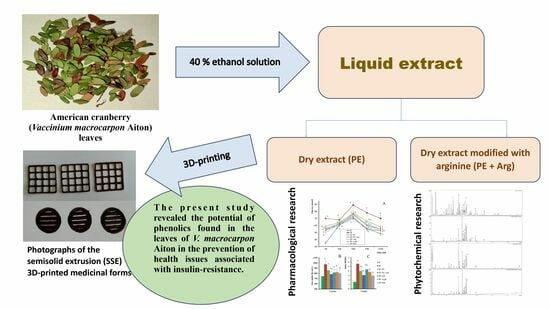
Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium macrocarpon Aiton, Ericaceae) Leaf Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and General Experiments
2.2. Plant Material
2.3. Preparation of Extracts
2.4. HPLC-DAD-MS Analysis of Extracts
2.5. Assay of Main Phytochemicals
2.6. The Pharmacological Activity of Extracts
2.7. Preparation of Gels Loaded with Cranberry Extracts for 3D Printing
2.8. Three-Dimensional Printing of Cranberry Extracts
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Analyses of Cranberry Leaf Extracts
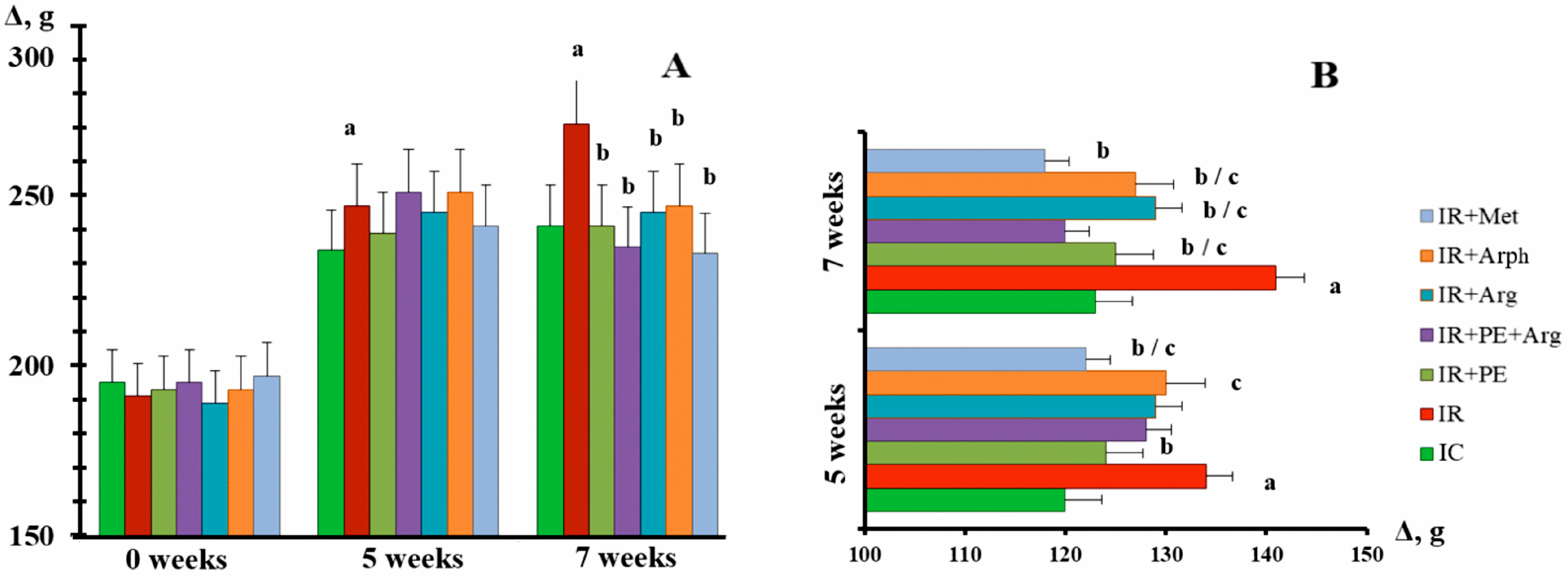
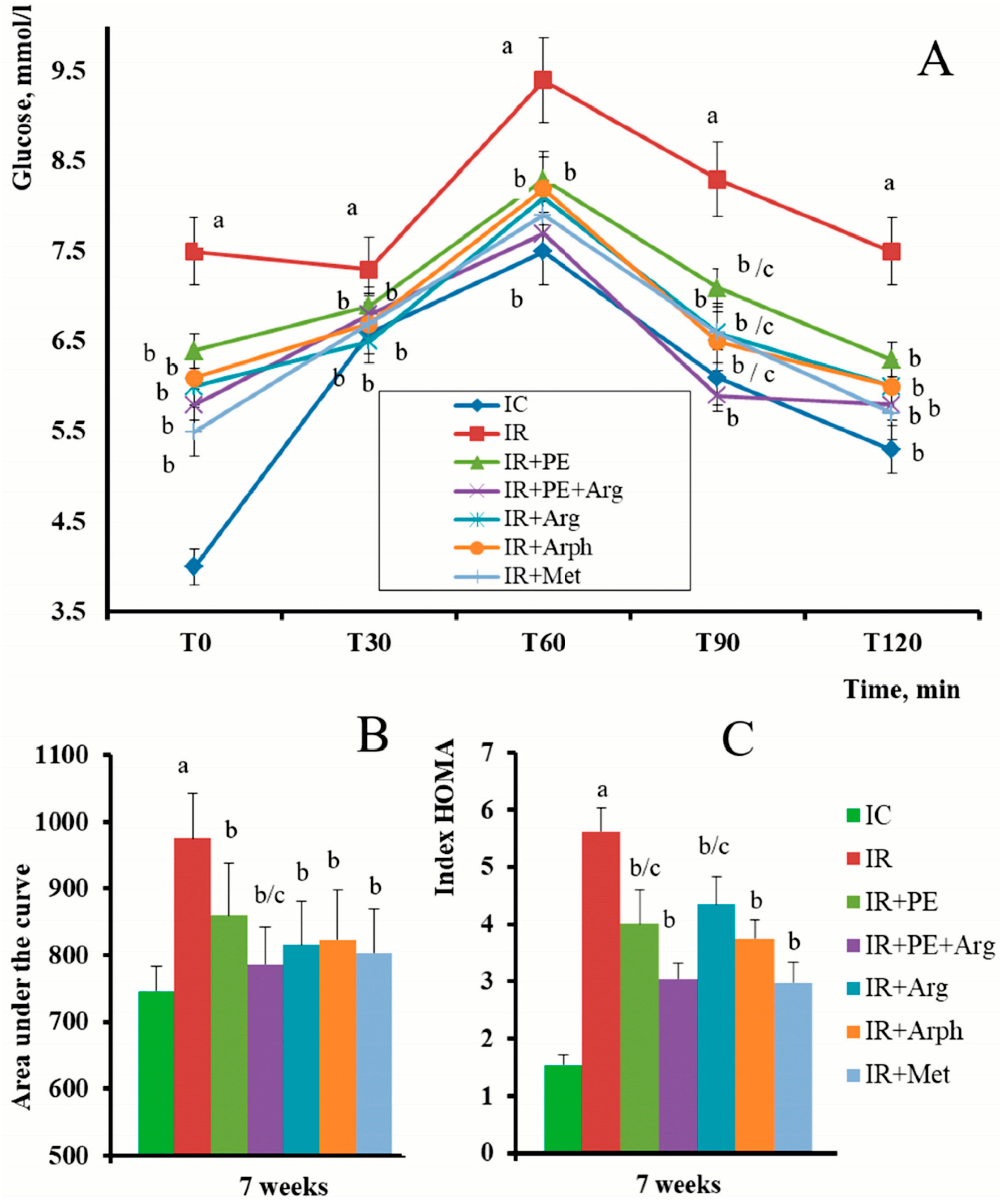
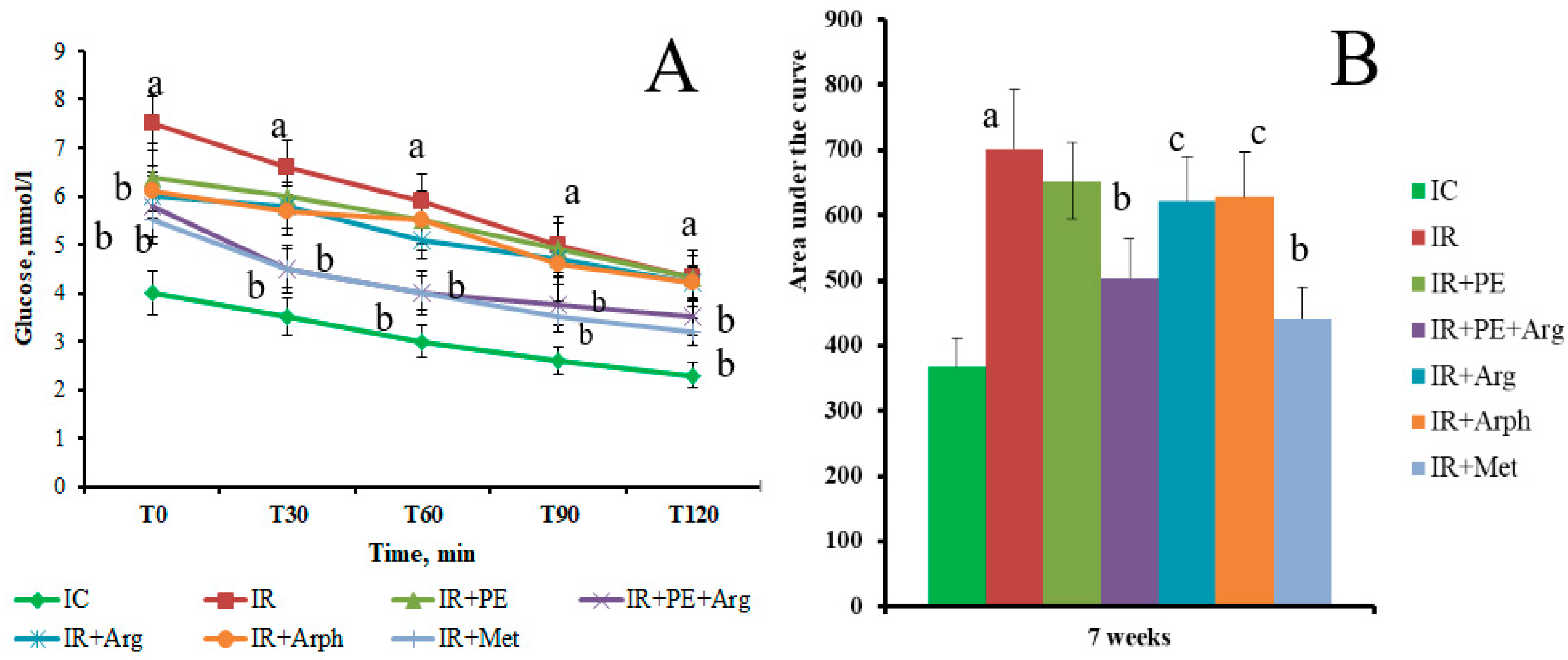
3.2. The Pharmacological Activity of Extracts
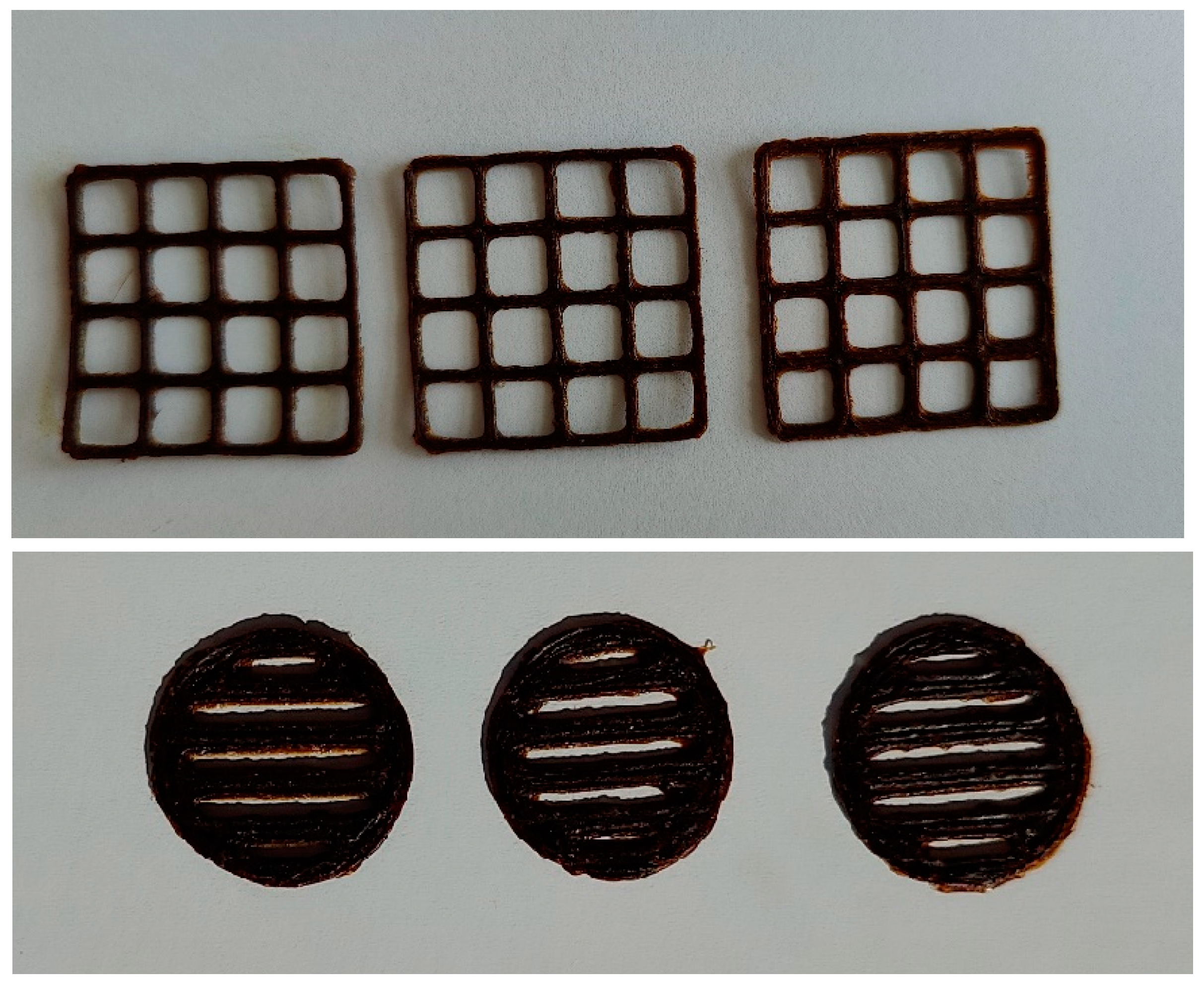
