The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Sample Size
2.3. Sample Preparation
2.3.1. Teeth Sample Preparation
2.3.2. Composite Sample Preparation
2.4. Medication Administration and Brushing Technique
2.5. Assessments
2.5.1. Microhardness
Teeth Samples
Restorative Composite Samples
2.5.2. Surface Roughness
2.5.3. Color
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Salbutamol and Budesonide significantly decreased the microhardness of enamel and flowable composite samples after 10 days of administration and brushing.
- After 10 days of Salbutamol or Budesonide administration and brushing, flowable composite showed lower microhardness and lower surface roughness.
- Packable composite showed more resistance to microhardness change.
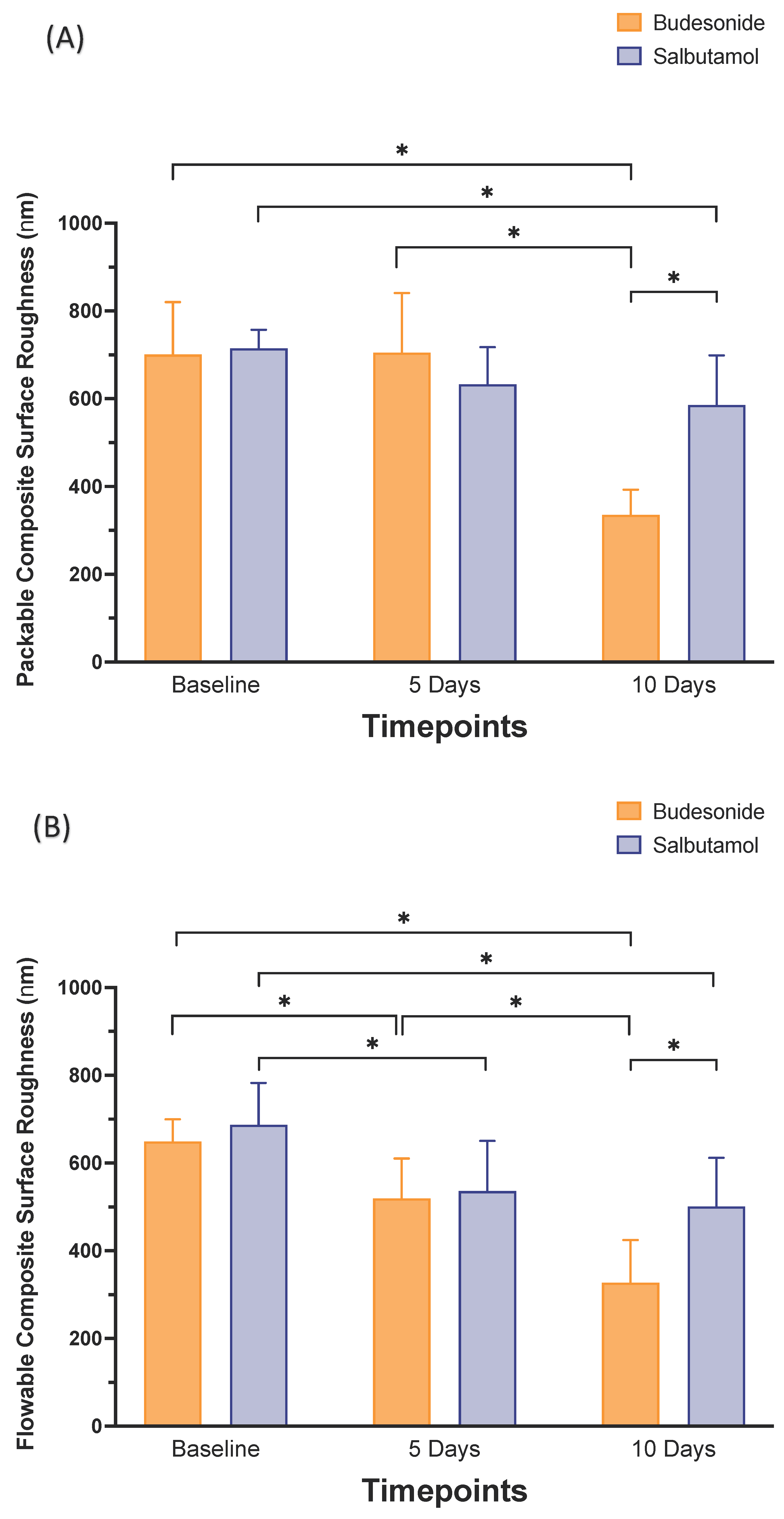
- Longer application of any of the medications resulted in lowering the surface roughness of both flowable and packable composites. Budesonide showed more reduction in surface roughness than Salbutamol after 10 days.
