Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Fabrication and Sterilization
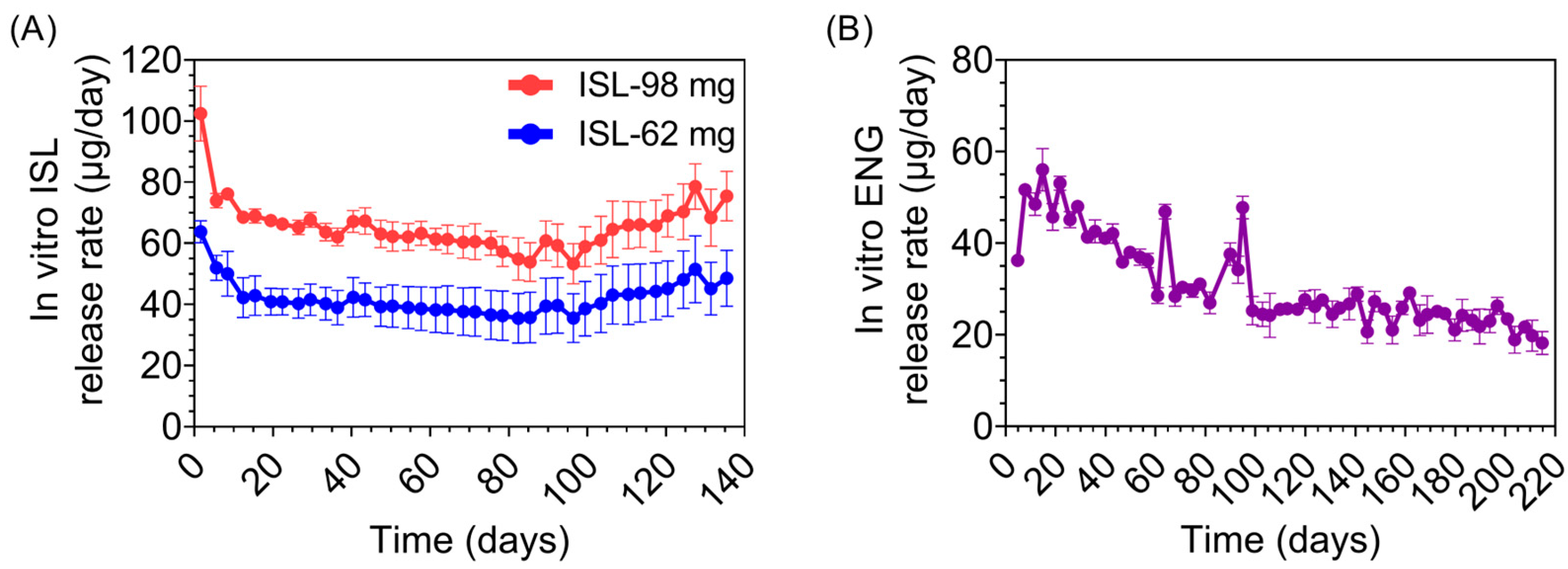
2.2. Determining Release Rates (In Vitro) and Drug Purity (In Vitro and In Vivo)
2.3. Animal Care Guidelines
2.4. Implantation and Removal
2.5. Pharmacokinetic Study Design
2.6. Blood, Tissue, and Swab Processing
2.7. Measurement of Intracellular ISL-TP and dATP from PBMCs and Tissue
2.8. Measurement of ISL and ENG in Plasma and Mucosal Fluids
