Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers
Abstract
:1. Introduction
2. Enriched H2O2-Powered Phototherapy
2.1. Hypoxia-Relieved Phototherapy via Enzyme-Catalyzed H2O2 Decomposition
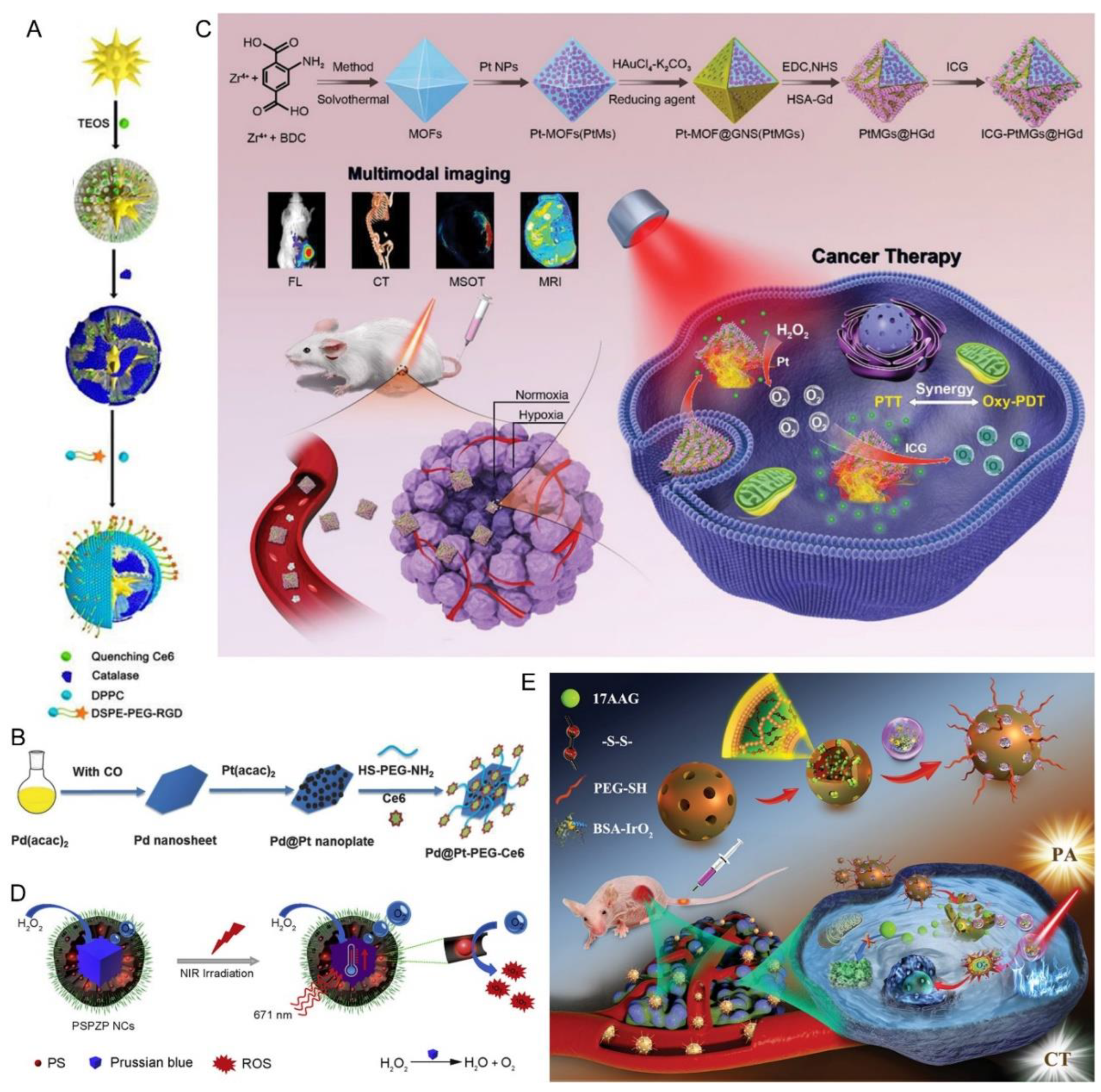
2.2. Hypoxia-Relieved Phototherapy via Metallic Nanomaterial-Catalyzed H2O2 Decomposition
3. pH-Responsive Phototherapy
3.1. pH-Responsive Drug Release
3.2. Low pH-Enhanced Cellular Uptake
3.3. pH-Responsive Drug Release and Cellular Uptake
3.4. Low pH-Activated Phototherapy
4. pH and H2O2 Dual-Responsive Phototherapy
4.1. Enriched H2O2-Powered Hypoxia Relief via MnO2 Nanomaterials
4.2. Enriched H2O2-Powered Hypoxia Relief and pH-Responsive Drug Release
4.3. Enriched H2O2-Powered Hypoxia Relief and pH-Responsive Enhanced Intratumoral Drug Penetration
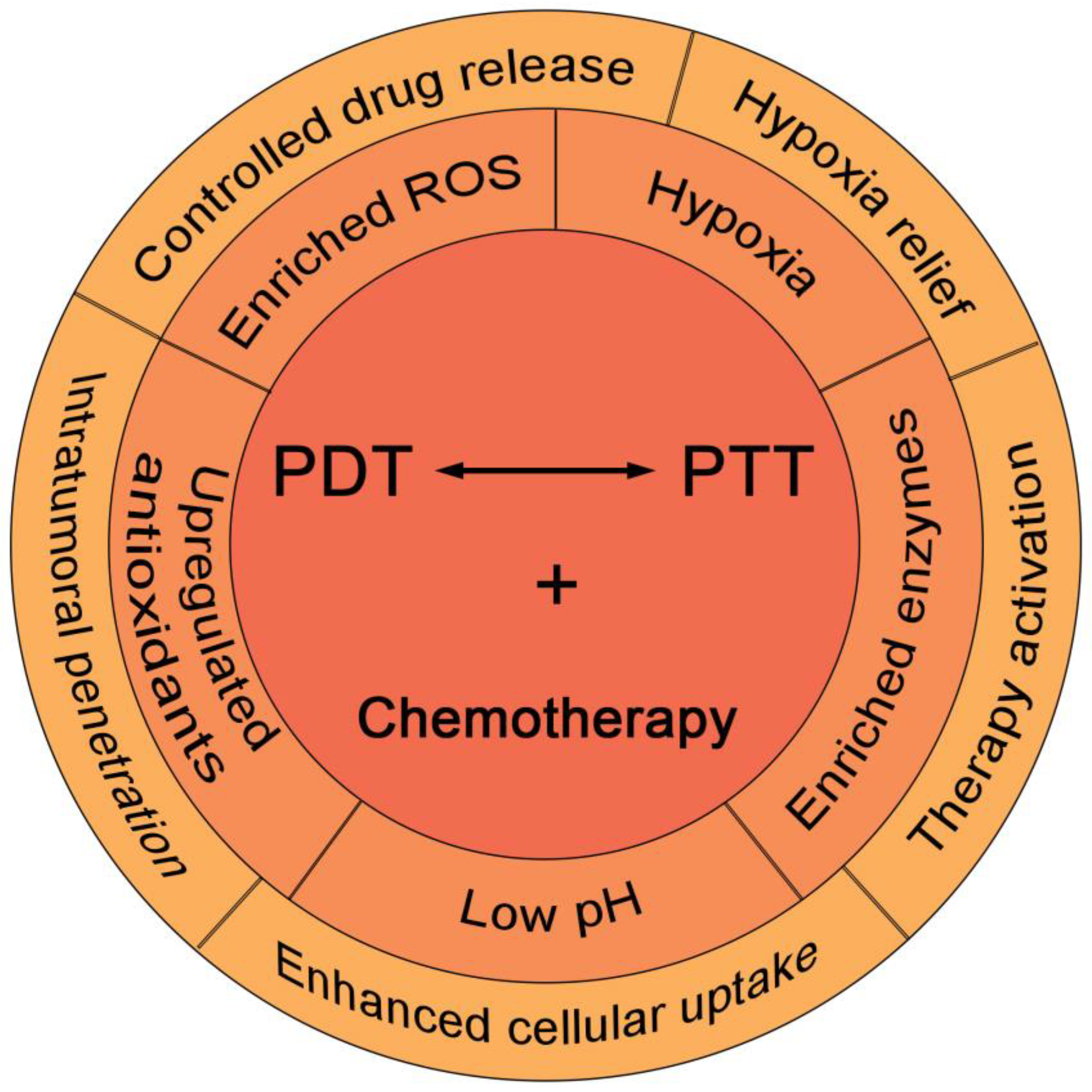
5. TME-Responsive PDT/PTT/Chemotherapy
5.1. TME-Responsive Hypoxia Relief and Drug Release for Enhanced PDT/PTT/Chemotherapy
5.2. TME-Responsive Drug Release for Enhanced PDT/PTT/Chemotherapy
5.3. TME-Responsive Drug Release and Treatment Activation for Enhanced PDT/PTT/Chemotherapy
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- He, M.Y.; Yang, T.; Wang, Y.H.; Wang, M.Y.; Chen, X.Y.; Ding, D.W.; Zheng, Y.R.; Chen, H.B. Immune Checkpoint Inhibitor-Based Strategies for Synergistic Cancer Therapy. Adv. Healthc. Mater. 2021, 10, e2002104. [Google Scholar] [CrossRef]
- Wen, S.G.; Wang, W.L.; Liu, R.M.; He, P.C. Amylase-Protected Ag Nanodots for in vivo Fluorescence Imaging and Photodynamic Therapy of Tumors. Int. J. Nanomed. 2020, 15, 3405–3414. [Google Scholar] [CrossRef]
- Li, X.S.; Lovell, J.F.; Yoon, J.; Chen, X.Y. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z.C. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Li, T.F.; Xu, H.Z.; Xu, Y.H.; Yu, T.T.; Tang, J.M.; Li, K.; Wang, C.; Peng, X.C.; Li, Q.R.; Sang, X.Y.; et al. Efficient Delivery of Chlorin e6 by Polyglycerol-Coated Iron Oxide Nanoparticles with Conjugated Doxorubicin for Enhanced Photodynamic Therapy of Melanoma. Mol. Pharm. 2021, 18, 3601–3615. [Google Scholar] [CrossRef]
- Yu, X.Z.; Zhu, W.W.; Di, Y.; Gu, J.C.; Guo, Z.Y.; Li, H.C.; Fu, D.L.; Jin, C. Triple-functional albumin-based nanoparticles for combined chemotherapy and photodynamic therapy of pancreatic cancer with lymphatic metastases. Int. J. Nanomed. 2017, 12, 6771–6785. [Google Scholar] [CrossRef]
- Li, X.S.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Hu, D.; Pan, M.; Yu, Y.; Sun, A.; Shi, K.; Qu, Y.; Qian, Z. Application of nanotechnology for enhancing photodynamic therapy via ameliorating, neglecting, or exploiting tumor hypoxia. View 2020, 1, e6. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.W.; Liang, C.; Feng, L.Z.; Dong, Z.L.; Song, X.J.; Song, G.S.; Liu, Z. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J. Control. Release 2017, 263, 79–89. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhang, Y.; Ju, E.G.; Liu, Z.; Cao, F.F.; Chen, Z.W.; Ren, J.S.; Qu, X.G. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 3334. [Google Scholar] [CrossRef]
- Zhang, X.C.; Feng, L.Z.; Dong, Z.L.; Xin, X.Q.; Yang, Z.J.; Deng, D.; Wagner, E.; Liu, Z.; Liu, X.W. Protein-drug conjugate programmed by pH-reversible linker for tumor hypoxia relief and enhanced cancer combination therapy. Int. J. Pharm. 2020, 582, 119321. [Google Scholar] [CrossRef]
- He, M.Y.; Wang, M.Y.; Xu, T.; Zhang, M.Y.; Dai, H.X.; Wang, C.; Ding, D.W.; Zhong, Z.Y. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J. Control. Release 2023, 356, 623–648. [Google Scholar] [CrossRef]
- Lan, J.S.; Liu, L.; Zeng, R.F.; Qin, Y.H.; Hou, J.W.; Xie, S.S.; Yue, S.; Yang, J.; Ho, R.J.Y.; Ding, Y.; et al. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021, 407, 127212. [Google Scholar] [CrossRef]