3.3. Formulation of the Gels and 3D-Printed Dosage Forms of Cranberry Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Rathmann, W.; Charbonnel, B.; Khunti, K.; Kosiborod, M.; Nicolucci, A.; Pocock, S.J.; Shestakova, M.V.; Shimomura, I.; Tang, F.; et al. Treatment of type 2 diabetes mellitus worldwide: Baseline patient characteristics in the global DISCOVER study. Diabetes Res. Clin. Pract. 2019, 151, 20–32. [Google Scholar] [CrossRef]
- Kovalenko, V.N. Compendium 2020—Medicines; MORION: Kyiv, Ukraine, 2020. [Google Scholar]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Koshovyi, O.; Vlasova, I.; Jakštas, V.; Vilkickyt, E.G.; Žvikas, V.; Hrytsyk, R.; Grytsyk, L.; Raal, A. American cranberry (Oxycoccus macrocarpus (Ait.) Pursh) leaves extract and its amino-acids preparation: The phytochemical and pharmacological study. Plants 2023, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Kõiva, M.; Kuperjanov, A.; Vilbaste, K.; Vlasova, I.; Koshovyi, O. Multi-use of cranberries (Vaccinium spp.): Heritage and pharmaceutical results. Folklore 2023, 89, 107–142. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Y.; Komissarenko, A.N. Phytochemical study of the dry extract from bilberry leaves. Azerbaijan Pharm. Pharmacother. J. 2016, 16, 18–23. [Google Scholar]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of anti-obesity and lipid-lowering properties of Vaccinium myrtillus leaves powder extract in a hamster model. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 697–703. [Google Scholar] [CrossRef]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush blueberry (Vaccinium corymbosum L.) leaves extract and its modified arginine preparation for the management of metabolic syndrome—Chemical analysis and bioactivity in rat model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Chaika, N.; Koshovyi, O.; Raal, A.; Kireyev, I.; Zupanets, A.; Odyntsova, V. Phytochemical profile and pharmacological activity of the dry extract from Arctostaphylos uva-ursi leaves modified with phenylalanine. Sci. Pharm. Sci. 2020, 6, 74–78. [Google Scholar] [CrossRef]
- Chaika, N.; Mazen, M.; Koshovyi, O.; Kravchenko, G.; Goryacha, O.; Kireyev, I.; Kovalenko, S.; Darmograi, R. Research in phytochemical composition and hypoglycemic activity screening of the dry extracts from bearberry leaves. Sci. Pharm. Sci. 2021, 3, 42–50. [Google Scholar] [CrossRef]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine; Naukova dumka: Kyiv, Ukraine, 1999. [Google Scholar]
- State Pharmacopoeia of Ukraine; SO «Ukrainian Scientific Pharmacopoeial Center of Drugs Quality»: Kharkiv, Ukraine, 2015.