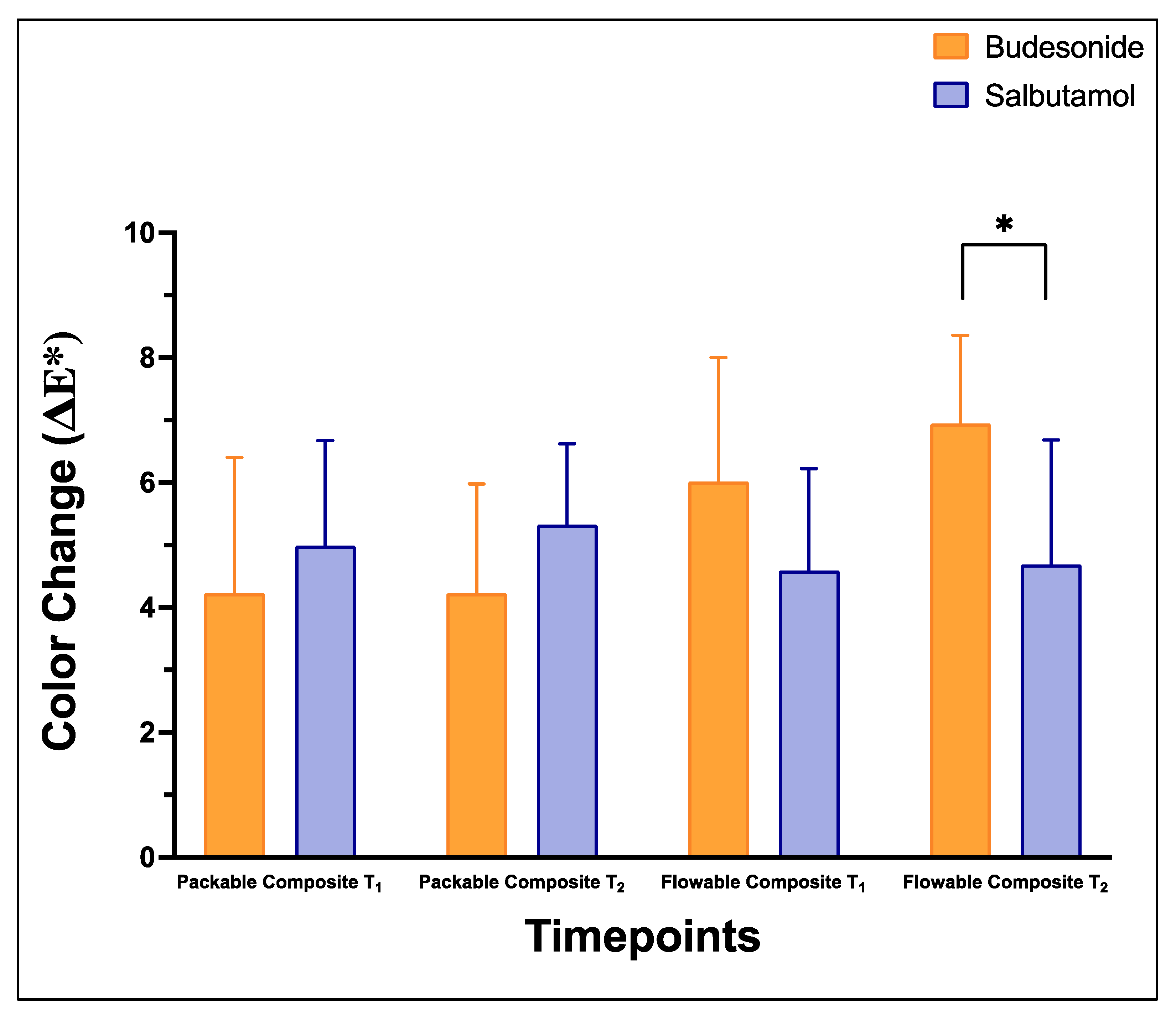
- Both medications showed a clinically detectable change in color of the packable and flowable composites, with Budesonide showing a greater change than Salbutamol after 10 days.
- Despite the improvements in composite resin materials, clinicians should consider the long-term durability of restorative materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Meeting on the Global Alliance against Chronic Respiratory Diseases (GARD); World Health Organization: Geneva, Switzerland, 2005; Available online: https://www.who.int/respiratory/publications/WHO_NMH_CHP_CPM_05.2_eng.pdf (accessed on 25 March 2020).