- Jana, D.; Zhao, Y. Strategies for enhancing cancer chemodynamic therapy performance. Exploration 2022, 2, 20210238. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Shen, P.; Wang, J.; Shen, Y.D.; Shen, Y.; Webster, T.J.; Deng, J.J. Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment. Int. J. Nanomed. 2020, 15, 1903–1914. [Google Scholar] [CrossRef]
- Wang, M.; He, M.; Zhang, M.; Xue, S.; Xu, T.; Zhao, Y.; Li, D.; Zhi, F.; Ding, D. Controllable hypoxia-activated chemotherapy as a dual enhancer for synergistic cancer photodynamic immunotherapy. Biomaterials 2023, 301, 122257. [Google Scholar] [CrossRef]
- Pan, Y.B.; Tang, W.; Fan, W.P.; Zhang, J.M.; Chen, X.Y. Development of nanotechnology-mediated precision radiotherapy for anti-metastasis and radioprotection. Chem. Soc. Rev. 2022, 51, 9759–9830. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, F.H.; Zhong, Z.Y. Emerging targeted drug delivery strategies toward ovarian cancer. Adv. Drug Deliv. Rev. 2021, 178, 113969. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Mao, L.S.; Dong, Y.L.; Zhao, Z.W.; Sun, Q.; Mazhar, M.; Ma, Y.N.; Yang, S.J.; Ren, W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics 2021, 13, 2070. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tao, H.Q.; Li, Q.G.; Sheng, W.L.; Xu, Y.J.; Hao, E.H.; Chen, M.W.; Liu, Z.; Feng, L.Z. Surfactant-stripped J-aggregates of azaBODIPY derivatives: All-in-one phototheranostics in the second near infrared window. J. Control. Release 2020, 326, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.Q.; Zhan, Y.Y.; Wang, L.G.; Luo, J.; Xu, J.; Zhan, L.L.; Li, Z.H.; Liu, Y.G.; Wei, J.C. Enhancing the Efficiency of Mild-Temperature Photothermal Therapy for Cancer Assisting with Various Strategies. Pharmaceutics 2022, 14, 2279. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, Z.C.; Fu, S.W.; Yu, X.; Sun, M.C.; Zhai, Y.L.; Sun, J. Emerging platinum(0) nanotherapeutics for efficient cancer therapy. J. Control. Release 2022, 352, 276–287. [Google Scholar] [CrossRef]
- Seo, S.H.; Joe, A.; Han, H.W.; Manivasagan, P.; Jang, E.S. Methylene Blue-Loaded Mesoporous Silica-Coated Gold Nanorods on Graphene Oxide for Synergistic Photothermal and Photodynamic Therapy. Pharmaceutics 2022, 14, 2242. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Junior, W.T.D.; Mundim, T.; Vale, C.L.C.; de Oliveira, J.V.; Ganassin, R.; Pacheco, T.J.A.; Morais, J.A.V.; Longo, J.P.F.; Azevedo, R.B.; et al. Induction of Immunogenic Cell Death by Photodynamic Therapy Mediated by Aluminum-Phthalocyanine in Nanoemulsion. Pharmaceutics 2022, 14, 2242. [Google Scholar] [CrossRef]
- Sekar, R.; Basavegowda, N.; Thathapudi, J.J.; Sekhar, M.R.; Joshi, P.; Somu, P.; Baek, K.H. Recent Progress of Gold-Based Nanostructures towards Future Emblem of Photo-Triggered Cancer Theranostics: A Special Focus on Combinatorial Phototherapies. Pharmaceutics 2023, 15, 433. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, S.; Li, S.; Lv, A.M.; Chen, Q.; Jiang, K.; Jiang, Z.Q.; Li, Z.J.; Wu, A.G.; Lin, H.W. Programmed Stimuli-Responsive Carbon Dot-Nanogel Hybrids for Imaging-Guided Enhanced Tumor Phototherapy. ACS Appl. Mater. Interfaces 2022, 14, 10142–10153. [Google Scholar] [CrossRef]
- Wang, X.W.; Cheng, L. Multifunctional two-dimensional nanocomposites for photothermal-based combined cancer therapy. Nanoscale 2019, 11, 15685–15708. [Google Scholar] [CrossRef]
- Li, Q.X.; Liu, X.; Yan, C.M.; Zhao, B.L.; Zhao, Y.X.; Yang, L.; Shi, M.Y.; Yu, H.; Li, X.F.; Luo, K.P. Polysaccharide-Based Stimulus-Responsive Nanomedicines for Combination Cancer Immunotherapy. Small 2023, 19, e2206211. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, C.; Wang, Y.G.; Chen, H.B.; Zhang, X.X.; Luo, C.; Zhou, W.H.; Li, L.L.; Teng, L.S.; Yu, H.J.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Liu, B.; Liu, Z. Tumor microenvironment-responsive dynamic inorganic nanoassemblies for cancer imaging and treatment. Adv. Drug Deliv. Rev. 2021, 179, 114004. [Google Scholar] [CrossRef] [PubMed]
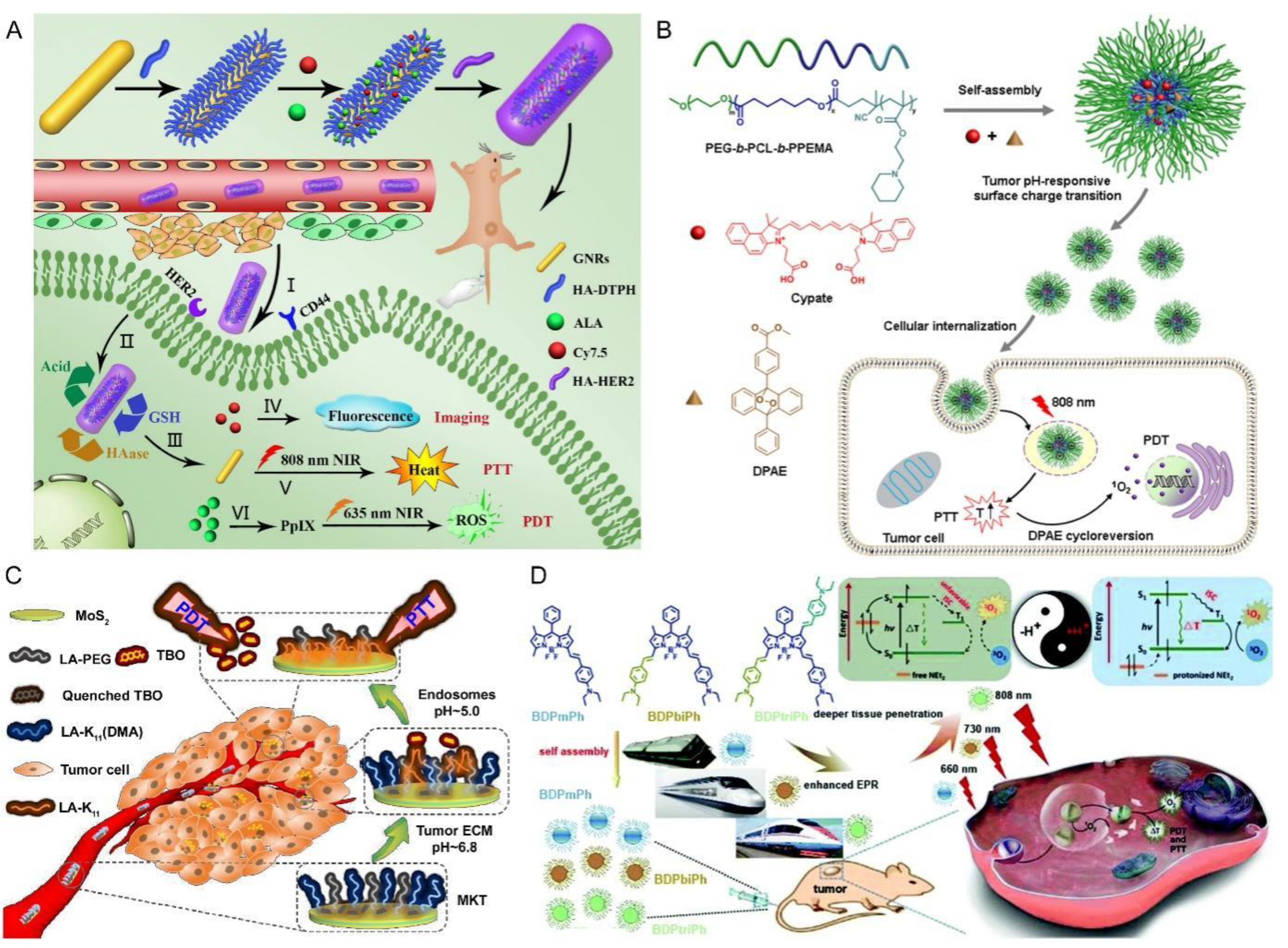
