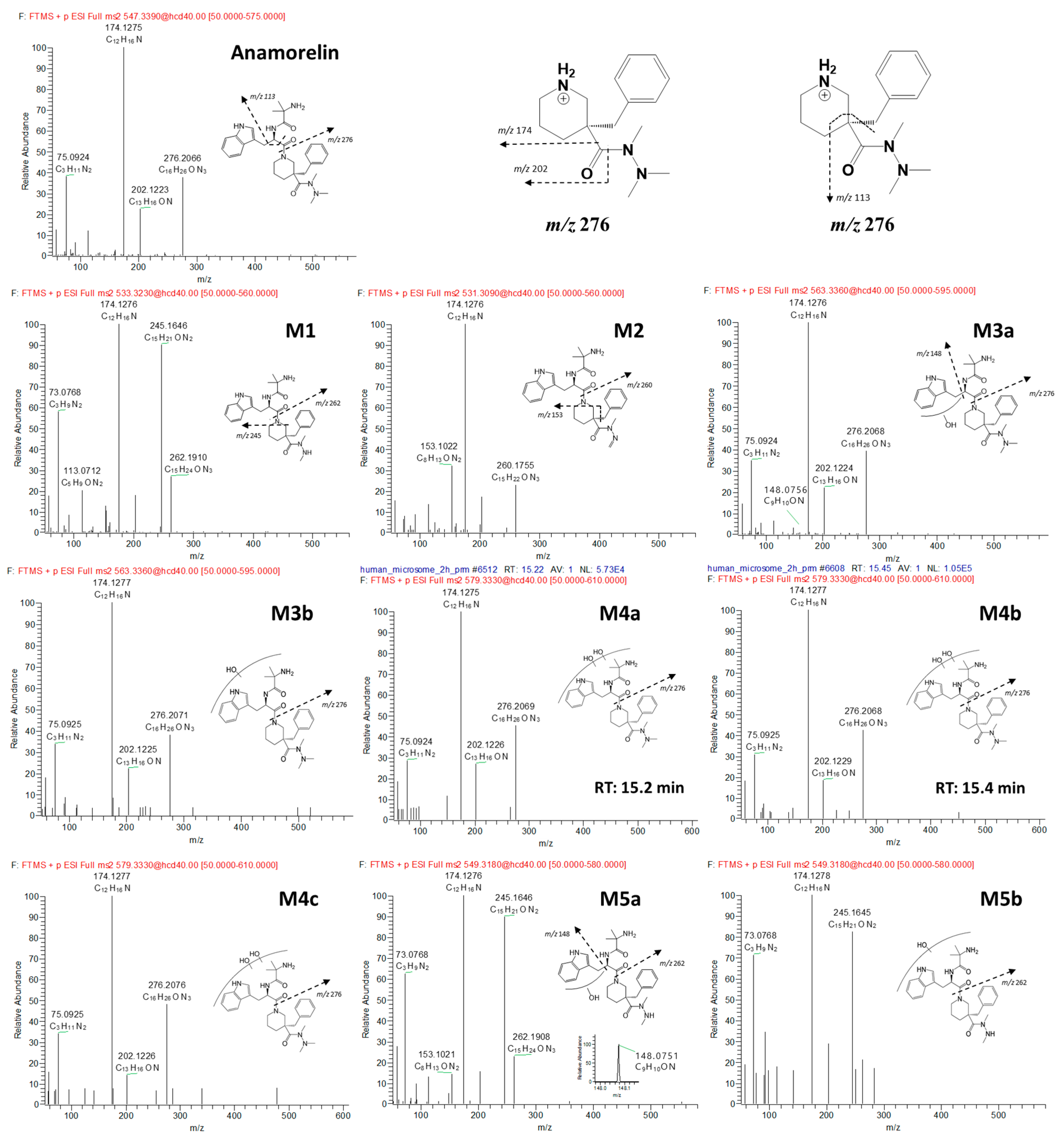
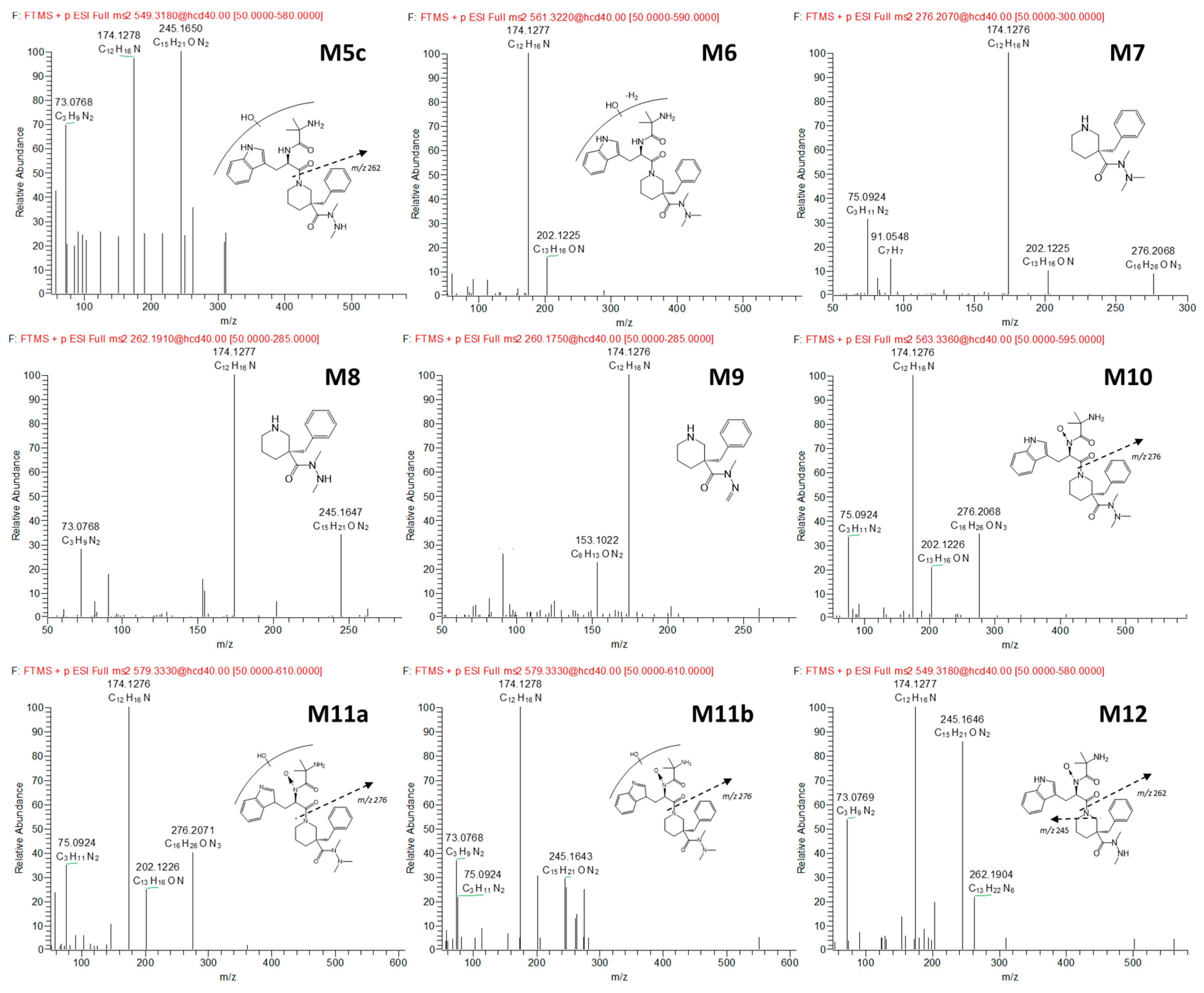
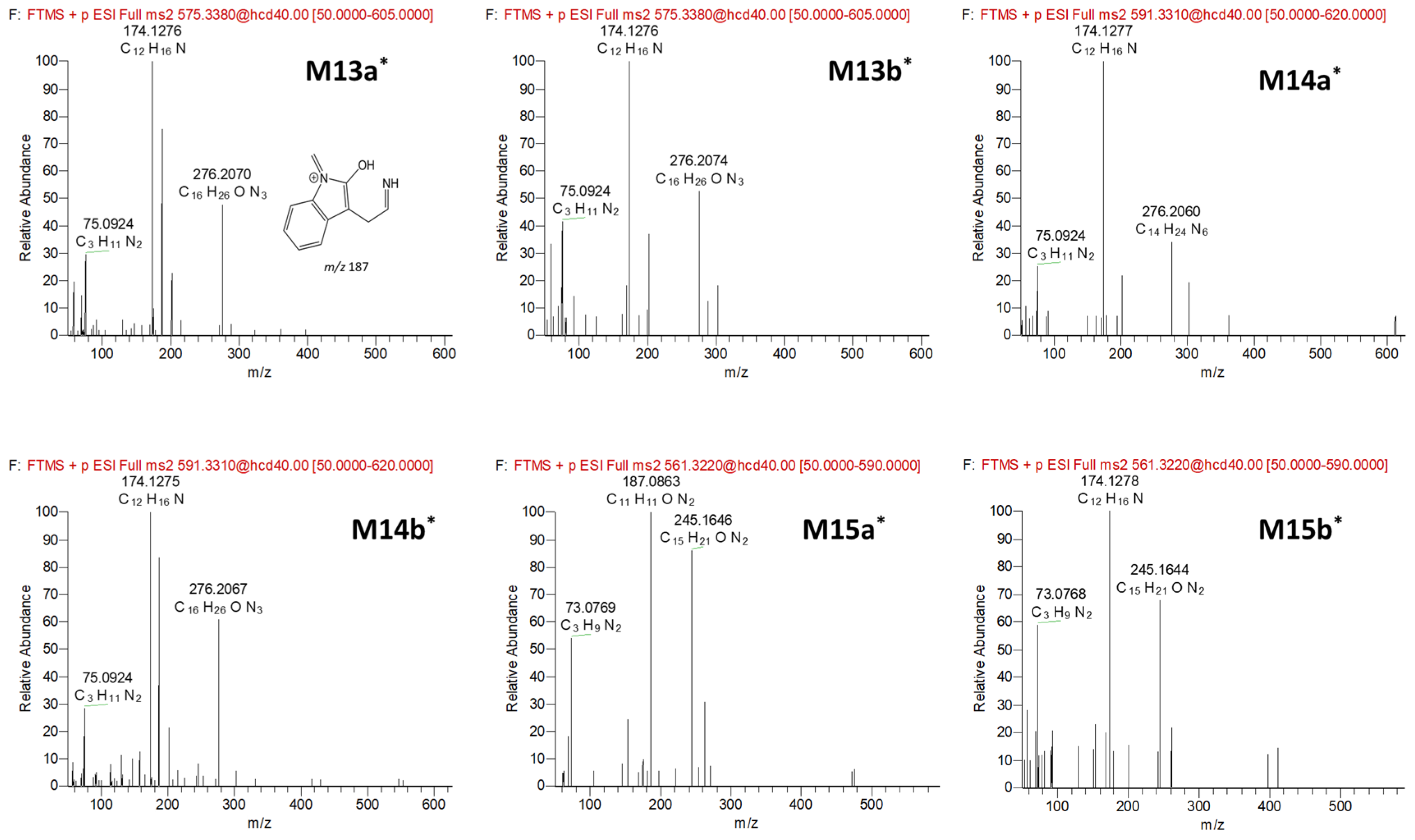
3.2. Anamorelin Metabolite Structural Elucidation
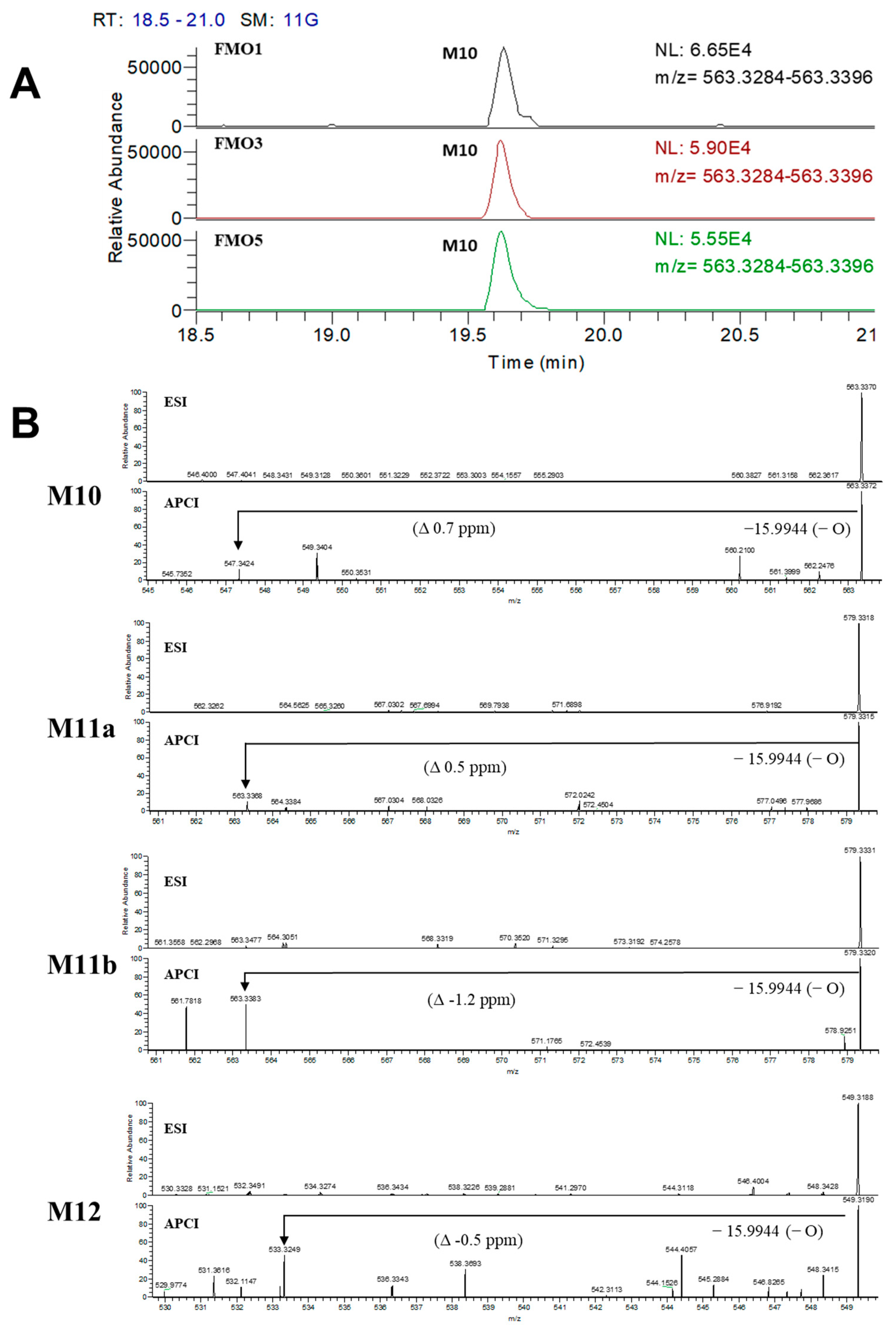
3.3. Confirmation of N-Oxide Metabolites and Identification of CYP3A4-Mediated Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishida, J.; Saitoh, M.; Ebner, N.; Springer, J.; Anker, S.D.; von Haehling, S. Growth Hormone Secretagogues: History, Mechanism of Action, and Clinical Development. JCSM Rapid Commun. 2020, 3, 25–37. [Google Scholar] [CrossRef]
- Garcia, J.M.; Boccia, R.V.; Graham, C.D.; Yan, Y.; Duus, E.M.; Allen, S.; Friend, J. Anamorelin for Patients with Cancer Cachexia: An Integrated Analysis of Two Phase 2, Randomised, Placebo-Controlled, Double-Blind Trials. Lancet Oncol. 2015, 16, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Polvino, W.J. Pharmacodynamic Hormonal Effects of Anamorelin, a Novel Oral Ghrelin Mimetic and Growth Hormone Secretagogue in Healthy Volunteers. Growth Horm. IGF Res. 2009, 19, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.A.; Garcia, J.M. Anamorelin Hydrochloride in the Treatment of Cancer