Inflammation-Related Immune-Modulatory SLURP1 Prevents the Proliferation of Human Colon Cancer Cells, and Its Delivery by Salmonella Demonstrates Cross-Species Efficacy against Murine Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Construction of Rough ST Mutant Secreting Human SLURP1 Protein
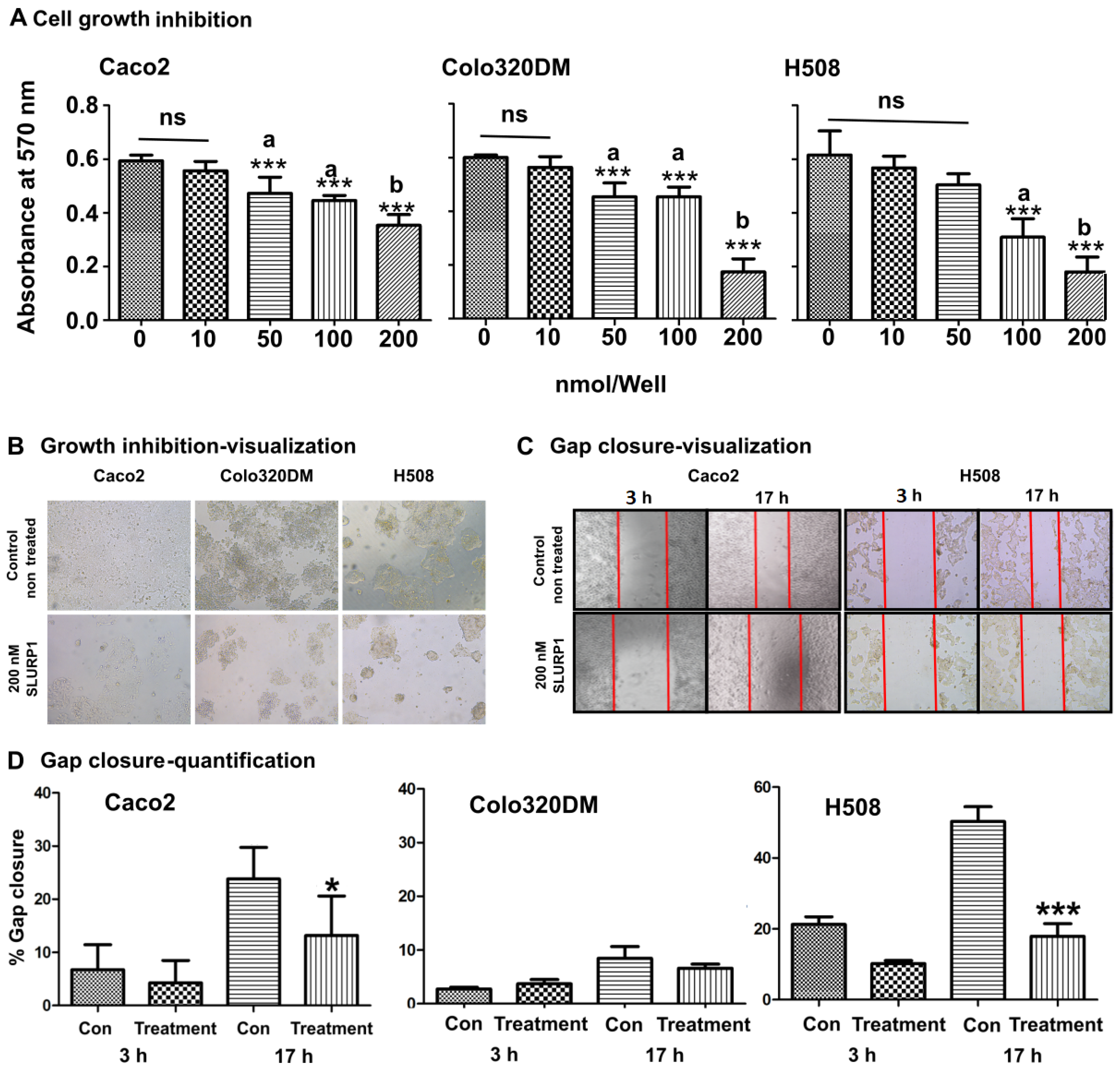
2.3. SLURP1-Derived Cancer Cell Proliferation Inhibition
2.4. Scratch Wound Healing
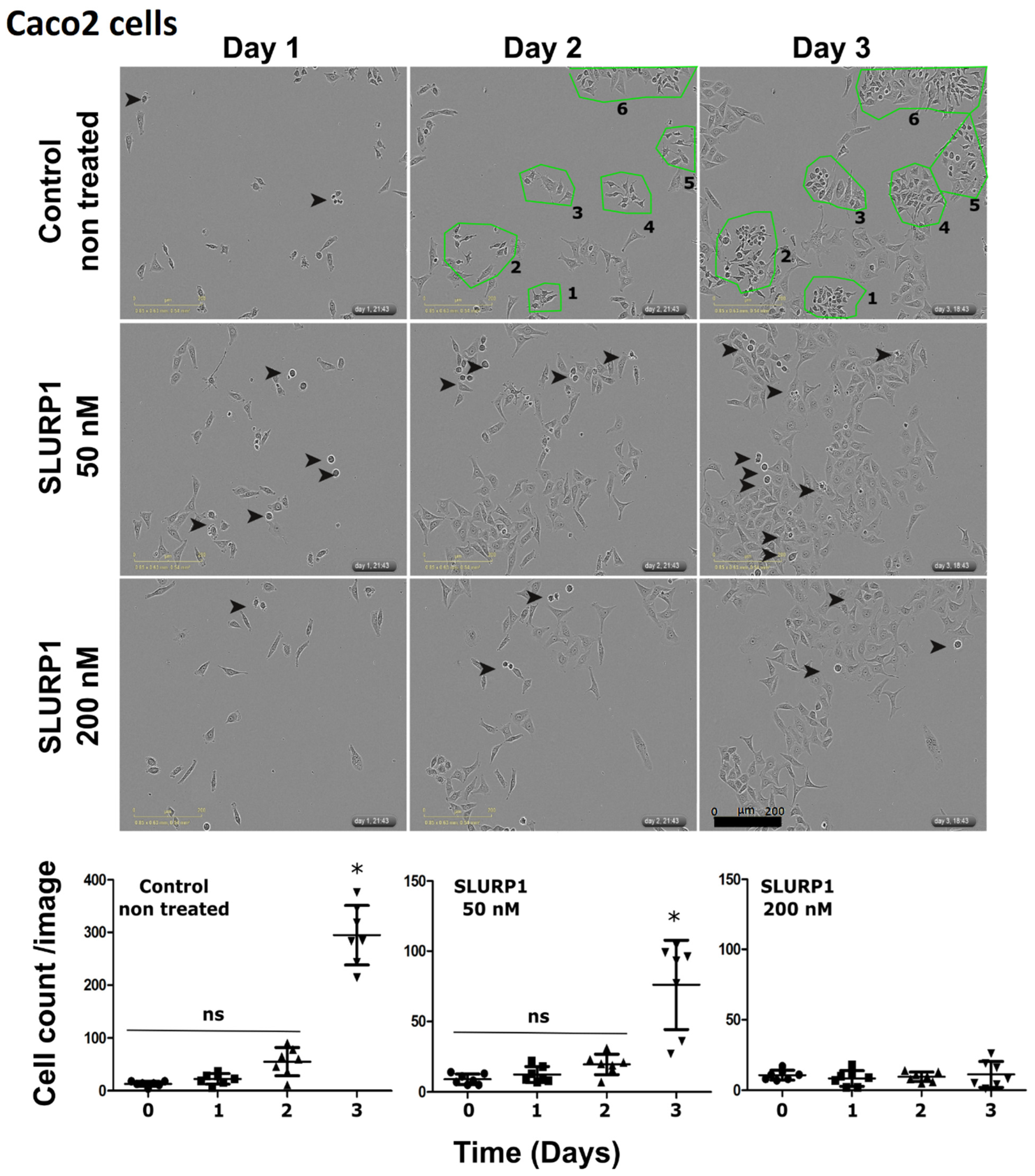
2.5. SLURP1-Induced Morphological Changes in Cancer Cells
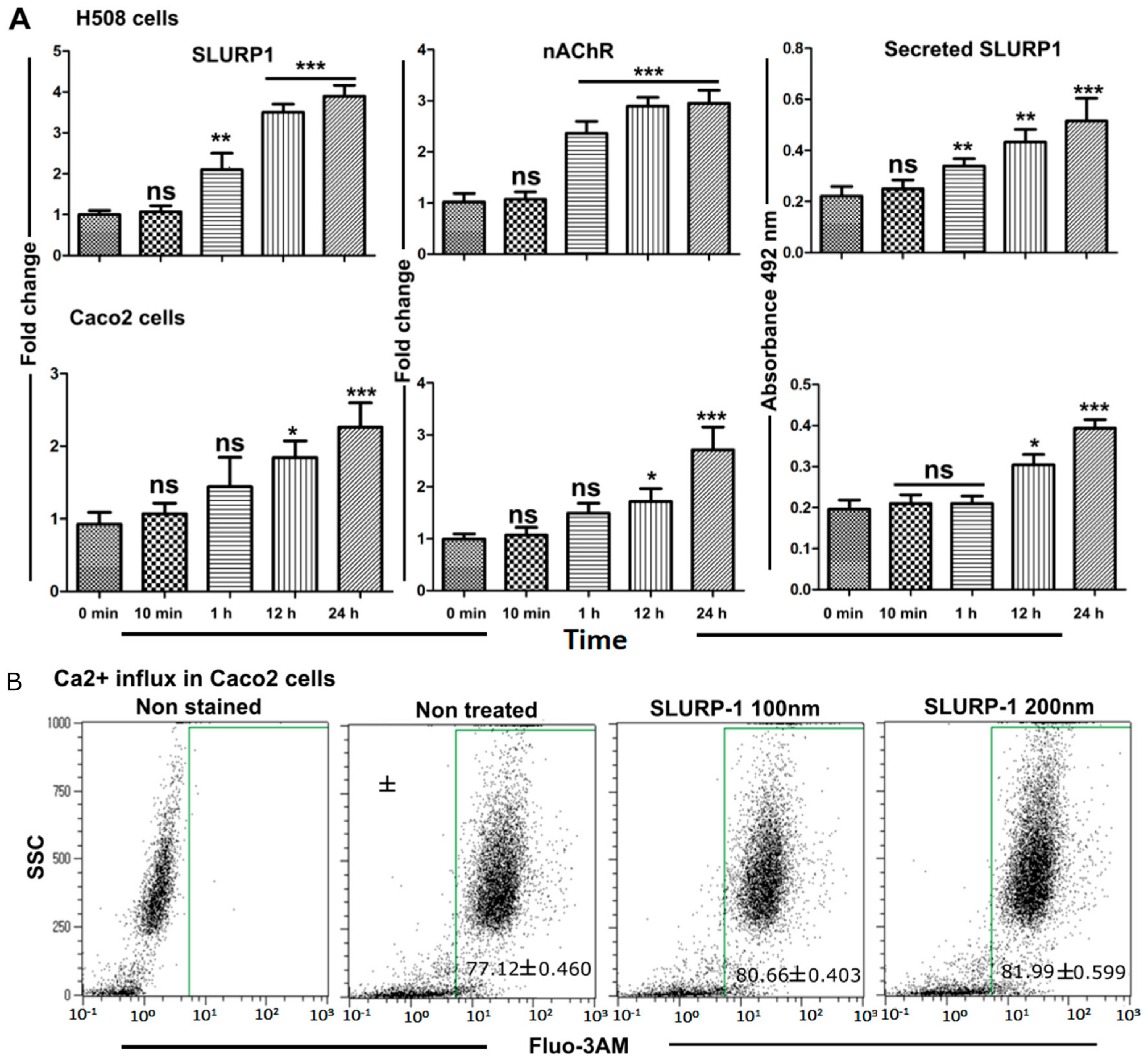
2.6. Effect of rSLURP1 on Endogenous Expression
2.7. Calcium Influx Assay
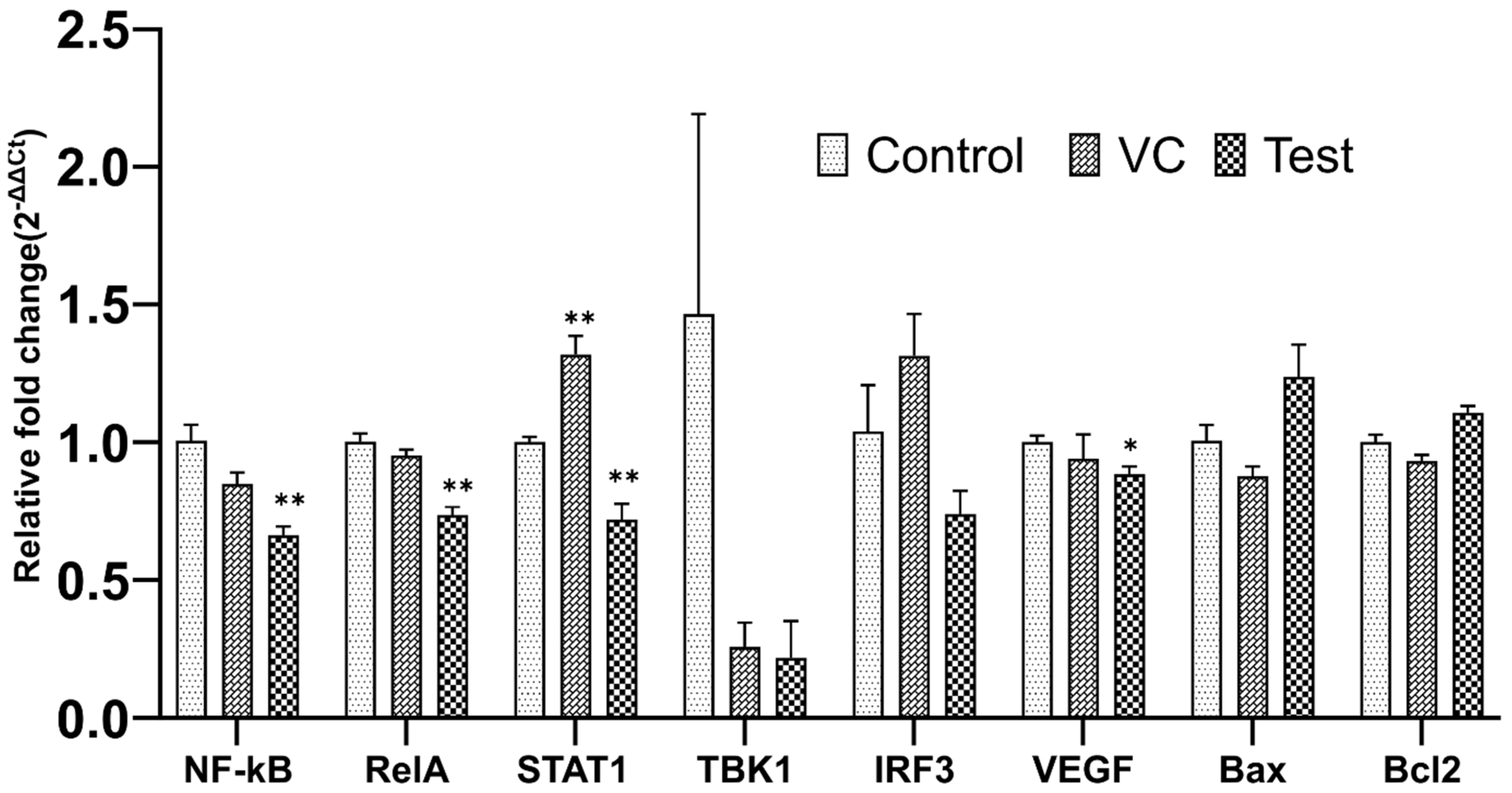
2.8. qRT-PCR Assay
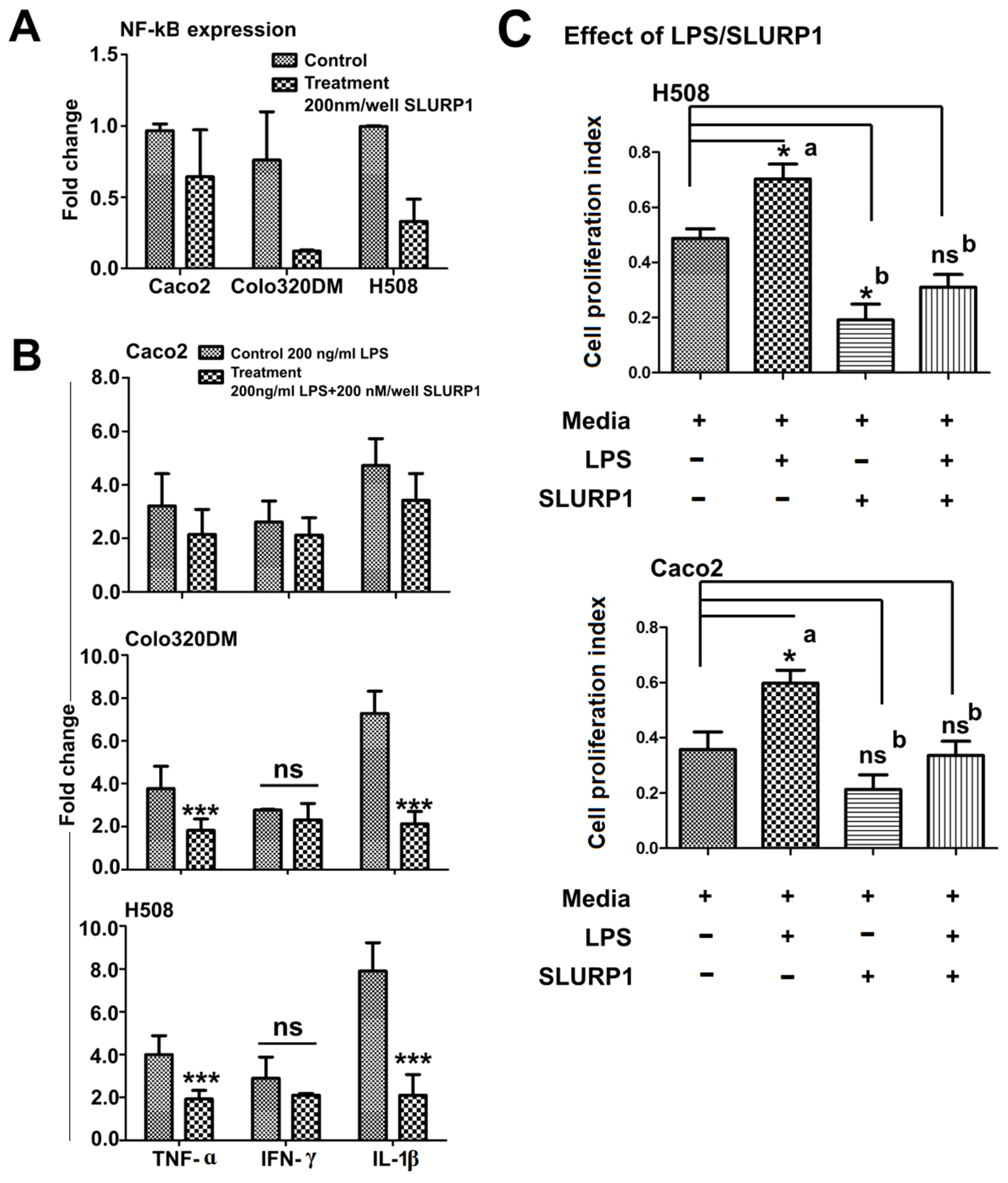
2.9. Effect of SLURP1 under Inflammation
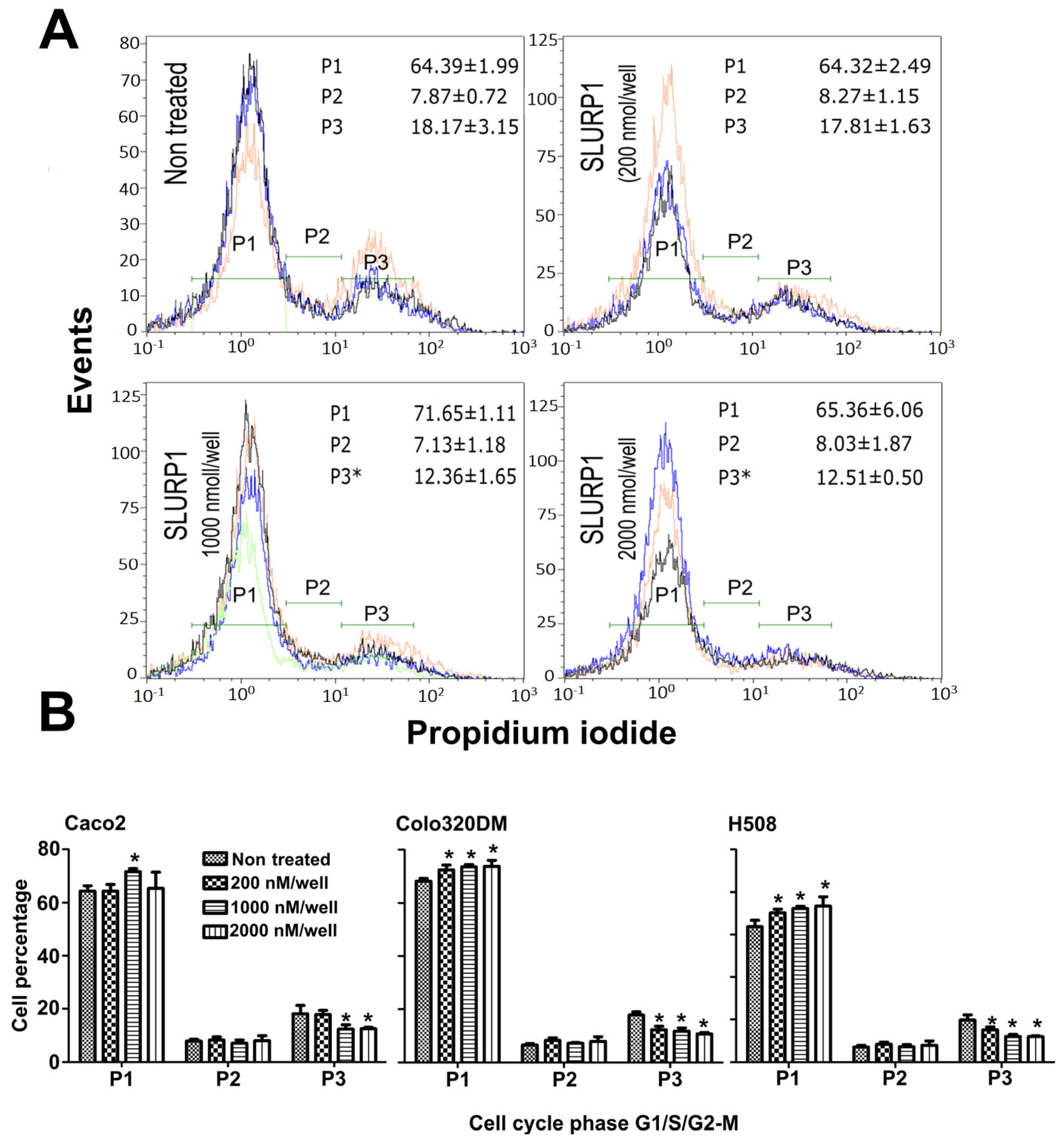
2.10. SLURP1-Induced Cell Cycle Analysis
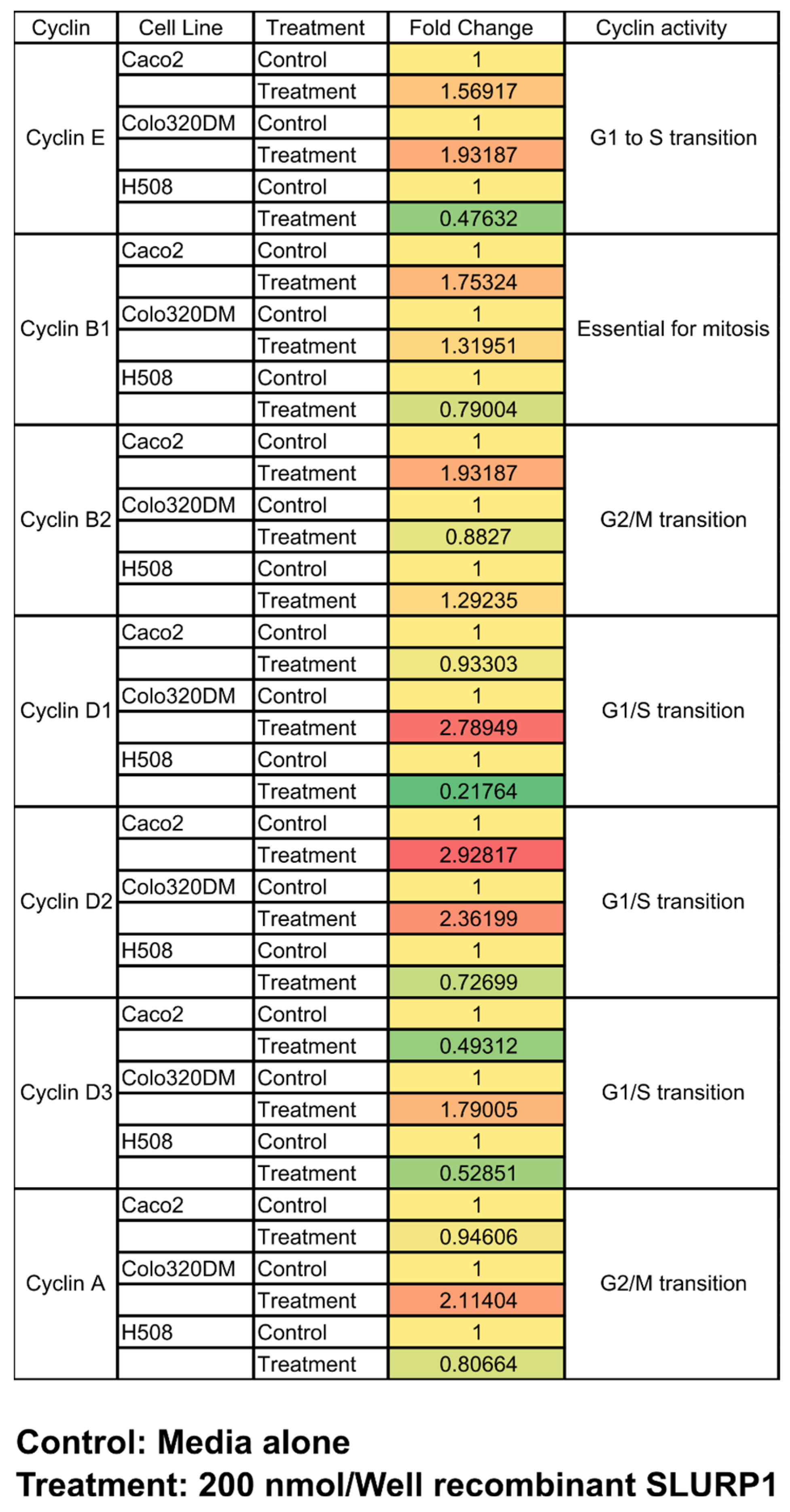
2.11. SLURP1 Effect on Cell Cyclins
2.12. Western Blot
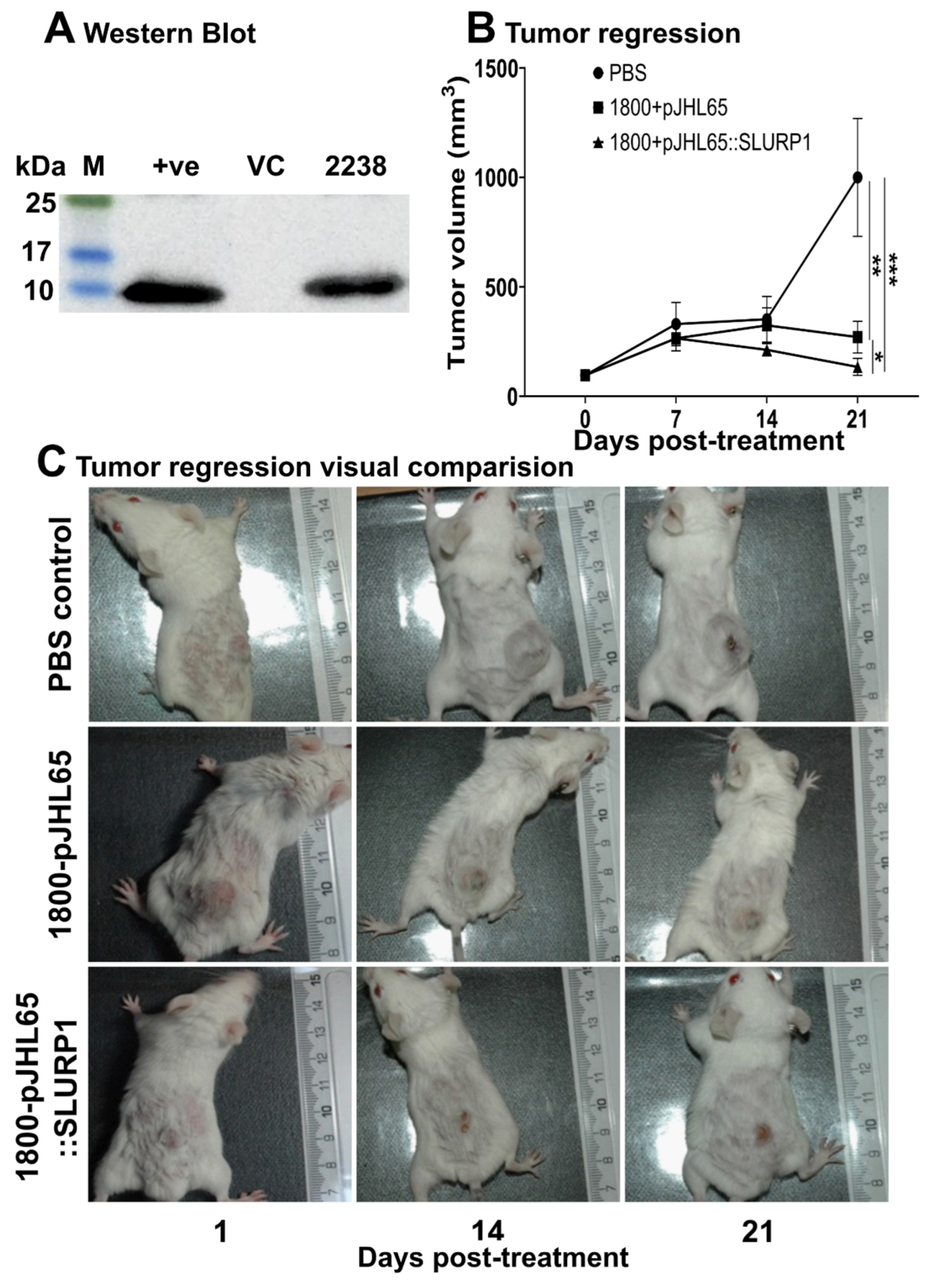
2.13. Anti-Tumor Effect in Mouse Model
2.14. Statistical Analysis
3. Results
3.1. SLURP1 Inhibits Cell Proliferation
3.2. SLURP1’s Effect on Cancer Cell Morphology and Proliferation
3.3. Effect of Recombinant SLURP1 on Endogenous Expression
3.4. SLURP1-Induced Anti-Inflammatory Responses
3.5. SLURP1-Induced Cell Cycle Arrest
3.6. qRT-PCR Analysis
3.7. Tumor Regression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J. Transl. Med. 2011, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Bychkov, M.L.; Sharonov, G.V.; Efremenko, A.V.; Shulepko, M.A.; Kulbatskii, D.S.; Shenkarev, Z.O.; Feofanov, A.V.; Dolgikh, D.A.; Kirpichnikov, M.P. Human secreted proteins SLURP-1 and SLURP-2 control the growth of epithelial cancer cells via interactions with nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1973–1986. [Google Scholar] [CrossRef]