- Zhang, H.B.; Zhang, Y.B.; Chen, Y.L.; Zhang, Y.; Wang, Y.G.; Zhang, Y.Y.; Song, L.; Jiang, B.L.; Su, G.H.; Li, Y.; et al. Glutathione-responsive self-delivery nanoparticles assembled by curcumin dimer for enhanced intracellular drug delivery. Int. J. Pharm. 2018, 549, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Q.; Zhang, W.; Fan, G.R.; Zou, C.M.; Zhang, J.; Wu, N.; Ding, J.H.; Zou, W.Q.; Xiao, H.J.; Tan, S.W. Paclitaxel-loaded ROS-responsive nanoparticles for head and neck cancer therapy. Drug Deliv. 2023, 30, 2189106. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Bian, Y.L.; Xiao, X.; Liu, B.; Ding, B.B.; Cheng, Z.Y.; Ma, P.A.; Lin, J. Tumor Microenvironment-Responsive Cu/CaCO3-Based Nanoregulator for Mitochondrial Homeostasis Disruption-Enhanced Chemodynamic/Sonodynamic Therapy. Small 2022, 18, e2204047. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Wu, J.R.; He, M.Y.; Hou, X.Y.; Wang, Y.; Cai, X.R.; Xin, H.L.; Gao, F.; Chen, Y.Z. Combined Cancer Chemo-Photodynamic and Photothermal Therapy Based on ICG/PDA/TPZ-Loaded Nanoparticles. Mol. Pharm. 2019, 16, 2172–2183. [Google Scholar] [CrossRef]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, X.Q.; Sun, C.S.; Liu, Y.X.; Zhao, Q.F.; Wang, S.L. Mesoporous carbon-manganese nanocomposite for multiple imaging guided oxygen-elevated synergetic therapy. J. Control. Release 2020, 319, 104–118. [Google Scholar] [CrossRef]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, T.T.; Kirillov, A.M.; Liu, W.S.; Tang, Y. NIR light/H2O2-triggered nanocomposites for a highly efficient and selective synergistic photodynamic and photothermal therapy against hypoxic tumor cells. Chem. Commun. 2016, 52, 7939–7942. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.Q.; Wei, J.S.; Li, X.; Wang, H.; Zhao, Y.D. Intelligent gold nanostars for in vivo CT imaging and catalase-enhanced synergistic photodynamic & photothermal tumor therapy. Theranostics 2019, 9, 5424–5442. [Google Scholar] [PubMed]
- Wei, J.P.; Li, J.C.; Sun, D.; Li, Q.; Ma, J.Y.; Chen, X.L.; Zhu, X.; Zheng, N.F. A Novel Theranostic Nanoplatform Based on Pd@Pt-PEG-Ce6 for Enhanced Photodynamic Therapy by Modulating Tumor Hypoxia Microenvironment. Adv. Funct. Mater. 2018, 28, 1706310. [Google Scholar] [CrossRef]
- Chen, Q.; He, S.; Zhang, F.J.; Cui, F.Z.; Liu, J.H.; Wang, M.; Wang, D.M.; Jin, Z.G.; Li, C.X. A versatile Pt-Ce6 nanoplatform as catalase nanozyme and NIR-II photothermal agent for enhanced PDT/PTT tumor therapy. Sci. China Mater. 2021, 64, 510–530. [Google Scholar] [CrossRef]
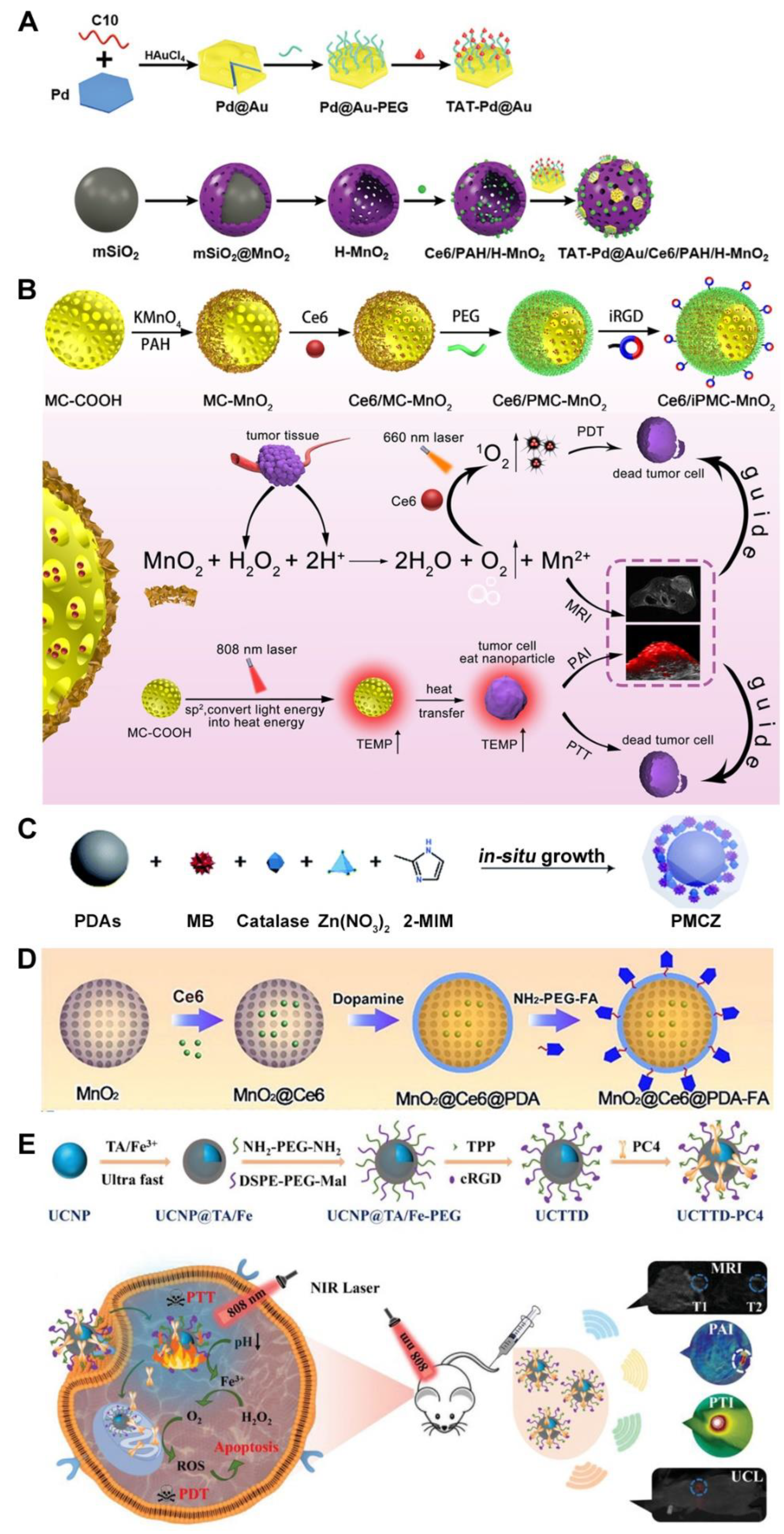
- Gu, W.X.; Hua, Z.Y.; Li, Z.; Cai, Z.H.; Wang, W.D.; Guo, K.J.; Yuan, F.; Gao, F.L.; Chen, H.L. Palladium cubes with Pt shell deposition for localized surface plasmon resonance enhanced photodynamic and photothermal therapy of hypoxic tumors. Biomater. Sci. 2021, 10, 216–226. [Google Scholar] [CrossRef]
- You, Q.; Zhang, K.Y.; Liu, J.Y.; Liu, C.L.; Wang, H.Y.; Wang, M.T.; Ye, S.Y.; Gao, H.Q.; Lv, L.T.; Wang, C.; et al. Persistent Regulation of Tumor Hypoxia Microenvironment via a Bioinspired Pt-Based Oxygen Nanogenerator for Multimodal Imaging-Guided Synergistic Phototherapy. Adv. Sci. 2020, 7, 1903341. [Google Scholar] [CrossRef]
- Wang, D.D.; Shi, R.H.; Zhou, J.J.; Shi, S.X.; Wu, H.H.; Xu, P.P.; Wang, H.; Xia, G.L.; Barnhart, T.E.; Cai, W.B.; et al. Photo-Enhanced Singlet Oxygen Generation of Prussian Blue-Based Nanocatalyst for Augmented Photodynamic Therapy. Iscience 2018, 9, 14. [Google Scholar] [CrossRef]
- Xu, P.P.; Wang, X.Y.; Li, T.W.; Wu, H.H.; Li, L.L.; Chen, Z.L.; Zhang, L.; Guo, Z.; Chen, Q.W. Biomineralization-inspired nanozyme for single-wavelength laser activated photothermal-photodynamic synergistic treatment against hypoxic tumors. Nanoscale 2020, 12, 4051–4060. [Google Scholar] [CrossRef]
- Wang, J.P.; Sun, J.Y.; Hu, W.; Wang, Y.H.; Chou, T.M.; Zhang, B.L.; Zhang, Q.; Ren, L.; Wang, H.J. A Porous Au@Rh Bimetallic Core-Shell Nanostructure as an H2O2-Driven Oxygenerator to Alleviate Tumor Hypoxia for Simultaneous Bimodal Imaging and Enhanced Photodynamic Therapy. Adv. Mater. 2020, 32, 2001862. [Google Scholar] [CrossRef]
