The Exploitation of pH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. Optimization of Formulation Components
2.2.3. Loading of TER into MSNs
2.2.4. Preparation of Eud-Coated TER-Loaded MSNs
2.2.5. Determination of EE
2.2.6. In Vitro Cumulative Release Study
2.2.7. In Vitro Characterization of the Optimal Formula
Kinetic Study of the Release Data
Measurement of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
Gas Adsorption Manometry
Fourier-Transform Infrared (FT-IR) Spectroscopy
Differential Scanning Calorimetry (DSC)
Polarized Light Microscopy (PLM)
Transmission Electron Microscopy (TEM)
2.2.8. Cytotoxicity Evaluation of the Optimal Formula
Cell Culture
In Vitro Cell Viability Assay
Apoptosis Assay by Flow Cytometry
Cell Cycle Study
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Eud/TER-MSNs
3.2. Experimental Analysis
3.2.1. Variables’ Influence on the EE (Y1)
3.2.2. Variables’ Influence on the Q2 in pH 1.2 (Y2)
3.2.3. Variables’ Influence on the Q24 in pH 7.4 (Y3)
3.3. Optimization and Validation of Variables
3.4. In Vitro Characterization of Optimal Formula
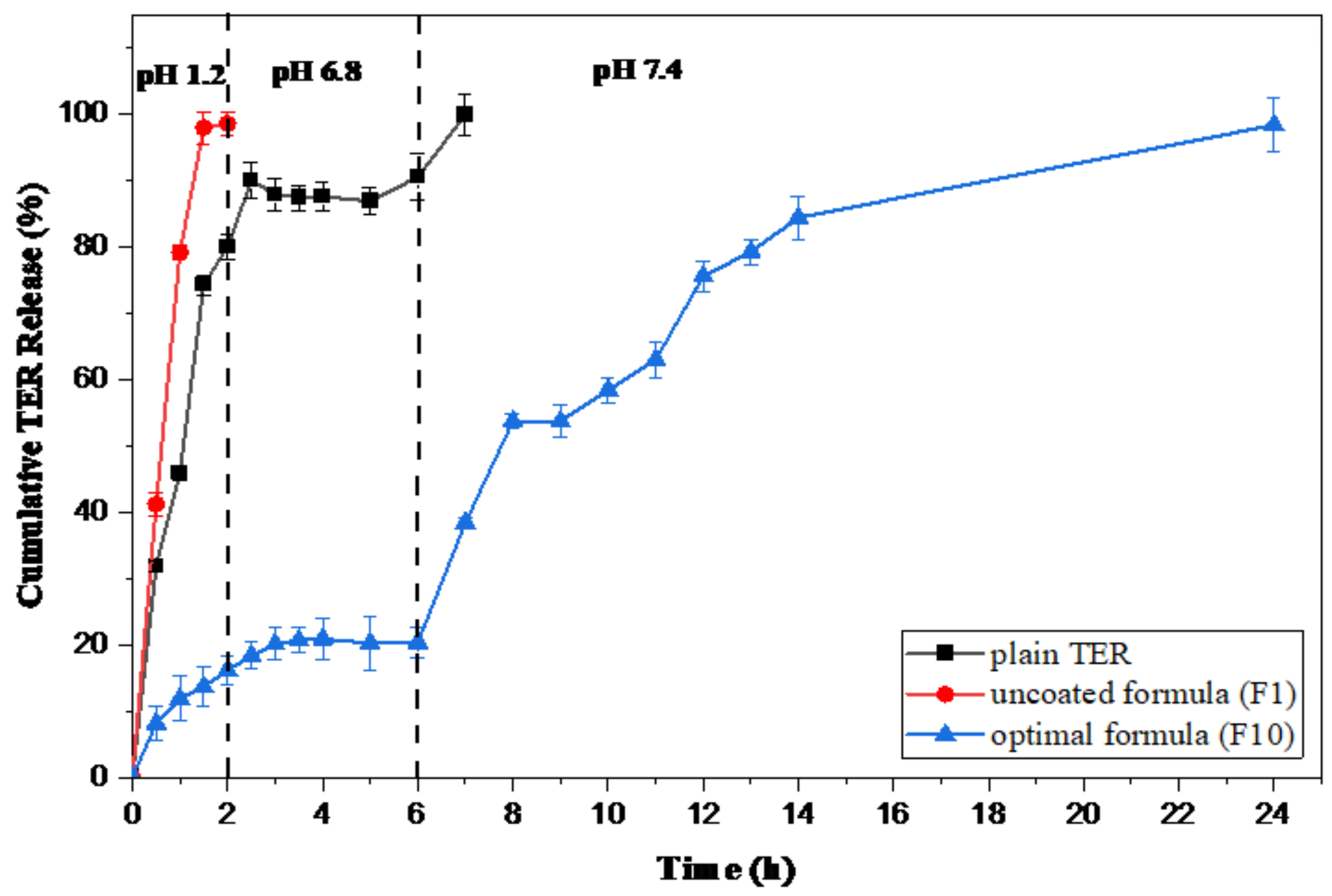
3.4.1. Kinetic Release Study
3.4.2. Measurement of PS, PDI, and ZP
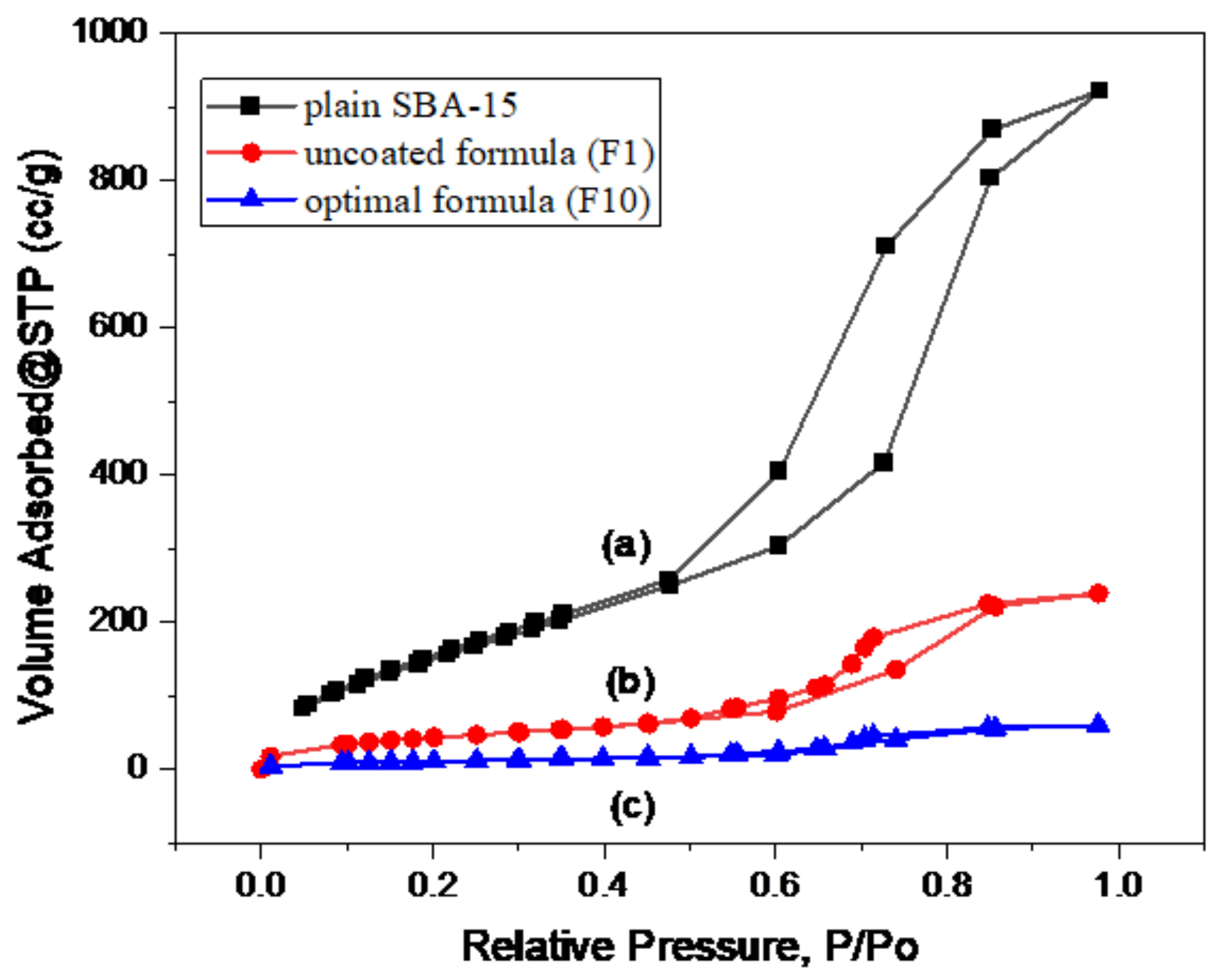
3.4.3. Gas Adsorption Manometry
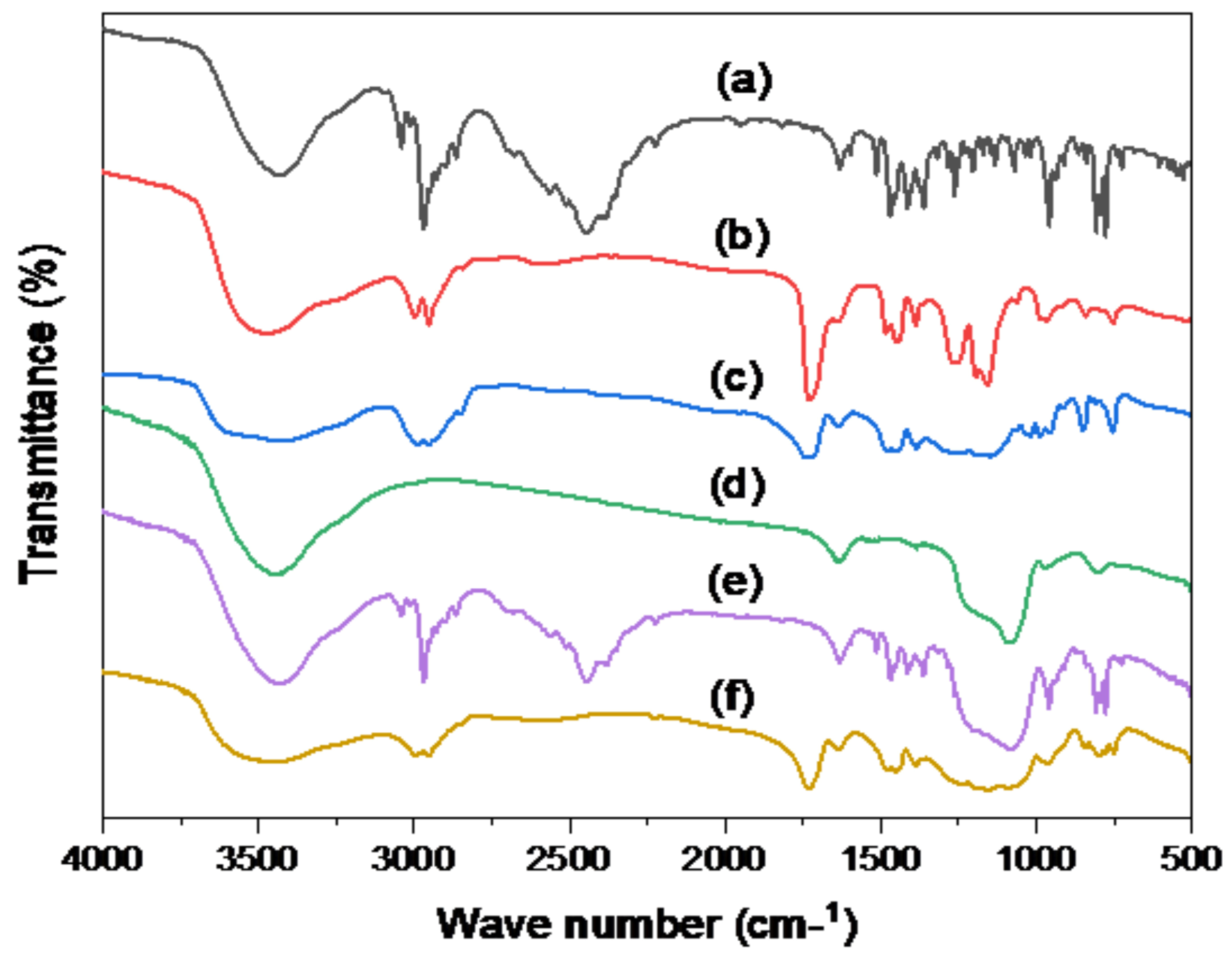
3.4.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.4.5. Differential Scanning Calorimetry (DSC) Study