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) pursh and Oxycoccus palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 3, 48–57. [Google Scholar] [CrossRef]
- Raal, A.; Jaama, M.; Utt, M.; Püssa, T.; Žvikas, V.; Jakštas, V.; Koshovyi, O.; Nguyen, K.V.; Nguyen, H.T. The phytochemical profile and anticancer activity of Anthemis tinctoria and Angelica sylvestris used in Estonian ethnomedicine. Plants 2022, 11, 994. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Vovk, G.V.; Akhmedov, E.Y.; Komissarenko, A.N. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharm. Pharmacother. J. 2015, 15, 30–34. [Google Scholar]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules. 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and pharmacological research in Agrimonia eupatoria l. herb extract with anti-inflammatory and hepatoprotective properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Ilina, T.; Skowronska, W.; Kashpur, N.; Granica, S.; Bazylko, A.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Immunomodulatory activity and phytochemical profile of infusions from cleavers herb. Molecules 2020, 25, 3721. [Google Scholar] [CrossRef]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Toryanyk, E.L.; Kryvoruchko, O.V. The immunomodulatory activity of the extracts and complexes of biologically active compounds of Galium verum L. herb. Ceska A Slov. Farm. 2018, 67, 25–29. [Google Scholar]
- Starchenko, G.; Hrytsyk, A.; Raal, A.; Koshovyi, O. Phytochemical profile and pharmacological activities of water and hydroethanolic dry extracts of Calluna vulgaris (L.) Hull. herb. Plants 2020, 9, 751. [Google Scholar] [CrossRef]
- Stefanov, O.V. Preclinical Studies of Medicinal Products; Avitsena: Kyiv, Ukraine, 2002. [Google Scholar]
- De Olivera, D.T.; Soursa-Silva, E.; Scand, T. Gingival vein punction: A new simple technique for drug administration or blood sampling in rats and mice. Scand. J. Lab. Anim. Sci. 2009, 36, 109–113. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Takeda, K.; Maeda, M.; Ogawa, W.; Sato, T.; Okada, S.; Ohnishi, Y.; Nakajima, H.; Kashiwagi, A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2015, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Miller, G.L. Protein determination for large numbers of samples. Anal. Chem. 1959, 31, 964–966. [Google Scholar] [CrossRef]
- Viidik, L.; Sera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-printability of aqueous poly(ethylene oxide) gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-printing of nanoemulsified eucalypt xtracts and their antimicrobial activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, S.N.; Grytsyk, A.; Raal, A. Semi-solid extrusion 3D-printing of eucalypt extract-loaded polyethylene oxide gels intended for pharmaceutical applications. Ann. 3D Print. Med. 2023, 12, 100123. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y. Van (2001–2021). FreeCAD (Version 0.19.24291). Available online: http://www.freecad.org (accessed on 12 October 2023).
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019.
