Copolymer Micelles: A Focus on Recent Advances for Stimulus-Responsive Delivery of Proteins and Peptides
Abstract
:1. Introduction
2. Non-Covalent Association to Micelles
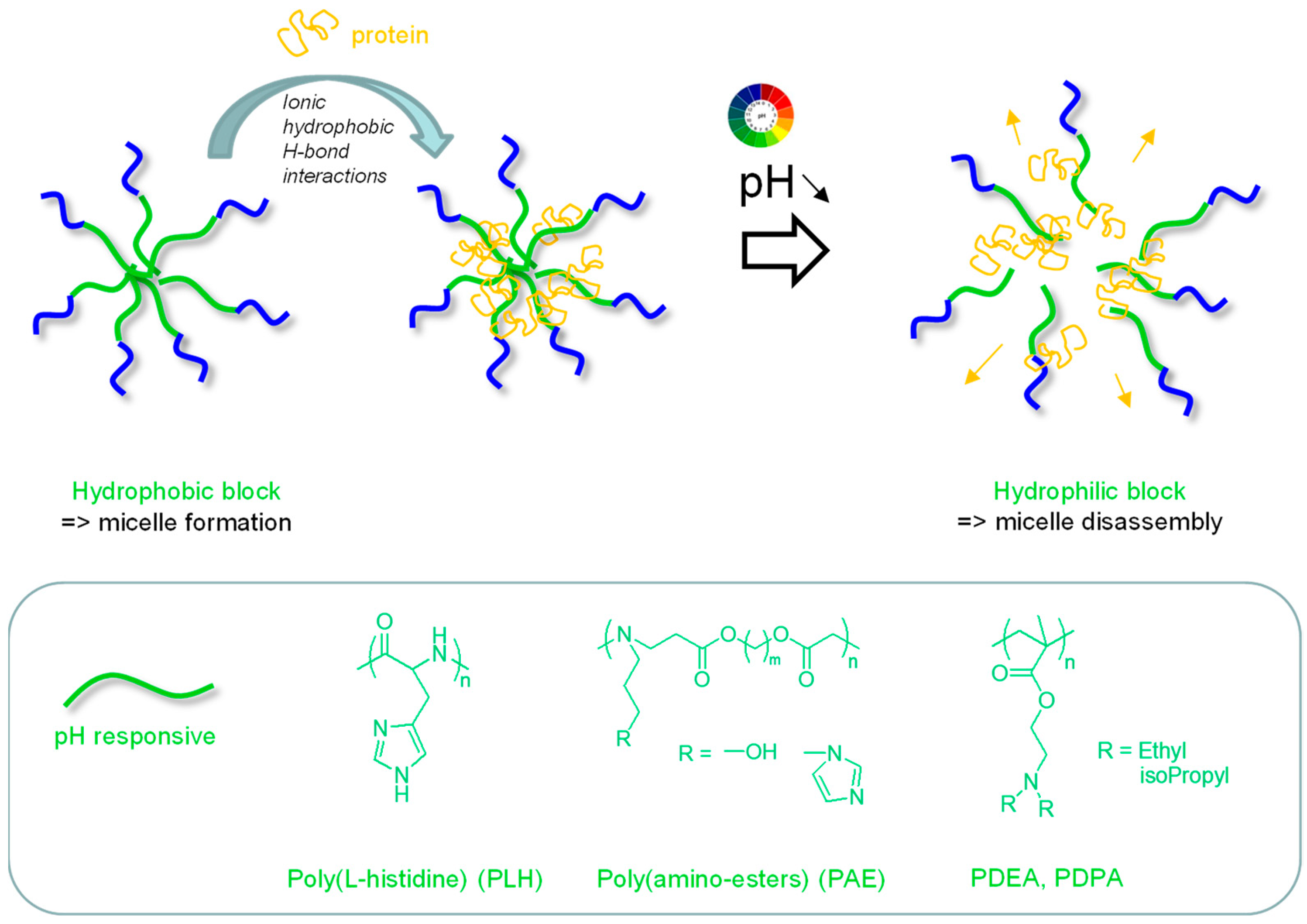
2.1. pH-Repsonsive Micelles
| Copolymer | Protein/Peptide (pI) | Interactions | Results | Ref. |
|---|---|---|---|---|
| PIC micelles | ||||
| pAsp(DET)-PEG | Cytochrome C a (3.7) | electrostatic | Improved cytoplasm delivery | [47] |
| Poly(L-lysine) a-PEG | Myoglobin (7) | electrostatic | Improved half-life in blood | [49] |
| Polycarboxylate-PEG | Lysozyme (11) | electrostatic | Improved release upon cell uptake | [50] |
| Standard micelles | ||||
| Poly(β-amino ester)-PEG | BSA (5) HSA (5) | hydrophobic/H-bond/electrostatic | Improved delivery in acidic tissues (ischemic area) | [53] [54] |
| Poly(urethane amino sulfamethazine) | SDF-1α (9.8) | hydrophobic/H-bond/electrostatic | Enhanced neurogenesis and angiogenesis (cerebral ischemia) | [55] |
| phosphatidylethanolamine-PEG | E5 peptide (11.4) b | electrostatic | Improved CXCR4 targeting by E5 antagonist (cancer) | [56] |
| Polyglutamate-PEG | BSA (5) | electrostatic | Efficient delivery in cells | [57] |
| Polyhistidine-polyglutamate | Granzyme B (9.6) c | electrostatic | Improved anti-tumor efficiency | [58] |
| Poly(lactide-co-glycolide)-PEI a | Antimicrobial peptide d | electrostatic | Biofilm eradication | [59] |
2.2. pH-Repsonsive Micelles in Biomaterials
2.3. Thermo-Repsonsive Micelles
2.4. Multiple Stimuli-Responsive Micelles
3. Covalent Association to Micelles
3.1. pH-Sensitive Linkages
3.2. Reduction-Sensitive Linkages
3.3. Enzyme-Sensitive Linkages
4. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Carton, J.M.; Strohl, W.R. Protein Therapeutics (Introduction to Biopharmaceuticals). In Introduction to Biological and Small Molecule Drug Research and Development; Elsevier: Amsterdam, The Netherlands, 2013; pp. 127–159. ISBN 978-0-12-397176-0. [Google Scholar]
- Ebrahimi, S.B.; Samanta, D. Engineering Protein-Based Therapeutics through Structural and Chemical Design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current Strategies in Extending Half-Lives of Therapeutic Proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for Protein Delivery: Overview and Perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Vila, A.; Sánchez, A.; Tobio, M.; Calvo, P.; Alonso, M.J. Design of Biodegradable Particles for Protein Delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- Lamalle-Bernard, D.; Munier, S.; Compagnon, C.; Charles, M.-H.; Kalyanaraman, V.S.; Delair, T.; Verrier, B.; Ataman-Önal, Y. Coadsorption of HIV-1 P24 and Gp120 Proteins to Surfactant-Free Anionic PLA Nanoparticles Preserves Antigenicity and Immunogenicity. J. Control. Release 2006, 115, 57–67. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-Based Nanoparticles for Protein Delivery: Design, Strategies and Applications. J. Mater. Chem. B 2016, 4, 4060–4071. [Google Scholar] [CrossRef]