Anorexia-Cachexia Syndrome: Design, Development, and Potential Place in Therapy. Drug Des. Dev. Ther. 2017, 11, 2325–2331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakabayashi, H.; Arai, H.; Inui, A. The Regulatory Approval of Anamorelin for Treatment of Cachexia in Patients with Non-Small Cell Lung Cancer, Gastric Cancer, Pancreatic Cancer, and Colorectal Cancer in Japan: Facts and Numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in Patients with Non-Small-Cell Lung Cancer and Cachexia (ROMANA 1 and ROMANA 2): Results from Two Randomised, Double-Blind, Phase 3 Trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M. What Is next after Anamorelin? Curr. Opin. Support. Palliat. Care 2017, 11, 266. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Shaikh, S.; You, H.J.; Lee, E.-Y.; Ali, S.; Lee, E.J.; Choi, I. Molecular Mechanisms and Current Treatment Options for Cancer Cachexia. Cancers 2022, 14, 2107. [Google Scholar] [CrossRef]
- Vincenti, F.; Montesano, C.; Di Ottavio, F.; Gregori, A.; Compagnone, D.; Sergi, M.; Dorrestein, P. Molecular Networking: A Useful Tool for the Identification of New Psychoactive Substances in Seizures by LC–HRMS. Front. Chem. 2020, 8, 572952. [Google Scholar] [CrossRef]