- Vázquez, E.M.; Vázquez, F.; Barrientos, M.C.; Córdova, J.A.; Lin, D.; Beltrán, F.J.; Vázquez, C.F. Association between asthma and dental caries in the primary dentition of Mexican children. World J. Pediatr. 2011, 7, 344–349. [Google Scholar] [CrossRef]
- Manuel, S.T.; Kundabala, M.; Shetty, N.; Parolia, A. Asthma and dental erosion. Kathmandu Univ. Med J (KUMJ) 2008, 6, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Rutter, C.E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; Ellwood, E.; Ellwood, P.; García-Marcos, L.; Marks, G.B.; Morales, E.; et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet 2021, 398, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Launch of Global Alliance Against Chronic Respiratory Diseases (GARD). Chin. Med. J. 2006, 119, 668. [CrossRef]
- Venkatesh, U. Allied health–3009. Comparative assessment of dental caries experience, oral hygiene status, gingival health status, salivary Streptococcus mutans count and Lactabacillus count between asthmatic and non-asthmatic children aged between 5–12 years in Davangere City, Karnataka, India. World Allergy Organ. J. 2013, 6 (Suppl. S1), 185. [Google Scholar] [CrossRef]
- Adams, N.; Bestall, J.; Jones, P.W. Budesonide at different doses for chronic asthma. Cochrane Database Syst. Rev. 2001, 2000, CD003271. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Parolia, A.; Kundabala, M.; Vikram, M. Asthma and oral health: A review. Aust. Dent. J. 2010, 55, 128–133. [Google Scholar] [CrossRef]
- Gutierrez, J.; Mendoza, R.; Mauricio, F.; Munive-Degregori, A.; Alvitez-Temoche, D.; Mayta-Tovalino, F. Comparison of the Microhardness of Surface Enamel Exposed to Anti-asthmatic Inhalants. J. Contemp. Dent. Pract. 2022, 23, 149–153. [Google Scholar]
- Gerits, E.; Van der Massen, I.; Vandamme, K.; De Cremer, K.; De Brucker, K.; Thevissen, K.; Cammue, B.P.A.; Beullens, S.; Fauvart, M.; Verstraeten, N.; et al. In vitro activity of the antiasthmatic drug zafirlukast against the oral pathogens Porphyromonas gingivalis and Streptococcus mutans. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Boskabady, M.; Nematollahi, H.; Boskabady, M.H. Effect of inhaled medication and inhalation technique on dental caries in asthmatic patients. Iran. Red. Crescent Med. J. 2012, 14, 816–821. [Google Scholar] [CrossRef]
- Scatena, C.; de Mesquita-Guimarães, K.S.F.; Galafassi, D.; Palma-Dibb, R.G.; Borsatto, M.C.; Serra, M.C. Effects of a potentially erosive antiasthmatic medicine on the enamel and dentin of primary teeth: An in situ study. Microsc. Res. Tech. 2018, 81, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Godara, N.; Khullar, M.; Godara, R.; Singh, V. Evaluation of cariogenic potential of dry powder inhalers: A case-control study. Lung India 2013, 30, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Town, G.I.; Herbison, G.P.; Boothman-Burrell, D.; Flannery, E.M.; Hancox, B.; Harré, E.; Laubscher, K.; Linscott, V.; Ramsay, C.M.; et al. Asthma control during long-term treatment with regular inhaled salbutamol and salmeterol. Thorax 1998, 53, 744–752. [Google Scholar] [CrossRef]
- Szefler, S.J.; Eigen, H. Budesonide inhalation suspension: A nebulized corticosteroid for persistent asthma. J. Allergy Clin. Immunol. 2002, 109, 730–742. [Google Scholar] [CrossRef] [PubMed]
- DiBlasi, R.M.; Crandall, C.N.; Engberg, R.J.; Bijlani, K.; Ledee, D.; Kajimoto, M.; Walther, F.J. Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP. Pharmaceutics 2023, 15, 2368. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Emeryk, A.; Janeczek, K.; Krzosa, R.; Pirożyński, M.; Sosnowski, T.R. Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer. Pharmaceutics 2023, 15, 752. [Google Scholar] [CrossRef]
- Puspitasari, D.; Wibowo, D. Effect of Salbutamol Sulfat Exposure To the Surface Roughness of Bioactive Resin. Dentino J. Kedokt. Gigi 2021, 6, 44–49. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 2020, 97, 103323. [Google Scholar] [CrossRef]