- Swamynathan, S.; Swamynathan, S.K. SLURP-1 modulates corneal homeostasis by serving as a soluble scavenger of urokinase-type plasminogen activator. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6251–6261. [Google Scholar] [CrossRef]
- Dass, K.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Sarkar, F.H. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008, 34, 122–136. [Google Scholar] [CrossRef]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef]
- Adermann, K.; Wattler, F.; Wattler, S.; Heine, G.; Meyer, M.; Forssmann, W.G.; Nehls, M. Structural and phylogenetic characterization of human SLURP-1, the first secreted mammalian member of the Ly-6/uPAR protein superfamily. Protein Sci. 1999, 8, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, F.; Hogg, R.C.; Plantard, L.; Lehmann, C.; Brakch, N.; Fischer, J.; Huber, M.; Bertrand, D.; Hohl, D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum. Mol. Genet. 2003, 12, 3017–3024. [Google Scholar] [CrossRef]
- Castro, N.G.; Albuquerque, E.X. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J. 1995, 68, 516–524. [Google Scholar] [CrossRef]
- Favre, B.; Plantard, L.; Aeschbach, L.; Brakch, N.; Christen-Zaech, S.; de Viragh, P.A.; Sergeant, A.; Huber, M.; Hohl, D. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda. J. Investig. Dermatol. 2007, 127, 301–308. [Google Scholar] [CrossRef]
- Fischer, J.; Bouadjar, B.; Heilig, R.; Huber, M.; Lefevre, C.; Jobard, F.; Macari, F.; Bakija-Konsuo, A.; Ait-Belkacem, F.; Weissenbach, J.; et al. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum. Mol. Genet. 2001, 10, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, R.; Donini, S.; Kelton, C.A.; Chaomei, H.; Bressan, A.; Milazzo, F.; Ciolli, V.; Borrelli, F.; Martelli, F.; Biffoni, M. ARS Component B: Structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda. Eur. J. Dermatol. 2003, 13, 560–570. [Google Scholar]