- Quinn, R.A.; Nothias, L.-F.; Vining, O.; Meehan, M.; Esquenazi, E.; Dorrestein, P.C. Molecular Networking As a Drug Discovery, Drug Metabolism, and Precision Medicine Strategy. Trends Pharmacol. Sci. 2017, 38, 143–154. [Google Scholar] [CrossRef]
- Yu, J.S.; Seo, H.; Kim, G.B.; Hong, J.; Yoo, H.H. MS-Based Molecular Networking of Designer Drugs as an Approach for the Detection of Unknown Derivatives for Forensic and Doping Applications: A Case of NBOMe Derivatives. Anal. Chem. 2019, 91, 5483–5488. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Le Daré, B.; Ferron, P.-J.; Allard, P.-M.; Clément, B.; Morel, I.; Gicquel, T. New Insights into Quetiapine Metabolism Using Molecular Networking. Sci. Rep. 2020, 10, 19921. [Google Scholar] [CrossRef]
- Rousu, T.; Tolonen, A. Characterization of Cyanide-Trapped Methylated Metabonates Formed during Reactive Drug Metabolite Screening in Vitro. Rapid Commun. Mass Spectrom. 2011, 25, 1382–1390. [Google Scholar] [CrossRef]
- Li, C.; Surapaneni, S.; Zeng, Q.; Marquez, B.; Chow, D.; Kumar, G. Identification of a Novel in Vitro Metabonate from Liver Microsomal Incubations. Drug Metab. Dispos. 2006, 34, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kang, M.J.; Jin, C.; In, M.K.; Kim, D.-H.; Yoo, H.H. Flavin-Containing Monooxygenase 1-Catalysed N,N-Dimethylamphetamine N-Oxidation. Xenobiotica 2009, 39, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.B.; Choi, M.S. Identification of a Metabolite for the Detection of the Hydrophilic Drug Diisopropylamine for Doping Control. J. Pharm. Biomed. Anal. 2023, 234, 115576. [Google Scholar] [CrossRef]
- Kim, I.S.; Rehman, S.U.; Choi, M.S.; Jang, M.; Yang, W.; Kim, E.; Yoo, H.H. Characterization of in Vitro Metabolites of Methylenedioxypyrovalerone (MDPV): An N-Oxide Metabolite Formation Mediated by Flavin Monooxygenase. J. Pharm. Biomed. Anal. 2016, 131, 160–166. [Google Scholar] [CrossRef]
- Okidono, Y.; Osada, J.; Otsu, K.; Kowase, S.; Aoki, H.; Yumoto, K. Two Cases of Wide QRS Complex Tachycardia Caused by Anamorelin. J. Cardiol. Cases 2022, 26, 212–216. [Google Scholar] [CrossRef]
- Prommer, E. Oncology Update: Anamorelin. Palliat. Care 2017, 10, 1178224217726336. [Google Scholar] [CrossRef]