- Vardaxi, A.; Kafetzi, M.; Pispas, S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers 2022, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Trimaille, T.; Verrier, B. Micelle-Based Adjuvants for Subunit Vaccine Delivery. Vaccines 2015, 3, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Chen, T.; Xian, W.; Zhang, H.; Wu, L.; Zhu, W.; Zeng, Q. Nanoscale Cationic Micelles of Amphiphilic Copolymers Based on Star-Shaped PLGA and PEI Cross-Linked PEG for Protein Delivery Application. J. Mater. Sci. Mater. Med. 2019, 30, 93. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, H.; Ren, Y.; Li, K.; Tian, B.; Han, J.; Feng, D. Preparation of Protein-Loaded PEG-PLA Micelles and the Effects of Ultrasonication on Particle Size. Colloid Polym. Sci. 2017, 295, 259–266. [Google Scholar] [CrossRef]
- Lamrayah, M.; Phelip, C.; Coiffier, C.; Lacroix, C.; Willemin, T.; Trimaille, T.; Verrier, B. A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response. Pharmaceutics 2022, 14, 107. [Google Scholar] [CrossRef]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef]
- Nomani, A.; Nosrati, H.; Manjili, H.; Khesalpour, L.; Danafar, H. Preparation and Characterization of Copolymeric Polymersomes for Protein Delivery. Drug Res. 2017, 67, 458–465. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-Based Colloidal Carriers for Peptide and Protein Delivery--Liposomes versus Lipid Nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. ISBN 978-0-12-814031-4. [Google Scholar]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mu, H. Lipid and PLGA Microparticles for Sustained Delivery of Protein and Peptide Drugs. Pharm. Nanotechnol. 2020, 8, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Scaletti, F.; Hardie, J.; Lee, Y.-W.; Luther, D.C.; Ray, M.; Rotello, V.M. Protein Delivery into Cells Using Inorganic Nanoparticle–Protein Supramolecular Assemblies. Chem. Soc. Rev. 2018, 47, 3421–3432. [Google Scholar] [CrossRef]
- van de Weert, M.; Hennink, W.E.; Jiskoot, W. Protein Instability in Poly(Lactic-Co-Glycolic Acid) Microparticles. Pharm. Res. 2000, 17, 1159–1167. [Google Scholar] [CrossRef]
- Mehnert, W. Solid Lipid Nanoparticles Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Ball, R.; Bajaj, P.; Whitehead, K. Achieving Long-Term Stability of Lipid Nanoparticles: Examining the Effect of pH, Temperature, and Lyophilization. Int. J. Nanomed. 2016, 12, 305–315. [Google Scholar] [CrossRef]
- Stevens, C.A.; Kaur, K.; Klok, H.-A. Self-Assembly of Protein-Polymer Conjugates for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Stenzel, M.H. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768. [Google Scholar] [CrossRef]
- Yildirim, I.; Weber, C.; Schubert, U.S. Old Meets New: Combination of PLA and RDRP to Obtain Sophisticated Macromolecular Architectures. Prog. Polym. Sci. 2018, 76, 111–150. [Google Scholar] [CrossRef]
- Palmiero, U.C.; Sponchioni, M.; Manfredini, N.; Maraldi, M.; Moscatelli, D. Strategies to Combine ROP with ATRP or RAFT Polymerization for the Synthesis of Biodegradable Polymeric Nanoparticles for Biomedical Applications. Polym. Chem. 2018, 9, 4084–4099. [Google Scholar] [CrossRef]