2.9. Measurement of Progesterone in Plasma
2.10. Implant-Site Reactions
2.11. Statistical Analysis
3. Results
3.1. Implant Characteristics and Macaque Study Design
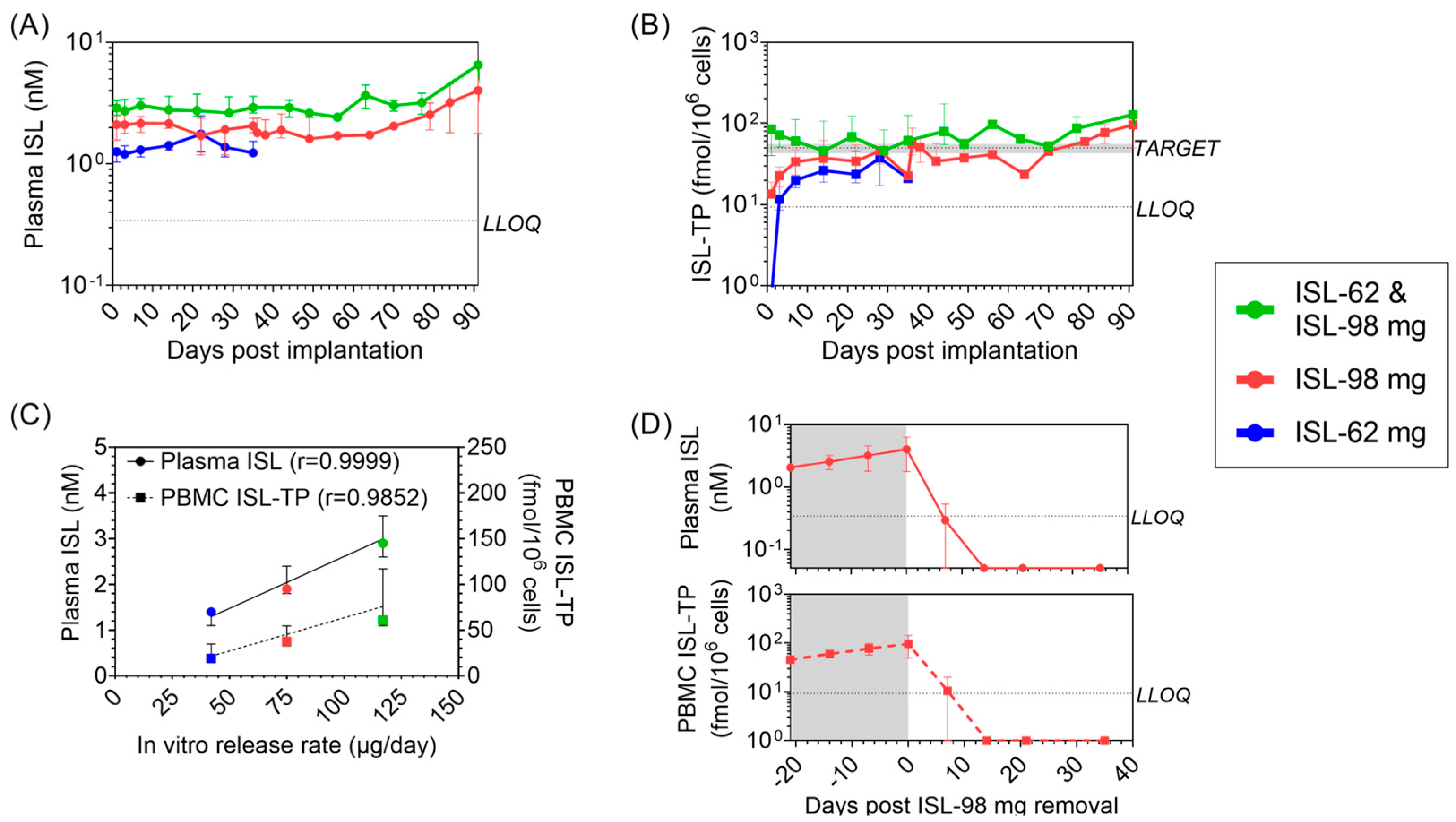
3.2. Pharmacokinetic Profile of ISL and ISL-TP in Blood
3.3. ISL Penetration in Rectal and Vaginal Tissues
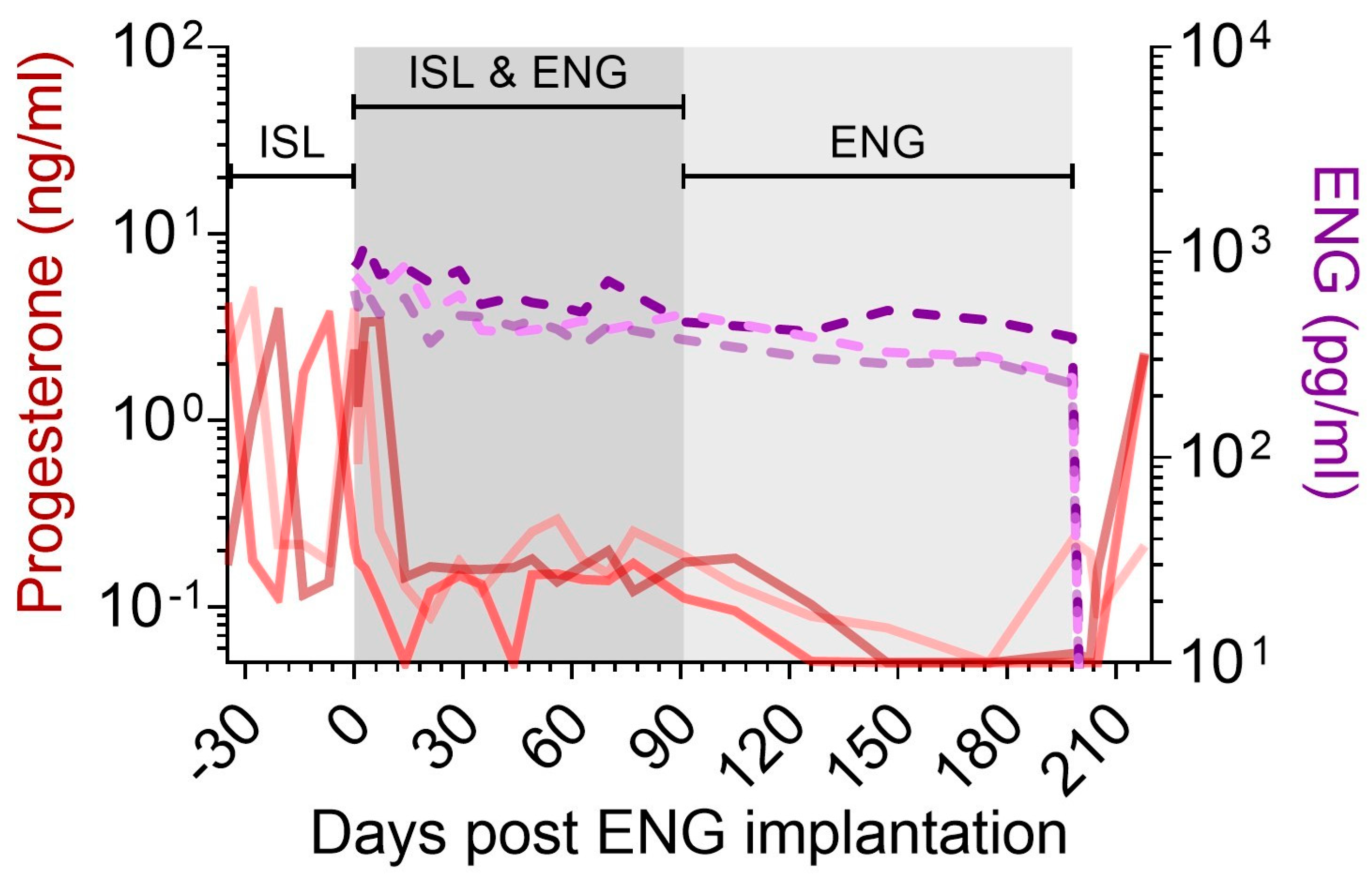
3.4. Suppression of Progesterone Production with ENG Implants
3.5. Effect of ISL and ENG Co-Implantation on ISL Pharmacokinetics
3.6. Implant Site Reactions
3.7. Purity of Drug and Predicted Duration of Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: The Global Burden of Disease Study 2015. Lancet HIV 2016, 3, e361–e387. [Google Scholar] [CrossRef]
- UNAIDS. Gender Every Minute. Available online: https://www.unaids.org/sites/default/files/20120607_infographic_gender-every-minute_en.pdf (accessed on 20 June 2023).