- Duus, E.; Matson, M.A.; Bernareggi, A. Effects of Strong Cytochrome P450 (CYP)3A4 Inducers/Inhibitors on the Pharmacokinetic (PK) Profile of Anamorelin in Healthy Volunteers. J. Clin. Oncol. 2018, 36, e22180. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, M.; Tang, W. Metabolite Identification and Profiling in Drug Design: Current Practice and Future Directions. Curr. Pharm. Des. 2009, 15, 2220–2235. [Google Scholar] [CrossRef]
- Kirchmair, J.; Göller, A.H.; Lang, D.; Kunze, J.; Testa, B.; Wilson, I.D.; Glen, R.C.; Schneider, G. Predicting Drug Metabolism: Experiment and/or Computation? Nat. Rev. Drug Discov. 2015, 14, 387–404. [Google Scholar] [CrossRef]
- Testa, B. Drug Metabolism for the Perplexed Medicinal Chemist. Chem. Biodivers. 2009, 6, 2055–2070. [Google Scholar] [CrossRef]
- Kirchmair, J.; Howlett, A.; Peironcely, J.E.; Murrell, D.S.; Williamson, M.J.; Adams, S.E.; Hankemeier, T.; van Buren, L.; Duchateau, G.; Klaffke, W.; et al. How Do Metabolites Differ from Their Parent Molecules and How Are They Excreted? J. Chem. Inf. Model. 2013, 53, 354–367. [Google Scholar] [CrossRef]
- European Medicine Agency. Assessment Report–Aldumiz. Available online: https://www.ema.europa.eu/en/documents/assessment-report/adlumiz-epar-refusal-public-assessment-report_en.pdf (accessed on 8 November 2023).