- Swamynathan, S.; Delp, E.E.; Harvey, S.A.; Loughner, C.L.; Raju, L.; Swamynathan, S.K. Corneal Expression of SLURP-1 by Age, Sex, Genetic Strain, and Ocular Surface Health. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7888–7896. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Watanabe, Y.; Shinagawa, T.; Kai, M.; Miyazawa, M.; Okuda, T.; Kawashima, K.; Yabashi, A.; Waguri, S.; Misawa, H. Primary sensory neuronal expression of SLURP-1, an endogenous nicotinic acetylcholine receptor ligand. Neurosci. Res. 2009, 64, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Throm, V.M.; Mannle, D.; Giese, T.; Bauer, A.S.; Gaida, M.M.; Kopitz, J.; Bruckner, T.; Plaschke, K.; Grekova, S.P.; Felix, K.; et al. Endogenous CHRNA7-ligand SLURP1 as a potential tumor suppressor and anti-nicotinic factor in pancreatic cancer. Oncotarget 2018, 9, 11734–11751. [Google Scholar] [CrossRef]
- Ray, R.; Schnoll, R.A.; Lerman, C. Nicotine dependence: Biology, behavior, and treatment. Annu. Rev. Med. 2009, 60, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Takaya, A.; Suzuki, M.; Matsui, H.; Tomoyasu, T.; Sashinami, H.; Nakane, A.; Yamamoto, T. Lon, a stress-induced ATP-dependent protease, is critically important for systemic Salmonella enterica serovar typhimurium infection of mice. Infect. Immun. 2003, 71, 690–696. [Google Scholar] [CrossRef]
- Humphreys, S.; Rowley, G.; Stevenson, A.; Anjum, M.F.; Woodward, M.J.; Gilbert, S.; Kormanec, J.; Roberts, M. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect. Immun. 2004, 72, 4654–4661. [Google Scholar] [CrossRef]