- Savić, R.; Eisenberg, A.; Maysinger, D. Block Copolymer Micelles as Delivery Vehicles of Hydrophobic Drugs: Micelle–Cell Interactions. J. Drug Target. 2006, 14, 343–355. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Sun, J.-H.; Liang, X.; Cai, M.; Yan, L.; Chen, Z.; Guo, L.; Jing, L.; Wang, Y.; Zhou, D. Protein-Crowned Micelles for Targeted and Synergistic Tumor-Associated Macrophage Reprogramming to Enhance Cancer Treatment. Nano Lett. 2022, 22, 4410–4420. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in Polymeric Micelles for Drug Delivery and Tumor Targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Lee, E. Polymeric Micelle for Tumor pH and Folate-Mediated Targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Re Ko, N.; Kwon Oh, J. Recent Advances in Stimuli-Responsive Degradable Block Copolymer Micelles: Synthesis and Controlled Drug Delivery Applications. Chem. Commun. 2012, 48, 7542. [Google Scholar] [CrossRef] [PubMed]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted Therapy for Cancer Using pH-Responsive Nanocarrier Systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, A.; Jia, Y.; Yang, L.; Ning, Y.; Xu, L.; Zhong, Y.; Zhuang, Z.; Guan, J.; Zhang, X.; et al. pH-Responsive Multifunctional Theranostic Rapamycin-Loaded Nanoparticles for Imaging and Treatment of Acute Ischemic Stroke. ACS Appl. Mater. Interfaces 2021, 13, 56909–56922. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Liu, G.; He, Y.; Gao, P.; Gou, S.; Wu, J.; Yu, J.; Liu, P.; Cai, K. Fabrication of a pH-Responsive Core–Shell Nanosystem with a Low-Temperature Photothermal Therapy Effect for Treating Bacterial Biofilm Infection. Biomater. Sci. 2021, 9, 7483–7491. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Lin, W.; Gao, J.; Shi, X.; Davaritouchaee, M.; Nielsen, A.E.; Mancini, R.J.; Wang, Z. pH-Responsive Nanoparticles Targeted to Lungs for Improved Therapy of Acute Lung Inflammation/Injury. ACS Appl. Mater. Interfaces 2019, 11, 16380–16390. [Google Scholar] [CrossRef]
- Tayo, L.L. Stimuli-Responsive Nanocarriers for Intracellular Delivery. Biophys. Rev. 2017, 9, 931–940. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Development of pH-Responsive Hyaluronic Acid-Based Antigen Carriers for Induction of Antigen-Specific Cellular Immune Responses. ACS Biomater. Sci. Eng. 2019, 5, 5790–5797. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block Copolymers with Poly(Ethylene Glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Lee, Y.; Kataoka, K. Biosignal-Sensitive Polyion Complex Micelles for the Delivery of Biopharmaceuticals. Soft Matter 2009, 5, 3810. [Google Scholar] [CrossRef]
- Lee, Y.; Ishii, T.; Cabral, H.; Kim, H.J.; Seo, J.-H.; Nishiyama, N.; Oshima, H.; Osada, K.; Kataoka, K. Charge-Conversional Polyionic Complex Micelles-Efficient Nanocarriers for Protein Delivery into Cytoplasm. Angew. Chem. Int. Ed. 2009, 48, 5309–5312. [Google Scholar] [CrossRef]