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, Y.B.; Seo, J.I.; Yoo, H.H. Exploring Metabolic Pathways of Anamorelin, a Selective Agonist of the Growth Hormone Secretagogue Receptor, via Molecular Networking. Pharmaceutics 2023, 15, 2700. https://doi.org/10.3390/pharmaceutics15122700
Kwak YB, Seo JI, Yoo HH. Exploring Metabolic Pathways of Anamorelin, a Selective Agonist of the Growth Hormone Secretagogue Receptor, via Molecular Networking. Pharmaceutics. 2023; 15(12):2700. https://doi.org/10.3390/pharmaceutics15122700
Chicago/Turabian StyleKwak, Young Beom, Jeong In Seo, and Hye Hyun Yoo. 2023. "Exploring Metabolic Pathways of Anamorelin, a Selective Agonist of the Growth Hormone Secretagogue Receptor, via Molecular Networking" Pharmaceutics 15, no. 12: 2700. https://doi.org/10.3390/pharmaceutics15122700
APA StyleKwak, Y. B., Seo, J. I., & Yoo, H. H. (2023). Exploring Metabolic Pathways of Anamorelin, a Selective Agonist of the Growth Hormone Secretagogue Receptor, via Molecular Networking. Pharmaceutics, 15(12), 2700. https://doi.org/10.3390/pharmaceutics15122700