- Wulandari, L.P.L.; He, S.Y.; Fairley, C.K.; Bavinton, B.R.; Marie-Schmidt, H.; Wiseman, V.; Guy, R.; Tang, W.; Zhang, L.; Ong, J.J. Preferences for pre-exposure prophylaxis for HIV: A systematic review of discrete choice experiments. EClinicalMedicine 2022, 51, 101507. [Google Scholar] [CrossRef]
- Quaife, M.; Eakle, R.; Cabrera Escobar, M.A.; Vickerman, P.; Kilbourne-Brook, M.; Mvundura, M.; Delany-Moretlwe, S.; Terris-Prestholt, F. Divergent Preferences for HIV Prevention: A Discrete Choice Experiment for Multipurpose HIV Prevention Products in South Africa. Med. Decis. Mak. 2018, 38, 120–133. [Google Scholar] [CrossRef]
- Browne, E.N.; Montgomery, E.T.; Mansfield, C.; Boeri, M.; Mange, B.; Beksinska, M.; Schwartz, J.L.; Clark, M.R.; Doncel, G.F.; Smit, J.; et al. Efficacy is Not Everything: Eliciting Women’s Preferences for a Vaginal HIV Prevention Product Using a Discrete-Choice Experiment. AIDS Behav. 2020, 24, 1443–1451. [Google Scholar] [CrossRef]
- Shapley-Quinn, M.K.; Manenzhe, K.N.; Agot, K.; Minnis, A.M.; van der Straten, A. “We are not the same”: African women’s view of multipurpose prevention products in the TRIO clinical study. Int. J. Womens Health 2019, 11, 97–107. [Google Scholar] [CrossRef]
- Dimitrov, D.T.; Mâsse, B.R.; Donnell, D. PrEP Adherence Patterns Strongly Affect Individual HIV Risk and Observed Efficacy in Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 2016, 72, 444–451. [Google Scholar] [CrossRef]
- Trussell, J. Contraceptive failure in the United States. Contraception 2011, 83, 397–404. [Google Scholar] [CrossRef]
- Trussell, J.A.A.; Micks, E.; Guthrie, K.A. Efficacy, safety, and personal considerations. In Contraceptive Technology, 21st ed.; Ayer Company Publishers, Inc.: New York, NY, USA, 2018. [Google Scholar]
- Nexplanon (Etonegestrel) FDA Insert, Reference ID 3808594; Application No. 021529/S11; Merck & Co, Inc.: Rahway, NJ, USA, 2001.
- Apretude (Cabotegravir) FDA Insert, Reference ID 4908014; ViiV Healthcare: London, UK, 2021.
- PrEPWatch an initiative of AVAC. The Dapivirine Vaginal Ring. Available online: https://www.prepwatch.org/products/the-dapivirine-vaginal-ring/ (accessed on 6 September 2023).
- Agrahari, V.; Anderson, S.M.; Peet, M.M.; Wong, A.P.; Singh, O.N.; Doncel, G.F.; Clark, M.R. Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: Recent advances, emerging technologies, and development challenges. Expert. Opin. Drug Deliv. 2022, 19, 1365–1380. [Google Scholar] [CrossRef]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Matthews, R.P.; Patel, M.; Barrett, S.E.; Haspeslagh, L.; Reynders, T.; Zhang, S.; Rottey, S.; Goodey, A.; Vargo, R.C.; Grobler, J.A.; et al. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: A randomized, placebo-controlled phase 1 trial. Nat. Med. 2021, 27, 1712–1717. [Google Scholar] [CrossRef]
- Matthews, R.P.; Zang, X.; Barrett, S.E.; Koynov, A.; Goodey, A.; Heimbach, T.; Weissler, V.L.; Leyssens, C.; Reynders, T.; Xu, Z.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Trial of Radiopaque Islatravir-Eluting Subdermal Implants for Pre-exposure Prophylaxis Against HIV-1 Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2023, 92, 310–316. [Google Scholar] [CrossRef]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal im-plant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef]
- Gengiah, T.N.; Karim, Q.A.; Harkoo, I.; Mansoor, L.; Zuma, N.Y.; Radebe, P.; Samsunder, N.; Baxter, C.; Maharaj, B.; Baum, M.M.; et al. CAPRISA 018: A phase I/II clinical trial study protocol to assess the safety, acceptability, tolerability and pharmacokinetics of a sustained-release tenofovir alafenamide subdermal implant for HIV prevention in women. BMJ Open 2022, 12, e052880. [Google Scholar] [CrossRef]