- Ding, M.L.; Miao, Z.H.; Zhang, F.; Liu, J.; Shuai, X.T.; Zha, Z.B.; Cao, Z. Catalytic rhodium (Rh)-based (mesoporous polydopamine) MPDA nanoparticles with enhanced phototherapeutic efficiency for overcoming tumor hypoxia. Biomater. Sci. 2020, 8, 4157–4165. [Google Scholar] [CrossRef]
- Wu, J.R.; Niu, S.W.; Bremner, D.H.; Nie, W.; Fu, Z.; Li, D.J.; Zhu, L.M. A Tumor Microenvironment-Responsive Biodegradable Mesoporous Nanosystem for Anti-Inflammation and Cancer Theranostics. Adv. Healthc. Mater. 2020, 9, e1901307. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, Q.; Luo, K.Y.; Zhu, C.P.; Xie, M.M.; Wang, S.G.; Fei, Z.W.; Zhao, J.L. Synthesis of iridium-based nanocomposite with catalase activity for cancer phototherapy. J. Nanobiotech. 2021, 19, 203. [Google Scholar] [CrossRef]
- Xu, J.T.; Han, W.; Yang, P.P.; Jia, T.; Dong, S.M.; Bi, H.T.; Gulzar, A.; Yang, D.; Gai, S.L.; He, F.; et al. Tumor Microenvironment-Responsive Mesoporous MnO2-Coated Upconversion Nanoplatform for Self-Enhanced Tumor Theranostics. Adv. Funct. Mater. 2018, 28, 1803804. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.J.; Nie, W.; Zhang, Q.Q.; Wang, W.Z.; Zhang, Y.Z.; He, C.L. Multifunctional Redox-Responsive Mesoporous Silica Nanoparticles for Efficient Targeting Drug Delivery and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef]
- Ruan, Y.; Jia, X.; Wang, C.; Zhen, W.; Jiang, X. Mn-Fe layered double hydroxide nanosheets: A new photothermal nanocarrier for O2-evolving phototherapy. Chem. Commun. 2018, 54, 11729–11732. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Gou, H.L.; Liu, Y.F.; Xi, K.; Jiang, D.C.; Jia, X.D. pH-responsive PEG-chitosan/iron oxide hybrid nanoassemblies for low-power assisted PDT/PTT combination therapy. Nanomedicine 2020, 15, 1097–1112. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Cui, J.; Hao, J. Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 561–569. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.P.; Zhao, H.; Zha, Z.S.; Ke, W.D.; Wang, Y.H.; Ge, Z.S. Oxygen-independent combined photothermal/photodynamic therapy delivered by tumor acidity-responsive polymeric micelles. J. Control. Release 2018, 284, 15–25. [Google Scholar] [CrossRef]
- Wang, W.Y.; Li, Z.M.; Nie, X.Z.; Zeng, W.F.; Zhang, Y.; Deng, Y.M.; Chen, H.Z.; Zeng, X.W.; Ma, H.L.; Zheng, Y.; et al. pH-Sensitive and Charge-Reversal Polymeric Nanoplatform Enhanced Photothermal/Photodynamic Synergistic Therapy for Breast Cancer. Front. Bioeng. Biotechnol. 2022, 10, 836468. [Google Scholar] [CrossRef]
- Bejjanki, N.K.; Zhong, Y.; Liu, J.; Li, Q.M.; Xu, H.F.; Shen, H.; Xie, M.Q. Surface charge transition nano-theranostics based on ultra-small Fe3O4 nanoparticles for enhanced photodynamic and photothermal therapy against nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2021, 557, 240–246. [Google Scholar] [CrossRef]
- Yu, M.; Guo, F.; Wang, J.P.; Tan, F.P.; Li, N. Photosensitizer-Loaded pH-Responsive Hollow Gold Nanospheres for Single Light-Induced Photothermal/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 17592–17597. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.Y.; Zheng, D.W.; Wang, S.B.; Cheng, S.X.; Zhang, X.Z. Multifunctional Nanosystem for Synergistic Tumor Therapy Delivered by Two-Dimensional MoS2. ACS Appl. Mater. Interfaces 2017, 9, 13965–13975. [Google Scholar] [CrossRef]
- Zou, J.H.; Wang, P.; Wang, Y.; Liu, G.Y.; Zhang, Y.W.; Zhang, Q.; Shao, J.J.; Si, W.L.; Huang, W.; Dong, X.C. Penetration depth tunable BODIPY derivatives for pH triggered enhanced photothermal/photodynamic synergistic therapy. Chem. Sci. 2019, 10, 268–276. [Google Scholar] [CrossRef]