- Almutairi, M.; Moussa, I.; Alsaeri, N.; Alqahtani, A.; Alsulaiman, S.; Alhajri, M. The Effects of Different Pediatric Drugs and Brushing on the Color Stability of Esthetic Restorative Materials Used in Pediatric Dentistry: An In Vitro Study. Children 2022, 9, 1026. [Google Scholar] [CrossRef]
- Candan, M.; Ünal, M. The effect of various asthma medications on surface roughness of pediatric dental restorative materials: An atomic force microscopy and scanning electron microscopy study. Microsc. Res. Tech. 2021, 84, 271–283. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Alabbas, M.S.; Alsomaly, K.U.; AlMansour, A.A.; Aljouie, A.A.; Alzahrani, M.M.; Asseri, A.A.; AlHumaid, J. Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants. Polymers 2021, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Yildiz, E.; Eren, M.M.; Ozel, S. Surface hardness evaluation of different composite resin materials: Influence of sports and energy drinks immersion after a short-term period. J. Appl. Oral Sci. 2013, 21, 124–131. [Google Scholar] [CrossRef]
- Ayaz, E.A.; Bagis, B.; Turgut, S. Effect of antiasthmatic medication on the surface roughness and color stability of dental restorative materials. Med. Princ. Pract. 2014, 23, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez Mdel, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.C.; Bohner, L.O.; de Godoi, A.P.; Colucci, V.; Corona, S.A.; Catirse, A.B. Surface roughness of composite resins subjected to hydrochloric acid. Braz. Dent. J. 2015, 26, 268–271. [Google Scholar] [CrossRef]
- Münchow, E.A.; Ferreira, A.C.; Machado, R.M.; Ramos, T.S.; Rodrigues-Junior, S.A.; Zanchi, C.H. Effect of acidic solutions on the surface degradation of a micro-hybrid composite resin. Braz. Dent. J. 2014, 25, 321–326. [Google Scholar] [CrossRef]
- Farag, Z.H.; Awooda, E.M. Dental Erosion and Dentin Hypersensitivity among Adult Asthmatics and Non-asthmatics Hospital-based: A Preliminary Study. Open Dent. J. 2016, 10, 587–593. [Google Scholar] [CrossRef]
- Attin, T.; Knöfel, S.; Buchalla, W.; Tütüncü, R. In situ evaluation of different remineralization periods to decrease brushing abrasion of demineralized enamel. Caries Res. 2001, 35, 216–222. [Google Scholar] [CrossRef]
- Valinoti, A.C.; Pierro, V.S.; Da Silva, E.M.; Maia, L.C. In vitro alterations in dental enamel exposed to acidic medicines. Int. J. Paediatr. Dent. 2011, 21, 141–150. [Google Scholar] [CrossRef]
- Voltarelli, F.R.; Santos-Daroz, C.B.; Alves, M.C.; Cavalcanti, A.N.; Marchi, G.M. Effect of chemical degradation followed by toothbrushing on the surface roughness of restorative composites. J. Appl. Oral Sci. 2010, 18, 585–590. [Google Scholar] [CrossRef]
- Pravallika, K.N.; Prakash, M.; Surapaneni, H.G. Effect of salbutamol-based nebulizer on surface roughness and color stability of different dental resin materials—An invitro study. J. Adv. Med. Dent. Sci. Res. 2021, 8. [Google Scholar] [CrossRef]
- Beltrami, R.; Ceci, M.; De Pani, G.; Vialba, L.; Federico, R.; Poggio, C.; Colombo, M. Effect of different surface finishing/polishing procedures on color stability of esthetic restorative materials: A spectrophotometric evaluation. Eur. J. Dent. 2018, 12, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N. The Effect of Finishing and Polishing Sequences on The Surface Roughness of Three Different Nanocomposites and Composite/Enamel and Composite/Cementum Interfaces. Nanomaterials 2020, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Bezgin, T.; Özer, L.; Tulga Öz, F.; Özkan, P. Effect of toothbrushing on color changes of esthetic restorative materials. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Commission Internationale de I’Eclairage. Recommendations on Uniform Color Space. Color Difference Equations. Psychometric Color Terms; Suppl 2 to CIE pub 15 (E-13.1) 1971/(TC-1.3); Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- Kale, Y.J.; Nalwade, A.V.; Dahake, P.T.; Dadpe, M.V.; Kendre, S.B. Effect of different pediatric drug formulations on color stability of composite, zirconia-reinforced glass ionomer cement, and glass ionomer cement. J. Indian. Soc. Pedod. Prev. Dent. 2019, 37, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud Shalan, H.; Alagami, R.A.; Hasan, M.A. Effect of Coloring Beverages on Different Esthetic Restorative Materials in Primary Teeth. Pediatr. Dent. Diploma 2019, 3, 64–68. [Google Scholar]