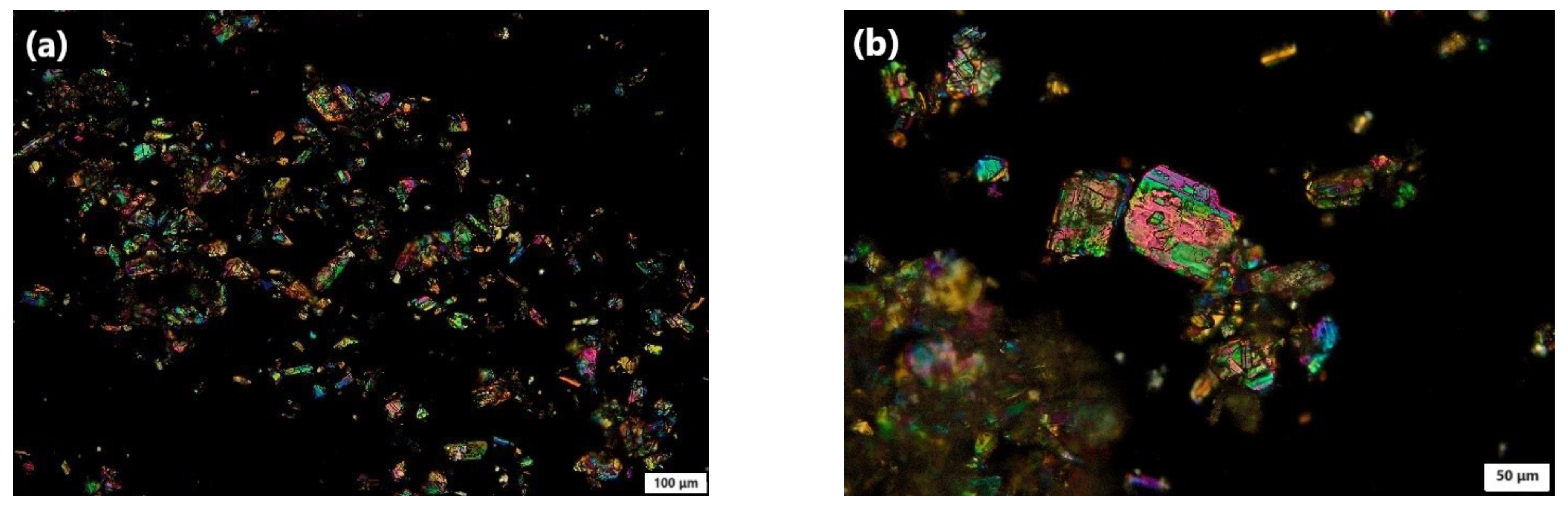
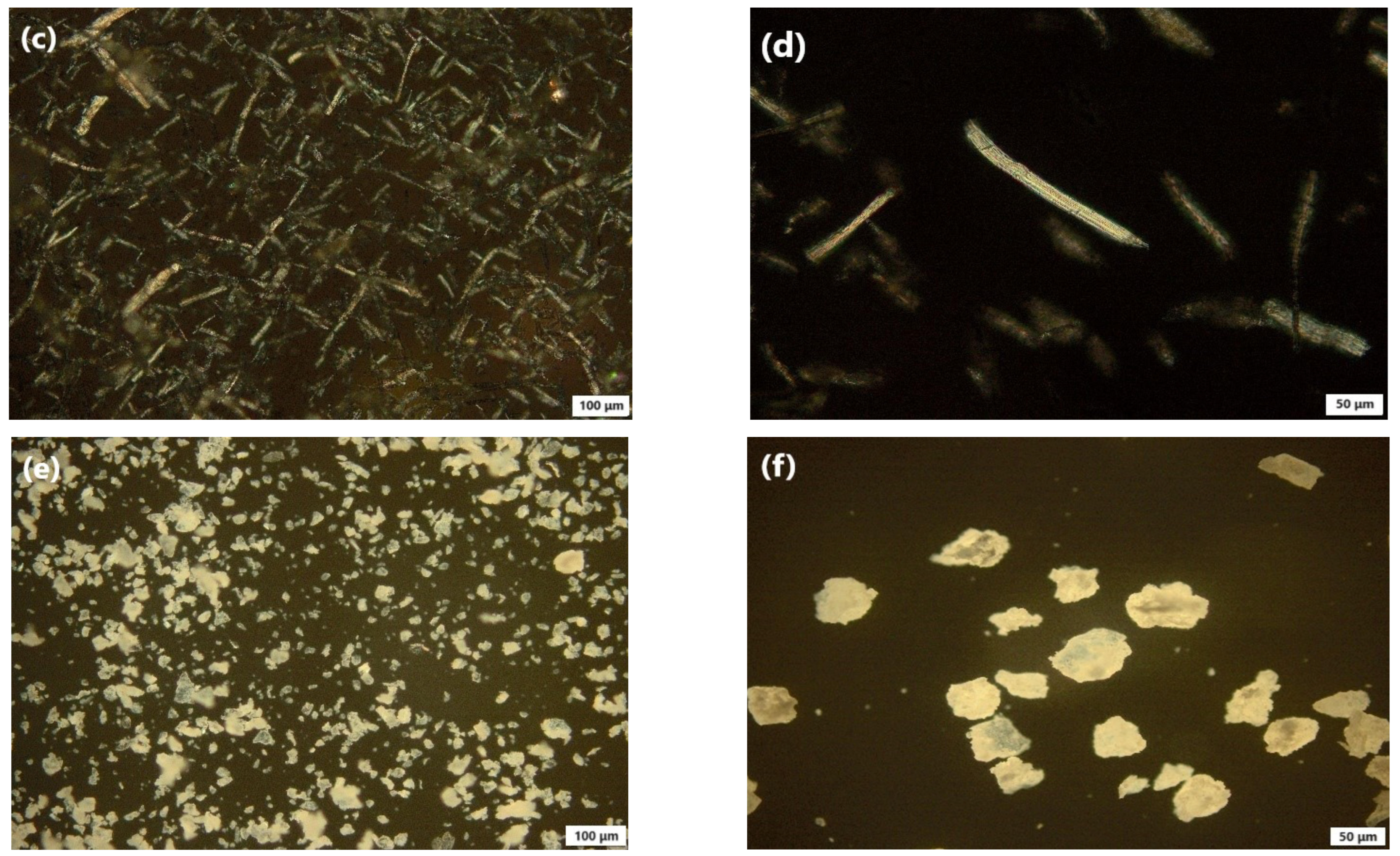
3.4.6. Polarized Light Microscopy (PLM) Study
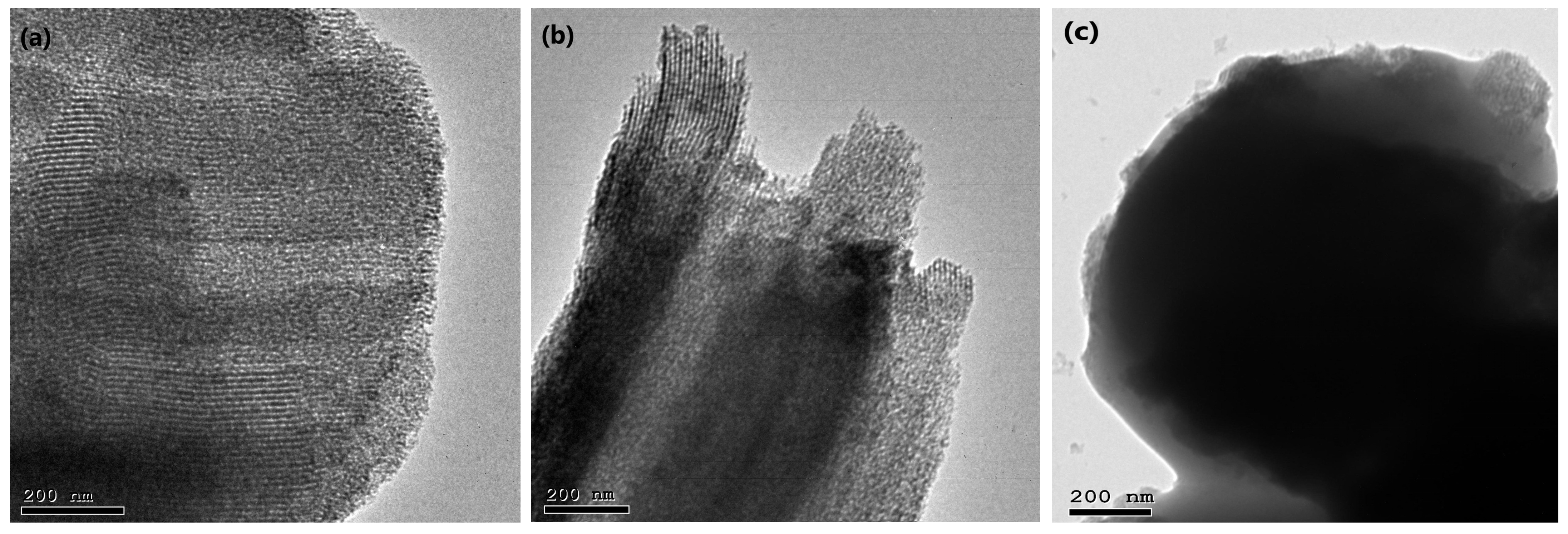
3.4.7. Transmission Electron Microscopy (TEM) Study
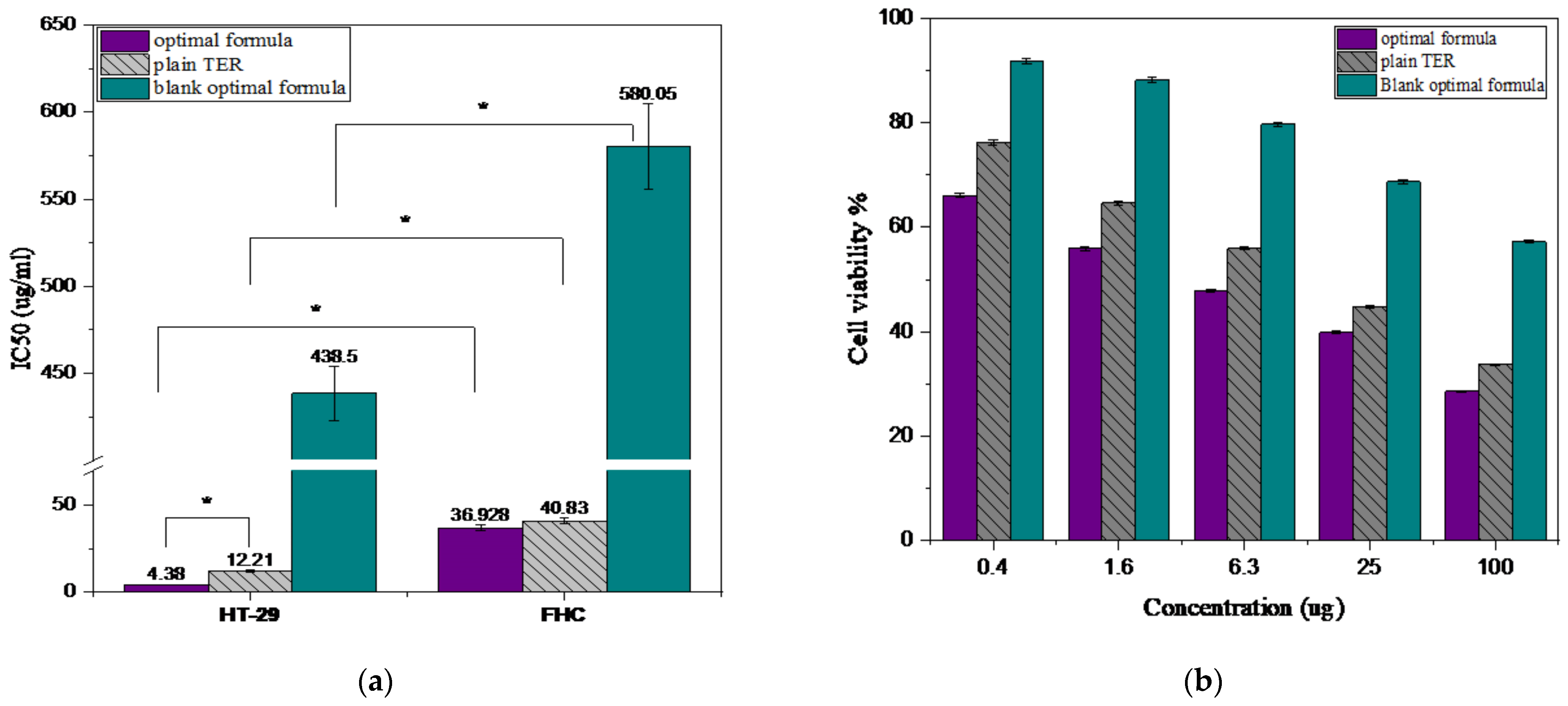
3.5. Cytotoxicity Evaluation of the Optimal Formula
3.5.1. Cell Viability Study
3.5.2. Apoptosis Assay by Flow Cytometry
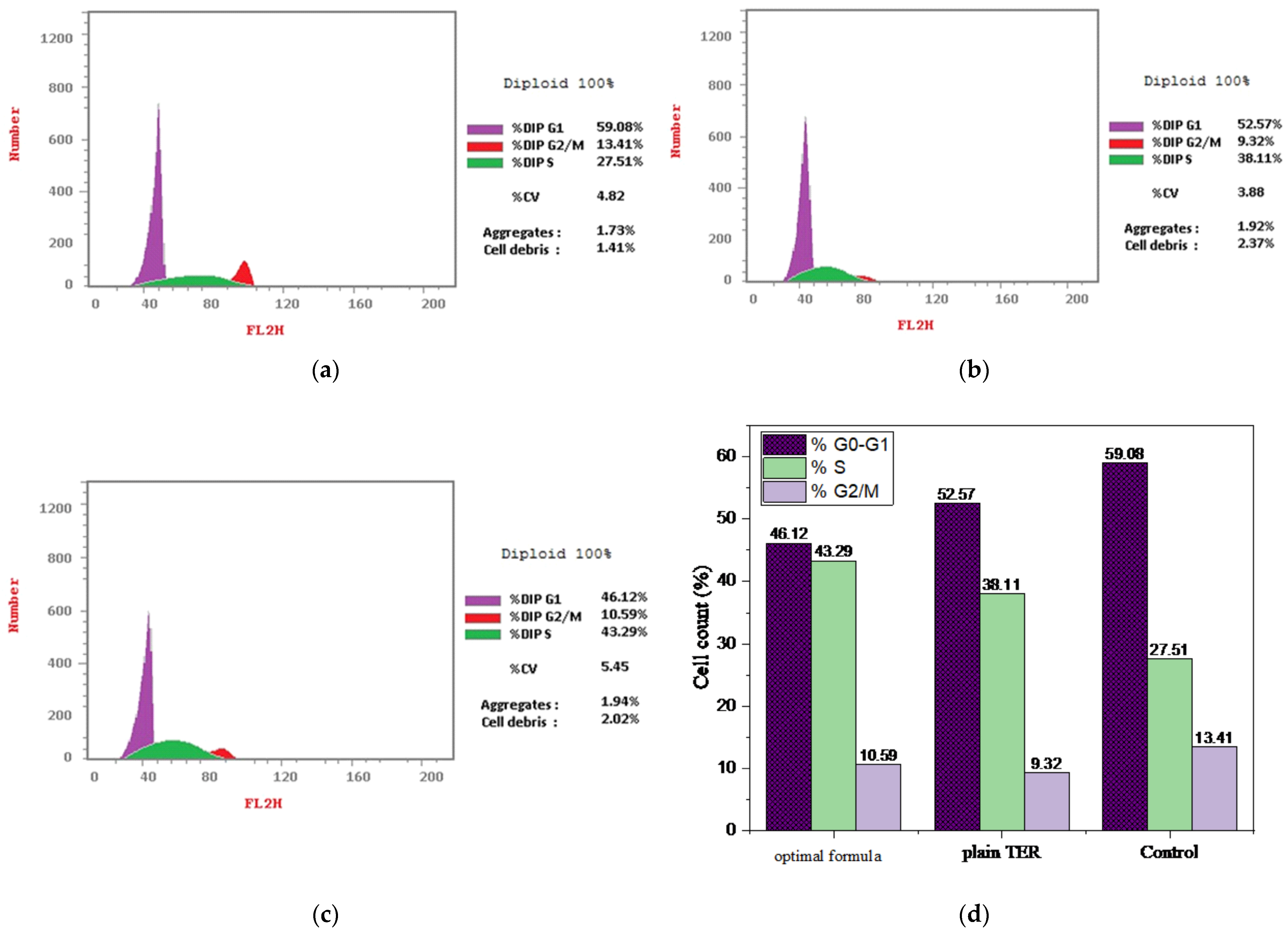
3.5.3. Cell Cycle Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 1 September 2023).
- Yu, Z.; Li, X.; Duan, J.; Yang, X.D. Targeted treatment of colon cancer with aptamer-guided albumin nanoparticles loaded with docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Albalawi, R.; Pottoo, F.H. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med. Res. Rev. 2022, 42, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Elsewedy, H.S.; Dhubiab, B.E.A.; Mahdy, M.A.; Elnahas, H.M. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv. 2020, 27, 1134–1146. [Google Scholar] [CrossRef]
- Rafati, H.; Taghavi, S.; Shahnani, M. Preparation and Characterization of Tamoxifen Loaded Silica and NH2 Functionalized Mesoporous Silica Nanoparticles as Delivery Systems against MCF-7 Breast Cancer Cells. Iran. J. Basic Med. Sci. 2023, 26, 1334–1341. [Google Scholar]
- Musallam, A.A.; Mahdy, M.A.; Elnahas, H.M.; Aldeeb, R.A.; Musallam, A.A.; Mahdy, M.A. Optimization of mirtazapine loaded into mesoporous silica nanostructures via Box-Behnken design: In-vitro characterization and in-vivo assessment Box-Behnken. Drug Deliv. 2022, 29, 1582–1594. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, P.; Wu, Y.; Tan, L.; Wei, W.; Liu, S. Materials Science & Engineering C Epsilon-poly-L-lysine decorated ordered mesoporous silica contributes to the synergistic antifungal effect and enhanced solubility of a lipophilic drug. Mater. Sci. Eng. C 2019, 99, 231–240. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L. pH-responsive poly(acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf. B Biointerfaces 2017, 154, 287–296. [Google Scholar] [CrossRef]