- Singh, V.V.; Kaufmann, K.; de Avila, B.E.F.; Karshalev, E.; Wang, J. Molybdenum Disulfide-Based Tubular Microengines: Toward Biomedical Applications. Adv. Funct. Mater. 2016, 26, 6270–6278. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lv, F.; Cheng, Y.R.; Yuan, Z.P.; Yang, F.; Liu, C.H.; Cao, Y.; Zhang, K.; Lu, H.T.; Zada, S.; et al. Pd@Au Bimetallic Nanoplates Decorated Mesoporous MnO2 for Synergistic Nucleus-Targeted NIR-II Photothermal and Hypoxia-Relieved Photodynamic Therapy. Adv. Healthc. Mater. 2020, 9, e1901528. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, H.H.; Sun, Z.Q.; Wang, B.; Tang, X.Y.; Dai, Y.N.; Li, M.X.; Shen, Q.M.; Zhang, H.; Fan, Q.L.; et al. Endogenous oxygen generating multifunctional theranostic nanoplatform for enhanced photodynamic-photothermal therapy and multimodal imaging. Theranostics 2019, 9, 7697–7713. [Google Scholar] [CrossRef]
- Zheng, W.J.; Zhou, Z.L.; Lv, Q.Y.; Song, X.J.; Zhang, W.X.; Cui, H.F. Oxygen-Generated Hierarchical-Structured AuNRs@MnO2@SiO2 Nanocarrier for Enhanced NIR- and H2O2-Responsive Mild-Hyperthermia Photodynamic/Photothermal Combined Tumor Therapy. Adv. Ther. 2022, 5, 2200108. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, T.; Sun, Q.; Kuang, Y.; Liu, B.; Xu, M.; Zhu, H.; He, F.; Gai, S.; Yang, P. Construction of Bi/phthalocyanine manganese nanocomposite for trimodal imaging directed photodynamic and photothermal therapy mediated by 808 nm light. Biomaterials 2020, 228, 119569. [Google Scholar] [CrossRef]
- Feng, J.; Yu, W.Q.; Xu, Z.; Wang, F. An intelligent ZIF-8-gated polydopamine nanoplatform for in vivo cooperatively enhanced combination phototherapy. Chem. Sci. 2020, 11, 1649–1656. [Google Scholar] [CrossRef]
- Zeng, W.W.; Zhang, H.J.; Deng, Y.M.; Jiang, A.T.; Bao, X.Y.; Guo, M.Q.; Li, Z.M.; Wu, M.Y.; Ji, X.Y.; Zeng, X.W.; et al. Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. Chem. Eng. J. 2020, 389, 124494. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Ren, J.H.; Ba, L.; He, W.S.; Wong, C.Y.E. Multifunctional oxygen-enriching nano-theranostics for cancer-specific magnetic resonance imaging and enhanced photodynamic/photothermal therapy. Nano Res. 2020, 13, 1389–1398. [Google Scholar] [CrossRef]
- Li, X.Y.; Ji, Q.; Yan, C.; Zhu, Z.Y.; Yan, Z.H.; Chen, P.; Wang, Y.S.; Song, L. H2O2/pH Dual-Responsive Biomimetic Nanoenzyme Drugs Delivery System for Enhanced Tumor Photodynamic Therapy. Nanoscale Res. Lett. 2022, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.N.; Liu, D.M.; Yu, C.; Niu, N.; Li, D.; Wang, J.; Wang, E.K. Tumor Microenvironment Stimuli-Responsive Single-NIR-Laser Activated Synergistic Phototherapy for Hypoxic Cancer by Perylene Functionalized Dual-Targeted Upconversion Nanoparticles. Adv. Sci. 2022, 9, e2203292. [Google Scholar] [CrossRef]
- Liu, X.; Tian, K.; Zhang, J.; Zhao, M.; Liu, S.; Zhao, Q.; Huang, W. Smart NIR-Light-Mediated Nanotherapeutic Agents for Enhancing Tumor Accumulation and Overcoming Hypoxia in Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1225–1232. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, X.D.; Wang, D.D.; Zhang, K.; Dai, W.H.; Dong, H.F.; Zhang, X.J. Intelligent MnO2/Cu2-xS for Multimode Imaging Diagnostic and Advanced Single-Laser Irradiated Photothermal/Photodynamic Therapy. ACS Appl. Mater. Interfaces. 2018, 10, 17732–17741. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.Z.; Chen, C.; Wang, D.D.; Wu, T.T.; Dong, H.F.; Zhang, X.J. Rattle-type Au@Cu2-xS hollow mesoporous nanocrystals with enhanced photothermal efficiency for intracellular oncogenic microRNA detection and chemo-photothermal therapy. Biomaterials 2018, 158, 23–33. [Google Scholar] [CrossRef]
- Ji, M.W.; Xu, M.; Zhang, W.; Yang, Z.Z.; Huang, L.; Liu, J.J.; Zhang, Y.; Gu, L.; Yu, Y.X.; Hao, W.C.; et al. Structurally Well-Defined Au@Cu2-xS Core-Shell Nanocrystals for Improved Cancer Treatment Based on Enhanced Photothermal Efficiency. Adv. Mater. 2016, 28, 3094–3101. [Google Scholar] [CrossRef]
- Satyavolu, N.S.; Tan, L.H.; Lu, Y. DNA-Mediated Morphological Control of Pd-Au Bimetallic Nanoparticles. J. Am. Chem. Soc. 2016, 138, 16542–16548. [Google Scholar] [CrossRef]