- Simpson, S.M.; Widanapathirana, L.; Su, J.T.; Sung, S.; Watrous, D.; Qiu, J.; Pearson, E.; Evanoff, A.; Karunakaran, D.; Chacon, J.E.; et al. Design of a Drug-Eluting Subcutaneous Implant of the Antiretroviral Tenofovir Alafenamide Fumarate. Pharm. Res. 2020, 37, 83. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Jain, P.; Ballerini, A.; Bruno, G.; Hood, R.L.; Gupte, M.; Gao, S.; Di Trani, N.; Susnjar, A.; Shelton, K.; et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J. Control. Release 2018, 286, 315–325. [Google Scholar] [CrossRef]
- Johnson, L.M.; Krovi, S.A.; Li, L.; Girouard, N.; Demkovich, Z.R.; Myers, D.; Creelman, B.; van der Straten, A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2019, 11, 315. [Google Scholar] [CrossRef]
- Li, L.; Gatto, G.J.; Brand, R.M.; Krovi, S.A.; Cottrell, M.L.; Norton, C.; van der Straten, A.; Johnson, L.M. Long-acting biodegradable implant for sustained delivery of antiretrovirals (ARVs) and hormones. J. Control. Release 2021, 340, 188–199. [Google Scholar] [CrossRef]
- Massud, I.; Krovi, A.; Nishiura, K.; Ruone, S.; Li, L.; Holder, A.; Gary, J.; Mills, P.; Mitchell, J.; Khalil, G.; et al. Safety and efficacy of a biodegradable implant releasing tenofovir alafenamide for vaginal protection in a macaque model. J. Antimicrob. Chemother. 2022, 77, 2964–2971. [Google Scholar] [CrossRef]
- Gatto, G.J.; Krovi, A.; Li, L.; Massud, I.; Holder, A.; Gary, J.; Mills, P.; Mitchell, J.; Luecke, E.; Demkovich, Z.R.; et al. Comparative Pharmacokinetics and Local Tolerance of Tenofovir Alafenamide (TAF) From Subcutaneous Implant in Rabbits, Dogs, and Macaques. Front. Pharmacol. 2022, 13, 923954. [Google Scholar] [CrossRef]
- Su, J.T.; Simpson, S.M.; Sung, S.; Tfaily, E.B.; Veazey, R.; Marzinke, M.; Qiu, J.; Watrous, D.; Widanapathirana, L.; Pearson, E.; et al. A Subcutaneous Implant of Tenofovir Alafenamide Fumarate Causes Local Inflammation and Tissue Necrosis in Rabbits and Macaques. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Zane, D.; Roller, S.; Shelton, J.; Singh, R.; Jain, R.; Wang, Y.; Yang, B.; Felx, M.; Alessi, T.; Feldman, P.L. A 28-Day Toxicity Study of Tenofovir Alafenamide Hemifumarate by Subcutaneous Infusion in Rats and Dogs. Microbiol. Spectr. 2021, 9, e0033921. [Google Scholar] [CrossRef]
- Romano, J.W.; Baum, M.M.; Demkovich, Z.R.; Diana, F.; Dobard, C.; Feldman, P.L.; Garcia-Lerma, J.G.; Grattoni, A.; Gunawardana, M.; Ho, D.K.; et al. Tenofovir Alafenamide for HIV Prevention: Review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development Meeting. AIDS Res. Hum. Retroviruses 2021, 37, 409–420. [Google Scholar] [CrossRef]
- Markowitz, M.; Grobler, J.A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1. Curr. Opin. HIV AIDS. 2020, 15, 27–32. [Google Scholar] [CrossRef]
- Hillier, S.; Bekker, L.-G.; Riddler, S.A.; Hendrix, C.W.; Badal-Faesen, S.; Rasmussen, S.; Schwartz, H.; Macdonald, P.; Lombaard, J.; Caraco, Y.; et al. Safety and Pharmacokinetics of Oral Islatravir Once Monthly for HIV Pre-exposure Prophylaxis (PrEP): Week 24 Analysis of a Phase 2a Trial. In Proceedings of the 11th IAS Con-ference on HIV Science, Virtual, 18–22 July 2021. [Google Scholar]
- Merck & Co., Inc. Merck Announces Clinical Holds on Studies Evaluating Islatravir for the Treatment and Pre-Vention of HIV-1 Infection. Available online: https://www.merck.com/news/merck-announces-clinical-holds-on-studies-evaluating-islatravir-for-the-treatment-and-prevention-of-hiv-1-infection/ (accessed on 20 June 2023).