- Miyata, K.; Oba, M.; Nakanishi, M.; Fukushima, S.; Yamasaki, Y.; Koyama, H.; Nishiyama, N.; Kataoka, K. Polyplexes from Poly(Aspartamide) Bearing 1,2-Diaminoethane Side Chains Induce pH-Selective, Endosomal Membrane Destabilization with Amplified Transfection and Negligible Cytotoxicity. J. Am. Chem. Soc. 2008, 130, 16287–16294. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Huang, G.L.; Igarashi, K.; Hong, T.; Liao, S.; Stellacci, F.; Matsumoto, Y.; Yamasoba, T.; Kataoka, K.; Cabral, H. Polymeric Micelles Loading Proteins through Concurrent Ion Complexation and pH-Cleavable Covalent Bonding for In Vivo Delivery. Macromol. Biosci. 2020, 20, 1900161. [Google Scholar] [CrossRef]
- Li, K.; Chen, F.; Wang, Y.; Stenzel, M.H.; Chapman, R. Polyion Complex Micelles for Protein Delivery Benefit from Flexible Hydrophobic Spacers in the Binding Group. Macromol. Rapid Commun. 2020, 41, 2000208. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Ba, S.; Zhu, J.; Zhang, J.; Hong, W.; Zhao, X.; Hu, H.; Qiao, M. Poly(l-Histidine) Based Triblock Copolymers: pH Induced Reassembly of Copolymer Micelles and Mechanism Underlying Endolysosomal Escape for Intracellular Delivery. Biomacromolecules 2014, 15, 4032–4045. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly(l-Histidine)–PEG Block Copolymer Micelles and pH-Induced Destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef]
- Kim, M.S.; Gao, G.H.; Kang, S.W.; Lee, D.S. Evaluation of pH-Sensitive Poly(β-Amino Ester)-Graft-Poly(Ethylene Glycol) and Its Usefulness as a pH-Sensor and Protein Carrier. Macromol. Biosci. 2011, 11, 946–951. [Google Scholar] [CrossRef]
- Gao, G.H.; Park, M.J.; Li, Y.; Im, G.H.; Kim, J.-H.; Kim, H.N.; Lee, J.W.; Jeon, P.; Bang, O.Y.; Lee, J.H.; et al. The Use of pH-Sensitive Positively Charged Polymeric Micelles for Protein Delivery. Biomaterials 2012, 33, 9157–9164. [Google Scholar] [CrossRef]
- Kim, D.H.; Seo, Y.K.; Thambi, T.; Moon, G.J.; Son, J.P.; Li, G.; Park, J.H.; Lee, J.H.; Kim, H.H.; Lee, D.S.; et al. Enhancing Neurogenesis and Angiogenesis with Target Delivery of Stromal Cell Derived Factor-1α Using a Dual Ionic pH-Sensitive Copolymer. Biomaterials 2015, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xie, H.; Duan, H.; Li, P.; Yousaf, M.; Xu, H.; Yang, Y.; Wang, C. Anti-Tumor Activity of Nanomicelles Encapsulating CXCR4 Peptide Antagonist E5. PLoS ONE 2017, 12, e0182697. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; She, Y.; Peng, Z.; Wei, J.; He, X. Enzyme-Mediated in Situ Formation of pH-Sensitive Nanogels for Proteins Delivery. RSC Adv. 2016, 6, 8032–8042. [Google Scholar] [CrossRef]
- Pang, X.; Liang, S.; Wang, T.; Yu, S.; Yang, R.; Hou, T.; Liu, Y.; He, C.; Zhang, N. Engineering Thermo-pH Dual Responsive Hydrogel for Enhanced Tumor Accumulation, Penetration, and Chemo-Protein Combination Therapy. Int. J. Nanomed. 2020, 15, 4739–4752. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-L.; Cheng, K.; Li, Y.; Zhong, Z.-T.; Hou, X.-L.; Song, L.-B.; Zhang, F.; Wang, J.-H.; Zhao, Y.-D.; Xu, Q.-R. The Eradication of Biofilm for Therapy of Bacterial Infected Chronic Wound Based on pH-Responsive Micelle of Antimicrobial Peptide Derived Biodegradable Microneedle Patch. Chem. Eng. J. 2023, 462, 142222. [Google Scholar] [CrossRef]