- Ding, X.G.; Liow, C.H.; Zhang, M.X.; Huang, R.J.; Li, C.Y.; Shen, H.; Liu, M.Y.; Zou, Y.; Gao, N.; Zhang, Z.J.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Li, A.; Li, X.; Yu, X.J.; Li, W.; Zhao, R.Y.; An, X.; Cui, D.X.; Chen, X.Y.; Li, W.W. Synergistic thermoradiotherapy based on PEGylated Cu3BiS3 ternary semiconductor nanorods with strong absorption in the second near-infrared window. Biomaterials 2017, 112, 164–175. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.; Gong, K.K.; Wang, J.; Ge, X.X.; Liu, X.Q.; Guo, S.J.; Wang, F. MnO2-Laden Black Phosphorus for MRI-Guided Synergistic PDT, PTT, and Chemotherapy. Matter 2019, 1, 496–512. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Cheng, Z.J.; Yang, N.; Li, Q.Z.; Wang, P.; Chen, D.P.; Wang, W.J.; Song, X.J.; Dong, X.C. Hydrangea-structured tumor microenvironment responsive degradable nanoplatform for hypoxic tumor multimodal imaging and therapy. Biomaterials 2019, 205, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, M.Y.; Li, R.H.; Zhou, L.L.; Wang, C.H.; Ye, P.T.; Hu, X.C.; Yang, J.X.; Sun, Y.T.; Zhu, Z.N.; et al. Biodegradable oxygen-producing manganese-chelated metal organic frameworks for tumor-targeted synergistic chemo/photothermal/photodynamic therapy. Acta Biomater. 2022, 138, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wang, D.G.; Yu, H.J.; Wang, M.W.; Liu, J.P.; Feng, B.; Zhou, F.Y.; Yin, Q.; Zhang, Z.W.; Huang, Y.Z.; et al. Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug-Resistant Tumor. Acs Nano 2016, 10, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.X.; Chen, Z.; Zhang, F.; Wang, Q.; Guo, W.; Wang, K.; Lin, H.M.; Qu, F.Y. Construct of MoSe2/Bi2Se3 nanoheterostructure: Multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials 2019, 217, 119282. [Google Scholar] [CrossRef]
- Tan, H.S.; Liu, Y.L.; Hou, N.; Cui, S.S.; Liu, B.; Fan, S.S.; Yu, G.P.; Han, C.; Zheng, D.C.; Li, W.Z.; et al. Tumor microenvironment pH-responsive pentagonal gold prism-based nanoplatform for multimodal imaging and combined therapy of castration-resistant prostate cancer. Acta Biomater. 2022, 141, 408–417. [Google Scholar] [CrossRef]
- Li, R.T.; Zhu, Y.D.; Li, W.Y.; Hou, Y.K.; Zou, Y.M.; Zhao, Y.H.; Zou, Q.; Zhang, W.H.; Chen, J.X. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core-shell AuNS@NMOFs nanocomposite encapsulated with gambogic acid. J. Nanobiotech. 2022, 20, 212. [Google Scholar] [CrossRef]
- Xu, W.J.; Qian, J.M.; Hou, G.H.; Wang, Y.P.; Wang, J.L.; Sun, T.T.; Ji, L.J.; Suo, A.L.; Yao, Y. PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomater. 2018, 82, 171–183. [Google Scholar] [CrossRef]
- Cheng, D.; Ji, Y.J.; Wang, B.; Wang, Y.Y.; Tang, Y.; Fu, Y.; Xu, Y.F.; Qian, X.H.; Zhu, W.P. Dual-responsive nanohybrid based on degradable silica-coated gold nanorods for triple-combination therapy for breast cancer. Acta Biomater. 2021, 128, 435–446. [Google Scholar] [CrossRef]
- Peng, Y.T.; Cheng, L.H.; Luo, C.Y.; Xiong, F.Q.; Wu, Z.P.; Zhang, L.; Zhan, P.; Shao, L.S.; Luo, W.H. Tumor microenvironment-responsive nanosystem achieves reactive oxygen species self-cycling after photothermal induction to enhance efficacy of antitumor therapy. Chem. Eng. J. 2023, 463, 142370. [Google Scholar] [CrossRef]
- Hu, X.C.; Lu, Y.L.; Shi, X.K.; Yao, T.M.; Dong, C.Y.; Shi, S. Integrating in situ formation of nanozymes with mesoporous polydopamine for combined chemo, photothermal and hypoxia-overcoming photodynamic therapy. Chem. Commun. 2019, 55, 14785–14788. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Lin, H.M.; Qu, F.Y. Hollow CuS nanocube as nanocarrier for synergetic chemo/photothermal/photodynamic therapy. Mater. Sci. Eng. C 2019, 96, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhen, W.Y.; Jin, L.H.; Zhang, S.T.; Sun, G.Y.; Zhang, T.Q.; Xu, X.; Song, S.Y.; Wang, Y.H.; Liu, J.H.; et al. All-in-One Theranostic Nanoagent with Enhanced Reactive Oxygen Species Generation and Modulating Tumor Microenvironment Ability for Effective Tumor Eradication. Acs Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.Y.; Ke, H.T.; Ding, D.W.; Deng, Y.B.; Chen, H.B. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Song, J.B.; Nie, L.M.; Chen, X.Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Tian, H.; Liu, L.L.; Chen, Z.K.; Liang, R.J.; Chen, Z.; Wu, Z.H.; Ma, A.Q.; Zheng, M.B.; Cai, L.T. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584–3596. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Cheng, H.; Jiang, C.X.; Qiu, X.F.; Wang, K.K.; Huan, W.; Yuan, A.; Wu, J.H.; Hu, Y.Q. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef]
- Liu, C.H.; Cao, Y.; Cheng, Y.R.; Wang, D.D.; Xu, T.L.; Su, L.; Zhang, X.J.; Dong, H.F. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat. Commun. 2020, 11, 1735. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent advances in strategies for overcoming hypoxia in photodynamic therapy of cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar]


| PS | Photothermal Agent | H2O2-Decomposition Entity | Function/Purpose | Tumor Model In Vitro | Tumor Model In Vivo | Ref. |
|---|---|---|---|---|---|---|
| MB | APMs | CAT | Controlled Release and hypoxia relief | PC-3 cells | / | [40] |
| Ce6 | GNS | CAT | Hypoxia relief, in vivo CT imaging | HeLa and MCF-7 cells | BALB/c nude mice with HeLa tumors | [41] |
| Ce6 | Pd@Pt | Pt | Hypoxia relief and tumor imaging | 4T1 cells | Balb/c mice with 4T1 tumors | [42] |
| Ce6 | PTAs | CAT | Hypoxia relief | HeLa cells | Balb/c mice with U14 tumors | [43] |
| Pt | Pd@Pt | Pt | Hypoxia relief | LM8 and L929 cells | Female Balb/c mice with LM8 tumors | [44] |
| ICG | GNSs | Pt | Hypoxia relief | 4T1 cells | Mice with 4T1 tumors | [45] |
| ZnPc | PB | PB | Hypoxia relief | 4T1 cells | Mice with 4T1 tumors | [46] |
| IR808-Br2 | RuO2@BSA | RuO2@BSA | Hypoxia relief and NIRF imaging | 4T1 cells | Mice with 4T1 tumors | [47] |
| ICG | Rh | Au@Rh | Hypoxia relief | MDA-MB-231 cells | Balb/c nude mice with MDA-MB-231 tumors | [48] |
| Ce6 | MPDAand Rh NPs | Rh NPs | Hypoxia relief | 4T1 cells | Female BALB/c mice with 4T1 tumors | [49] |
| BSA-IrO2 | BSA-IrO2 | BSA-IrO2 | Hypoxia relief and low-temperature PTT | L929 cells | Mice with MDA-MB-231 tumors | [50] |
| Ce6 | PDA and IrO2 NPs | IrO2 | Hypoxia relief | HT29 cells | Balb/c nude mice with HT29 tumors | [51] |
| PS | Photothermal Agent | pH-Responsive Entity | Function/Purpose | Tumor Model In Vitro | Tumor Model In Vivo | Reference |
|---|---|---|---|---|---|---|
| MB | IONPs | Chitosan. | Controlled drug release | HeLa, A549 and MCF-7 cells | BALB/c nude mice with A549 tumors | [55] |
| ALA | GNRs | hydrazone bonds | Controlled drug release | MCF-7 cells | Mice with MCF-7 tumors | [56] |
| ICG | ICG | DMMA | Enhanced cellular uptake | HeLa cells | / | [57] |
| DPAE | Cypate | C/O@N-Micelle | Enhanced tumor accumulation and improved cellular uptake | 4T1 cells | BALB/c mice with 4T1 tumors | [58] |
| Ce6 | PDA | PAH-DMMA | Enhanced cellular uptake | MCF-7 cells | / | [59] |
| ICG | ICG | PEG-b-PAEMA-DMA | Enhanced cellular uptake | HNE-1 cells | BALB/c mice with HNE-1 tumors | [60] |
| CDs | CDs | MAA | Enhanced cellular uptake | 4T1 and MCF-7 cells | Nude mice with 4T1 tumors | [28] |
| Ce6 | HAuNS | pHLIP | Drug release and cellular uptake | Hela cells | Nude mice with Hela tumors | [61] |
| TBO | MoS2 | LA-K11 (DMA) | Drug release and cellular uptake | SCC-7 cells | BALB/c nude mice with SCC-7 tumors | [62] |
| BDPmPh, BDPbiPh and BDPtriPh NPs | BDPmPh, BDPbiPh and BDPtriPh NPs | Diethylamino groups | Low pH-activated phototherapy | HeLa cells | Nude mice with HeLa tumors | [63] |
| PS | Photothermal Agent | pH-Responsive Entity | H2O2-Responsive Entity | Function/Purpose | Tumor Model In Vitro | Tumor Model In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| Ce6 | Pd@Au nanoplates | MnO2 | MnO2 | Hypoxia relief | MCF-7 cells | BALB/c mice with MCF-7 tumors | [66] |