- Hur, J.; Lee, J.H. Immune responses to new vaccine candidates constructed by a live attenuated Salmonella Typhimurium delivery system expressing Escherichia coli F4, F5, F6, F41 and intimin adhesin antigens in a murine model. J. Vet. Med. Sci. 2011, 73, 1265–1273. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Hajam, I.A.; Lalsiamthara, J.; Lee, J.H. Intranasally administered anti-Brucella subunit vaccine formulation induces protective immune responses against nasal Brucella challenge. Vet. Microbiol. 2019, 228, 112–118. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, K.; Lim, D.; Jeong, K.; Hong, Y.; Nguyen, V.H.; Kim, T.H.; Ryu, S.; Lim, J.A.; Kim, J.I.; et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS ONE 2014, 9, e80050. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Gurusinghe, S.; Chen, H.; Chen, S.; Wang, L.X.; Lim, S.Y.; Strappe, P. Minicircle DNA-mediated endothelial nitric oxide synthase gene transfer enhances angiogenic responses of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 48. [Google Scholar] [CrossRef]
- McGuffee, L.J.; Little, S.A.; Mercure, J.V.; Skipper, B.J.; Wheeler-Clark, E.S. Effects of temperature and buffer composition on calcium sequestration by sarcoplasmic reticulum and plasma membrane of rabbit renal artery. Anat. Rec. 1990, 228, 288–296. [Google Scholar] [CrossRef]
- Sui, H.; Luo, M.; Miao, Y.; Cheng, W.; Wen, S.; Zhao, B.; Li, Y.; Qiao, Z.; Liu, Y.; Xu, C. Cystic fibrosis transmembrane conductance regulator ameliorates lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/AKT/mTOR pathway in mice. Respir. Physiol. Neurobiol. 2020, 273, 103338. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Galitovskiy, V.; Qian, J.; Grando, S.A. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am. J. Physiol. Cell Physiol. 2010, 299, C903–C911. [Google Scholar] [CrossRef]
- Pradel, N.; Delmas, J.; Wu, L.F.; Santini, C.L.; Bonnet, R. Sec- and Tat-dependent translocation of beta-lactamases across the Escherichia coli inner membrane. Antimicrob. Agents Chemother. 2009, 53, 242–248. [Google Scholar] [CrossRef]