- Tang, N.; Du, G.; Wang, N.; Liu, C.; Hang, H.; Liang, W. Improving Penetration in Tumors with Nanoassemblies of Phospholipids and Doxorubicin. J. Natl. Cancer Inst. 2007, 99, 1004–1015. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Zeng, W.; Huang, F.; Qin, L.; Zhang, C.; Liang, W. Stability Influences the Biodistribution, Toxicity, and Anti-Tumor Activity of Doxorubicin Encapsulated in PEG-PE Micelles in Mice. Pharm. Res. 2012, 29, 1977–1989. [Google Scholar] [CrossRef]
- Wittemann, A.; Haupt, B.; Ballauff, M. Adsorption of Proteins on Spherical Polyelectrolyte Brushes in Aqueous Solution. Phys. Chem. Chem. Phys. 2003, 5, 1671–1677. [Google Scholar] [CrossRef]
- Pan, M.; Lu, C.; Zhang, W.; Huang, H.; Shi, X.; Tang, S.; Liu, D. Poly(l-Ornithine)-Based Polymeric Micelles as pH-Responsive Macromolecular Anticancer Agents. Pharmaceutics 2023, 15, 1307. [Google Scholar] [CrossRef]
- Seo, J.-W.; Hwang, J.-Y.; Shin, U.S. Ionic Liquid-Doped and p-NIPAAm-Based Copolymer (p-NIBIm): Extraordinary Drug-Entrapping and -Releasing Behaviors at 38–42 °C. RSC Adv. 2014, 4, 26738–26747. [Google Scholar] [CrossRef]
- Bonacucina, G.; Cespi, M.; Mencarelli, G.; Giorgioni, G.; Palmieri, G.F. Thermosensitive Self-Assembling Block Copolymers as Drug Delivery Systems. Polymers 2011, 3, 779–811. [Google Scholar] [CrossRef]
- Huynh, C.T.; Nguyen, M.K.; Lee, D.S. Injectable Block Copolymer Hydrogels: Achievements and Future Challenges for Biomedical Applications. Macromolecules 2011, 44, 6629–6636. [Google Scholar] [CrossRef]
- Determan, M.D.; Guo, L.; Lo, C.-T.; Thiyagarajan, P.; Mallapragada, S.K. P H- and Temperature-Dependent Phase Behavior of a PEO-PPO-PEO-Based Pentablock Copolymer in Aqueous Media. Phys. Rev. E 2008, 78, 021802. [Google Scholar] [CrossRef]
- Adams, J.R.; Haughney, S.L.; Mallapragada, S.K. Effective Polymer Adjuvants for Sustained Delivery of Protein Subunit Vaccines. Acta Biomater. 2015, 14, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Darling, R.J.; Loh, D.; Schneider, I.C.; Wannemuehler, M.J.; Narasimhan, B.; Mallapragada, S.K. Pentablock Copolymer Micelle Nanoadjuvants Enhance Cytosolic Delivery of Antigen and Improve Vaccine Efficacy While Inducing Low Inflammation. ACS Biomater. Sci. Eng. 2019, 5, 1332–1342. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.-H. A Supramolecular Host-Guest Interaction-Mediated Injectable Hydrogel System with Enhanced Stability and Sustained Protein Release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Y.; Zhang, J.; Gao, H.; Liu, G.; Ma, R.; An, Y.; Kong, D.; Shi, L. pH/Sugar Dual Responsive Core-Cross-Linked PIC Micelles for Enhanced Intracellular Protein Delivery. Biomacromolecules 2013, 14, 3434–3443. [Google Scholar] [CrossRef]
- Xia, H.; Qin, M.; Wang, Z.; Wang, Y.; Chen, B.; Wan, F.; Tang, M.; Pan, X.; Yang, Y.; Liu, J.; et al. A pH-/Enzyme-Responsive Nanoparticle Selectively Targets Endosomal Toll-like Receptors to Potentiate Robust Cancer Vaccination. Nano Lett. 2022, 22, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Zhao, H.; Liu, L. Ca 2+ -Chelation-Induced Fabrication of Multistimuli-Responsive Charged Nanogels from Phospholipid–Polymer Conjugates and Use for Drug/Protein Loading. Langmuir 2022, 38, 6612–6622. [Google Scholar] [CrossRef] [PubMed]