- Squires, K.E.; Correll, T.A.; Robertson, M.N.; Klopfer, S.O.; Hwang, P.M.T.; Zhou, Y.-P.; Martin, E.; Rhee, E.G. Effect of ISL on Total Lymphocytes and Lymphocytes Subset Counts. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 19–22 February 2023. [Google Scholar]
- Vargo, R.C.; Robey, S.; Zang, X.; Du, L.; Kandala, B.; Roberts, J.; Klopfer, S.; Squires, K. Modeling to optimize islatravir QW dose in HIV virologically supressed PWH. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 19–22 February 2023. [Google Scholar]
- Patel, M.; Zang, X.; Cao, Y.; Matthews, R.P.; Plank, R.; Sklar, P.; Grobler, J.; Robertson, M.; Vargo, R. Islatravir PK Threshold & Dose Selection for Monthly Oral PrEP. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021. [Google Scholar]
- Institute of Laboratory Animal Resources (US); Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Kuklenyik, Z.; Martin, A.; Pau, C.P.; Holder, A.; Youngpairoj, A.S.; Zheng, Q.; Cong, M.E.; Garcia-Lerma, J.G.; Heneine, W.; Pirkle, J.L.; et al. On-line coupling of anion exchange and ion pair chromatography for measurement of intracel-lular triphosphate metabolites of reverse transcriptase inhibitors. J. Chromatogr. B 2009, 877, 3659–3666. [Google Scholar] [CrossRef]
- Sykes, C.; Van Horne, B.; Jones, J.; Kashuba, A.D.M.; Gatto, G.; Van Der Straten, A.; Johnson, L.; Cottrell, M.L. Intracellular islatravir pharmacology differs between species in an in vitro model: Implications for preclinical study design. J. Antimicrob. Chemother. 2022, 77, 1000–1004. [Google Scholar] [CrossRef]
- Farage, M.A.; Maibach, H.I.; Andersen, K.E.; Lachapelle, J.M.; Kern, P.; Ryan, C.; Ely, J.; Kanti, A. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermatitis 2011, 65, 65–75. [Google Scholar] [CrossRef]
- ISO 10993-6:2016; Biological Evaluation of Medical Devices. Part 6: Tests for Local Effects after Implantation. ISO: Geneva, Switzerland, 2016.
- Shen, Z.; Rodriguez-Garcia, M.; Patel, M.V.; Bodwell, J.; Kashuba, A.D.M.; Wira, C.R. Hormonal Contraceptives Differentially Suppress TFV and TAF Inhibition of HIV Infection and TFV-DP in Blood and Genital Tract CD4+ T cells. Sci. Rep. 2017, 7, 17697. [Google Scholar] [CrossRef]
- Cottrell, M.L.; Prince, H.M.A.; Schauer, A.P.; Sykes, C.; Maffuid, K.; Poliseno, A.; Chun, T.W.; Huiting, E.; Stanczyk, F.Z.; Peery, A.F.; et al. Decreased Tenofovir Diphosphate Concentrations in a Transgender Female Cohort: Implications for Human Immunodeficiency Virus Preexposure Prophylaxis. Clin. Infect. Dis. 2019, 69, 2201–2204. [Google Scholar] [CrossRef]
- Daly, M.B.; Sterling, M.; Holder, A.; Dinh, C.; Nishiura, K.; Khalil, G.; García-Lerma, J.G.; Dobard, C. The effect of depot medroxyprogesterone acetate on tenofovir alafenamide in rhesus macaques. Antivir. Res. 2021, 186, 105001. [Google Scholar] [CrossRef]
- Bershteyn, A.; Resar, D.; Kim, H.Y.; Platais, I.; Mullick, S. Optimizing the pipeline of multipurpose prevention technologies: Opportunities across women’s reproductive lifespans. Front. Reprod. Health 2023, 5, 1169110. [Google Scholar] [CrossRef]