- Popova, M.; Trendafilova, I.; Zgureva, D.; Kalvachev, Y.; Boycheva, S.; Novak Tušar, N. Polymer-coated mesoporous silica nanoparticles for controlled release of the prodrug sulfasalazine. J. Drug Deliv. Sci. Technol. 2018, 44, 415–420. [Google Scholar] [CrossRef]
- Qu, Z.; Wong, K.Y.; Moniruzzaman, M.; Begun, J.; Santos, H.A.; Hasnain, S.Z. One-Pot Synthesis of pH-Responsive Eudragit-Mesoporous Silica Nanocomposites Enable Colonic Delivery of Glucocorticoids for the Treatment of Inflammatory Bowel Disease. Adv. Ther. 2021, 4, 2000165. [Google Scholar] [CrossRef]
- Borderwala, K.; Rathod, S.; Yadav, S.; Vyas, B.; Shah, P. Eudragit S-100 Surface Engineered Nanostructured Lipid Carriers for Colon Targeting of 5-Fluorouracil: Optimization and In Vitro and In Vivo Characterization. AAPS PharmSciTech 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, K.; Ka, S.; Wielgus, E.; Potrzebowski, M.J. Microporous and Mesoporous Materials DiSupLo-New extremely easy and efficient method for loading of active pharmaceutical ingredients into the pores of MCM-41 mesoporous silica particles. Microporous Mesoporous Mater. 2020, 308, 110506. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-ouda, A.; Bak-sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Eita, A.S.; Makky, A.M.A.; Anter, A.; Khalil, I.A. Repurposing of atorvastatin emulsomes as a topical antifungal agent. Drug Deliv. 2022, 29, 3414–3431. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Shadab, M. Repurposing itraconazole loaded PLGA nanoparticles for improved antitumor efficacy in non-small cell lung cancers. Pharmaceutics 2019, 11, 685. [Google Scholar] [CrossRef]
- Eita, A.S.; Makky, A.M.A.; Anter, A.; Khalil, I.A. Atorvastatin-loaded emulsomes foam as a topical antifungal formulation. Int. J. Pharm. X 2022, 4, 100140. [Google Scholar] [CrossRef]