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Medica 2012, 78, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Dávalos, A.; Latasa, M.-J.; Sala-Vila, A.; Sanchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Sheedy, K. Effects of polyphenols on insulin resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef] [PubMed]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. Biotecholgy 2019, 9, 4. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Kartha, C.C. Quercetin improves oxidative stress-induced pancreatic beta cell alterations via mTOR-signaling. Mol. Cell. Biochem. 2021, 476, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of polyphenols in the management of dyslipidemia: A focus on clinical studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Stull, A.J. Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants 2016, 5, 44. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Sreeja, S.; Geetha, R.; Radika, M.K.; Anuradha, C.V. Grape seed proanthocyanidin rescues rats from steatosis: A comparative and combination study with metformin. J. Lipids 2013, 2013, 153897. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural polyphenols in metabolic syndrome: Protective mechanisms and clinical applications. Int. J. Mol. Sci. 2021, 22, 6110. [Google Scholar] [CrossRef]
- Meshkani, R.; Adeli, K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 2009, 42, 1331–1346. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual intake of dietary L-arginine in relation to risk of type 2 diabetes: A prospective study. BMC Endocr. Disord. 2021, 21, 113. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kravchenko, G.B.; Fylymonenko, V.P.; Krasilnikova, O.A. Effect of apple polyphenol concentrate on lipid metabolism in rats under experimental insulin resistance. Wiad. Lek. 2017, 70, 200–204. [Google Scholar] [PubMed]
- Hur, J.H.; Park, S.Y.; Dall’Armi, C.; Lee, J.S.; Di Paolo, G.; Lee, H.Y.; Yoon, M.S.; Min, D.S.; Choi, C.S. Phospholipase D1 deficiency in mice causes nonalcoholic fatty liver disease via an autophagy defect. Sci. Rep. 2016, 6, 39170. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Devlin, C.; Tabas, I.; Vance, D.E. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004, 279, 47402–47410. [Google Scholar] [CrossRef] [PubMed]