- McCann, J.R.; McDonough, J.A.; Pavelka, M.S.; Braunstein, M. Beta-lactamase can function as a reporter of bacterial protein export during Mycobacterium tuberculosis infection of host cells. Microbiology 2007, 153, 3350–3359. [Google Scholar] [CrossRef]
- Selas Castineiras, T.; Williams, S.G.; Hitchcock, A.; Cole, J.A.; Smith, D.C.; Overton, T.W. Development of a generic beta-lactamase screening system for improved signal peptides for periplasmic targeting of recombinant proteins in Escherichia coli. Sci. Rep. 2018, 8, 6986. [Google Scholar] [CrossRef]
- Egleton, R.D.; Brown, K.C.; Dasgupta, P. Nicotinic acetylcholine receptors in cancer: Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol. Sci. 2008, 29, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Alsharari, S.D.; Freitas, K.; Damaj, M.I. Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: Studies in transgenic mice. Biochem. Pharmacol. 2013, 86, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, J.; Chernyavsky, A.I.; Marubio, L.M.; Beaudet, A.L.; Jolkovsky, D.L.; Pinkerton, K.E.; Grando, S.A. Receptor-mediated tobacco toxicity: Regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells. Am. J. Pathol. 2005, 166, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, J.; Chernyavsky, A.I.; Grando, S.A. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol. Ther. 2006, 5, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shulepko, M.A.; Bychkov, M.L.; Shenkarev, Z.O.; Paramonov, A.S.; Chugunov, A.O.; Arseniev, A.S.; Dolgikh, D.A.; Kirpichnikov, M.P. Human SLURP-1 and SLURP-2 Proteins Acting on Nicotinic Acetylcholine Receptors Reduce Proliferation of Human Colorectal Adenocarcinoma HT-29 Cells. Acta Naturae 2014, 6, 60–66. [Google Scholar] [CrossRef]