- Uzun, C.; Gü ven, O. Controlled Release of Terbinafine Hydrochloride from pH Sensitive Poly(Acrylamide/Maleic Acid) Hydrogels. 2000, p. 203. Available online: www.elsevier.com/locate/ijpharm (accessed on 25 August 2023).
- Huang, C.S.; Ho, W.L.; Lee, W.S.; Sheu, M.T.; Wang, Y.J.; Tu, S.H. SP1-regulated p27/Kip1 gene expression is involved in terbinafine-induced human A431 cancer cell differentiation: An in vitro and in vivo study. Biochem. Pharmacol. 2008, 75, 1783–1796. [Google Scholar] [CrossRef]
- Aldeeb, R.A.E.; Mahdy, M.A.E.G.; El-Nahas, H.M.; Musallam, A.A. Design of mirtazapine solid dispersion with different carriers’ systems: Optimization, in vitro evaluation, and bioavailability assessment. Drug Deliv. Transl. Res. 2023, 13, 2340–2352. [Google Scholar] [CrossRef]
- Attia, M.S.; Radwan, M.F.; Ibrahim, T.S.; Ibrahim, T.M. Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance. Pharmaceutics 2023, 15, 1425. [Google Scholar] [CrossRef]
- Trendafilova, I.; Szegedi, Á.; Yoncheva, K.; Shestakova, P.; Mihály, J.; Ristić, A.; Konstantinov, S.; Popova, M. A pH dependent delivery of mesalazine from polymer coated and drug-loaded SBA-16 systems. Eur. J. Pharm. Sci. 2016, 81, 75–81. [Google Scholar] [CrossRef]
- Tang, H.; Guo, J.; Sun, Y.; Chang, B.; Ren, Q.; Yang, W. Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int. J. Pharm. 2011, 421, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Sabry, S.A.; Abd El Razek, A.M.; Nabil, M.; Khedr, S.M.; El-Nahas, H.M.; Eissa, N.G. Brain-targeted delivery of Valsartan using solid lipid nanoparticles labeled with Rhodamine B; a promising technique for mitigating the negative effects of stroke. Drug Deliv. 2023, 30, 2179127. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Eissa, R.G.; El-Megrab, N.A.; El-Nahas, H.M. Morphological characterization of optimized risperidone-loaded in-situ gel forming implants with pharmacokinetic and behavioral assessments in rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102195. [Google Scholar] [CrossRef]