- Vanparijs, N.; De Coen, R.; Laplace, D.; Louage, B.; Maji, S.; Lybaert, L.; Hoogenboom, R.; De Geest, B.G. Transiently Responsive Protein–Polymer Conjugates via a ‘Grafting-from’ RAFT Approach for Intracellular Co-Delivery of Proteins and Immune-Modulators. Chem. Commun. 2015, 51, 13972–13975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vanparijs, N.; Louage, B.; De Geest, B.G.; Hoogenboom, R. Dual pH- and Temperature-Responsive RAFT-Based Block Co-Polymer Micelles and Polymer–Protein Conjugates with Transient Solubility. Polym. Chem. 2014, 5, 1140–1144. [Google Scholar] [CrossRef]
- Lacroix, C.; Humanes, A.; Coiffier, C.; Gigmes, D.; Verrier, B.; Trimaille, T. Polylactide-Based Reactive Micelles as a Robust Platform for mRNA Delivery. Pharm. Res. 2020, 37, 30. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Hou, C.-Y.; Zhang, D.; Liu, Y.; Lin, Y.-X.; An, H.-W.; Li, X.-J.; Wang, H. Self-Assembly of Cytotoxic Peptide Conjugated Poly(β-Amino Ester)s for Synergistic Cancer Chemotherapy. J. Mater. Chem. B 2015, 3, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Florinas, S.; Liu, M.; Fleming, R.; Van Vlerken-Ysla, L.; Ayriss, J.; Gilbreth, R.; Dimasi, N.; Gao, C.; Wu, H.; Xu, Z.-Q.; et al. A Nanoparticle Platform to Evaluate Bioconjugation and Receptor-Mediated Cell Uptake Using Cross-Linked Polyion Complex Micelles Bearing Antibody Fragments. Biomacromolecules 2016, 17, 1818–1833. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, X.; Fan, J.; Wang, G.; Liu, S. Aldehyde Surface-Functionalized Shell Cross-Linked Micelles with pH-Tunable Core Swellability and Their Bioconjugation with Lysozyme. Macromolecules 2007, 40, 9074–9083. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Drug-Conjugated PLA–PEG–PLA Copolymers: A Novel Approach for Controlled Delivery of Hydrophilic Drugs by Micelle Formation. Pharm. Dev. Technol. 2017, 22, 947–957. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Ding, X.; Shen, W.; Li, M.; Wagner, E.; Xiao, C.; Chen, X. A Multistage Cooperative Nanoplatform Enables Intracellular Co-Delivery of Proteins and Chemotherapeutics for Cancer Therapy. Adv. Mater. 2020, 32, 2000013. [Google Scholar] [CrossRef]
- Sevimli, S.; Knight, F.C.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants. ACS Biomater. Sci. Eng. 2017, 3, 179–194. [Google Scholar] [CrossRef]
- Keller, S.; Wilson, J.T.; Patilea, G.I.; Kern, H.B.; Convertine, A.J.; Stayton, P.S. Neutral Polymer Micelle Carriers with pH-Responsive, Endosome-Releasing Activity Modulate Antigen Trafficking to Enhance CD8+ T Cell Responses. J. Control. Release 2014, 191, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Keller, S.; Manganiello, M.J.; Cheng, C.; Lee, C.-C.; Opara, C.; Convertine, A.; Stayton, P.S. pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano 2013, 7, 3912–3925. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Song, K.; Yen, A.; Peeler, D.J.; Nguyen, D.C.; Olshefsky, A.; Sylvestre, M.; Srinivasan, S.; Stayton, P.S.; Pun, S.H. Well-Defined Mannosylated Polymer for Peptide Vaccine Delivery with Enhanced Antitumor Immunity. Adv Healthc. Mater. 2022, 11, 2101651. [Google Scholar] [CrossRef]