- Hendrix, C.W.; Hillier, S.L.; Bekker, L.-G.; Badal-Faesen, S.; Riddler, S.A.; Fuchs, E.J.; Macdonald, P.J.; Edick, S.; Plank, R.M.; Chavez-Eng, C.M. Islatravir distribution in mucosal tissues, PBMC & plasma after monthly oral dosing. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 12–16 February 2022. [Google Scholar]
- Matthews, R.P.; Jackson Rudd, D.; Zhang, S.; Fillgrove, K.L.; Sterling, L.M.; Grobler, J.A.; Vargo, R.C.; Stoch, S.A.; Iwamoto, M. Safety and Pharmacokinetics of Once-Daily Multiple-Dose Administration of Islatravir in Adults Without HIV. J. Acquir. Immune Defic. Syndr. 2021, 88, 314–321. [Google Scholar] [CrossRef]
- Matthews, R.P.; Ankrom, W.; Friedman, E.; Jackson Rudd, D.; Liu, Y.; Mogg, R.; Panebianco, D.; De Lepeleire, I.; Petkova, M.; Grobler, J.A.; et al. Safety, tolerability, and pharmacokinetics of single- and multiple-dose administration of islatravir (MK-8591) in adults without HIV. Clin. Transl. Sci. 2021, 14, 1935–1944. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Di Trani, N.; Capuani, S.; Campa-Carranza, J.N.; Nehete, B.; Sharma, S.; Shelton, K.A.; Bushman, L.R.; Abdelmawla, F.; Williams, M.; et al. Long-acting refillable nanofluidic implant confers protection against SHIV infection in nonhuman primates. Sci. Transl. Med. 2023, 15, eadg2887. [Google Scholar] [CrossRef]
- Markowitz, M.; Gettie, A.; St Bernard, L.; Andrews, C.D.; Mohri, H.; Horowitz, A.; Grasperge, B.F.; Blanchard, J.L.; Niu, T.; Sun, L.; et al. Once-Weekly Oral Dosing of MK-8591 Protects Male Rhesus Macaques from Intrarectal Challenge with SHIV109CP3. J. Infect. Dis. 2020, 221, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Kim, D.; Li, L.; Krovi, A.; Norton, C.; Forero, C.; Brake, M.; Dinh, C.; Edwards, T.I.; Mitchell, J.; et al. Vagi-nal PrEP efficacy of biodegradable islatravir implants in macaques. In Proceedings of the Conference on Retro-viruses and Opportunistic Infections, Seattle, WA, USA, 19–22 February 2023. [Google Scholar]
- Bennink, H.J. The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant. Eur. J. Contracept. Reprod. Health Care 2000, 5 (Suppl. S2), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Slayden, O.D.; Luo, F.; Bishop, C.V. Physiological Action of Progesterone in the Nonhuman Primate Oviduct. Cells 2022, 11, 1534. [Google Scholar] [CrossRef] [PubMed]
- Bleasby, K.; Houle, R.; Hafey, M.; Lin, M.; Guo, J.; Lu, B.; Sanchez, R.I.; Fillgrove, K.L. Islatravir Is Not Expected to Be a Victim or Perpetrator of Drug-Drug Interactions via Major Drug-Metabolizing Enzymes or Transporters. Viruses 2021, 13, 1566. [Google Scholar] [CrossRef]
- Ankrom, W.; Jackson Rudd, D.; Zhang, S.; Fillgrove, K.L.; Gravesande, K.N.; Matthews, R.P.; Brimhall, D.; Stoch, S.A.; Iwamoto, M.N. A phase 1, open-label study to evaluate the drug interaction between islatravir (MK-8591) and the oral contraceptive levonorgestrel/ethinyl estradiol in healthy adult females. J. Int. AIDS Soc. 2021, 24, e25858. [Google Scholar] [CrossRef]



| Implant | Length (mm) | Wall Thickness (µm) | In Vitro Release Rate (µg/Day) | In Vitro Purity (%) | In Vivo Purity (%) |
|---|---|---|---|---|---|
| ISL-62 mg 1 | 25 | 100 | 41.9 ± 5.5 | 97.1 ± 0.04 | 97.1 ± 0.09 |