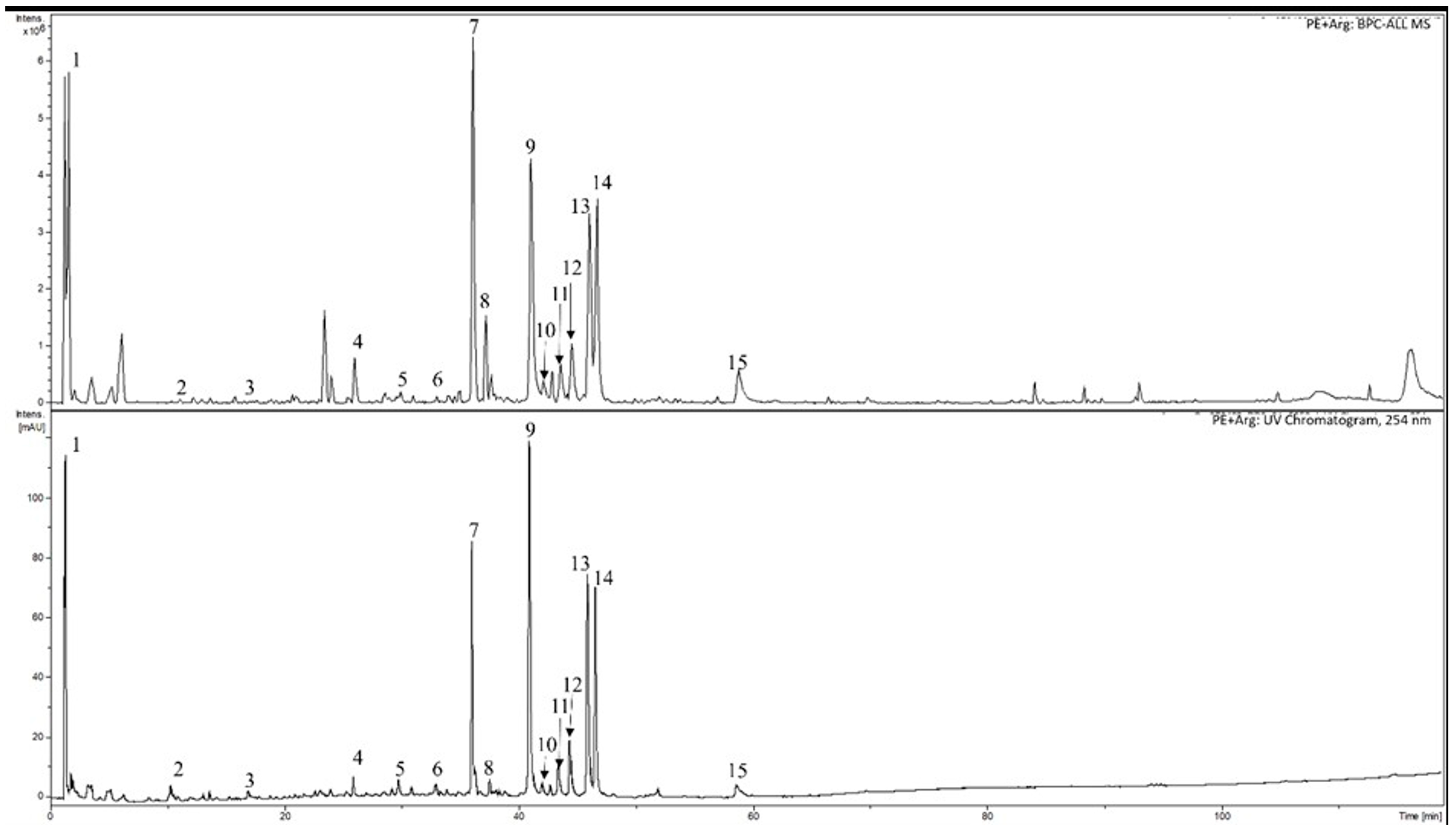
| Substances | Retention Time [min] | PE | PE + Arg |
|---|---|---|---|
| Quinic acid | 1.5 | + | + |
| ND | 10.4 | + | |
| 3-O-Caffeoylquinic acid (chlorogenic acid) | 16.8 | + | + |
| ND | 26.0 | + | + |
| (+)-Catechin | 29.0 | + | |
| p-Coumaroylquinic acid | 29.9 | + | |
| Proanthocyanidin A type | 32.9 | + | |
| ND | 33.0 | + | |
| ND | 36.0 | + | + |
| ND | 37.6 | + | + |
| Quercetin 3-O-galactoside | 40.7 | + | + |
| Quercetin-3-O-glucoside | 41.8 | + | + |
| Procyanidin dimer A2 type | 42.7 | + | |
| Quercetin-3-O-xyloside | 43.1 | + | + |
| Quercetin-3-O-arabino pyranoside | 44.2 | + | + |
| Quercetin-3-O-arabino furanoside | 45.7 | + | + |
| Quercetin 3-O-rhamnoside | 46.4 | + | + |
| Quercetin-O-p-coumaroyl hexoside-1 | 50.6 | + | |
| Kaempferol 3-O-rhamnoside | 51.9 | + | |
| Quercetin-O-p-coumaroyl hexoside-2 | 58.2 | + | + |
| Quercetin derivative | 69.6 | + |
| BAS Group | Spectrophotometric Method | Assay, % | |
|---|---|---|---|
| PE | PE + Arg | ||
| Hydroxycinnamic acids | In terms of chlorogenic acid (λ = 327 nm) | 11.54 ± 0.11 | 7.10 ± 0.07 |
| In terms of chlorogenic acid (λ = 525 nm); chromogenic reagent: sodium nitrite and sodium molybdate | 13.59 ± 0.63 | 8.10 ± 0.37 | |
| Flavonoids | In terms of rutin (λ = 417 nm) | 4.01 ± 0.26 | 2.53 ± 0.14 |
| In terms of hyperoside (λ = 425 nm) | 4.94 ± 0.46 | 3.19 ± 0.45 | |
| Total polyphenols | In terms of gallic acid (λ = 270 nm) | 17.16 ± 0.29 | 4.94 ± 0.30 |
| Chromogenic reagent: Folin & Ciocalteu′s Phenol Reagent (λ = 765 nm) | 19.18 ± 0.43 | 7.59 ± 0.56 | |
| Amino acids | In terms of leucine (λ = 573 nm); chromogenic reagent: ninhydrin solution | 0.88 ± 0.09 | 5.60 ± 0.38 |
| Indices | Experimental Groups | ||||||
|---|---|---|---|---|---|---|---|
| IC | IR | IR + PE | IR + PE + Arg | IR + Arg | IR + Arph | IR + Met | |
| Blood serum | |||||||
| TG, mmol/L | 1.45 ± 0.19 | 2.35 ± 0.24 a | 1.69 ± 0.15 b | 1.58 ± 0.18 b | 2.15 ± 0.21 c | 1.75 ± 0.47 | 1.63 ± 0.35 b |
| TCh, mmol/L | 3.21 ± 0.19 | 6.99 ± 0.24 a | 4.09 ± 0.37 b | 3.57 ± 0.54 b | 5.44 ± 0.67 c | 4.95 ± 1.63 b | 3.38 ± 0.94 b |