- Grozio, A.; Paleari, L.; Catassi, A.; Servent, D.; Cilli, M.; Piccardi, F.; Paganuzzi, M.; Cesario, A.; Granone, P.; Mourier, G.; et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: A promising prospective in anti-cancer drug development. Int. J. Cancer 2008, 122, 1911–1915. [Google Scholar] [CrossRef]
- Arredondo, J.; Chernyavsky, A.I.; Grando, S.A. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J. Cancer Res. Clin. Oncol. 2006, 132, 653–663. [Google Scholar] [CrossRef]
- Wu, C.H.; Lee, C.H.; Ho, Y.S. Nicotinic acetylcholine receptor-based blockade: Applications of molecular targets for cancer therapy. Clin. Cancer Res. 2011, 17, 3533–3541. [Google Scholar] [CrossRef]
- Uteshev, V.V. alpha7 nicotinic ACh receptors as a ligand-gated source of Ca(2+) ions: The search for a Ca(2+) optimum. Adv. Exp. Med. Biol. 2012, 740, 603–638. [Google Scholar] [CrossRef]
- Dang, N.; Meng, X.; Song, H. Nicotinic acetylcholine receptors and cancer. Biomed. Rep. 2016, 4, 515–518. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.S.; Chen, C.S.; Tu, S.H.; Wang, Y.J.; Chang, Y.J.; Tam, K.W.; Wei, P.L.; Cheng, T.C.; Chu, J.S.; et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010, 102, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Sanner, T.; Grimsrud, T.K. Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment—A Review. Front. Oncol. 2015, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, J.; Chernyavsky, A.I.; Webber, R.J.; Grando, S.A. Biological effects of SLURP-1 on human keratinocytes. J. Investig. Dermatol. 2005, 125, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.C.; Ooi, J.; Goonewardene, M.S. A novel molecule, SLURP-1, enhances the survival of periodontal ligament fibroblasts. J. Periodontal Res. 2010, 45, 331–336. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Qian, J.; Galitovskiy, V.; Grando, S.A. Coupling of ionic events to protein kinase signaling cascades upon activation of alpha7 nicotinic receptor: Cooperative regulation of alpha2-integrin expression and Rho kinase activity. J. Biol. Chem. 2009, 284, 22140–22148. [Google Scholar] [CrossRef]