- Sedki, M.; Khalil, I.A.; El-Sherbiny, I.M. Hybrid nanocarrier system for guiding and augmenting simvastatin cytotoxic activity against prostate cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S641–S650. [Google Scholar] [CrossRef] [PubMed]
- Sedeky, A.S.; Khalil, I.A.; Hefnawy, A.; El-Sherbiny, I.M. Development of core-shell nanocarrier system for augmenting piperine cytotoxic activity against human brain cancer cell line. Eur. J. Pharm. Sci. 2018, 118, 103–112. [Google Scholar] [CrossRef]
- Lehto, V.; Riikonen, J. Drug Loading and Characterization of Porous Silicon Materials; Woodhead Publishing Limited: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- Palanikumar, L.; Choi, E.S.; Cheon, J.Y.; Joo, S.H.; Ryu, J.H. Noncovalent polymer-gatekeeper in mesoporous silica nanoparticles as a targeted drug delivery platform. Adv. Funct. Mater. 2015, 25, 957–965. [Google Scholar] [CrossRef]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater. Nanotechnol. 2014, 4, 1–15. [Google Scholar] [CrossRef]
- Biswas, N. Modified mesoporous silica nanoparticles for enhancing oral bioavailability and antihypertensive activity of poorly water soluble valsartan. Eur. J. Pharm. Sci. 2017, 99, 152–160. [Google Scholar] [CrossRef]
- Mccarthy, C.A.; Ahern, R.J.; Dontireddy, R.; Ryan, K.B.; Crean, A.M. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opin. Drug Deliv. 2016, 13, 93–108. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Nanostructured mesoporous silica matrices in nanomedicine. J. Intern. Med. 2010, 267, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.M.; Shi, M. PLGA/mesoporous silica hybrid structure for controlled drug release. J. Control. Release 2004, 98, 209–217. [Google Scholar] [CrossRef]
- Badhana, S.; Garud, N.; Garud, A. Colon Specific Drug Delivery of Mesalamine Using Eudragit S100-Coated Chitosan Microspheres for the Treatment of Ulcerative Colitis. 2013, p. 2. Available online: http://www.icpjonline.com/documents/Vol2Issue3/01.pdf (accessed on 3 August 2023).