| ISL-98 mg 1 | 40 | 100 | 65.6 ± 8.2 | 97.1 ± 0.02 | 97.1 ± 0.12 |
| ENG-33 mg 2 | 16 | 300 | 31.1 ± 9.6 | 99.3 ± 0.01 | 98.9 ± 0.01 |
| ISL-62 mg (n = 3) | ISL-98 mg ± ENG-33 (n = 3) | ISL-62 mg + ISL-98 mg (n = 3) | |
|---|---|---|---|
| Plasma | |||
| Islatravir (nM) | 1.3 [1.0–2.5] | 1.9 [1.2–6.3] | 2.8 [2.3–11.6] |
| ISL Cmax (nM) | 1.9 [1.5–2.5] | 3.7 [2.2–6.3] | 6.1 [2.9–11.6] |
| ISL Tmax (days) | 26 [22–35] | 37 [7–91] | 43 [7–91] |
| Etonogestrel (pg/mL) | n/a | 495 [229–1100] | n/a |
| ENG Cmax (nM) | n/a | 875 [660–1110] | n/a |
| ENG Tmax (days) | n/a | 12 [7–22] | n/a |
| Peripheral blood mononuclear cells | |||
| Islatravir-triphosphate (fmol/106 cells) | 20.5 [7.8–45.4] | 37.7 [13.3–143.0] | 63.6 [40.3 -229.0] |
| ISL-TP Cmax (fmol/106 cells) | 36.4 [26.3–45.4] | 96.8 [59.7–143.0] | 164.2 [89.5–229.0] |
| ISL-TP Tmax (days) | 21 [14–28] | 69 [36–91] | 67 [44–100] |
| Mucosal tissues | |||
| Vaginal ISL-TP (pmol/g) | 9.6 a [8.5–11.6] | 13.5 a [8.4–21.6] | 30.5 b [10.7–64.1] |
| Rectal ISL-TP (pmol/g) | 18.5 a [8.6–27.3] | 20.5 a [12.5–34.0] | 36.9 b [26.0–45.4] |
| Macaque ID | PT-4 | PT-5 | PT-6 | |||
|---|---|---|---|---|---|---|
| Biopsy Site | Medial | Distal | Medial (at Break) | Distal | Medial | Distal |
| Lymphocytes | 1 | 0 | 3 | 1 | 0 | 0 |
| Plasma cells | 2 | 0 | 3 | 0 | 0 | 0 |
| Histiocytes/Macrophages | 1 | 0 | 3 | 0 | 0 | 0 |
| Multinucleated giant cells | 0 | 0 | 1 | 0 | 0 | 1 |
| Polymorphonuclear cells | 1 | 0 | 3 | 0 | 0 | 0 |
| Fibrosis | 0 | 0 | 3 | 0 | 0 | 0 |
| Reactive fibroblasts | 1 | 0 | 3 | 0 | 0 | 0 |
| Hemorrhage | 0 | 0 | 2 | 0 | 0 | 0 |
| Neovascularization | 0 | 0 | 2 | 0 | 0 | 0 |
| Necrosis + type | 0 | 0 | 2 | 0 | 0 | 0 |
| Edema | 0 | 0 | 3 | 0 | 0 | 0 |
| Total Score | 6 | 0 | 27 | 1 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daly, M.B.; Wong-Sam, A.; Li, L.; Krovi, A.; Gatto, G.J.; Norton, C.; Luecke, E.H.; Mrotz, V.; Forero, C.; Cottrell, M.L.; et al. Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques. Pharmaceutics 2023, 15, 2676. https://doi.org/10.3390/pharmaceutics15122676
Daly MB, Wong-Sam A, Li L, Krovi A, Gatto GJ, Norton C, Luecke EH, Mrotz V, Forero C, Cottrell ML, et al. Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques. Pharmaceutics. 2023; 15(12):2676. https://doi.org/10.3390/pharmaceutics15122676
Chicago/Turabian StyleDaly, Michele B., Andres Wong-Sam, Linying Li, Archana Krovi, Gregory J. Gatto, Chasity Norton, Ellen H. Luecke, Victoria Mrotz, Catalina Forero, Mackenzie L. Cottrell, and et al. 2023. "Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques" Pharmaceutics 15, no. 12: 2676. https://doi.org/10.3390/pharmaceutics15122676
APA StyleDaly, M. B., Wong-Sam, A., Li, L., Krovi, A., Gatto, G. J., Norton, C., Luecke, E. H., Mrotz, V., Forero, C., Cottrell, M. L., Schauer, A. P., Gary, J., Nascimento-Seixas, J., Mitchell, J., van der Straten, A., Heneine, W., Garcίa-Lerma, J. G., Dobard, C. W., & Johnson, L. M. (2023). Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques. Pharmaceutics, 15(12), 2676. https://doi.org/10.3390/pharmaceutics15122676