- Scheuer, T.; Auld, V.J.; Boyd, S.; Offord, J.; Dunn, R.; Catterall, W.A. Functional properties of rat brain sodium channels expressed in a somatic cell line. Science 1990, 247, 854–858. [Google Scholar] [CrossRef]
- Shen, J.X.; Yakel, J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Zhou, Z.; Liu, Y.; Bai, Y.; Xing, X.; Wang, X. Nicotine induces the production of IL-1beta and IL-8 via the alpha7 nAChR/NF-kappaB pathway in human periodontal ligament cells: An in vitro study. Cell Physiol. Biochem. 2014, 34, 423–431. [Google Scholar] [CrossRef]
- Lau, P.P.; Li, L.; Merched, A.J.; Zhang, A.L.; Ko, K.W.; Chan, L. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor(−/−) mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 143–149. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Galitovskiy, V.; Shchepotin, I.B.; Grando, S.A. Anti-inflammatory effects of the nicotinergic peptides SLURP-1 and SLURP-2 on human intestinal epithelial cells and immunocytes. Biomed. Res. Int. 2014, 2014, 609086. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Yoshikawa, K.; Fukuda, H.; Fujii, Y.X.; Misawa, H.; Kawashima, K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007, 80, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Nylund, G.; Khorram-Manesh, A.; Nordgren, S.; Delbro, D.S. Nicotine induced modulation of SLURP-1 expression in human colon cancer cells. Auton. Neurosci. 2009, 148, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Y.; Zhang, Z.; Sun, X. Advances in Salmonella Typhimurium-based drug delivery system for cancer therapy. Adv. Drug Deliv. Rev. 2022, 185, 114295. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Pan, Z.; Zheng, L.; Li, N.; Danielpour, D.; Ma, J.J. Overexpression of Bax sensitizes prostate cancer cells to TGF-beta induced apoptosis. Cell Res. 2005, 15, 160–166. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef]