- Pande, V.V.; Jadhav, K.S.; Giri, M.A.; Kendre, P.N.; Vibhute, S.K.; Borawake, D.D. Design and development of paliperidone mesoporous silica template as a platform for surge dose drug delivery system. Mater. Technol. 2019, 34, 117–125. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Thakral, N.K.; Ray, A.R.; Majumdar, D.K. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Güven, U.M.; Öztürk, A.A.; Yenilmez, E. Evaluation of carvedilol-loaded eudragit® nanoparticles. J. Res. Pharm. 2020, 24, 71–81. [Google Scholar] [CrossRef]
- Soltani, S.; Zakeri-Milani, P.; Barzegar-Jalali, M.; Jelvehgari, M.; Design, J.M. Design of Eudragit RL Nanoparticles by Nanoemulsion Method as Carriers for Ophthalmic Drug Delivery of Ketotifen Fumarate. Iran J Basic Med Sci. 2016, 19, 550–560. [Google Scholar]
- Elgendy, H.A.; Makky, A.M.A.; Elakkad, Y.E.; Ismail, R.M.; Younes, N.F. Syringeable atorvastatin loaded eugenol enriched PEGylated cubosomes in-situ gel for the intra-pocket treatment of periodontitis: Statistical optimization and clinical assessment. Drug Deliv. 2023, 30, 2162159. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Wathoni, N.; Nguyen, A.N.; Rusdin, A.; Umar, A.K.; Mohammed, A.F.A.; Motoyama, K. Enteric-coated strategies in colorectal cancer nanoparticle drug delivery system. In Drug Design, Development and Therapy; Dove Medical Press Ltd.: Macclesfield, UK, 2020; pp. 4387–4405. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Żeglinski, J.; Szewczyk, A.; Skwira, A.; Walker, G. Surface-Activated Fibre-Like SBA-15 as Drug Carriers for Bone Diseases. AAPS PharmSciTech 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Malkawi, A.; Jalil, A.; Nazir, I.; Matuszczak, B.; Kennedy, R. Self-emulsifying drug delivery systems: Hydrophobic drug polymer complexes provide a sustained release in vitro. Mol. Pharm. 2020, 17, 3709–3719. [Google Scholar] [CrossRef]
- Abd-Elbary, A.; El Nabarawi, M.A.; Hassen, D.H.; Taha, A.A. Inclusion and characterization of ketoprofen into different mesoporous silica nanoparticles using three loading methods. Int. J. Pharm. Pharm. Sci. 2014, 6, 183–191. [Google Scholar]
- Hassan, S.u.; Khalid, I.; Hussain, L.; Barkat, K.; Khan, I.U. Development and Evaluation of pH-Responsive Pluronic F 127 Co-Poly-(Acrylic Acid) Biodegradable Nanogels for Topical Delivery of Terbinafine HCL. Dose-Response 2022, 20. [Google Scholar] [CrossRef]
- Kokunešoski, M.; Gulicovski, J.; Matović, B.; Logar, M.; Milonjić, S.K.; Babić, B. Synthesis and surface characterization of ordered mesoporous silica SBA-15. Mater. Chem. Phys. 2010, 124, 1248–1252. [Google Scholar] [CrossRef]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khan, R.U.; Iqbal, M.S.; Khan, H.A. In-vitro and in-vivo evaluation of velpatasvir-loaded mesoporous silica scaffolds. A prospective carrier for drug bioavailability enhancement. Pharmaceutics 2020, 12, 307. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M. Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iran. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar] [CrossRef]
- Lai, J.; Lin, W.; Scholes, P.; Li, M. Investigating the effects of loading factors on the in vitro pharmaceutical performance of mesoporous materials as drug carriers for Ibuprofen. Materials 2017, 10, 150. [Google Scholar] [CrossRef]
- Budiman, A. Characterization of drugs encapsulated into mesoporous silica. Int. J. Appl. Pharm. 2019, 11, 7–11. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Zhu, Y.; Li, X.; Liu, X.; Pang, J. Galactosylated chitosan-functionalized mesoporous silica nanoparticle loading by calcium leucovorin for colon cancer cell-targeted drug delivery. Molecules 2018, 23, 3082. [Google Scholar] [CrossRef]
- Iranshahy, M.; Hanafi-Bojd, M.Y.; Aghili, S.H.; Iranshahi, M.; Nabavi, S.M.; Saberi, S. Curcumin-loaded mesoporous silica nanoparticles for drug delivery: Synthesis, biological assays and therapeutic potential—A review. RSC Adv. 2023, 13, 22250–22267. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Wu, C.C.; Wu, C.H.; Chen, J.H.; Chu, C.H.; Chen, C.H. Involvement of proapoptotic Bcl-2 family members in terbinafine-induced mitochondrial dysfunction and apoptosis in HL60 cells. Food Chem. Toxicol. 2006, 44, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shen, J.; Li, Z.; Zhang, D.; Zhang, Q.; Liu, G. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int. J. Pharm. 2013, 445, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Pan, C.; Hua, Y.; Peng, J.; Zhou, Z. Squalene epoxidase promotes colorectal cancer cell proliferation through accumulating calcitriol and activating CYP24A1-mediated MAPK signaling. Cancer Commun. 2021, 41, 726–746. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Liu, D.; Wong, C.C.; Coker, O.O.; Zhang, X. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 2022, 71, 2253–2265. [Google Scholar] [CrossRef]