| Ch-LDL, µmol/mg protein | 2.33 ± 0.45 | 4.67 ± 0.87 a | 3.09 ± 0.68 b | 2.12 ± 0.45 b | 4.02 ± 0.63 | 3.82 ± 0.74 b | 2.57 ± 0.85 b |
| Ch-HDL, µmol/mg protein | 0.99 ± 0.08 | 0.54 ± 0.11 a | 0.96 ± 0.10 b | 1.12 ± 0.13 b | 1.06 ± 0.19 b | 1.03 ± 0.12 b | 0.99 ± 0.08 b |
| Liver homogenate | |||||||
| PL, nmol/mg protein | 115.7 ± 11.3 | 82.9 ± 7.3 a | 93.6 ± 8.7 | 108.5 ± 9.4 b | 90.8 ± 8.5 | 90.4 ± 10.7 | 105.9 ± 8.4 b |
| DG, nmol/mg protein | 14.23 ± 1.56 | 19.36 ± 1.75 a | 18.38 ± 2.11 | 16.54 ± 1.43 b | 17.28 ± 1.33 | 18.41 ± 0.96 | 15.81 ± 1.96 b |
| Ch, nmol/mg protein | 10.26 ± 0.96 | 34.28 ± 4.59 a | 24.31 ± 1.94 | 15.94 ± 2.83 | 29.52 ± 4.59 c | 27.05 ± 3.81 c | 19.53 ± 1.79 b |
| TG, nmol/mg protein | 57.34 ± 4.42 | 68.52 ± 5.17 a | 62.71 ± 4.25 | 58.47 ± 3.29 b | 67.41 ± 4.83 c | 5.91 ± 6.18 | 55.68 ± 3.93 b |
| FFA, nmol/mg protein | 22.83 ± 1.45 | 35.33 ± 2.04 a | 29.45 ± 1.47 | 25.33 ± 0.94 b | 30.05 ± 1.23 c | 27.42 ± 1.11 b | 24.97 ± 1.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshovyi, O.; Vlasova, I.; Laur, H.; Kravchenko, G.; Krasilnikova, O.; Granica, S.; Piwowarski, J.P.; Heinämäki, J.; Raal, A. Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium macrocarpon Aiton, Ericaceae) Leaf Extracts. Pharmaceutics 2023, 15, 2528. https://doi.org/10.3390/pharmaceutics15112528
Koshovyi O, Vlasova I, Laur H, Kravchenko G, Krasilnikova O, Granica S, Piwowarski JP, Heinämäki J, Raal A. Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium macrocarpon Aiton, Ericaceae) Leaf Extracts. Pharmaceutics. 2023; 15(11):2528. https://doi.org/10.3390/pharmaceutics15112528
Chicago/Turabian StyleKoshovyi, Oleh, Inna Vlasova, Heleriin Laur, Ganna Kravchenko, Oksana Krasilnikova, Sebastian Granica, Jakub P. Piwowarski, Jyrki Heinämäki, and Ain Raal. 2023. "Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium macrocarpon Aiton, Ericaceae) Leaf Extracts" Pharmaceutics 15, no. 11: 2528. https://doi.org/10.3390/pharmaceutics15112528
APA StyleKoshovyi, O., Vlasova, I., Laur, H., Kravchenko, G., Krasilnikova, O., Granica, S., Piwowarski, J. P., Heinämäki, J., & Raal, A. (2023). Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium macrocarpon Aiton, Ericaceae) Leaf Extracts. Pharmaceutics, 15(11), 2528. https://doi.org/10.3390/pharmaceutics15112528