| Composite Type | Manufacture | Composition |
|---|---|---|
| Nanohybrid packable composite | Tetric® N-Ceram, TBF, Ivoclar/Vivadent | Dimethacrylates (19–20 wt.%) The fillers are barium glass, ytterbium trifluoride, mixed oxide and copolymers (80–81 wt.%) |
| Nanofilled flowable composite | Tetric® N-Flow, TBF, Ivoclar/Vivadent |
Monomethacrylates and dimethacrylates (28 wt%) The fillers are barium glass, ytterbium trifluoride and copolymers (71 wt%). Additives, initiators, stabilizers and pigments are additional ingredients (<1.0 wt%). |
| Group | Inhaler Type | Composition | Tested Dose |
|---|---|---|---|
| 1 | Salbutamol (Ventolin Nebules, Glaxo Smith Kline, Boronia, Vic., Australia) (100 mcg/actuation) | Salbutamol, sodium chloride, sulfuric acid, distilled water | 0.6 mL/6 mL saline |
| 2 | Budesonide, Pulmicort® Nebulising Suspension (0.5 mcg/actuation) | Budesonide, disodium edetate, sodium chloride, polysorbate, citric acid, monohydrate, sodium citrate, distilled water | 2 mL/2 mL saline |
| Medication | Group | Microhardness (KH for Enamel or VH for Composite) | ||
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| Salbutamol | Enamel samples | 498.98 ± 24.25 a | 120.43 ± 59 b | 92.86 ± 54.40 b |
| Packable composite | 95.16 ± 22.01 a | 84.73 ± 12.87 ab | 64.67 ± 14.12 b | |
| Flowable composite | 71.25 ± 13.37 a | 62.18 ± 10.62 a | 43.28 ± 9.44 b | |
| Budesonide | Enamel samples | 420.90.57 ± 34.80 a | 169.65 ± 79.62 b | 127.15 ± 84.30 b |
| Packable composite | 89.26 ± 18.03 a | 75.35 ± 12.55 ab | 69.91 ± 12.63 b | |
| Flowable composite | 76.41 ± 13.97 a | 52.82 ± 10.54 b | 51.95 ± 11.82 b | |
| Medication | Group | Surface Roughness (nm) | ||
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| Salbutamol | Packable composite | 714.94 ± 42.34 a | 632.89 ± 84.58 ab | 585.93 ± 112.90 bA |
| Flowable composite | 686.47 ± 95.95 a | 535.74 ± 114.32 b | 500.71 ± 110.95 bA | |
| Budesonide | Packable composite | 700.89 ± 119.35 a | 705.33 ± 135.59 a | 335.32 ± 57.58 bB |
| Flowable composite | 649.28 ± 50.39 a | 519.34 ± 91.08 b | 372.22 ± 97.20 cB | |
| Medication | Group | Color Change (ΔE*) | |
|---|---|---|---|
| T1 | T2 | ||
| Salbutamol | Packable composite | 4.99 ± 1.68 | 5.32 ± 1.30 |
| Flowable composite | 4.59 ± 1.63 | 4.68 ± 2.00 A | |
| Budesonide | Packable composite | 4.23 ± 2.17 | 4.23 ± 1.76 |
| Flowable composite | 6.02 ± 1.98 | 6.94 ± 1.42 B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.S.; Alatiyyah, F.M.; Mohammed, K.A.; Alhawaj, H.N.; Balhaddad, A.A.; Ibrahim, A.S. The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color. Pharmaceutics 2023, 15, 2527. https://doi.org/10.3390/pharmaceutics15112527
Ibrahim MS, Alatiyyah FM, Mohammed KA, Alhawaj HN, Balhaddad AA, Ibrahim AS. The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color. Pharmaceutics. 2023; 15(11):2527. https://doi.org/10.3390/pharmaceutics15112527
Chicago/Turabian StyleIbrahim, Maria Salem, Fatimah Mohammed Alatiyyah, Khawla Abbas Mohammed, Hibah Nouh Alhawaj, Abdulrahman A. Balhaddad, and Ahmed Salem Ibrahim. 2023. "The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color" Pharmaceutics 15, no. 11: 2527. https://doi.org/10.3390/pharmaceutics15112527
APA StyleIbrahim, M. S., Alatiyyah, F. M., Mohammed, K. A., Alhawaj, H. N., Balhaddad, A. A., & Ibrahim, A. S. (2023). The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color. Pharmaceutics, 15(11), 2527. https://doi.org/10.3390/pharmaceutics15112527