| Factors | Actual Levels (Coded) | ||
|---|---|---|---|
| Low Limit (−1) | Central Limit (0) | High Limit (+1) | |
| A: Drug/MSN (%) | 25 | 33.33 | 50 |
| B: Coat concentration (%) | 0 | 5 | 10 |
| C: MSN type | MCM-41 | SBA-15 | |
| Responses | Goals | ||
| Y1: EE (%) | Maximize | ||
| Y2: Q2 in pH 1.2 (%) | Minimize | ||
| Y3: Q24 in pH 7.4 (%) | Maximize | ||
| F | A: Drug/MSN (%) | B: Coat Concentration (%) | C: MSN Type | Y1: EE (%) | Y2: Q2 in pH 1.2 (%) | Y3: Q24 in pH 7.4 (%) |
|---|---|---|---|---|---|---|
| 1 | 50 | 0 | SBA-15 | 99.35 ± 3.16 | 98.65 ± 1.85 | 81.06 ± 2.96 |
| 2 | 33.33 | 0 | SBA-15 | 98.34 ± 2.87 | 62.44 ± 2.21 | 57.80 ± 2.06 |
| 3 | 50 | 0 | MCM-41 | 96.73 ± 0.88 | 44.87 ± 1.12 | 54.33 ± 2.21 |
| 4 | 25 | 0 | MCM-41 | 95.28 ± 3.22 | 38.82 ± 2.21 | 46.19 ± 1.98 |
| 5 | 50 | 5 | MCM-41 | 94.84 ± 1.67 | 30.27 ± 1.12 | 59.74 ± 2.37 |
| 6 | 33.33 | 5 | MCM-41 | 93.97 ± 2.64 | 28.52 ± 2.21 | 56.45 ± 3.21 |
| 7 | 25 | 10 | MCM-41 | 91.94 ± 4.65 | 12.59 ± 2.21 | 57.95 ± 2.06 |
| 8 | 50 | 5 | SBA-15 | 95.81 ± 3.55 | 42.04 ± 3.11 | 65.99 ± 3.21 |
| 9 | 25 | 5 | SBA-15 | 92.26 ± 2.33 | 15.68 ± 1.12 | 66.93 ± 3.06 |
| 10 | 50 | 10 | SBA-15 | 96.49 ± 3.15 | 16.15 ± 2.21 | 78.09 ± 4.06 |
| 11 | 33.33 | 10 | SBA-15 | 93.28 ± 1.26 | 19.79 ± 1.85 | 63.27 ± 3.06 |
| 12 | 50 | 10 | MCM-41 | 95.24 ± 2.76 | 21.08 ± 2.21 | 66.16 ± 4.16 |
| Responses | Model | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | p-Value | F-Ratio | Significance |
|---|---|---|---|---|---|---|---|---|
| Y1: EE (%) | Quadratic | 0.99 | 0.98 | 0.94 | 37.76 | 0.0001 | 131.02 | significant |
| Y2: Q2 in pH 1.2 (%) | Quadratic | 0.94 | 0.89 | 0.54 | 14.5 | 0.0002 | 18.01 | significant |
| Y3: Q24 in pH 7.4 (%) | Quadratic | 0.85 | 0.71 | −0.12 | 7.73 | 0.0095 | 6.13 | significant |
| Factors | Optimized Level | ||
|---|---|---|---|
| A: Drug/MSN (%) | 50% | ||
| B: Coat concentration (%) | 10% | ||
| C: Type of MSN | SBA-15 | ||
| Responses | Observed | Predicted | Prediction error (%) |
| Y1: EE (%) | 96.49 | 96.05 | −0.004 |
| Y2: Q2 in pH 1.2 (%) | 16.15 | 22.100 | 0.269 |
| Y3: Q24 in pH 7.4 (%) | 78.09 | 74.64 | −0.046 |
| Formulas | Zero Order Model | First Order Model | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | |
| Plain TER | −0.0140 | 0.9605 | 0.7967 | 0.9320 | 0.9075 | 0.300 |
| Uncoated F1 | 0.8594 | 0.9695 | 0.9665 | 0.9896 | 0.9687 | 0.554 |
| Optimal coated F10 | 0.8931 | 0.9142 | 0.8369 | 0.9391 | 0.9315 | 0.791 |
| Material | PS (nm) | PDI | ZP (mV) |
|---|---|---|---|
| Plain SBA-15 | 126.6 ± 3.40 | 0.476 ± 0.09 | −23.7 ± 3.42 |
| Uncoated F1 | 160.3 ± 4.43 | 0.420 ± 0.074 | −20.2 ± 4.62 |
| Optimal F10 | 176.1 ± 6.09 | 0.422 ± 0.06 | −15.8 ± 2.72 |
| Material | Specific Surface Area (m2/g) | Pore Volume (cc/g) |
|---|---|---|
| Plain SBA-15 | 621.13 | 1.42 |
| Uncoated formula (F1) | 156.58 | 0.42 |
| Optimal formula (F10) | 39.07 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyami, M.H.; Musallam, A.A.; Ibrahim, T.M.; Mahdy, M.A.; Elnahas, H.M.; Aldeeb, R.A. The Exploitation of pH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment. Pharmaceutics 2023, 15, 2677. https://doi.org/10.3390/pharmaceutics15122677
Alyami MH, Musallam AA, Ibrahim TM, Mahdy MA, Elnahas HM, Aldeeb RA. The Exploitation of pH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment. Pharmaceutics. 2023; 15(12):2677. https://doi.org/10.3390/pharmaceutics15122677
Chicago/Turabian StyleAlyami, Mohammad H., Abeer A. Musallam, Tarek M. Ibrahim, Mahmoud A. Mahdy, Hanan M. Elnahas, and Reem A. Aldeeb. 2023. "The Exploitation of pH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment" Pharmaceutics 15, no. 12: 2677. https://doi.org/10.3390/pharmaceutics15122677
APA StyleAlyami, M. H., Musallam, A. A., Ibrahim, T. M., Mahdy, M. A., Elnahas, H. M., & Aldeeb, R. A. (2023). The Exploitation of pH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment. Pharmaceutics, 15(12), 2677. https://doi.org/10.3390/pharmaceutics15122677