- Peeler, D.J.; Yen, A.; Luera, N.; Stayton, P.S.; Pun, S.H. Lytic Polyplex Vaccines Enhance Antigen-Specific Cytotoxic T Cell Response through Induction of Local Cell Death. Adv. Ther. 2021, 4, 2100005. [Google Scholar] [CrossRef]
- Song, K.; Nguyen, D.C.; Luu, T.; Yazdani, O.; Roy, D.; Stayton, P.S.; Pun, S.H. A Mannosylated Polymer with Endosomal Release Properties for Peptide Antigen Delivery. J. Control. Release 2023, 356, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Berguig, G.Y.; Convertine, A.J.; Frayo, S.; Kern, H.B.; Procko, E.; Roy, D.; Srinivasan, S.; Margineantu, D.H.; Booth, G.; Palanca-Wessels, M.C.; et al. Intracellular Delivery System for Antibody–Peptide Drug Conjugates. Mol. Ther. 2015, 23, 907–917. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Cruz-Montañez, A.; Figueroa, C.M.; González-Robles, T.; Davila, J.; Inyushin, M.; Loza-Rosas, S.A.; Molina, A.M.; Muñoz-Perez, L.; Kucheryavykh, L.Y.; et al. Combining Stimulus-Triggered Release and Active Targeting Strategies Improves Cytotoxicity of Cytochrome c Nanoparticles in Tumor Cells. Mol. Pharm. 2016, 13, 2844–2854. [Google Scholar] [CrossRef]
- Kern, H.B.; Srinivasan, S.; Convertine, A.J.; Hockenbery, D.; Press, O.W.; Stayton, P.S. Enzyme-Cleavable Polymeric Micelles for the Intracellular Delivery of Proapoptotic Peptides. Mol. Pharm. 2017, 14, 1450–1459. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, Z.; Huang, J.; Wang, Y.; An, Y.; Xiao, H.; Peng, Y.; Pang, P.; Han, S.; Zhu, K.; et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 12845–12856. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.-P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef]
- Liang, Y.; Sullivan, H.L.; Carrow, K.; Mesfin, J.M.; Korpanty, J.; Worthington, K.; Luo, C.; Christman, K.L.; Gianneschi, N.C. Inflammation-Responsive Micellar Nanoparticles from Degradable Polyphosphoramidates for Targeted Delivery to Myocardial Infarction. J. Am. Chem. Soc. 2023, 145, 11185–11194. [Google Scholar] [CrossRef]
- Heffernan, M.J.; Murthy, N. Disulfide-Crosslinked Polyion Micelles for Delivery of Protein Therapeutics. Ann. Biomed. Eng. 2009, 37, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Wei, J.; Yan, L.; Jing, X. Application of the Biodegradable Diblock Copolymer Poly(L-Lactide)-Block-Poly(L-Cysteine): Drug Delivery and Protein Conjugation. J. Appl. Polym. Sci. 2010, 118, 1738–1742. [Google Scholar] [CrossRef]
- Eby, J.K.; Dane, K.Y.; O’Neil, C.P.; Hirosue, S.; Swartz, M.A.; Hubbell, J.A. Polymer Micelles with Pyridyl Disulfide-Coupled Antigen Travel through Lymphatics and Show Enhanced Cellular Responses Following Immunization. Acta Biomater. 2012, 8, 3210–3217. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme Responsive Drug Delivery Systems in Cancer Treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, Y.; Ochbaum, G.; Lin, R.; Bitton, R.; Cui, H.; Azevedo, H.S. Enzymatic Activation of Cell-Penetrating Peptides in Self-Assembled Nanostructures Triggers Fibre-to-Micelle Morphological Transition. Chem. Commun. 2017, 53, 7037–7040. [Google Scholar] [CrossRef]