| Ce6 | MC | MnO2 | MnO2 | Hypoxia relief | 4T1 cells | BALB/c mice with 4T1 tumors | [38] |
| Ce6 | Au/Ag alloy | MnO2 | MnO2 | Hypoxia relief | HeLa cells | Mice with HeLa tumors | [67] |
| ICG | AuNRs | MnO2 | MnO2 | Modulate the tumor microenvironment | MCF-7 cells | BALB/c mice with 4T1 tumors | [68] |
| MnPcE4 | Bi | MnO2 | Mn2+ | Hypoxia relief | HeLa cells | Kunming mice with U14 tumors | [69] |
| MB | PDA | ZIF-8 | CAT | Hypoxia relief and lowpH-triggered drug release | HeLa cells | Mice with HeLa tumors | [70] |
| Ce6 | MnO2@Ce6@PDA-FA NPs | PDA | MnO2 | Hypoxia relief and low pH-triggered drug release | MCF-7 and NP69 cells | BALB/c nude mice with MCF-7 tumors | [71] |
| Ce6 | CuS | PDA | MnO2 | Hypoxia relief and low pH-triggered drug release | 4T1 cells | BALB/c mice with 4T1 tumors | [72] |
| ICG | ICG | ZIF-8 | MnO2 | Hypoxia relief and low pH-triggered drug release | 4T1 cells | BALB/c mice with 4T1 tumors | [73] |
| PC4 | TA/Fe3+ nanofilms | UCTTD | Fe3+ | Hypoxia relief and low pH-triggered drug release | Capan-1 cells | BALB/c-nude mice with Capan-1 tumors | [74] |
| IR780 | IR780 | MnO2 | MnO2 | Hypoxia relief and enhanced intratumoral drug penetration | HepG2 and 3T3 cells | Nude mice with HepG2 tumors | [75] |
| / | Cu2–xS | MnO2 | MnO2 | Hypoxia relief | A549 and MCF-7 cells | Female BALB/c nude mice with B16 tumors | [76] |
| / | Au@Cu2−xS | Au@Cu2−xS/DOX-PEG | / | Drug delivery | MCF-7 cells | BALB/c nude mice with A549 tumors | [77] |
| PS | Photothermal Agent | Chemotherapeutic Drug | TME Involved | Entities Responsive to TME | Purpose | Tumor Model In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| BPNs | BPN/MnO2 | DOX | High level of H2O2, low pH | MnO2 | Oxygen generation for hypoxia relief and PDT enhancement, controlled drug release | Mice with HeLa tumors | [82] |
| aza-BODIPY | aza-BODIPY | DOX | High level of H2O2, low pH | MnO2 | Hypoxia relief and drug release | / | [83] |
| PCN-224(Mn) | PDA | Iniparib | High level of H2O2 | Mn-TCPP | Hypoxia relief, drug release and PDT enhancement | BALB/c nude mice with MDA-MB-231 tumors | [84] |
| Ce6 | PDPC micelles | PDOX | Low pH | PEG-b-PDPA | Controlled drug release | Nude mice with MCF-7/ADR tumors | [85] |
| MoSe2 | Bi2Se3/MoSe2 | DOX | Low pH | Bi-M-3@PEG-Dox | Controlled drug release | Mice with U14 tumors | [86] |
| IR820 | IR820 | DTX | Low pH | CaCO3 | Controlled drug release | PC-3 xenograft tumor-bearing nude mice | [87] |
| TCPP | AuNS | GA | Low pH | ZrTCPP | Controlled drug release | BALB/c mice with 4T1 tumors | [88] |
| PpIX | GNRs | DOX | Low pH | MPH | Controlled drug release | BALB/c nude mice with MCF-7 tumors | [89] |
| IR820 | IR820 | DOX | GSH and HAase | HA and organosilica | Controlled drug release | Nude mice with 4T1 tumors | [90] |
| IR825 | IR825 | RESV | ROS | MND-IR@RESV | Enhanced cellular uptake | Nude mice with U14 tumors | [91] |
| ICG | PDA | TPZ | Low pH | CaCO3 | Controlled drug release | Mice with U87MG tumors | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Yao, Y.; Xue, S.; Zhang, M.; Li, D.; Xu, T.; Zhi, F.; Liu, Y.; Ding, D. Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers. Pharmaceutics 2023, 15, 2480. https://doi.org/10.3390/pharmaceutics15102480
Liu K, Yao Y, Xue S, Zhang M, Li D, Xu T, Zhi F, Liu Y, Ding D. Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers. Pharmaceutics. 2023; 15(10):2480. https://doi.org/10.3390/pharmaceutics15102480
Chicago/Turabian StyleLiu, Kehan, Yao Yao, Shujuan Xue, Mengyao Zhang, Dazhao Li, Tao Xu, Feng Zhi, Yang Liu, and Dawei Ding. 2023. "Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers" Pharmaceutics 15, no. 10: 2480. https://doi.org/10.3390/pharmaceutics15102480
APA StyleLiu, K., Yao, Y., Xue, S., Zhang, M., Li, D., Xu, T., Zhi, F., Liu, Y., & Ding, D. (2023). Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers. Pharmaceutics, 15(10), 2480. https://doi.org/10.3390/pharmaceutics15102480