- Swamynathan, S.; Loughner, C.L.; Swamynathan, S.K. Inhibition of HUVEC tube formation via suppression of NFkappaB suggests an anti-angiogenic role for SLURP1 in the transparent cornea. Exp. Eye Res. 2017, 164, 118–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senevirathne, A.; Aganja, R.P.; Hewawaduge, C.; Lee, J.H. Inflammation-Related Immune-Modulatory SLURP1 Prevents the Proliferation of Human Colon Cancer Cells, and Its Delivery by Salmonella Demonstrates Cross-Species Efficacy against Murine Colon Cancer. Pharmaceutics 2023, 15, 2462. https://doi.org/10.3390/pharmaceutics15102462
Senevirathne A, Aganja RP, Hewawaduge C, Lee JH. Inflammation-Related Immune-Modulatory SLURP1 Prevents the Proliferation of Human Colon Cancer Cells, and Its Delivery by Salmonella Demonstrates Cross-Species Efficacy against Murine Colon Cancer. Pharmaceutics. 2023; 15(10):2462. https://doi.org/10.3390/pharmaceutics15102462
Chicago/Turabian StyleSenevirathne, Amal, Ram Prasad Aganja, Chamith Hewawaduge, and John Hwa Lee. 2023. "Inflammation-Related Immune-Modulatory SLURP1 Prevents the Proliferation of Human Colon Cancer Cells, and Its Delivery by Salmonella Demonstrates Cross-Species Efficacy against Murine Colon Cancer" Pharmaceutics 15, no. 10: 2462. https://doi.org/10.3390/pharmaceutics15102462
APA StyleSenevirathne, A., Aganja, R. P., Hewawaduge, C., & Lee, J. H. (2023). Inflammation-Related Immune-Modulatory SLURP1 Prevents the Proliferation of Human Colon Cancer Cells, and Its Delivery by Salmonella Demonstrates Cross-Species Efficacy against Murine Colon Cancer. Pharmaceutics, 15(10), 2462. https://doi.org/10.3390/pharmaceutics15102462