- Cox, F.; Khalib, K.; Conlon, N. PEG That Reaction: A Case Series of Allergy to Polyethylene Glycol. J. Clin. Pharmacol. 2021, 61, 832–835. [Google Scholar] [CrossRef]
- Liu, M.; Chu, Y.; Liu, H.; Su, Y.; Zhang, Q.; Jiao, J.; Liu, M.; Ding, J.; Liu, M.; Hu, Y.; et al. Accelerated Blood Clearance of Nanoemulsions Modified with PEG-Cholesterol and PEG-Phospholipid Derivatives in Rats: The Effect of PEG-Lipid Linkages and PEG Molecular Weights. Mol. Pharm. 2020, 17, 1059–1070. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]

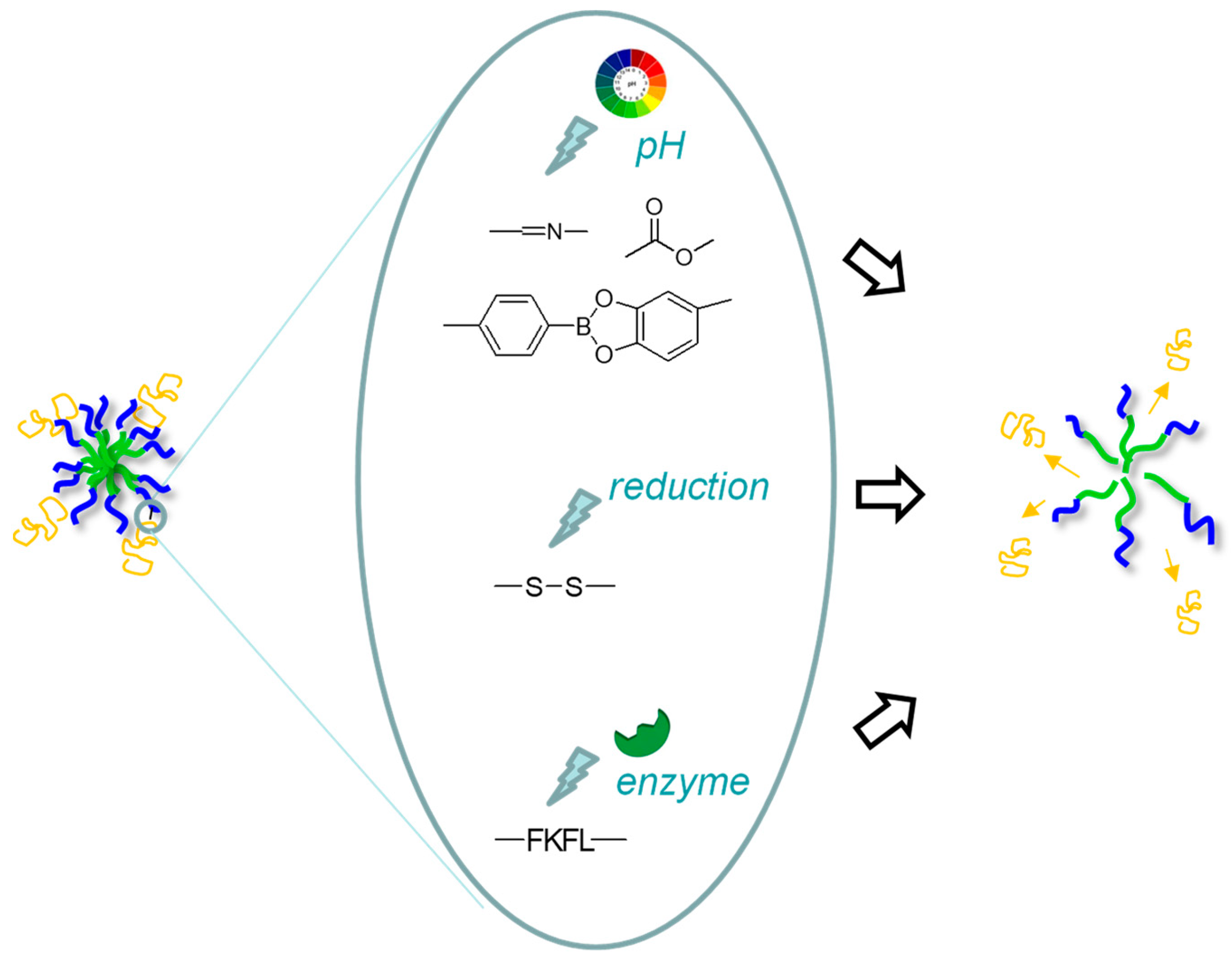
| Copolymer | Protein/Peptide | Stimulus | Linkage | Results | Ref. |
|---|---|---|---|---|---|
| POEGMA-PDMA-PDEA | Lysozyme | pH | Imine | Bioconjugation assessment | [79] |
| PLA-PEG-PLA | Lisinopril | pH | Ester | Improved drug loading stability at pH 7.4 (and release at pH 4) | [80] |
| Polyglutamate-PEG based | Ribonuclease A a | pH | phenylboronic acid–catechol | Enhanced intracellular release, enhanced anticancer efficacy | [81] |
| PDPA/PDMA based | Ovalbumin Lytic peptide | Reduction | Disulfide | Improved immune responses (vaccines) | [82,83,84,85,86,87] |
| Proapoptotic peptide (BIM) | Reduction | Disulfide | Suppression of tumor growth | [88] | |
| Poly(lactide-co-glycolide)-PEG | Cytochrome C | Reduction | Disulfide | Endosomal escape, potential for antitumor therapy (brain model) | [89] |
| PDMA/PDPA based | Proapoptotic peptide (BIM) | Enzyme (cathepsin B) | FKFL cleavable sequence | Improved intracellular delivery, Successful cancer cell apoptosis | [90] |
| PAsp(Bz)-PEG | PD-L1 antibody b | Enzyme (MMP2) | GGPLGVRGG cleavable sequence | Improved intracellular delivery, improved antitumor effect | [91] |
| Polynorbornene based | - | Enzyme (MMP2/9) | GPLGLAG cleavable sequence | Specific accumulation in infarcted heart | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimaille, T.; Verrier, B. Copolymer Micelles: A Focus on Recent Advances for Stimulus-Responsive Delivery of Proteins and Peptides. Pharmaceutics 2023, 15, 2481. https://doi.org/10.3390/pharmaceutics15102481
Trimaille T, Verrier B. Copolymer Micelles: A Focus on Recent Advances for Stimulus-Responsive Delivery of Proteins and Peptides. Pharmaceutics. 2023; 15(10):2481. https://doi.org/10.3390/pharmaceutics15102481
Chicago/Turabian StyleTrimaille, Thomas, and Bernard Verrier. 2023. "Copolymer Micelles: A Focus on Recent Advances for Stimulus-Responsive Delivery of Proteins and Peptides" Pharmaceutics 15, no. 10: 2481. https://doi.org/10.3390/pharmaceutics15102481
APA StyleTrimaille, T., & Verrier, B. (2023). Copolymer Micelles: A Focus on Recent Advances for Stimulus-Responsive Delivery of Proteins and Peptides. Pharmaceutics, 15(10), 2481. https://doi.org/10.3390/pharmaceutics15102481







